Abstract
Dengue virus (DENV) is the principal arthropod-borne viral pathogen afflicting human populations. While repertoires of antibodies to DENV have been linked to protection or enhanced infection, the role of T lymphocytes in these processes remains poorly defined. This study provides a comprehensive overview of CD4+ and CD8+ T cell epitope reactivities against the DENV 2 proteome in adult patients experiencing secondary DENV infection. Dengue virus-specific T cell responses directed against an overlapping 15mer peptide library spanning the DENV 2 proteome were analyzed ex vivo by enzyme-linked immunosorbent spot assay, and recognition of individual peptides was further characterized in specific T cell lines. Thirty novel T cell epitopes were identified, 9 of which are CD4+ and 21 are CD8+ T cell epitopes. We observe that whereas CD8+ T cell epitopes preferentially target nonstructural proteins (NS3 and NS5), CD4+ epitopes are skewed toward recognition of viral components that are also targeted by B lymphocytes (envelope, capsid, and NS1). Consistently, a large proportion of dengue virus-specific CD4+ T cells have phenotypic characteristics of circulating follicular helper T cells (CXCR5 expression and production of interleukin-21 or gamma interferon), suggesting that they are interacting with B cells in vivo. This study shows that during a dengue virus infection, the protein targets of human CD4+ and CD8+ T cells are largely distinct, thus highlighting key differences in the immunodominance of DENV proteins for these two cell types. This has important implications for our understanding of how the two arms of the human adaptive immune system are differentially targeted and employed as part of our response to DENV infection.
INTRODUCTION
Dengue virus (DENV) belongs to the Flaviviridae family, is transmitted by infected mosquitoes, and cocirculates as four infectious serotypes (DENV 1 to 4) that are endemic to more than 100 countries worldwide (1). DENV infection can cause a range of clinical symptoms, from asymptomatic to self-limiting fever or severe and often fatal manifestations, termed dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Immunity to DENV is serotype specific, thus secondary infections are common in areas where multiple serotypes cocirculate (2). The reported association between secondary infections and severe disease strongly implicates the host immune response in dengue virus pathology. While antibodies have been linked to protection and enhanced infection (3, 4), the role of T cells in protection versus immune pathology remains poorly defined.
Previous studies of mice lacking the alpha/beta interferon receptor (IFN-α/β R−/−) have indicated an important protective role of CD8+ T cells during primary and secondary heterotypic dengue virus infections (5). In contrast, CD4+ T cells were dispensable in these mice during primary DENV infections but contributed significantly to viral clearance when induced by immunization (6). However, a study based on a dengue virus patient cohort suggested that human CD8+ memory T cells play a role in the pathogenesis of DHF during secondary infections in a process termed original antigenic sin (7). This concept implies that a secondary DENV infection is dominated by the proliferation of cross-reactive memory cells generated during the primary response. Because these cells have a lower affinity for the secondary infecting virus, they are unable to control this infection but may contribute to the cytokine storm that is proposed to underlie dengue virus immunopathology.
The role of CD4+ T cells in human dengue virus infections is unclear. DENV-specific CD4+ T cells have been characterized principally in individuals who received live attenuated DENV vaccines. After in vitro expansion, these cells displayed a Th1 phenotype and high proliferative and cytotoxic potential (8–10). In addition, DENV-specific CD4+ T cells from vaccinated volunteers displayed an altered cytokine profile toward heterologous viral serotypes with a higher ratio of tumor necrosis factor alpha (TNF-α) to IFN-γ production. The data presented in this study support a possible role of CD4+ T cells in immunopathology during secondary heterologous infections (11).
The genome of DENV is composed of a single-stranded RNA of 10.7 kb in length that is translated into a single polypeptide and is subsequently cleaved into the constituent viral proteins. These include two surface glycoproteins (envelope [E] and premembrane [preM/M]) that mediate host cell attachment/fusion, one capsid protein (C) that forms the nucleocapsid in association with the RNA genome, and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) that regulate viral replication.
A comprehensive overview of T cell epitope reactivities during clinical dengue virus infection is needed to understand the impact and role of T cells in protection and/or pathogenesis. Previous studies aimed at identifying DENV T cell epitopes have focused on specific viral proteins as opposed to the entire DENV proteome (12, 13). A recent study identified DENV-specific T cell epitopes across 9 out of 10 DENV proteins. Peptides were designed based on predictive binding algorithms to chosen human HLA types and tested both in HLA-transgenic mouse models and human peripheral blood mononuclear cells (PBMCs) (14). The only comprehensive study to date profiled the T cell response to the entire DENV genome and focused on defining immunodominance of total viral proteins, therefore it did not provide information on specific T cell epitope recognition (15).
CD4+ and CD8+ T lymphocytes have been shown to play a critical role in other acute viral infections. While virus-specific CD8+ T cells are important for viral clearance, CD4+ T cells are required for the elicitation of protective antibody responses and for the generation of both B cell and CD8+ T cell memory responses (16), and they can also directly kill virus-infected cells through expression of IFN-γ and cytotoxic effector functions (17). The B cell helper activity of CD4+ T cells resides within a subset of cells expressing CXCR5, named follicular helper cells (TFH). These are localized in B cell germinal centers, where they promote B cell differentiation and production of high-affinity, class-switched antibodies (18, 19). CXCR5 also identifies a subset of circulating CCR7+ central memory T cells (20, 21) that was recently shown to provide B cell help through production of IL-21, thus representing the circulating counterpart of TFH cells (22, 23).
In this report, we identify 30 new DENV-specific T cell epitopes in a proteome-wide epitope screen. Our results show that during a dengue virus infection, CD8+ T cells mainly target nonstructural proteins (NS3 and NS5), while CD4+ T cells preferentially target proteins recognized by B cells (E, C, and NS1). A proportion of DENV-specific CD4+ T cells displayed a phenotype characteristic of circulating TFH cells, suggesting that they are interacting with B cells in vivo. The key differences in immunodominance of dengue virus-specific CD4+ and CD8+ T cell responses highlighted by these results are discussed.
MATERIALS AND METHODS
Ethics statement.
Human blood samples were obtained after written informed consent from all participants. The study was conducted in accordance with the Declaration of Helsinki and approved by the Singapore National Healthcare Group ethical review board (DSRB 2008/00293).
Patients.
Blood samples were collected from patients with confirmed dengue virus diagnosis in EDTA Vacutainer tubes at three different time points from fever onset: acute (day 4 to 8), postfebrile (day 14 to 21), and convalescent (day 60 to 120). Diagnosis was confirmed on the basis of either detection of DENV RNA by reverse transcription-PCR (RT-PCR) or of NS1 antigen by enzyme-linked immunosorbent assay (ELISA) (Bio-Rad). Some patients with positive IgM and IgG acute serology (Panbio Dengue Duo Cassette) were also included if they fulfilled the World Health Organization (24) criteria for probable dengue virus. This includes having a compatible clinical syndrome consisting of fever with any two of the following manifestations: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, and leukopenia. These parameters were evaluated by clinicians of the dengue virus team at Tan Tock Seng Hospital in Singapore. To further confirm and characterize the DENV infection, we performed DENV serotype-specific IgG/IgM ELISA in acute, postfebrile, and convalescent plasma samples (using an in-house ELISA kit) and plaque reduction neutralization assays (PRNT) for all serotypes on postfebrile plasma samples (data not shown). Secondary infections were defined as a DENV 2-specific IgM/IgG ratio of <1.8 in paired acute and convalescent plasma samples, as assessed by IgM and IgG capture ELISA. According to this definition, seven patients were classified as primary infections (Table 1) (24). All patients were classified as nonsevere dengue based on the 2009 WHO guidelines (24). Patient details are provided in Tables 1 and 4. Healthy individuals seronegative for DENV and with no clinical history of dengue virus infection were recruited from laboratory staff.
Table 1.
