Abstract
Background. Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections induce robust, generalized inflammatory responses that begin during acute infection and lead to pathological systemic immune activation, fibrotic damage of lymphoid tissues, and CD4+ T-cell loss, pathogenic processes that contribute to disease progression.
Methods. To better understand the contribution of tumor necrosis factor (TNF), a key regulator of acute inflammation, to lentiviral pathogenesis, rhesus macaques newly infected with SIVmac239 were treated for 12 weeks in a pilot study with adalimumab (Humira), a human anti-TNF monoclonal antibody.
Results. Adalimumab did not affect plasma SIV RNA levels or measures of T-cell immune activation (CD38 or Ki67) in peripheral blood or lymph node T cells. However, compared with untreated rhesus macaques, adalimumab-treated rhesus macaques showed attenuated expression of proinflammatory genes, decreased infiltration of polymorphonuclear cells into the T-cell zone of lymphoid tissues, and weaker antiinflammatory regulatory responses to SIV infection (ie, fewer presumed alternatively activated [ie, CD163+] macrophages, interleukin 10–producing cells, and transforming growth factor β–producing cells), along with reduced lymphoid tissue fibrosis and better preservation of CD4+ T cells.
Conclusions. While HIV/SIV replication drives pathogenesis, these data emphasize the contribution of the inflammatory response to lentiviral infection to overall pathogenesis, and they suggest that early modulation of the inflammatory response may help attenuate disease progression.
Keywords: SIV, rhesus macaque, Sooty mangabey, lymph node, inflammation, adalimumab, TNF, macrophage, fibrosis, collagen, TGFb
(See the editorial commentary by Michael on pages 875–6.)
Persistent immune activation is a hallmark of human immunodeficiency virus type 1 (HIV-1) infection in humans and simian immunodeficiency virus (SIV) infection in rhesus macaques. In progressive HIV/SIV infections, the level of immune activation has been demonstrated to be a stronger predictor of disease progression than either plasma viral load or peripheral CD4+ T-cell count [1, 2]. Acute infection is associated with the development of intense immune activation that remains abnormally high throughout the course of infection in the absence of antiretroviral treatment (ART). This persistent pathological immune activation is associated with elevated proinflammatory cytokine and coagulation markers that correlate with an increased risk of death among HIV-positive patients [3]. In SIV-infected monkeys and HIV-infected humans, early augmentation of proinflammatory cytokines and chemokines in lymphoid tissues may play a key role in modulating disease progression [4, 5]. Notably, early high expression of both proinflammatory and antiinflammatory molecules in lymphoid tissues has been proposed to contribute to enhanced immune activation, resulting in tissue damage rather than viral control, thus driving disease progression [4, 6]. Dissecting the elements of the acute inflammatory response that lead to chronic immune activation and disease progression may provide insights that can aid the development of specific adjunctive therapies that may limit inflammation-induced tissue damage and thereby improve the prognosis of HIV-infected individuals.
The inflammatory response to infections reflects the aggregate effects of various cytokines and other soluble factors produced by multiple cellular populations. Tumor necrosis factor (TNF) has been identified as a key regulator and amplifier of inflammatory responses, with effects on the innate and adaptive immune systems [7–9]. TNF has been proposed to contribute to the overall pathogenesis of HIV/SIV infection, enhancing viral propagation, lymphocyte depletion, and clinical manifestations of disease. High TNF levels have been shown to be associated with all stages of HIV-1 infection, with increased TNF expression in the plasma and lymphoid tissues during acute HIV-1 infection [10, 11]. Sequential plasma samples collected during the eclipse and exponential viral expansion phases of acute HIV-1 infection demonstrated rapid and robust inflammatory responses, including early and sustained increases in TNF, that likely have immunopathological consequences by promoting immune activation, viral replication, and CD4+ T-cell loss [12]. In nonhuman-primate models, elevated TNF expression has been shown in the serum, peripheral blood mononuclear cells, lymph nodes, and brain of infected animals in temporal association with SIV replication during acute infection [13–15]. In contrast, studies of nonprogressive and apathogenic SIV infection in natural hosts of SIV have shown a lack of TNF upregulation throughout the acute and chronic stages of SIV infection [16], suggesting that differences in the host acute inflammatory response may be critically important in pathogenesis and disease progression.
Since TNF inhibitors are used to treat a range of inflammatory conditions [17, 18], and given the data suggesting that TNF may play a key role in the acute inflammatory response to HIV/SIV infection, we evaluated the effect of anti-TNF antibody (adalimumab; Humira) treatment during the first 12 weeks of SIV infection in a small, nonhuman-primate study. While treatment did not affect viral replication dynamics or systemic T-cell activation profiles, inhibition of the biological activity of TNF reduced proinflammatory gene expression in lymphoid tissues during acute infection and attenuated antiinflammatory responses to SIV associated with pathological damage of lymphoid tissues [6]. Specifically, TNF blockade attenuated 3 manifestations of inflammation associated with SIV infection: (1) infiltration of CD163+ macrophages and neutrophils into the paracortical T-cell zone, (2) transforming growth factor β (TGF-β) expression in the T-cell zone, and (3) lymphoid tissue fibrosis, which was associated with less CD4+ T-cell loss, demonstrating that modulation of inflammatory responses to HIV/SIV infection may beneficially affect pathogenesis, independent of any direct effects on viral replication.
METHODS
Animals and Tissues
Rhesus macaques (Indian-origin Macaca mulatta) were seronegative for simian retrovirus type D, simian T-cell lymphotropic virus, and SIV at study initiation and did not express protective major histocompatibility complex I alleles (MamuB*08, MamuB*17). Rhesus macaques were infected with 20 monkey median infectious doses of SIVmac239 (a kind gift from R. Desrosiers, Harvard Medical School/New England Primate Research Center) by intravenous infusion. Peripheral blood and lymph node biopsy specimens were obtained at specified time points (Figure 1). An additional cross-sectional cohort was studied that included 3 SIV-negative rhesus macaques, 6 chronically SIV-positive rhesus macaques (infected with either SIVmac239 or SIVsmE543), 5 SIV-negative sooty mangabeys (Cercocebus atys), and 5 chronically SIV-positive sooty mangabeys (Table 1). Animals were housed and cared for in accordance with American Association for Accreditation of Laboratory Animal Care (AAALAC) standards in AAALAC-accredited facilities, and all animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees of the National Cancer Institute, National Institutes of Health, or the Yerkes National Primate Research Center.