Characteristics of the dengue virus patient cohort used for the epitope reactivity study
| Patient no. | Age (yr) | Gender | Ethnicity | HLA type | Type of infectiona | Severe dengue virusb (WS/NWS) |
|---|---|---|---|---|---|---|
| 09 | 36 | Male | Indian | A*2402 A*3101-B*4006 B*5501-Cw*1502 Cw*0102 | Secondary | No (WS) |
| 11 | 42 | Male | Indian | A*1101 A*0101-B*1501 B*0702-Cw*0401 Cw*0702 | Unknown | No (NWS) |
| 13 | 24 | Male | Bangladeshi | A*6801 A*0301-B*3503 B*3503-Cw*0401 Cw*1203 | Secondary | No (NWS) |
| 14 | 41 | Male | Chinese | A*1101 A*3303-B*5701 B*4403-Cw*1403 Cw*0602 | Secondary | No (WS) |
| 15 | 59 | Female | Chinese | A*1101 A*1101-B*4060 B*4001-Cw*0702 Cw*0102 | Secondary | No (WS) |
| 16 | 45 | Male | Chinese | A*1102 A*3101-B*2704 B*3511-Cw*1202 Cw*0401 | Secondary | No (WS) |
| 17 | 30 | Female | Chinese | A*1101 A*0201-B*4001 B*1511-Cw*0702 Cw*0303 | Primary | No (NWS) |
| 18 | 42 | Male | Chinese | A*0203 A*3303-B*3802 B*5801-Cw*0302 Cw*0702 | Secondary | No (WS) |
| 24 | 26 | Male | Malay | A*0101 A*0101-B*5701 B*3701-Cw*0602 Cw*0602 | Unknown | No (WS) |
| 25 | 38 | Male | Chinese | A*1101 A*2402-B*5102 B*5801-Cw*0302 Cw*1502 | Secondary | No (WS) |
| 26 | 24 | Male | Chinese | A*1101 A*1101-B*4601 B*1502-Cw*0801 Cw*0102 | Secondary | No (WS) |
| 27 | 27 | Male | Chinese | A*2901 A*2402-B*4601 B*0702-Cw*1505 Cw*0102 | Primary | No (WS) |
| 28 | 39 | Female | Eurasian | A*0201A*3303-B*4001B *5801-Cw*0302 Cw*0702 | Secondary | No (WS) |
| 29 | 32 | Male | Chinese | A*3303 A*2402-B*5801 B*5101-Cw*1402 Cw*0302 | Secondary | No (WS) |
| 32 | 33 | Male | Chinese | A*1101 A*2402-B*1302 B*4002-Cw*0304 Cw*0602 | Secondary | No (NWS) |
| 33 | 25 | Male | Chinese | A*0101 A*1101-B*3901 B*5701-Cw*0602 Cw*0702 | Secondary | No (WS) |
| 35 | 34 | Male | Chinese | A*1101 A*2402-B*1512 B*5201-Cw*0303 Cw*1202 | Secondary | No (NWS) |
| 38 | 48 | Male | Chinese | A*0101 A*3001-B*1302 B*3701-Cw*0602 Cw*0602 | Secondary | No (NWS) |
| 39 | 46 | Male | Chinese | A*2402- A*240-B*3505 B*5401-Cw*0102 Cw*0401 | Secondary | No (WS) |
| 43 | 40 | Male | Indian | A*0101 A*0101-B*3701 B*5501-Cw*0102 Cw*0602 | Secondary | No (WS) |
| 47 | 32 | Male | Bangladeshi | A*0301 A*1101-B*3501 B*5201-Cw*0401 Cw*0501 | Primary | No (NWS) |
| 92 | 27 | Male | Indian | A*0301 A*1101-B*3501 B*3503-Cw*0401 Cw*1203 | Primary | No (WS) |
| 96 | 32 | Male | Chinese | A*0203 A*3001-B*1302 B*4001-Cw*0403 Cw*0602 | Primary | No (NWS) |
| 97 | 28 | Male | Chinese | A*1102 A*3101-B*1518 B*5401-Cw*0102 Cw*0801 | Primary | No (NWS) |
| 118 | 21 | Male | Chinese | A*0301 A*2601-B*0801 B*1801-Cw*0702 Cw*1203 | Primary | No (NWS) |
Secondary infections were defined as a DENV 2-specific IgM/IgG ratio of <1.8 as assessed by capture ELISA.
Cases were classified according to the revised WHO guidelines (24). WS, with warning signs; NWS, without warning signs.
Table 4.
Characteristics of the dengue virus patient cohort used for the CXCR5 experiments
| Patient no. | Age (yr) | Gender | Ethnicity | Severe DENV statusa |
|---|---|---|---|---|
| 08 | 52 | Male | Chinese | NWS |
| 37 | 29 | Male | Chinese | WS |
| 47 | 32 | Male | Bangladeshi | NWS |
| 49 | 27 | Male | Indian | NWS |
| 52 | 59 | Male | Chinese | NWS |
| 53 | 40 | Male | Chinese | NWS |
| 190 | 53 | Female | Malay | ND |
| 192 | 33 | Female | Chinese | ND |
Cases were classified according to the 2009 WHO guidelines (24). WS, with warning signs; NWS, without warning signs; ND, not determined.
Synthetic peptide library.
Peptides were designed based on the sequence of DENV 2 virus (strain D2/SG/05K4155DK1/2005) (25) and were purchased from Mimotopes (Australia). The peptide library consists of 660 15mer peptides overlapping by 10 amino acids and spanning the entire DENV 2 sequence. The purity of the peptides was above 80%, and their composition was confirmed by mass spectrometry analysis. Peptides were pooled in a matrix so that each individual peptide was present in 2 different pools as described previously (26). Proteins are indicated as capsid (C or CAP), envelope (E or Env), precursor-membrane/membrane (preM/M), and nonstructural proteins 1 to 5 (NS 1 to 5). CAP and M were pooled in a 9-by-6 matrix containing 6, 8, or 9 peptides/pool; E was pooled in a 10-by-10 matrix containing 10 peptides/pool; NS1, NS2A/B, and NS4A/B were pooled in a 9-by-8 matrix containing 9 or 8 peptides/pool; NS3 was pooled in a 12-by-11 matrix containing 11 or 12 peptides; and NS5 was pooled in a 14-by-13 matrix containing 14 or 13 peptides/pool. All peptides were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 40 mg/ml, and intermediate working dilutions were performed in RPMI supplemented with 200 U/ml penicillin and 200 μg/ml streptomycin.
PBMC isolation and T cell culture.
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood by Ficoll gradient purification and cryopreserved. Cells were thawed on the day of the experiment and were used directly for the ex vivo assay. For the in vitro assays, T cell lines were generated as follows. Twenty percent of PBMCs were pulsed with 10 μg/ml of the overlapping DENV peptides for 1 h at 37°C, subsequently washed, and cocultured with the remaining cells in AIM-V medium (Gibco) supplemented with 2% AB human serum (Gibco). T cell lines were cultured for 10 days in the presence of 20 U/ml of recombinant interleukin-2 (IL-2; R&D Systems).
ICS.
T cell lines were left unstimulated or were stimulated with the peptide of interest (5 μg/ml) for 5 h at 37°C in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich). To assess degranulation, CD107a fluorescein isothiocyanate (FITC) antibody was added to the cells at the beginning of the stimulation. Cells were surface stained with anti-CD3 peridinin chlorophyll protein (PerCP) CY5.5 and anti-CD8 PE-CY7 and then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Pharmingen). Cells were then stained with anti-IFN-γ phycoerythrin (PE) and analyzed on a BD-Canto or Fortessa FACS Scan. For the CXCR5 experiments, thawed ex vivo PBMCs were used. Twenty percent of cells were stimulated with the total peptide pools (10 μg/ml) for 1 h at 37°C, washed, and cocultured with the remaining cells for 5 h in the presence of brefeldin A. As a positive control, cells were stimulated with anti-CD3/28 beads (Invitrogen) according to the manufacturer's instructions. Cells were surface stained with anti-CD3 Pacific blue, anti-CD4 V500, anti-CD45RA PerCP CY5.5 (eBioscience), and anti-CXCR5 biotin, followed by PE- or allophycocyanin (APC)-conjugated streptavidin. In most experiments the NIR viability stain (Invitrogen) was included. Intracellular staining (ICS) was performed with anti-CD40L FITC (eBioscience), anti-IL-21 PE, and IFN-γ PE-CY7. Antibodies were purchased from BD Pharmingen unless otherwise stated.
HLA restriction.
Epstein-Barr virus (EBV)-transformed B cell lines matched for one or more HLA molecules with the patient (kindly provided by Chan Soh Ha, WHO Immunology Training and Research Centre, NUS) were grown and maintained in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich), 20 mM HEPES, 0.5 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, minimum essential medium (MEM) amino acids with l-glutamine, and MEM nonessential amino acids (all purchased from Gibco). EBV B cells were left unstimulated or were pulsed with the peptide of interest (1 μg/ml) for 1 h at 37°C, washed, and then cocultured with the T cell lines for 5 h at 37°C in the presence of CD107a FITC antibodies and brefeldin A. Surface and intracellular stainings were performed as described in the previous section.
IFN-γ ELISPOT assay.
Enzyme-linked immunosorbent spot (ELISPOT) assays for the detection of IFN-γ-producing cells were performed using the panel of 660 dengue virus peptides pooled in the described mixtures. Assays were performed using thawed, ex vivo-isolated PBMCs. T cell lines were used to determine the minimal epitope of NS3 68. Briefly, 96-well plates (Multiscreen HTS; Millipore) were coated overnight at 4°C with 5 μg/ml of capture mouse anti-human IFN-γ antibody (clone 1-DK1; Mabtech). Plates were washed with phosphate-buffered saline (PBS) and blocked with AIM-V supplemented with 10% heat-inactivated FBS for 1 h at room temperature. The blocking solution was then removed, and a total of 1 × 105 PBMCs (or 5,000 cells from the NS3 68 T cell line) were plated per well in the presence or absence of the dengue virus peptide pools at a concentration of 5 μg/ml (or in the presence of 10-fold serial dilutions of NS3 68 peptide, from 0.0001 to 10 μM). Cells were incubated for 16 to 18 h, after which plates were washed and 100 μg/ml of biotinylated anti-human IFN-γ (clone 7-B6-1; Mabtech) was added for 2 h at room temperature. After washing, 100 μl of streptavidin-alkaline phosphatase (Mabtech) was added and plates were incubated in the dark for 1 h at room temperature. Plates were washed and 50 μl of alkaline-phosphatase substrate (5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium chloride[BCIP-NBT]; KPL) was added. After 10 to 15 min, the colorimetric reaction was stopped with running tap water. Spots were counted using an automated ELISPOT reader (Immunospot; Cellular Technology Limited). The number of IFN-γ-producing cells was expressed as spot-forming cells (SFC) relative to 1 × 105 PBMCs. Values were calculated by subtracting the number of spots detected in the nonstimulated control wells. Values were considered positive if they were equal or greater than 5 spots and at least 2 times above the means of the unstimulated control wells. As a positive control, cells were stimulated with staphylococcal enterotoxin B (SEB).
Peptide ELISA.
For peptide ELISA, Maxisorp Immunoplates (Nunc) were coated with DENV E protein peptides (10 μg/ml in PBS) overnight at 4°C. Plates were washed with PBS–0.05% Tween and blocked with PBS–5% bovine serum albumin (BSA) (Sigma-Aldrich) for 2 h at room temperature. Plasma patient samples were then incubated at a 1:200 dilution for 2 h at room temperature, and human E protein-specific IgGs were detected by adding 50 μl of goat anti-human IgG antibody conjugated with horseradish peroxidase (HRP) (Thermo Scientific Pierce) according to the manufacturer's instructions. Plates were washed and incubated with 50 μl of TMB substrate (BD Pharmingen) for 10 to 15 min. The colorimetric reaction was stopped by adding 50 μl of 1 M H2SO4, and plates were read at 450 nm (PerkinElmer reader).
Capture ELISA.
Maxisorp Immunoplates (Nunc) were coated with 5 μg/ml of mouse HB112 capturing antibody (ATTC hybridoma) overnight at 4°C. Plates were washed with PBS–0.01% Tween and blocked with PBS–5% milk (Sigma-Aldrich) for 2 h at room temperature. Plates were washed, and 50 μl of DENV 2 virus stock was added for 1 h at room temperature. After washing the plates, plasma samples were added at a 1:200 dilution in PBS–5% milk and incubated for 1 h at room temperature. Plates were washed, and anti-human IgG-HRP (Pierce) or goat anti-human IgM-HRP (Bethyl) was added according to the manufacturer's instructions. Plates were washed, and 50 μl of TMB substrate (BD Pharmingen) was added for 10 to 15 min. The colorimetric reaction was stopped by adding 50 μl of 1 M H2SO4, and plates were read at 450 nm (PerkinElmer reader).
Statistics.
Statistical significance was determined using the nonparametric one-way analysis of variance (ANOVA) test (see Fig. 1C to E), the two-tailed Fischer's exact test (see Fig. 4B and C), or the nonparametric, two-tailed Mann-Whitney test (see Fig. 2A to D, 4A, and 5D and F). For the analyses of the CD4+ and B cell epitope overlap (see Fig. 6B), a permutation test was designed as follows. The number of CD4+ and antibody epitopes was based on the observed numbers in 5 patients. The positions of these epitopes were then randomly distributed within the protein in 97 possible positions. The number of CD4+ epitopes overlapping or lying side by side with the antibody epitopes was then counted. This was scored as a success case if there were 4 or more CD4+ epitopes (of a total of 6) overlapping or lying side by side with the antibody epitopes. This process was repeated 1,000,000 times, and the number of success cases was expressed as a probability of observing 4 of 6 CD4+ epitopes overlapping or lying next to an antibody epitope. The permutation test makes the assumption that the epitopes are equally likely to be at the 97 possible sites (where 97 is the total number of E protein peptides).
Fig 1.
Methodology used for the identification of T cell epitopes across the entire DENV proteome. Dengue virus-specific T cell responses were assessed using a peptide library of 660 15mers, overlapping by 10 amino acids, spanning the entire DENV proteome. (A) The DENV polyprotein showing the amino acid length of each constituent. aa, amino acids. (B) PBMCs derived from dengue virus patients at three time points of disease were screened ex vivo against the peptide pools, and IFN-γ production was measured by ELISPOT assay. IFN-γ production, expressed as spot-forming cells (SFC) relative to 105 PBMCs, is shown for the NS5 peptide pools. (C to E) Results from 27 patients during the acute (C), postfebrile (D), and convalescent (E) phases of disease are summarized for each viral protein. Statistics were calculated using the nonparametric one-way ANOVA test. *, P ≤ 0.05; **, P ≤ 0.01: ***, P ≤ 0.001. (F) Recognition of peptide NS5 69 was further confirmed by ICS. The patient's T cell line was stimulated in the presence of brefeldin A with or without the peptide of interest or with a positive control, and ICS was performed for detection of IFN-γ production and CD107a expression by T cells. The plot is gated on CD3+ CD8+ cells. Results from one representative patient are shown (patient 13). w/o, without stimulation.
Fig 4.
DENV-specific CD4+ and CD8+ T cells target distinct viral proteins. (A) Ex vivo frequency of CD4+ and CD8+ T cell epitopes in acute, postfebrile, and convalescent patients as determined by ELISPOT assay. Results are expressed as SFC relative to 105 PBMCs. PF and Conv indicate postfebrile and convalescent, respectively. Bars indicate mean values. Statistics were calculated using the nonparametric, two-tailed Mann-Whitney test. The distribution of CD4+ (B) and CD8+ (C) T cell epitopes across the DENV proteins is analyzed in 27 patients. The differential recognition of structural/surface proteins versus nonstructural proteins in each subset was compared using a two-tailed Fischer's exact test. Skewing of the CD4+ response toward recognition of structural/surface proteins and of the CD8+ response toward recognition of nonstructural proteins was statistically significant (P < 0.0001).
Fig 2.