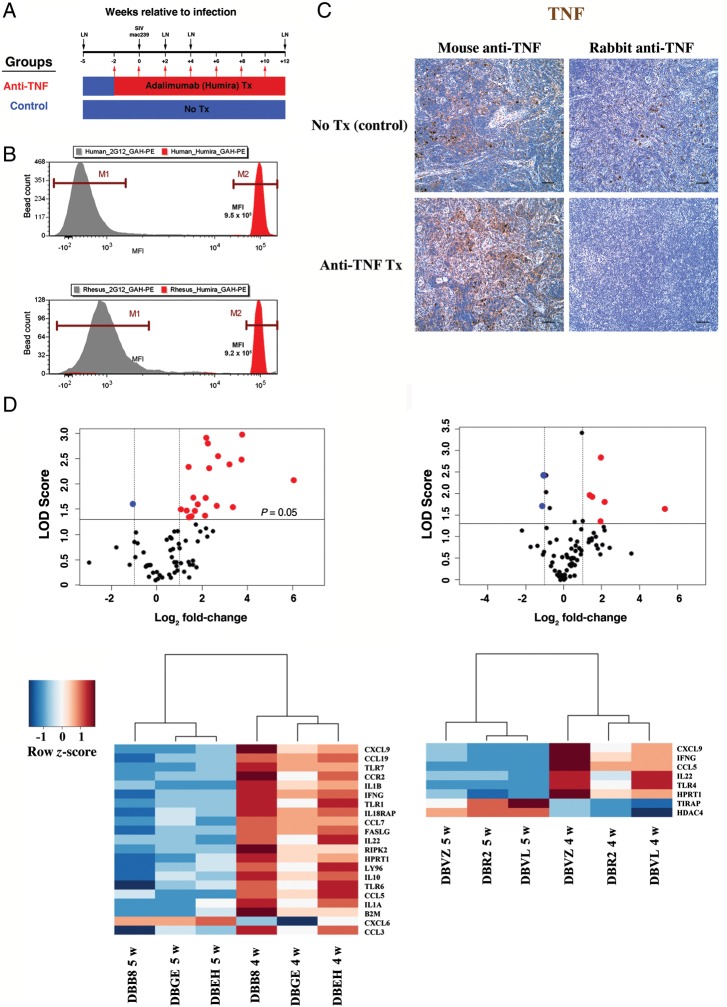
Figure 1.
Study design, adalimumab binding to rhesus tumor necrosis factor (TNF), and attenuation of early simian immunodeficiency virus (SIV)–mediated inflammation by anti-TNF treatment. A, Three rhesus macaques (blue) received no treatment, while 3 rhesus macaques (red) received adalimumab 2 weeks prior to SIVmac239 infection and every 2 weeks thereafter, to 10 weeks after infection. Black arrows show the times of SIV inoculation and of lymph node biopsies. B, Binding of adalimumab to rhesus macaque rTNF and human rTNF is comparable. Abbreviation: MFI, mean fluorescence intensity. C, Immunohistochemical analysis was performed on lymph node sections obtained 4 weeks after infection from untreated (top panel) and adalimumab-treated (bottom panel) rhesus macaques, using a mouse monoclonal anti-TNF antibody (left column) that targets a region outside any putative TNF-receptor (TNFR) binding domains, and using a rabbit polyclonal anti-TNF antibody (right column) that maps to the C-terminal end of TNF, encompassing residues known to be important for TNF receptor binding (described in Material and Methods). D, Volcano plots (top panel) and associated heat maps with dendrograms (bottom panel) depicting inflammatory genes that were either upregulated (red circles and red-shaded blocks) or downregulated (blue circles with blue-shaded blocks) in lymph nodes (5 weeks before infection vs 4 weeks after infection) from untreated (left column) and adalimumab-treated (right column) rhesus macaques. Heat maps depict inflammatory genes that were either upregulated (red hues) or downregulated (blue hues) in lymph nodes 4 weeks after infection versus 5 weeks before infection in control untreated and anti-TNF treatment macaques. Color key (scale) represents level of gene expression normalized for each gene (row z-score). The genes that are represented in the heat maps had an absolute fold-change of >2 and also had a P value of < .05 in a t test comparing the expression of genes in each animal at the 2 different time points. Scale bars = 50 µm. Abbreviation: LOD, logarithim of the odds [-(log10) P-value].
Table 1.
Animal Characteristics
| Animal ID | Species | Virus | Time(s) of LN Biopsya | Disease Stage | Plasma VLb | Peripheral CD4+ T-Cell Countc | Treatment |
|---|---|---|---|---|---|---|---|
| DBB8 | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 1.2 × 107 | 554 | … |
| DBEH | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 5.1 × 107 | 830 | … |
| DBGE | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 1.4 × 107 | 1603 | … |
| DBR2 | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 7.6 × 107 | 1894 | Adalimumab |
| DBVL | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 1.2 × 107 | 1407 | Adalimumab |
| DBVZ | RM | SIVmac239 | –5 w to 12 w | Acute-chronic | 2.8 × 107 | 1866 | Adalimumab |
| R451 | RM | None | –4 w | NA | … | 668d | … |
| RH495 | RM | None | … | NA | … | 574 | … |
| RH769 | RM | None | … | NA | … | 465 | … |
| R451 | RM | SIVmac239 | 21 w | Chronic | 4.1 × 107 | 242e | … |
| RH760 | RM | SIVsmE543 | 52 w | Chronic | 5.0 × 103 | 296 | … |
| RH764 | RM | SIVsmE543 | 52 w | Chronic | 2.2 × 104 | 134 | … |
| RPp6 | RM | SIVmac239 | 12 w | Chronic | 4.4 × 106 | 677 | … |
| RWv8 | RM | SIVmac239 | 52 w | Chronic | 9.0 × 106 | 239f | … |
| RZz8 | RM | SIVmac239 | 52 w | Chronic | 1.3 × 106 | 352f | … |
| FFk | SM | None | … | NA | … | 805 | … |
| FKu | SM | None | … | NA | … | 130g | … |
| FKv | SM | None | … | NA | … | 956 | … |
| FOz | SM | None | … | NA | … | 863 | … |
| FZr | SM | None | … | NA | … | 958 | … |
| FAv | SM | SIVsmm | Naturally infected | Chronic | 1.0 × 104 | 811 | … |
| FDu | SM | SIVsmm | Naturally infected | Chronic | 3.7 × 104 | 441 | … |
| FDv | SM | SIVsmm | Naturally infected | Chronic | 2.7 × 104 | 821 | … |
| FEy | SM | SIVsmm | Naturally infected | Chronic | 2.8 × 105 | 929 | … |
| FWv | SM | SIVsmm | 54 w | Chronic | 2.7 × 104 | 357 | … |
Abbreviations: LN, lymph node; NA, not applicable; RM, rhesus macaque; SIV, simian immunodeficiency virus; SM, sooty mangabey; VL, viral load.