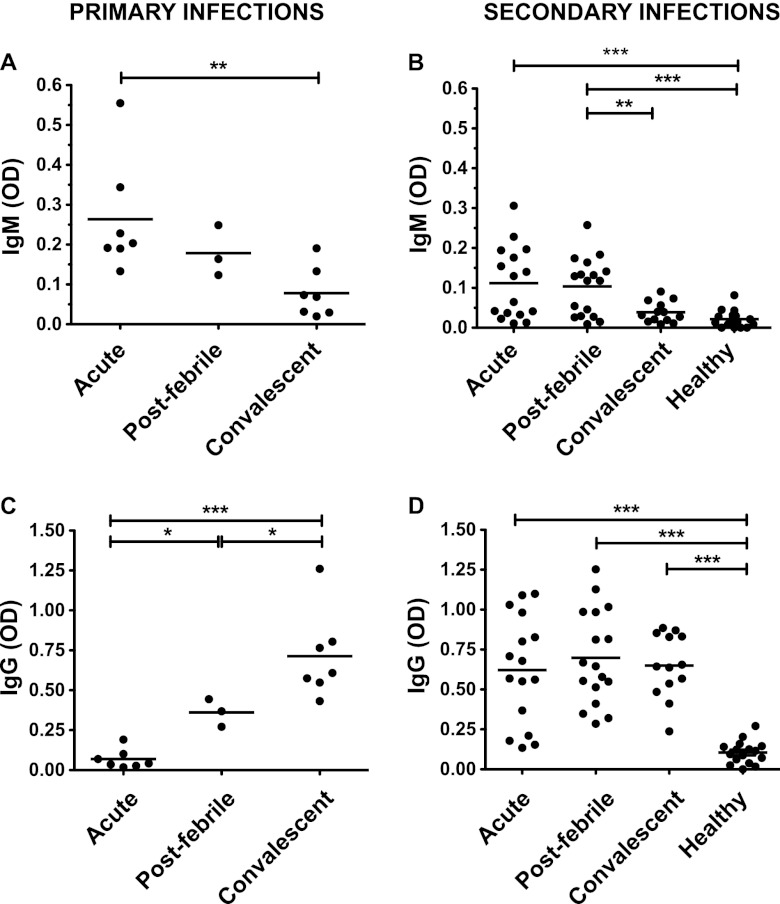
Dengue virus-specific IgM and IgG titers in dengue virus patients. Dengue virus-specific IgM (A and B) and IgG (C and D) titers were measured by ELISA in matched plasma samples from patients at different time points of disease. Results are shown separately for patients with IgM/IgG ratios of <1.8 that were defined as undergoing secondary infections (B and D) or for those defined as primary infections (A and C). Healthy patients were Singaporean residents of the same age group with no clinical history of dengue virus infection. Statistics were calculated using the nonparametric, two-tailed Mann-Whitney test.
Fig 5.
Dengue virus-specific CD4+ T cells have characteristics of circulating T follicular helper (TFH) cells. PBMCs from convalescent dengue virus patients were stimulated ex vivo in the presence of brefeldin A for 5 h with or without DENV peptides (DEN). Staining profiles for two representative patients are shown: patient 37 (A and B) and patient 8 (C). (A) CXCR5+ (TFH) and CXCR5− populations were identified according to the relative expression of CD45RA and CXCR5. The plot is gated on live CD3+ CD4+ cells. (B) CD40L and IFN-γ expression were determined by ICS in CXCR5+ and CXCR5− populations that were left unstimulated or after stimulation with DENV peptides. (C) CXCR5+ and CXCR5− populations were analyzed for expression of CD40L, IFN-γ, and IL-21. (D to F) Results for 8 patients are summarized. (D) Percentages of CD3+ CD4+ CD45RA− cells that expressed CD40L after stimulation with DEN. (E) CD40L+ cells were analyzed for their expression of CXCR5. Shown are the percentages of CD40L+ cells that were CXCR5+ or CXCR5−. (F) CD40L+ cells were analyzed for their capacity to produce IL-21 or IFN-γ following stimulation with DEN. Statistics were calculated using the nonparametric, two-tailed Mann-Whitney test.
Fig 6.
Matched E protein-specific CD4+ T and B cell specificities. CD4+ T cell and IgG responses directed against peptides spanning the E protein were assessed in postfebrile dengue virus patient samples by IFN-γ ELISPOT assay and IgG ELISA, respectively. (A and B) Responses to peptides Env 41 to Env 50 are shown for one representative patient (patient 11 [Pt 11]). IgG titers (A) and T cell responses (B) are expressed as OD (optical density) values or SFC relative to 105 PBMCs, respectively. (C) Location of CD4+ T cell (empty bar) and IgG (black bar) epitopes within the E protein in 5 patients. Numbers indicate the amino acid positions. Statistics were calculated using a permutation test (P = 0.0045).
For all tests, differences were considered significant at P ≤ 0.05.
RESULTS
Identification of novel T cell epitopes.
Virus-specific T cell responses against DENV were assessed using a peptide library spanning the entire DENV 2 proteome, consisting of 660 15mer peptides overlapping by 10 amino acids. The DENV proteome is represented schematically (Fig. 1A). Peptides were employed in a matrix so that each individual peptide was present in two different pools (one designated by a letter and one by a number), as described previously (26). Blood was collected from adult patients with confirmed DENV infection at three time points from fever onset (acute day 4 to 8, postfebrile day 14 to 21, and convalescent day 60 to 120). The Singaporean patient cohort employed as part of this study was defined as having mainly secondary DENV infections based upon a DENV 2-specific IgM/IgG ratio of <1.8 in paired acute and convalescent plasma samples, as assessed by IgM and IgG capture ELISA (Fig. 2). Details of the patients are listed in Table 1.
PBMCs derived from patient blood samples were tested ex vivo against the peptide pools for antigen specificity by IFN-γ ELISPOT assay (Fig. 1B). Collectively, our data from the ELISPOT assays, which were performed on 27 patients, indicate that DENV T cell specificity is broad, as it targets all viral proteins, although preferential recognition of NS3 and NS5 was observed at all time points of disease. Recognition of the viral proteins was similar across the three time points, with the highest frequencies being observed during the acute phase of disease (Fig. 1C to E). Dengue virus-specific T cells were undetectable ex vivo in healthy individuals (data not shown).
The specific peptides that elicited a response in the ex vivo ELISPOT assay then were confirmed through in vitro stimulation and expansion of the responding T cell lines for 10 days, followed by ICS for a peptide antigen-induced cytokine response. Briefly, T cell lines were stimulated in the presence of brefeldin A for 5 h with or without the peptide of interest or with anti-CD3/28 as a positive control, and expression of CD107a and IFN-γ was assessed by ICS. We illustrate this approach for the identification of a new peptide epitope derived from NS5 (Fig. 1B and F). The peptide epitope, defined as NS5 69, was contained in pools NS5-6 and NS5-D and elicited an IFN-γ response ex vivo at all time points of disease (Fig. 1B). This epitope was further recognized in T cell lines generated from the patient's PBMC (Fig. 1F). Employing this approach, we identified 30 novel DENV-specific T cell epitopes, 9 of which are CD4+ and 21 are CD8+ T cell epitopes. The complete list of CD4+ and CD8+ T cell peptides identified by ELISPOT assay and confirmed by ICS is shown in Tables 2 and 3, respectively.
Table 2.
CD4+ T cell epitopes identified in this study
| CD4+ peptide (amino acid position) | Sequence | No. of responding patients | ELISPOT frequencya | Source or reference |
|---|---|---|---|---|
| CAP 10 (46–60) | LFMALVAFLRFLTIP | 2 | 49.5 | This study |
| CAP 15 (71–85) | TIKSKAINVLRGFR | 6 | 27.3 | 12 |
| M 25 (121–135) | QRIETWILRHPGFTM | 1 | 5.5 | This study |
| Env 9 (41–55) | LDFELIKTEAKQPAT | 1 | 35 | This study |
| Env 42 (206–220) | WLVHRQWFLDLPLPW | 1 | 17.5 | This study |
| Env 48 (236–250) | TLVTFKNPHAKKQDV | 2 | 29.3 | This study |
| Env 49 (241–255) | KNPHAKKQDVVVLGS | 1 | 31.5 | 12 |
| Env 50 (246–260) | KKQDVVVLGSQEGAM | 1 | 15 | 12 |
| NS1 23 (111–125) | LKYSWKTWGKAKMLS | 2 | 10 | This study |
| NS1 66–68 (326–350) | EDGCWYGMEIRPLKEKEENLNSLV | 1 | In vitrob | This study |
| NS1 42 (206–220) | LNDTWKIEKASFIEV | 1 | 10 | This study |
| NS3 56 (276–290) | PNYNLIIMDEAHFTD | 1 | 19 | 12 |
| NS3 71 (351–365) | VTDFKGKTVWFVPSI | 1 | 5.5 | 11 |
| NS5 61 (301–315) | KTWAYHGSYETKQTG | 1 | In vitro | This study |
ELISPOT frequencies are expressed as SPC/105 cells; mean values are shown when n > 1.
Peptides were identified only after in vitro expansion of T cells.
Table 3.
CD8+ T cell epitopes identified in this study
| CD8+ peptide (amino acid position) | Sequence | No. of responding patients | ELISPOT frequencya | Source or reference (HLA restrictionb) |
|---|---|---|---|---|
| M 26 (126–140) | WILRHPGFTMMAAIL | 1 | 17.5 | This study |
| Env 21 (101–115) | WGNGCGLFGKGGIVT | 1 | In vitroc | This study |
| Env 41 (201–215) | MENKAWLVHRQWFLD | 2 | 20.3 | 12 (HLA B*3701) |
| Env 83 (411–425) | RMAILGDTAWDFGSL | 1 | 64.5 | 12 (HLA B*07) |
| Env 92 (456–470) | KILIGVIITWIGMNS | 1 | 17.5 | This study |
| NS1 33 (161–175) | GVFTTNIWLKLKEKQ | 1 | In vitro | This study |
| NS1 39 (191–205) | NRAVHADMGYWIESA | 1 | 8.5 | This study |
| NS2A 6 (26–40) | RVGTKHAILLVAVSF | 1 | 46 | This study |
| NS2A 11 (51–65) | RDLGRVMVMVGATMT | 3 | 118.7 | This study (HLA A*0101 or C*0602) |
| NS2B 22 (106–120) | ISGLFPVSIPITAAA | 1 | 39.5 | This study |
| NS3 18 (86–100) | EGEWKEGEEVQVLAL | 1 | 24 | This study |
| NS3 27 (131–145) | SPGTSGSPIIDKKGK | 6 | 22.5 | 7 (HLA A*1101) |
| NS3 41 (201–215) | KRYLPAIVREAIKRG | 2 | 26.6 | This study (HLA A*3101) |
| NS3 45 (221–235) | LAPTRVVAAEMEEAL | 1 | 15.5 | 27 (HLA B*07) |
| NS3 47 (231–245) | MEEALRGLPIRYQTP | 1 | 363 | 12 |
| NS3 51 (251–265) | HTGREIVDLMCHATF | 3 | 38.7 | 28 |
| NS3 61 (301–315) | STRVEMGEAAGIFMT | 1 | 9.5 | 12 (HLA B*4006) |
| NS3 68 (336–350) | EREIPERSWNSGHEW | 6 | 54.4 | This study (HLA B*5801) |
| NS3 100 (496–510) | DNINTPEGIIPSMFE | 4 | 233.5 | 12 (HLA B*35) |
| NS3 106 (526–540) | RGEARKTFVDLMRRG | 1 | 11.5 | 29 |
| NS3 108 (536–550) | LMRRGDLPVWLAYRV | 1 | In vitro | 12 (HLA B*5502) |
| NS3 111 (551–565) | AAEGINYADRRWCFD | 2 | 18.5 | 12 (HLA A*24) |
| NS3 120 (596–610) | LDARIYSDPLALKEF | 2 | 20.3 | This study (HLA A*2402) |
| NS4B 31 (151–165) | TVIDLDPIPYDPKFE | 1 | 60 | This study |
| NS4B 39 (191–205) | CEALTLATGPISTLW | 4 | 10.5 | This study |
| NS5 24 (116–130) | MSTYGWNLVRLQSGV | 1 | 111.5 | This study |
| NS5 33 (161–175) | TLRVLNLVENWLNNN | 1 | 19.5 | This study |
| NS5 59 (291–305) | SWHYDQDHPYKTWAY | 4 | 165.4 | 29 |
| NS5 61 (301–315) | KTWAYHGSYETKQTG | 1 | 9.5 | 29 |
| NS5 66 (326–340) | RLLTKPWDVVPMVTQ | 1 | In vitro | 29 (HLA B*5502) |
| NS5 69 (341–355) | MAMTDTTPFGQQRVF | 4 | 152 | This study (HLA A*6801 or B*3503) |
| NS5 78 (386–400) | GKKKTPRMCTREEFT | 1 | 34.5 | This study |
| NS5 113 (561–575) | HKKLAEAIFKLTYQN | 2 | 10.5 | This study |
| NS5 152 (756–770) | KSYAQMWSLMYEHRR | 1 | 60 | This study |
| NS5 176 (876–890) | NEEYTDYMPSMKRFR | 3 | 78.3 | This study |
ELISPOT frequencies are expressed as SPC/105 cells, and mean values are shown when n > 1.
HLA restriction is shown in boldface when it was identified in this study.
Peptides were identified only after in vitro expansion of T cells.
The characterization of the HLA restriction of the identified CD8+ T cell epitopes is shown for two representative patients (Fig. 3A to C, patient 32, and D to F, patient 29). Patient 32 responded to the CD4+ peptide CAP 15 and to the novel CD8+ peptide NS3 120 (Fig. 3A). The flanking peptides NS3 119 and NS3 121 did not elicit a response, suggesting that the immunogenic CD8+ epitope is contained within the NS3 120 15mer sequence (Fig. 3B). The HLA restriction of NS3 120 was characterized by evaluating the ability of HLA-matched EBV-transformed B cell lines to present the peptide of interest to CD8+ T cells. The EBV lines were left unstimulated or were pulsed with NS3 120, subsequently washed, and cocultured with the patient's T cell line. ICS was performed for IFN-γ and CD107a production by CD8+ T cells. Only EBV lines that shared HLA A*2402 with the patient (CF498 and WGB) were capable of presenting the peptide to CD8+ T cells, demonstrating that this peptide is restricted in HLA A*2402 (Fig. 3C). Patient 29 displayed a CD8+ T cell response directed toward the novel peptide NS3 68 restricted in HLA B*5801 (Fig. 3D and E). This peptide was commonly recognized among our patient cohort (6 out of 27 patients and 86% of patients with the relevant HLA). Because the immunogenic epitope was contained within the NS3 68 sequence (Fig. 3E), we evaluated the capacity of truncated variants of the NS3 68 15mer peptide to elicit an IFN-γ response. The highest response was triggered by the 9mer peptide RSWNSGHEW at all peptide concentrations, ranging from 0.001 to 10 μM, clearly indicating this peptide as the optimal CD8+ T cell epitope (Fig. 3F).
Fig 3.
Characterization of novel CD4+ and CD8+ T cell epitopes. Results from two representative patients are shown. (A to C) Patient 32. (D to F) Patient 29. (A) IFN-γ and CD107a production elicited by peptides NS3 120 and CAP 15 were assessed by ICS in T cell lines as described in the legend to Fig. 1. Plots are gated on CD3+ CD8+ (left) or CD3+ CD4+ (right) cells. The novel CD8+ peptide NS3 120 was further characterized for recognition of the flanking peptides (B) and HLA restriction by testing HLA-matched, peptide-pulsed EBV cell lines for their ability to stimulate peptide-specific T cell lines (C). The name of the EBV cell line and the HLA type that is shared with the patient are indicated above each plot. Similarly, the novel peptide NS3 68 was characterized for HLA restriction (D) and recognition of the flanking peptides (E). (F) Serial dilutions of truncated versions of peptide NS3 68 were evaluated for their capacity to elicit an IFN-γ response by ELISPOT in the patient's T cell line. Results are expressed as SPC/105 cells. The minimal 9mer epitope that elicited the highest production of IFN-γ is underlined. The total length and the first three amino acids are used to identify each truncated peptide.
In this study, we have identified 30 novel T cell epitopes in 9 out of 10 viral proteins. CD8+ T cell epitopes within NS3 and NS5 proteins were confirmed in 80 and 65% of patients, respectively. CD8+ T cell epitopes were less frequently distributed within E and NS2A proteins (each detected in 16% of patients), followed by NS4B (12% of patients). Two epitopes were commonly recognized by CD8+ T cells in the patient cohort: the novel B*5801-restricted NS3 68 and the previously described A*1101-restricted NS3 27 epitope. Consistent with previous observations, a third of HLA A*1101+ patients responded to NS3 27 (7). NS3 27 and NS3 68 epitopes each were recognized in 6 out of 27 patients and together provided coverage of 11 out of 27 (41%) patients, thus providing excellent tools to study the functionality and phenotype of DENV-specific CD8+ T cells ex vivo in this cohort.
The small 44-amino-acid region of the E protein (amino acids 206 to 260) is enriched for CD4+ epitopes, as it was recognized by 40% of patients in whom we were able to detect a CD4+ response. The peptide CAP 15 was also commonly recognized (in 43% of patients with detectable CD4+ responses).
Dengue virus-specific CD4+ T cells recognize antibody virion protein targets while CD8+ T cells recognize nonstructural proteins.
We next investigated the relative contribution of CD4+ and CD8+ T cell responses to the antiviral response and analyzed the two T cell subsets individually for preferential recognition of DENV viral proteins. CD8+ T cell epitopes dominated the DENV-specific T cell response, as they accounted for 75% of all identified epitopes. Consistent with previous studies in other viral infections (30), the ex vivo average frequency of dengue virus-specific CD8+ T cells was also higher than that of dengue virus-specific CD4+ T cells, in particular during the postfebrile phase. Shown in Fig. 4A are the number of IFN-γ SFC of peptide-specific CD4+ or CD8+ T cells as assessed in the ex vivo ELISPOT assay.