a Data are weeks relative to the time of SIV infection. The exact date and duration of infection for the naturally infected SMs in this study was not known. However, all were known to be SIV positive for >1 year at the time of LN biopsy.
b For animals in the anti-TNF study, plasma VL was measured 12 weeks after infection. All other measurements were performed at the time of LN biopsy.
c Determined 12 weeks after infection or at the time of LN biopsy, unless otherwise indicated.
d Determined 0 weeks after infection.
e Determined 20 weeks after infection.
f Determined 238 days after infection.
g Determined >2 months after LN biopsy was performed.
Adalimumab Treatment
Adalimumab (Humira, Abbott Laboratories) is Food and Drug Administration–approved for treatment of various autoimmune diseases and was chosen among other anti-TNF agents because of its reported cross-reactivity to macaque species TNF [19]. Because all rhesus macaques in this pilot study were the same sex (male) and of similar age and weight, we randomly assigned animals into treated and control groups (3 animals per group). Because of the size and age of our animals, we chose an adalimumab dosing strategy that was based on the recommended maintenance dosing strategy (after an initial 40-mg dose) for treatment of juvenile idiopathic arthritis for children 4–17 years of age (20 mg every other week for individuals weighing <30 kg). Adalimumab treatment began 2 weeks prior to SIV infection and continued into the chronic phase of disease (12 weeks after infection). Animals were injected subcutaneously with an initial 40-mg dose of adalimumab (5.9 mg/kg to 6.35 mg/kg) at 2 weeks before infection and subsequently with a maintenance dose of 20 mg (2.6 mg/kg to 3.1 mg/kg) every 2 weeks thereafter, through 12 weeks after infection. When this study began, several small controlled trials of anti-TNF–based therapies in patients with HIV infection with and without secondary infections had been reported with no serious adverse events observed [20–24].
Immunohistochemical Analysis and Quantitative Image Analysis
Immunohistochemical staining and quantitative image analysis were performed as described elsewhere [25, 26] and in the Supplementary Materials.
Plasma Viral Loads
Plasma samples were analyzed for SIV RNA, using a quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay that, as used, provides a threshold sensitivity of 30 copy Eq/mL, as previously described [27].
Lymph Node RNA Isolation, qRT-PCR, and PCR Array
Total RNA was prepared from approximately 100 mg of frozen lymph nodes using the FastRNA Pro Green Kit and FastPrep Instrument (MP Biomedicals) for 40 seconds at a speed setting of 6. One-tenth volume, approximately 75 µL, of 3M NaOAc (pH 5.2) was added to the initial tissue extract prior to addition of chloroform to reduce carbohydrate contamination. The final RNA preparations were dissolved in 100 µL of DEPC-water. The Inflammatory Response and Autoimmunity PCR array kit from SA Biosciences (catalog no. PAHS-077) was used for analysis of extracted RNA, following the instructions for complementary DNA synthesis and qPCR analysis provided and indicated for an Applied Biosystems 7500 qPCR instrument (Life Technologies/Applied Biosystems). Data were analyzed with the RT² Profiler PCR Array Data Analysis (spreadsheet) Template v3.2 from SA Biosciences.
Flow Cytometry
Freshly isolated cells were immunophenotyped using the following antibody panel: CD4 Pacific Blue (clone OKT4; BioLegend), CCR5 PE (clone 3A9; BD Biosciences), CD28 ECD (clone CD28.2; Beckman Coulter), CD95 PE-Cy5 (clone DX2; BD Biosciences), CD8 PE-Cy7 (clone SK1: BD Biosciences), CD38 APC (clone OK10; NIH Nonhuman Primate Reagent Resource), CD3 APC-Cy7 (clone SP34–2; BD Biosciences), and Ki67 FITC (clone B56; BD Biosciences). Surface and intracellular staining was performed using the BD Cytofix/Cytoperm reagents and protocol. Adalimumab binding to rhesus macaque TNF is described in detail in the Supplementary Materials.
Statistical Analysis
Linear hierarchical mixed-effects and random-coefficient longitudinal regression models were used to determine treatment differences between adalimumab-treated rhesus macaques and untreated control rhesus macaques on a wide variety of criterion measures (eg, viral RNA load, TGF-β, etc.). This modeling approach recognizes multiple levels of random variation, including (1) among-animal variation within each treatment condition, (2) within-animal variation across time, and (3) within-animal replicate variation, and it takes into account within-macaque dependencies. Models were routinely tested to satisfy assumptions regarding homogeneity of variance and covariance. For gene expression studies, genome-scale analyses were performed on preinfection and postinfection RT-PCR data to determine upregulated and downregulated genes under adalimumab-treated and untreated conditions. Further parametric and nonparametric analyses included standard correlation and regression, analysis of variance, and follow-up t tests. P values of <.05 were considered statistically significant.
RESULTS
To assess the importance of the early proinflammatory cascade in SIV-infected rhesus macaques, we studied inhibition of TNF biological activity because of its importance as an early proinflammatory amplifier and because of the availability of pharmaceutical grade anti-TNF antibody (adalimumab) that could be used for in vivo nonhuman-primate studies [19]. This initial study included an untreated control group (n = 3) and an adalimumab treatment group (n = 3) (Figure 1A). In addition to the longitudinal specimens from these animals, individual samples from a separate cross-sectional cohort were analyzed (Table 1).
Verified Human Anti-TNF Cross-Reactivity With Rhesus Macaque TNF
We evaluated the cross-reactivity of adalimumab with rhesus macaque TNF and the adequacy of our adalimumab treatment in achieving in vivo blockade of TNF. In a bead-based flow cytometry assay, adalimumab comparably bound to rhesus macaque and human TNF, validating the use of adalimumab to block TNF in rhesus macaques (Figure 1B). We next performed TNF immunohistochemical analysis on lymph node sections from both untreated and adalimumab-treated rhesus macaques, using 2 antibodies that target different regions of TNF (Figure 1C). One antibody (mouse monoclonal anti-TNF P/T2) targets a region outside any identified TNF receptor–binding domains (amino acids 115–130), while the second antibody (rabbit polyclonal anti-TNF) maps to the C-terminal end of TNF, encompassing residues shown to be important for TNF receptor binding (amino acids 217–233) [28, 29]. TNF immunoreactivity was seen in the lymph nodes from both untreated and adalimumab-treated rhesus macaques when the mouse monoclonal antibody was used (Figure 1C). However, only the untreated rhesus macaques showed TNF immunoreactivity when the rabbit polyclonal antibody was used, indicating that adalimumab treatment effectively blocked the TNF receptor–binding domain of TNF in the lymphoid tissues of treated rhesus macaques.
Limited Expression of Inflammatory Response Genes in Lymphoid Tissues With Adalimumab Treatment
After we confirmed that adalimumab inhibited rhesus TNF, we assessed the effects of TNF blockade in rhesus macaques on inflammatory responses to SIV infection in lymphoid tissue. We compared RNA isolated from lymph node biopsy specimens obtained before and 4 weeks after infection (ie, 6 weeks after initiation of anti-TNF treatment), using a quantitative RT-PCR array that monitored 84 key inflammatory response genes, including known TNF responsive genes. In untreated rhesus macaques, 20 proinflammatory genes were significantly upregulated in lymph nodes at 4 weeks after infection, compared with biopsy specimens obtained before infection (Figure 1D). Upregulated mediators included proinflammatory chemokines and cytokines, the antiinflammatory cytokine interleukin 10 (IL-10), and other molecules important in innate and adaptive immune activation. In the adalimumab-treated rhesus macaques, only 6 proinflammatory genes were significantly upregulated (5 of which were also upregulated in control rhesus macaques), confirming attenuation of TNF activity with adalimumab treatment (Figure 1D). Importantly, of the 15 proinflammatory genes upregulated in untreated rhesus macaques but not in the adalimumab-treated rhesus macaques, expression of 10 genes (CCL3, CCL7, CCL19, CCR2, IL1A, IL1B, IL10, IL18RAP, FASLG, and RIPK2) have been demonstrated in various in vitro and in vivo models to be directly enhanced by TNF [30–35].
Viral Load and T-Cell Activation
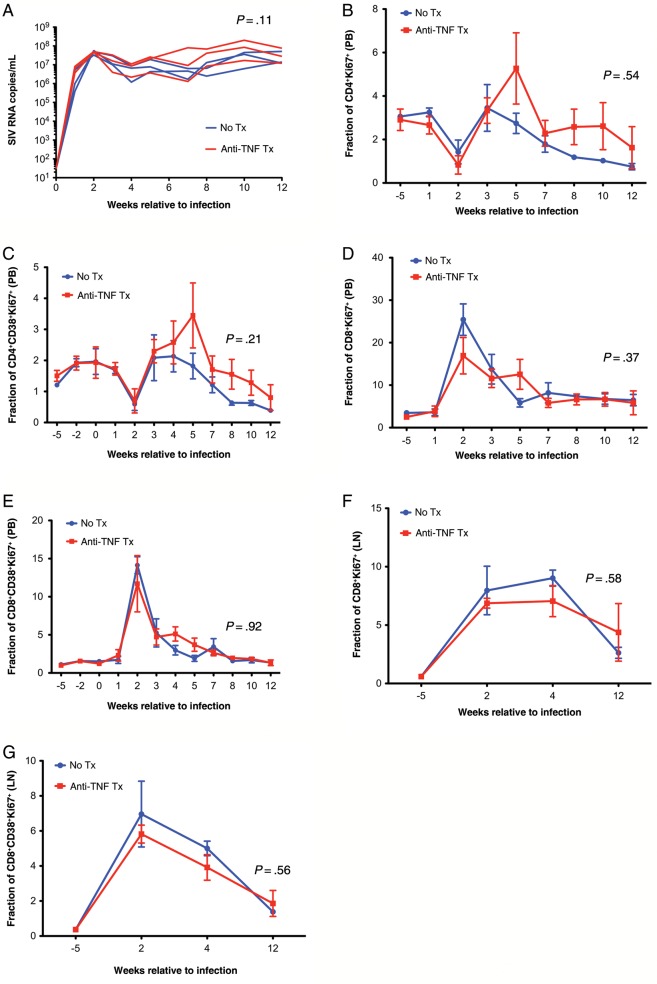
While proinflammatory cytokines, including TNF, may upregulate HIV replication in vitro via the NF-κB pathway [36], we found no apparent difference in either plasma viral load or tissue viral burden between adalimumab-treated and untreated animals (Figure 2 and data not shown), despite the clear antiinflammatory effects of the TNF blockade. We also evaluated immune activation in T cells during SIV infection. Surprisingly, flow cytometric analysis showed no differences between adalimumab-treated and untreated rhesus macaques in the frequencies of peripheral blood or lymph node T cells (CD3+CD4+ and CD3+CD8+) expressing classical markers of activation/proliferation (Ki67+ or CD38+Ki67+) throughout treatment (ie, 12 weeks after infection; Figure 2).
Figure 2.