Results from 27 patients showed that CD4+ and CD8+ T cells target distinct viral proteins. Results were similar for patients experiencing primary (7 patients) and secondary (16 patients) dengue virus infections. While CD8+ T cells mainly recognized the nonstructural proteins NS3 and NS5, CD4+ T cells preferentially targeted the structural proteins E and CAP and the secreted nonstructural protein NS1 (Fig. 4B and C). Previous studies suggest that these proteins also are the main targets of the B cell response. In fact, the majority of B cell epitopes described in human dengue virus infections are directed against the E protein followed by NS1, with a small number of reports on CAP-specific antibodies (13, 31, 32). Recently, prM-specific antibodies with poor in vitro neutralizing capacities were also proposed to play an important role in dengue virus pathology (3, 33). We could detect significant amounts of IgG and IgM antibodies directed against whole live DENV 2 in the plasma of dengue virus patients, demonstrating that at least part of their antibody response is directed against the surface proteins E and/or prM/M (Fig. 1). All 27 patients were classified as nonsevere dengue based on the revised 2009 WHO classification guidelines (24).
In conclusion, here we show that in adult dengue virus infections, CD4+ and CD8+ T cells preferentially recognized distinct viral proteins. CD4+ T cells targeted the same proteins believed to be recognized by B cells, which are present in the viral particle (E and CAP) or are secreted/displayed on the surface of virus-infected cells (NS1). In contrast, CD8+ T cells recognized mainly the nonstructural proteins NS3 and NS5.
Dengue virus-specific CD4+ T cells have characteristics of follicular helper cells.
Based on the similarity between the antigenic targets of CD4+ T and B cells, we hypothesized that CD4+ T cells are recognizing peptides in the context of an antigen-specific B cell. Because the interaction between CD4+ T and B cells is reciprocal, CD4+ T cells may also have a role in providing B cell help. Recent reports have demonstrated the existence in human peripheral blood of a CD4+ T cell subset of circulating TFH cells that express CXCR5 and are specialized in providing B cell help, which is mediated primarily by IL-21. In addition to IL-21, circulating CXCR5+ cells are able to produce IFN-γ, although at lower levels than those of CXCR5− Th1 cells (20, 23). Based on these findings, we investigated whether DENV-specific CD4+ T cells were contained within the circulating TFH population. As CXCR5 can be transiently upregulated on non-TFH cells during prolonged T cell receptor (TCR) stimulation (data not shown), we chose to use PBMCs from convalescent dengue virus patients to avoid an artificial increase in the frequency of CXCR5+ cells. Details of the patients are listed in Table 4. PBMCs were left unstimulated or were stimulated ex vivo for 5 h with DENV peptides, and CD4+ T cells were analyzed for expression of CXCR5 together with intracellular CD40L, which allows the detection of antigen-specific CD4+ T cells (23). The gating strategy and staining profiles for two representative patients are shown in Fig. 5A to C. The frequency of DENV-specific CD4+ T cells as assessed by CD40L expression was low but consistent with the ELISPOT assay results (Fig. 5D). A significant proportion of DENV-specific CD4+ T cells were CXCR5+ (Fig. 5E). Furthermore, a fraction of DENV-specific CXCR5+ cells was capable of producing IL-21 or IFN-γ following stimulation with DENV peptides (Fig. 5F). To our knowledge, this is the first report describing IL-21 production by virus-specific T cells during an acute viral infection. IL-21 was shown to play an important role during chronic but not acute infections in mice (34–36).
These data suggest that a fraction of DENV-specific CD4+ T cells are involved in supporting B cell antibody production. In addition to the conventional helper activity, our results also suggest that DENV-specific CD4+ T cells play a direct role in viral clearance through production of IFN-γ and expression of CD107a (Fig. 3A).
Matched E protein-reactive CD4+ T and B cell specificities in dengue virus.
Based on our data, we speculate that during a dengue virus infection, the high-affinity virus-specific B cells preferentially expand CD4+ T cells of paired antigen specificity, which in turn support B cell antibody production through CD40L expression and IL-21 production. The presentation of particular epitopes with increased efficiency by antigen-specific B cells was previously described in vitro (37–40) and was shown to be due to protection of the antibody-bound antigen fragments from the action of proteolytic enzymes (40–43). In a mouse model of vaccinia virus infection, the antibody repertoire was shown to greatly influence CD4+ T cell specificities. Moreover, the protein specificities of new CD4+ T cell epitopes could be predicted based on the antibody repertoire (44).
To understand whether a similar mechanism was playing a role in our system, we compared the E protein-specific IgG and CD4+ T cell response in postfebrile dengue virus patient samples by using 15mer overlapping peptides spanning the E protein sequence. IgG responses to individual E protein peptides were assessed by ELISA, while T cell responses were assessed by IFN-γ ELISPOT assay as described in the legend to Fig. 1. Antibody and CD4+ T cell responses to peptides Env 41 to Env 50, covering a total of 60 amino acids of the E protein sequence, are shown for one representative patient (patient 11) (Fig. 6A and B). In this patient, an Env 48-specific CD4+ T cell response was matched by an antibody response directed against Env 46. We analyzed 5 patients that displayed CD4+ T cell responses against the E protein, and our results showed that 4 out of 6 CD4+ epitopes were matched by IgG responses directed against immediately adjacent (Env 46/49; patient 35), overlapping (Env 46/48; patients 09 and 11), or identical (Env 42; patient 26) peptides (Fig. 6C). Despite the small sample size, this pattern was statistically significant in a permutation test (P = 0.0045).
In summary, for the 5 patients analyzed, we show that there is a high degree of overlap between the portions of the E protein recognized by CD4+ T cells and those targeted by IgG molecules. We speculate that antibodies play a role in skewing the repertoire of dengue virus-specific CD4+ T cells.
In conclusion, this report provides a comprehensive study of T cell epitopes in adult dengue virus patients and identifies novel epitopes that will be useful for the characterization of T cells in dengue virus patients. Our data show that CD4+ and CD8+ T cell epitopes recognize distinct viral proteins. While CD8+ T cells recognize nonstructural proteins, CD4+ T cells mainly target structural/surface proteins in a manner similar to that of B cells. Consistent with this, a significant proportion of CD4+ T cells display a phenotype that suggests their in vivo interaction with B cells. Our results highlight key differences in the immunodominance of DENV proteins in the context of CD4+ and CD8+ T cell responses.
DISCUSSION
This report comprehensively characterizes T cell epitope reactivities toward the entire DENV genome in adult populations that live in areas where dengue is endemic. Previous studies identifying epitopes from DENV have focused on a few viral proteins or on sets of peptides predicted by computer algorithms to bind chosen HLA types (12, 14). Therefore, published T cell epitope sequences may not be totally representative of in vivo epitope dominance. To date, only one study has comprehensively analyzed dengue virus-specific T cell responses by using whole dengue viral proteins, hence it does not provide information on T cell epitope recognition (15).
In this study, we report several novel findings. First, we identify 30 new T cell epitopes in dengue virus patients (9 CD4+ and 21 CD8+ T cell epitopes), thus providing new tools for the characterization of the phenotype and functionality of the T cell components of a dengue virus-specific adaptive response. Second, we show that DENV-specific CD4+ and CD8+ T cells target distinct viral proteins. CD4+ T cells target the same proteins believed to be recognized by B cells, which are present in the viral particle (E and CAP) or are secreted/displayed on the cell surface of virus-infected cells (NS1). In contrast, CD8+ T cells mainly recognize the nonstructural proteins NS3 and NS5. Our results differ from those reported in a previous study performed on a Thai pediatric cohort (15). The authors report NS3 as being immunodominant for both CD4+ and CD8+ T cells, although significant responses to NS5 and E protein were also detected. In addition, they observed a correlation between immunodominant responses to NS3 and induction of DHF (15). In contrast, only a few CD4+ epitopes directed against NS3 were detected in our study. Of the 33 NS3 peptides identified in the ELISPOT assays, 31 were recognized by CD8+ T cells and 2 by CD4+ T cells as determined by ICS. All of our ELISPOT assays were performed ex vivo and therefore reflect in the closest possible way the human in vivo response, and most of the epitopes that had triggered a significant amount of IFN-γ ex vivo were successfully confirmed by ICS. A number of factors could account for the differences observed in the two studies. First, the sensitivity of the methodologies used to assess T cell responses differs in the two studies (ICS in the Thai study versus ELISPOT assay in our study; the sensitivity of ICS is considerably lower than that of the ELISPOT assay). Furthermore, the two patient cohorts differ considerably in age (children versus adults) and ethnicity (Thai versus Chinese with Indian and Malay minorities). Patient details are listed in Table 1. Genetic differences in the host HLA/TCR repertoires or in the antigen-presenting machinery will invariably influence the immunodominance of the viral proteins. Furthermore, the patients recruited in our study were all classified as nonsevere dengue virus on the basis of the 2009 WHO guidelines (n = 10 without warning signs and n = 15 with warning signs) (24). No significant differences were observed in patients experiencing primary or secondary infections.