No significant differences in viral load or CD4+ and CD8+ T-cell activation levels associated with anti–tumor necrosis factor (TNF) treatment. A, Viral load profiles in untreated (blue) and adalimumab-treated (red) rhesus macaques. Longitudinal flow cytometric measurements of the frequency of peripheral blood (PB) CD4+ or CD8+ T-cell subsets expressing the activation markers CD4+Ki67+ (B), CD4+CD38+Ki67+ (C), CD8+Ki67+ (D), and CD8+CD38+Ki67+ (E) in untreated (blue) and adalimumab-treated (red) rhesus macaques. Longitudinal flow cytometric measurements of the frequency of lymph node (LN) CD4+ or CD8+ T-cell subsets expressing the activation markers CD8+Ki67+ (F) and CD8+CD38+Ki67+ (G) in untreated (blue) and adalimumab-treated (red) rhesus macaques. Data points show mean ± standard error of the mean; n = 3 per group.
Limited Macrophage Infiltration Into the T-Cell Zone of Lymph Nodes With Adalimumab Treatment
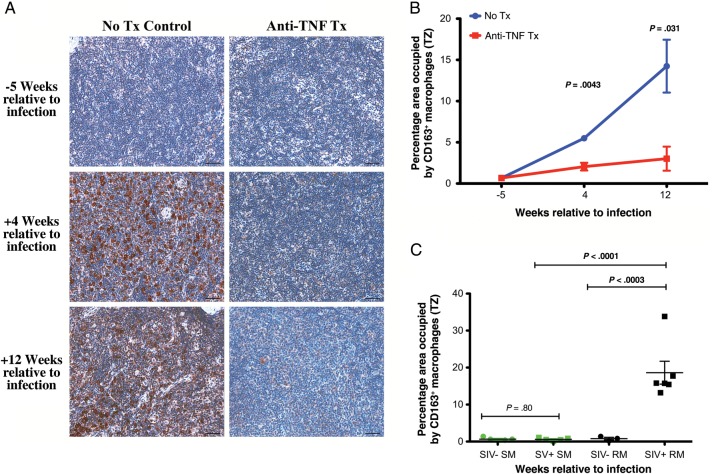
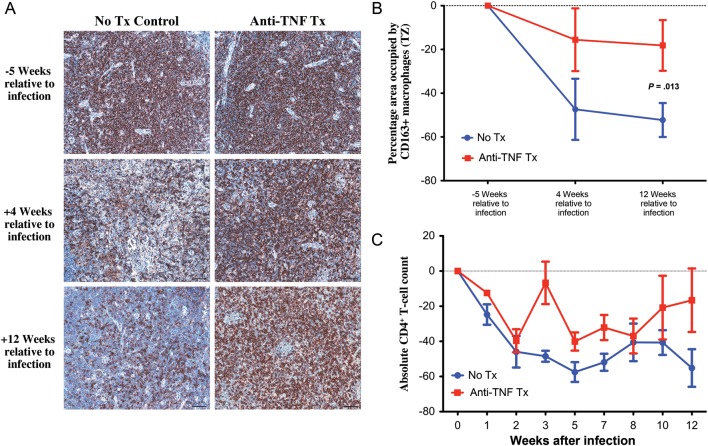
Because TNF is an important effector cytokine for the activation of macrophages, cells thought to play an important role in tissue pathogenesis in chronic inflammatory diseases [37, 38], we evaluated macrophage population dynamics in lymph nodes of SIV-infected rhesus macaques. We performed immunohistochemical and quantitative image analysis of lymph node sections for classical macrophages (CD68+) and presumed alternatively activated antiinflammatory (CD163+) macrophages, since our previous data demonstrated robust heterogeneous antiinflammatory responses to SIV infection [39]. We compared macrophage populations before infection and at 4 and 12 weeks after infection, focusing on the T-cell zone, a principal site of viral replication, CD4+ T-cell loss, and pathologic tissue damage [40]. The frequency of CD68+ classical macrophages increased in the T-cell zone from preinfection values in both the untreated and adalimumab-treated rhesus macaques, with no difference between untreated and adalimumab-treated rhesus macaques in the frequency of CD68+ macrophages in the lymph nodes at any time point (Supplementary Figure 1A and 1B). Consistent with the induction of an immunoregulatory response to acute SIV-triggered inflammation, untreated rhesus macaques demonstrated a progressive increase in CD163+ macrophages through 12 weeks after infection. In striking contrast, adalimumab-treated rhesus macaques had no significant change in the frequency of CD163+ macrophages, which was significantly lower compared with that in the untreated controls (Figure 3A and 3B). Furthermore, adalimumab-treated rhesus macaques also demonstrated a lack of polymorphonuclear leukocyte infiltration into the T-cell zone of lymph nodes, compared with the significant infiltration seen in untreated control rhesus macaques (Supplementary Figure 1C and 1D).
Figure 3.
Attenuated recruitment of CD163+ macrophages in the T-cell zone (TZ) of lymph nodes with anti–tumor necrosis factor (TNF) treatment. A, Representative images of the TZ of lymph node sections at 5 weeks before infection and 4 and 12 weeks after infection from untreated (left column) and adalimumab-treated (right column) rhesus macaques immunohistochemically stained for CD163 (presumed alternatively activated macrophages). B, Longitudinal changes in the percentage area of the TZ occupied by CD163+ macrophages from untreated (blue) and adalimumab-treated (red) rhesus macaques. Data points show mean ± standard error of the mean; n = 3 per group. C, No increase or difference in the percentage area of the TZ occupied by CD163+ macrophages from simian immunodeficiency virus (SIV)–negative (green circles) and SIV-infected (green squares) sooty mangabeys but significantly increased CD163+ macrophages in chronically SIV-infected rhesus macaques (black squares), compared with SIV-negative rhesus macaques (black circles) or chronically SIV-infected sooty mangabeys (green squares). Scale bars = 50 µm.
Despite limited reports of circulating CD163+ monocytes or CD163+ microglial cells in relation to central nervous system manifestations of SIV infection [41, 42], alternative activation of macrophages has not been a generally appreciated aspect of HIV/SIV pathogenesis. We built on the results of our adalimumab treatment study by comparing the frequency of CD163+ macrophages in the T-cell zone of lymph nodes from uninfected and chronically SIV-infected sooty mangabeys and rhesus macaques, using specimens from a separate cross-sectional cohort. Chronic-stage SIV-positive rhesus macaques had a significant increase in CD163+ macrophages within the T-cell zone, compared with uninfected animals (Figure 3C). However, SIV infection status did not influence this parameter in sooty mangabeys, a natural host species for SIV in which SIV infection does not result in pathologic chronic inflammation (Figure 3C). These data suggest that adalimumab administration alters the proinflammatory cytokine environment during acute SIV infection, thus limiting the antiinflammatory response, including limiting the recruitment of CD163+ presumed alternatively activated macrophages and polymorphonuclear leukocytes into the T-cell zone of lymph nodes.
Limited Lymphoid Tissue Fibrosis With Adalimumab Treatment
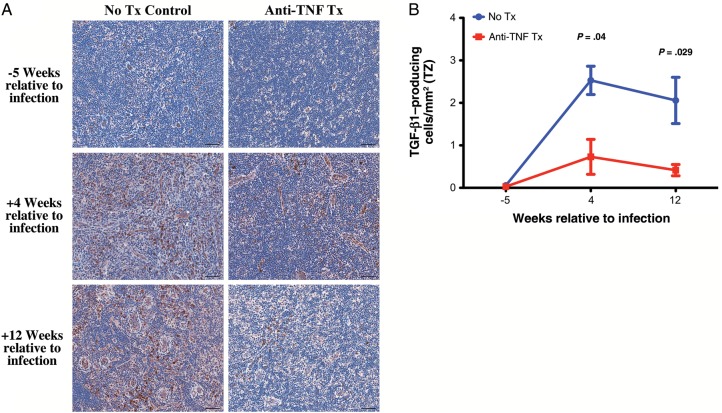
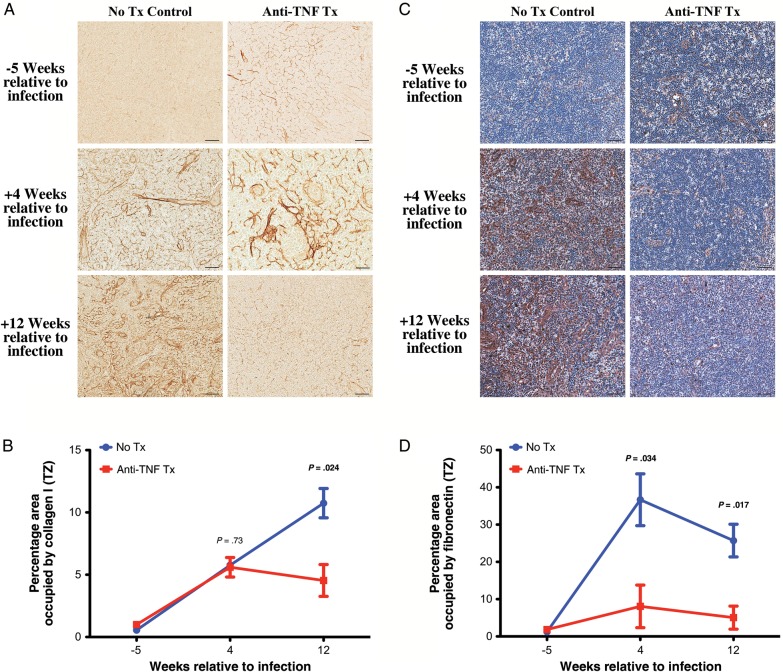
We previously demonstrated induction of an early immunosuppressive regulatory response to SIV infection that includes TGF-β–producing cells, particularly TGF-β–producing regulatory T cells, that is strongly associated with increased collagen deposition in lymphoid tissues and loss of CD4+ T cells [6, 39]. We therefore determined whether adalimumab treatment also attenuated this immunosuppressive regulatory response to SIV-triggered inflammation and limited the associated lymphoid tissue fibrotic damage. In untreated rhesus macaques, TGF-β–producing cells (Figure 4A and 4B) and IL-10–producing cells (data not shown) rapidly increased in the T-cell zone of lymph nodes after SIV infection. In stark contrast, in the adalimumab-treated animals, there was a limited population of TGF-β–producing cells (Figure 4A and 4B) and IL-10–producing cells (data not shown) in the T-cell zone. As previously reported [39], the majority of the TGF-β–producing cells within the T-cell zone were T cells (presumptively regulatory T cells), whereas the remaining TGF-β–producing cells consisted of a heterogeneous population of macrophages and lymphocytic non–T cells, likely B cells (data not shown). Importantly, associated with limited TGF-β expression, we found that adalimumab-treated rhesus macaques showed attenuated deposition of collagen type I (Figure 5A and 5B) and fibronectin (Figure 5C and 5D) as compared to untreated rhesus macaques that showed progressive lymph node fibrosis through 12 weeks after infection. Collectively, these data suggest that attenuating the early acute inflammatory responses to SIV infection additionally attenuates aspects of the early immunosuppressive and antiinflammatory regulatory responses, thus dramatically limiting progressive secondary lymphoid tissue fibrotic damage.
Figure 4.
Attenuated transforming growth factor β (TGF-β) expression in the T-cell zone (TZ) of lymph nodes with anti–tumor necrosis factor (TNF) treatment. A, Representative images of the TZ of lymph node sections at 5 weeks before infection and 4 and 12 weeks after infection from untreated (left column) and adalimumab-treated (right column) rhesus macaques immunohistochemically stained for TGF-β. B, Longitudinal changes in the percentage area of the TZ occupied by TGF-β–producing cells from untreated (blue) and adalimumab-treated (red) rhesus macaques. Scale bars = 50 µm. Data points show mean ± standard error of the mean; n = 3 per group.
Figure 5.
Attenuated collagen deposition in the T-cell zone (TZ) of lymph nodes with anti-tumor necrosis factor (TNF) treatment. A, Representative images of the TZ of lymph node sections at 5 weeks before infection and 4 and 12 weeks after infection from untreated (left column) and adalimumab-treated (right column) rhesus macaques immunohistochemically stained for collagen type I (no counter stain). B, Longitudinal changes in the percentage area of the TZ occupied by collagen I from untreated (blue) and adalimumab-treated (red) rhesus macaques. C, Representative images of the TZ of lymph node sections at 5 weeks before infection and 4 and 12 weeks after infection from untreated (left column) and adalimumab-treated (right column) rhesus macaques immunohistochemically stained for fibronectin. D, Longitudinal changes in the percentage area of the TZ occupied by fibronectin from untreated (blue) and adalimumab treated (red) rhesus macaques. Scale bars = 50 µm. Data points show mean ± standard error of the mean; n = 3 per group.