We must take into account that, similar to previous studies of dengue virus, we are defining DENV-specific CD4+ T cell specificities based on production of IFN-γ under the assumption that antiviral responses generally are mediated by Th1 cells. However, we cannot exclude that we are looking at only a portion of the CD4+ response, and further studies are currently addressing this issue.
Of note, in this study we used a DENV 2 peptide library. Although DENV 2 is still the prevalent serotype in Singapore (45 and our unpublished data), we were not able to identify the currently infecting serotype by RT-PCR in the majority of these patients (as the virus is undetectable in the plasma after day 5 from fever onset and blood samples were mostly taken from the patients on days 6 and 7). It is therefore possible that for some patients the DENV 2 library does not match the serotype of the currently infecting virus, and in these cases we are mostly detecting serotype cross-reactive T cell responses. The T cell responses identified in this study include both conserved and serotype-specific epitopes. Of the 14 15mers that elicited a CD4+ T cell response, 4 displayed a high degree of conservation across all serotypes, as they were either identical or differed in a single amino acid. Four epitopes were conserved only between specific serotypes. Two 15mers were likely to be serotype specific, as they displayed 3 to 5 amino acid differences. (Table 5). Of the 35 CD8+ T cell peptides detected, 11 had a high degree of conservation, as they were either identical for all serotypes (2 epitopes) or displayed single-amino-acid differences. Five 15mers were conserved only for specific serotypes. Four epitopes were significantly different across the serotypes, with 4 to 11 amino acid differences. Interestingly, three of these were detected only in patients experiencing primary infections (Table 6). Of note, T cells will recognize epitopes of 8 to 10 amino acids, thus analysis of the 15mer may not be highly informative. In addition, it is often difficult to predict whether mutations in single amino acid positions will affect T cell recognition. Previous studies have shown that one or two amino acid mutations within the NS3 27 epitope derived from different DENV serotypes did not alter peptide recognition but greatly affected the avidity at which it was recognized by T cells specific for a heterologous peptide. This is the theory underlying original antigenic sin (7).
Table 5.
Number of amino acid mutations in DENV 2 CD4+ T cell 15mer peptides across the DENV serotypes
| CD4+ peptide | No. of amino acid mutations in DENV type: |
||
|---|---|---|---|
| 1 | 3 | 4 | |
| CAP 10 (46–60) | 3 | 3 | 4 |
| CAP 15 (71–85) | 2 | 2 | 3 |
| M 25 (121–135) | 2 | 2 | 2 |
| Env 9 (41–55) | 5 | 4 | 5 |
| Env 42 (206–220) | 0 | 2 | 1 |
| Env 48 (236–250) | 3 | 1 | 3 |
| Env 49 (241–255) | 2 | 1 | 2 |
| Env 50 (246–260) | 0 | 0 | 1 |
| NS1 23 (111–125) | 3 | 3 | 2 |
| NS1 66–68 (326–350) | 0 | 1 | 1 |
| NS1 42 (206–220) | 2 | 3 | 3 |
| NS3 56 (276–290) | 1 | 0 | 0 |
| NS3 71 (351–365) | 1 | 1 | 1 |
| NS5 61 (301–315) | 2 | 1 | 2 |
Table 6.
Number of amino acid mutations in DENV 2 CD8+ T cell peptides across the DENV serotypes
| CD8+ peptidea | No. of amino acid mutations in DENV type: |
||
|---|---|---|---|
| 1 | 3 | 4 | |
| M 26 (126–140) | 2 | 3 | 1 |
| Env 21 (101–115) | 1 | 1 | 0 |
| Env 41 (201–215) | 2 | 3 | 2 |
| Env 83* (414–422) | 0 | 0 | 0 |
| Env 92 (456–470) | 2 | 2 | 2 |
| NS1 39 (191–205) | 2 | 2 | 1 |
| NS1 33 (161–175) | 2 | 2 | 4 |
| NS2A 6 (26–40) | 11 | 7 | 7 |
| NS2A 11 (51–65) | 4 | 4 | 4 |
| NS2B 22 (106–120) | 5 | 6 | 3 |
| NS3 18 (86–100) | 4 | 4 | 6 |
| NS3 27* (133–143) | 1 | 1 | 1 |
| NS3 41 (201–215) | 1 | 1 | 2 |
| NS3 45* (221–232) | 2 | 0 | 0 |
| NS3 47 (231–245) | 2 | 0 | 0 |
| NS3 51 (251–265) | 0 | 0 | 0 |
| NS3 61 (301–315) | 1 | 1 | 0 |
| NS3 68* (342–350) | 1 | 1 | 1 |
| NS3 100 (496–510) | 2 | 2 | 3 |
| NS3 106 (526–540) | 0 | 1 | 1 |
| NS3 108 (536–550) | 1 | 1 | 2 |
| NS3 111* (556–564) | 1 | 1 | 3 |
| NS3 120 (596–610) | 1 | 1 | 2 |
| NS4B 31 (151–165) | 1 | 1 | 2 |
| NS4B 39 (191–205) | 1 | 0 | 2 |
| NS5 24 (116–130) | 3 | 2 | 2 |
| NS5 33 (161–175) | 5 | 4 | 5 |
| NS5 59 (291–305) | 2 | 2 | 1 |
| NS5 61 (301–315) | 0 | 1 | 1 |
| NS5 66 (326–340) | 1 | 0 | 1 |
| NS5 69 (341–355) | 1 | 0 | 1 |
| NS5 78 (386–400) | 4 | 3 | 3 |
| NS5 113 (561–575) | 3 | 2 | 2 |
| NS5 152 (756–770) | 1 | 1 | 1 |
| NS5 176 (876–890) | 3 | 1 | 4 |
If the minimal epitope is known (indicated by an asterisk), the numbers refer to mutations within this portion. Otherwise, numbers refer to mutations within the 15mer peptide.
The tendency of CD8+ T cells to target epitopes derived from NS3 and NS5 has been previously reported, but the reasons underlying the immunodominance of these proteins is still not fully understood. Immunodominance was shown to be driven by, among other factors, the dose and timing of production of the antigen. These factors have also been linked to a differential recognition of viral proteins between CD4+ and CD8+ T cells (30). The DENV proteome initially is synthesized as a single continuous polypeptide that is subsequently cleaved into the 10 constituent proteins. One defining feature of NS3 and NS5 is that they are released into the infected cells' cytoplasm as part of the normal viral replicative cycle (46) and thus will have direct access to the ubiquitin/proteasome machinery and other components of the major histocompatibility complex class I pathway (47). The skewed recognition by CD8+ T cells of nonstructural proteins that are expressed only following viral infection of cells confirms that these cells recognize antigen on the surface of antigen-presenting cells that are either directly infected by DENV or have acquired antigens from virus-infected cells. In contrast, the preferential recognition by CD4+ T cells of proteins that are secreted or present in the viral particle is consistent with virions being the major source of antigens. Based on the similarity between the antigenic targets of CD4+ T and B cells and on previous studies showing that B cells are extremely efficient antigen-presenting cells, we hypothesized that CD4+ T cells are recognizing peptides in the context of an antigen-specific B cell. According to this scenario, we predicted that B cells would present DENV antigens derived from proteins that are secreted or present in the virion to CD4+ T cells which in turn would support B cell antibody production. Here, we report that a fraction of DENV-specific CD4+ T cells have a phenotype characteristic of TFH, as they express CXCR5 and produce IL-21 or IFN-γ following encounter of dengue antigens. Approximately half of the DENV-specific CD4+ T cells expressed CXCR5. This result was surprising given the difficulty that we and others have previously encountered in detecting the very low frequencies of antigen-specific cells within the CXCR5+ population (20, 23). Due to limited sample sizes, we were unable to functionally assess the B cell helper activity of the dengue virus-specific TFH cells in these patients. However, previous studies have clearly shown that cells with this phenotype are indeed circulating TFH cells that possess a B cell helper activity (22, 23).
Previous studies performed in vitro in the human system have showed that antigen-specific B cells can present particular epitopes with increased efficiency to CD4+ T cells and thus skew the CD4+ T cell repertoire (37–40). Studies in the mouse model further reported that the antibody repertoire greatly influenced CD4+ T cell specificities (44). We show that within the E protein, a major target of dengue virus-specific B cell responses, CD4+ T and B cell epitopes largely overlap, suggesting that the antibody repertoire does indeed affect the expansion of antigen-specific CD4+ T cells. We are aware of the simplification of our assay, as the use of a linear peptide library allows the detection of linear but not conformational epitopes. Recent studies by us and others have highlighted the importance of antibodies binding to discontinuous epitopes spanning the adjacent surface of envelope protein dimers in both dengue virus (32) and in the related West Nile virus (48). However, most likely because of the technical difficulties of assessing conformational epitopes, the large majority of human DENV-specific B cell epitopes described in the literature are linear in nature (352 linear versus 43 discontinuous DENV-specific human B cell epitopes) (13).
In conclusion, in this study we dissected the human T cell response during a natural dengue virus infection and identified 30 novel T cell epitopes in Asian individuals from a region in which dengue virus is endemic. Our results highlight the fundamentally distinct protein targets of CD4+ and CD8+ T cells during a natural secondary dengue virus infection. Our study has important implications for vaccine design and evaluation. It highlights the need to include nonstructural proteins (in particular NS3 and NS5) in the vaccine formulation if the induction of a CD8+ T cell response is desired. These data will also aid the investigation of T cell responses in dengue virus infection and contribute to understanding their role in protection versus immunopathology.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation (STOP-Dengue TCR R-182-003-220-275, NUS-HUJI consortium), from the National University of Singapore (R182-000-192-133 and R182-005-172-281), and from the Life Sciences Institute, Singapore (R711-000-024-101).
We thank Bernett Lee from the Bioinformatics Platform at the Singapore Immunology Network, A*STAR, for statistical analyses of the CD4+ T/B cell epitope overlap.
We have no conflicting financial interests.
Footnotes
Published ahead of print 19 December 2012
REFERENCES
- 1. Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halstead SB. 2007. Dengue. Lancet 370:1644–1652 [DOI] [PubMed] [Google Scholar]
- 3. Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. 2010. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect. Dis. 10:712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. 2009. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 182:4865–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. 2010. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 185:5405–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921–927 [DOI] [PubMed] [Google Scholar]
- 8. Gagnon SJ, Ennis FA, Rothman AL. 1999. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J. Virol. 73:3623–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Green S, Kurane I, Edelman R, Tacket CO, Eckels KH, Vaughn DW, Hoke CH, Jr, Ennis FA. 1993. Dengue virus-specific human CD4+ T-lymphocyte responses in a recipient of an experimental live-attenuated dengue virus type 1 vaccine: bulk culture proliferation, clonal analysis, and precursor frequency determination. J. Virol. 67:5962–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurane I, Innis BL, Nisalak A, Hoke C, Nimmannitya S, Meager A, Ennis FA. 1989. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J. Clin. Investig. 83:506–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mangada MM, Rothman AL. 2005. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 175:2676–2683 [DOI] [PubMed] [Google Scholar]
- 12. Simmons CP, Dong T, Chau NV, Dung NT, Chau TN, Thao L, Hien TT, Rowland-Jones S, Farrar J. 2005. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 79:5665–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughan K, Greenbaum J, Blythe M, Peters B, Sette A. 2010. Meta-analysis of all immune epitope data in the Flavivirus genus: inventory of current immune epitope data status in the context of virus immunity and immunopathology. Viral Immunol. 23:259–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, Peters B, Shresta S, Sette A. 2011. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 187:4268–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. 2010. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. U. S. A. 107:16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856 [DOI] [PubMed] [Google Scholar]
- 17. Jellison ER, Kim SK, Welsh RM. 2005. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J. Immunol. 174:614–618 [DOI] [PubMed] [Google Scholar]
- 18. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. 2004. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200:725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 [DOI] [PubMed] [Google Scholar]
- 22. Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. 2011. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 186:5556–5568 [DOI] [PubMed] [Google Scholar]
- 23. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34:108–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO 2009. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. WHO, Geneva, Switzerland: [PubMed] [Google Scholar]
- 25. Schreiber MJ, Holmes EC, Ong SH, Soh HS, Liu W, Tanner L, Aw PP, Tan HC, Ng LC, Leo YS, Low JG, Ong A, Ooi EE, Vasudevan SG, Hibberd ML. 2009. Genomic epidemiology of a dengue virus epidemic in urban Singapore. J. Virol. 83:4163–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan AT, Loggi E, Boni C, Chia A, Gehring AJ, Sastry KS, Goh V, Fisicaro P, Andreone P, Brander C, Lim SG, Ferrari C, Bihl F, Bertoletti A. 2008. Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J. Virol. 82:10986–10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL. 2002. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol 168:5959–5965 [DOI] [PubMed] [Google Scholar]
- 28. Sanchez V, Gimenez S, Tomlinson B, Chan PK, Thomas GN, Forrat R, Chambonneau L, Deauvieau F, Lang J, Guy B. 2006. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3). Vaccine 24:4914–4926 [DOI] [PubMed] [Google Scholar]
- 29. Imrie A, Meeks J, Gurary A, Sukhbataar M, Kitsutani P, Effler P, Zhao Z. 2007. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J. Virol. 81:10081–10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, Pasquetto V, Crotty S, Croft M, Lefkowitz EJ, Grey H, Sette A. 2007. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J. Immunol. 178:6814–6820 [DOI] [PubMed] [Google Scholar]
- 31. Chan AH, Tan HC, Chow AY, Lim AP, Lok SM, Moreland NJ, Vasudevan SG, MacAry PA, Ooi EE, Hanson BJ. 2012. A human PrM antibody that recognizes a novel cryptic epitope on dengue E glycoprotein. PLoS One 7:e33451 doi:10.1371/journal.pone.0033451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, Macary PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 4:139ra83 doi:10.1126/scitranslmed.3003888 [DOI] [PubMed] [Google Scholar]
- 33. Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elsaesser H, Sauer K, Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580 [DOI] [PubMed] [Google Scholar]
- 36. Yi JS, Du M, Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berzofsky JA. 1983. T-B reciprocity. An Ia-restricted epitope-specific circuit regulating T cell-B cell interaction and antibody specificity. Surv. Immunol. Res. 2:223–229 [DOI] [PubMed] [Google Scholar]
- 38. Manca F, Fenoglio D, Kunkl A, Cambiaggi C, Sasso M, Celada F. 1988. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J. Immunol. 140:2893–2898 [PubMed] [Google Scholar]
- 39. Manca F, Kunkl A, Fenoglio D, Fowler A, Sercarz E, Celada F. 1985. Constraints in T-B cooperation related to epitope topology on E. coli beta-galactosidase. I. The fine specificity of T cells dictates the fine specificity of antibodies directed to conformation-dependent determinants. Eur. J. Immunol. 15:345–350 [DOI] [PubMed] [Google Scholar]
- 40. Ozaki S, Berzofsky JA. 1987. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J. Immunol. 138:4133–4142 [PubMed] [Google Scholar]
- 41. Davidson HW, Watts C. 1989. Epitope-directed processing of specific antigen by B lymphocytes. J. Cell Biol. 109:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lanzavecchia A. 1985. Antigen-specific interaction between T and B cells. Nature 314:537–539 [DOI] [PubMed] [Google Scholar]
- 43. Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. 1995. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J. Exp. Med. 181:1957–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, Peters B, Rafii-El-Idrissi Benhnia M, Hoffmann J, Su HP, Singh K, Garboczi DN, Head S, Grey H, Felgner PL, Crotty S. 2008. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 28:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee KS, Lo S, Tan SS, Chua R, Tan LK, Xu H, Ng LC. 2012. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect. Genet. Evol. 12:77–85 [DOI] [PubMed] [Google Scholar]
- 46. Perera R, Kuhn RJ. 2008. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 11:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yewdell JW, Anton LC, Bennink JR. 1996. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J. Immunol. 157:1823–1826 [PubMed] [Google Scholar]
- 48. Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]