CD4+ T-Lymphocyte Preservation With Adalimumab Treatment
HIV/SIV-mediated fibrotic damage to lymph node architecture is associated with CD4+ T-lymphocyte loss and limited reconstitution during ART [6, 9, 40, 43, 44]. We examined the effects of adalimumab treatment on CD4+ T lymphocytes in peripheral blood and lymph node sections. In immunohistochemical studies of lymph node sections (Figure 6), untreated SIV-infected rhesus macaques had a significant, progressive loss of CD4+ T cells in the T-cell zone. In contrast, adalimumab-treated animals had no significant change in the fraction of cells that were CD4+ in the T-cell zone, which is consistent with the preservation of lymph node architecture and the limited T-cell zone fibrosis observed in these animals. While direct comparisons of the number of CD4+ T cells per microliter in peripheral blood showed no statistically significant difference across treatment arms, the trends over time differed between the 2 groups. Untreated rhesus macaques had a significant downward trend from baseline in peripheral blood CD4+ T-lymphocyte counts (P = .0068), while the adalimumab-treated group had no significant change from baseline (P = .32; Figure 6C). Collectively, these data suggest that modulation of the proinflammatory response to acute SIV infection can have a substantial impact on lymphoid tissue damage and CD4+ T-cell preservation, 2 indicators of disease progression, even with no amelioration of viral replication or conventional measures of T-cell immune activation.
Figure 6.
Preservation of CD4+ T cells in the T-cell zone (TZ) of lymph nodes with anti–tumor necrosis factor (TNF) treatment. A, Representative images of the TZ of lymph node sections at 5 weeks before infection and 4 and 12 weeks after infection from untreated (left column) and adalimumab-treated (right column) rhesus macaques immunohistochemically stained for CD4. B, Longitudinal changes from baseline in the percentage area of the TZ occupied by CD4+ T cells from untreated (blue) and adalimumab-treated (red) rhesus macaques. C, Longitudinal change from baseline in absolute peripheral blood CD4+ T cells in untreated control (blue) and adalimumab-treated (red) rhesus macaques. Each group was analyzed with a linear mixed effects model. Untreated rhesus macaques had a significant decrease over time (P = .011). Anti-TNF treated rhesus macaques had no significant change (P = .17) and were significantly different from untreated rhesus macaques (P = .013). Scale bars = 50 µm. Data points show mean ± standard error of the mean; n = 3 per group.
DISCUSSION
We studied SIV-infected rhesus macaques to ascertain the influence of the acute inflammatory response to the infection on pathogenesis and disease progression. Because the early inflammatory cascade initiated by most pathogens is typically complex and redundant, achieving complete blockade of the inflammatory response in acute HIV/SIV infection is likely impossible. However, we reasoned that blocking TNF, an important amplifier of the initial inflammatory responses to SIV, might impact immune activation and modulate downstream immunopathogenic effects.
Intriguingly, blockade of the biological activity of TNF through the first 12 weeks of infection did not affect viral replication dynamics or classical measures of T-cell activation, but it did limit immunopathogenic damage to lymphoid tissues and resulted in preserved CD4+ T-cell populations, which are important predictors of disease progression. This suggests that early inflammatory responses may critically influence disease progression. Consistent with this interpretation, natural hosts (ie, sooty mangabeys and African green monkeys) of SIV infection that do not progress to disease quickly down-modulate their robust acute inflammatory responses, whereas in pathogenic infection of rhesus macaques immune activation remains high throughout all stages of infection [45–48]. Strategies targeting other proinflammatory pathways, including those leading to T-cell activation, could be fruitful areas for future research. Animals in this study were subjected to intravenous exposure, to ensure that 100% of the animals were infected synchronously, as opposed to mucosal exposure, which is the more common route of infection in humans. It will be important to determine in subsequent studies how altering the acute inflammatory response effects mucosal transmission efficiency and viral dissemination and how important the early inflammatory response is in shaping the adaptive antiviral responses.
In this pilot study, attenuation of proinflammatory responses was reflected not only in the genes that were expressed in lymph node cells, but also in the attenuation of polymorphonuclear leukocyte infiltration in adalimumab-treated rhesus macaques, compared with untreated animals. Adalimumab treatment also attenuated several components of the heterogeneous immunosuppressive response induced after SIV infection. The specific significance of the recruitment of presumed “alternatively activated,” antiinflammatory (CD163+) macrophages into the T-cell zone of secondary lymphoid tissues remains unclear, but it is striking that these cells were abundant in the T-cell zone of untreated, SIV-infected control rhesus macaques but not in adalimumab-treated, SIV-infected rhesus macaques or in SIV-infected sooty mangabeys. Limited accumulation of CD163+ macrophages in adalimumab-treated rhesus macaques may reflect reduced recruitment of monocytes due to attenuated levels of proinflammatory monocyte/macrophage chemoattractants in lymphoid tissues. Importantly, CD163+ macrophage infiltration has been seen in many other inflammatory conditions, and decreased CD163+ macrophage populations have been described with anti-TNF therapy in arthritis [49]. Collectively, these data suggest a potential role for CD163+ macrophages in the pathogenesis of HIV/SIV infections and that limiting recruitment and activation of these cells in the parenchyma of lymphoid tissues may be beneficial.
In the absence of either viral clearance (which would remove the initial inflammatory stimulus) or active downmodulation of the inflammatory cascade (as is seen in SIV infections in natural hosts) both proinflammatory and antiinflammatory responses persist and escalate, leading to significant ongoing lymphoid tissue damage. One aspect of the antiinflammatory response (ie, TGF-β expression) is associated with significant lymphoid tissue fibrotic damage, CD4+ T-cell loss, and disease progression [39]. Adalimumab-mediated downmodulation of the acute inflammatory responses dramatically attenuated TGF-β expression and secondary lymphoid tissue fibrosis in treated animals, resulting in preserved lymphoid tissue CD4+ T cells. TNF can directly upregulate TGF-β expression [50], suggesting a role for early inflammatory responses in general and for TNF in particular in driving the accumulation of TGF-β–producing cells in the T-cell zone of lymphoid tissues in SIV infection. The limited CD4+ T-cell loss in adalimumab-treated rhesus macaques is remarkable in the context of no difference in viral loads between the treated and untreated groups.
These data suggest that targeted early antiinflammatory adjunctive treatment in combination with ART may be beneficial in HIV-infected patients by reducing HIV-associated inflammatory responses. This initial study highlights the importance of early inflammatory responses to lentiviral infections and underscores the need for additional studies to ascertain the potential clinical benefits of adjunctive therapies to attenuate these responses and to improve patient outcomes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge the expert support from the Pathology/Histotechnology Laboratory, ACVP Specimen Support Core, and the animal care provided by the Laboratory Animal Science Program staff at the Frederick National Laboratory for Cancer Research (Frederick, MD).
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 2.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83:12229–40. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, Andersson J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS. 2007;21:565–74. doi: 10.1097/QAD.0b013e3280117204. [DOI] [PubMed] [Google Scholar]
- 6.Estes JD, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–61. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. Interleukin-1 and tumor necrosis factor: effector cytokines in autoimmune diseases. Semin Immunol. 1992;4:133–45. [PubMed] [Google Scholar]
- 8.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 9.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–602. [PubMed] [Google Scholar]
- 10.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:375–80. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 11.Barcellini W, Rizzardi GP, Poli G, et al. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:325–31. doi: 10.1089/aid.1996.12.325. [DOI] [PubMed] [Google Scholar]
- 12.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayette P, Le Grand R, Noack O, et al. Tumor necrosis factor-alpha in serum of macaques during SIVmac251 acute infection. J Med Primatol. 1995;24:94–100. doi: 10.1111/j.1600-0684.1995.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 14.Khatissian E, Chakrabarti L, Hurtrel B. Cytokine patterns and viral load in lymph nodes during the early stages of SIV infection. Res Virol. 1996;147:181–9. doi: 10.1016/0923-2516(96)80233-0. [DOI] [PubMed] [Google Scholar]
- 15.Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76:5797–802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornfeld C, Ploquin MJ, Pandrea I, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton K, Clair EW. Tumour necrosis factor-alpha blockade: a new era for effective management of rheumatoid arthritis. Expert Opin Pharmacother. 2000;1:1041–52. doi: 10.1517/14656566.1.5.1041. [DOI] [PubMed] [Google Scholar]
- 18.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152–67. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 19.Lin PL, Myers A, Smith L, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–50. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker RE, Spooner KM, Kelly G, et al. Inhibition of immunoreactive tumor necrosis factor-alpha by a chimeric antibody in patients infected with human immunodeficiency virus type 1. J Infect Dis. 1996;174:63–8. doi: 10.1093/infdis/174.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Wallis RS, Kyambadde P, Johnson JL, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18:257–64. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 22.Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197–200. doi: 10.1016/j.jbspin.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Cepeda EJ, Williams FM, Ishimori ML, Weisman MH, Reveille JD. The use of anti-tumour necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710–2. doi: 10.1136/ard.2007.081513. [DOI] [PubMed] [Google Scholar]
- 24.Sha BE, Valdez H, Gelman RS, et al. Effect of etanercept (Enbrel) on interleukin 6, tumor necrosis factor alpha, and markers of immune activation in HIV-infected subjects receiving interleukin 2. AIDS Res Hum Retroviruses. 2002;18:661–5. doi: 10.1089/088922202760019365. [DOI] [PubMed] [Google Scholar]
- 25.Estes JD, Gordon SN, Zeng M, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes JD, Harris LD, Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001052. e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 28.Mukai Y, Nakamura T, Yoshikawa M, et al. Solution of the structure of the TNF-TNFR2 complex. Sci Signal. 2010;3:ra83. doi: 10.1126/scisignal.2000954. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher H, Stueber D, Banner D, Mackay F, Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993;268:26350–7. [PubMed] [Google Scholar]
- 30.Ramos CD, Canetti C, Souto JT, et al. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78:167–77. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate JW, van Deventer SJ. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1994;180:1985–8. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected] Brain Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia L, Lu J, Xiao W. Blockage of TNF-alpha by infliximab reduces CCL2 and CCR2 Levels in patients with rheumatoid arthritis. J Investig Med. 2011;59:961–3. doi: 10.2310/JIM.0b013e31821c0242. [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekar B, Colston JT, de la Rosa SD, Rao PP, Freeman GL. TNF-alpha and H2O2 induce IL-18 and IL-18R beta expression in cardiomyocytes via NF-kappa B activation. Biochem Biophys Res Commun. 2003;303:1152–8. doi: 10.1016/s0006-291x(03)00496-0. [DOI] [PubMed] [Google Scholar]
- 35.Bonetti B, Valdo P, Ossi G, et al. T-cell cytotoxicity of human Schwann cells: TNFalpha promotes fasL-mediated apoptosis and IFN gamma perforin-mediated lysis. Glia. 2003;43:141–8. doi: 10.1002/glia.10235. [DOI] [PubMed] [Google Scholar]
- 36.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner AR, Arthur MJ, Wright R. Macrophage activation, chronic inflammation and gastrointestinal disease. Gut. 1984;25:760–83. doi: 10.1136/gut.25.7.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valledor AF, Comalada M, Santamaria-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol. 2010;108:1–20. doi: 10.1016/B978-0-12-380995-7.00001-X. [DOI] [PubMed] [Google Scholar]
- 39.Estes JD, Li Q, Reynolds MR, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–12. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 40.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris LD, Tabb B, Sodora DL, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–91. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosinger SE, Li Q, Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect. 2011;13:14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baeten D, Demetter P, Cuvelier CA, et al. Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J Pathol. 2002;196:343–50. doi: 10.1002/path.1044. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med. 2009;13:1866–76. doi: 10.1111/j.1582-4934.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.