Abstract
Background. Genital human papillomavirus (HPV) infection is believed to be primarily sexually transmitted. Few studies have documented the detection of HPV in the vagina before first vaginal intercourse.
Methods. We used a longitudinally followed cohort of adolescent females without prior vaginal intercourse to examine the frequency of detection of vaginal HPV and the association between first reported HPV detection and noncoital sexual behaviors.
Results. HPV was detected in 45.5% of subjects (10 of 22) before first vaginal sex. Seven of these 10 subjects reported noncoital behaviors that, in part, might have explained genital transmission.
Conclusions. HPV can be detected in the vagina before first sexual intercourse, highlighting the need for early vaccination.
Keywords: human papillomavirus, sexual behaviors, adolescents
The primary mode of transmission for infection of the genital tract with human papillomavirus (HPV) is believed to be vaginal intercourse. Many studies have documented a high prevalence of HPV among adolescent females who have reported vaginal intercourse [1, 2]. Detection of HPV within the genital tract or oral mucosa of young women without a history of voluntary or involuntary sexual intercourse has been documented without clear elucidation of factors that may have resulted in viral transmission [3–5]. Most studies have been cross-sectional and tested for HPV at a single time point. Thus, such studies are not ideal for determining the time sequence of vaginal intercourse and infection acquisition. Understanding of the factors associated with detection of HPV during adolescence has implications for the timing of HPV vaccination. In this study, data from a longitudinal cohort of adolescent females were analyzed. The timing of first HPV detection, first reported vaginal intercourse, and the association between noncoital sexual behaviors and first reported HPV detection were examined to gain insights into possible mechanisms of HPV transmission.
METHODS
Participants
A longitudinal study of risk and protective factors associated with sexually transmitted infections (STIs) in 387 females aged 14–17 years at enrollment was reported in 2001 [6]. All study subjects were recruited from inner-city, university-affiliated clinics. Inclusion criteria included the ability to understand English, provision of written consent, and the availability of at least 1 parent to provide consent for participation at the time of enrollment; parents were made aware that questions and responses about sexual behaviors and STI testing were kept confidential. None of the subjects received HPV vaccination during the study period because vaccination was unavailable at that time. The study was approved by the Indiana University Institutional Review Board; participants were compensated for their time.
Sexual Behavior Assessments
Face-to-face interviews, written self-completed surveys, and daily diaries were used to construct the subjects' history of sexual exposures and sexual behaviors. Interviews were conducted at enrollment and approximately every 3 months thereafter. Written questionnaires were completed at enrollment and annually thereafter. Finally, subjects completed written daily diaries about sexual behaviors during alternating 3-month periods between interviews (ie, two 3-month periods per year). Yearly questionnaires asked subjects whether they ever had sexual intercourse and, if so, what their age was at first intercourse. Subjects identified their sex partners (defined as “someone you have had sex with, someone that you had sexual contact with in other ways or someone important to you”) during the last 3 months before the interview. For each partner identified, subjects reported whether they had touched their partner's genitals, their partner had touched the subject's breasts or genitals, they participated in deep kissing (explained as “French kissing”), their partner had put his penis in the subject's vagina, or their partner had put his mouth on the subject's genitals. Diary periods included daily reporting of vaginal intercourse (recorded as “yes” or “no”). The date of first lifetime vaginal intercourse was determined by either the first report at a quarterly interview or by a daily diary report.
HPV Detection
All subjects had either a vaginal swab sample obtained by a clinician or provided self-collected vaginal samples at enrollment and every 3 months thereafter, at the time of the quarterly interview. Dry vaginal swab samples were transported to the laboratory after collection and vortexed in a tube containing 1 mL of sterile water. The solution was subsequently stored at −20°C until processing for HPV testing. For HPV testing and genotyping, DNA was extracted from samples, using QIAamp MinElute Media Kit (Qiagen, Valencia, CA). The Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Indianapolis, IN) was used for HPV detection and genotyping [7]. This assay detects 37 HPV types, using 5′ biotin-labeled primer pools for polymerase chain reaction (PCR) amplification within the L1 region of the HPV genome. The 37 individual HPV types detected by the Linear Array HPV test comprise high-risk HPV (HR-HPV) types and low-risk HPV (LR-HPV) types. Assessment of types on the basis of the International Agency for Research on Cancer (IARC) definition of HR-HPV types was also performed (Table 1).
Table 1.
Demographic Characteristics of and Human Papillomavirus (HPV) Types Found in Adolescent Females Before First Vaginal Sex
| Variable | Swab Samples Positive for HPV, No. |
Days Between First and Second Positive Swab Sample, Median (IQR) | |
|---|---|---|---|
| ≥1 (n = 10) | ≥2 (n = 7) | ||
| Demographic characteristic | |||
| Age, y, mean ± SD | 14.9 ± 1.2 | 14.9 ± 1.1 | … |
| African American race | 10 (100) | 7 (100) | … |
| Age at first vaginal sex, y, mean ± SD | 17.2 ± 1.3 | 16.7 ± 0.8 | … |
| Age at first HPV detection, y, mean ± SD | 16.6 ± 1.6 | 16.2 ± 0.8 | … |
| HPV type detected | |||
| Any | 29 | 19 | 273 (1200) |
| High risk | |||
| Overalla | 16 | 9 | 273 (360) |
| IARC-defined carcinogenb | 10 | 5 | 231 (444) |
| Low riskc | 13 | 5 | 347 (1379) |
| 6, 11, 16, and/or 18 | 3 | 2 | 1180 (1323) |
| 16 and 18 | 1 | 1 | 1841 |
Data are no. or no. (%) of subjects, unless otherwise indicated.
Abbreviation: IQR, interquartile range.
a Types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, and IS39.
b Types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. All are considered group 1 or 2 carcinogens, according to the International Agency for Research on Cancer (IARC).
c Types 6, 11, 40, 42, 54, 55, 61, 62, 64, 72, 81, 83, 84, and CP6108.
All PCR reactions were amplified in a PerkinElmer TC9600 Thermal Cycler (PerkinElmer) and interpreted as previously described. A positive control reaction (provided by Qiagen) and a negative control reaction (no DNA) were performed with each assay. The GH20/PC04 human β-globin target was coamplified to determine sample adequacy. Identification of specific HPV types was performed as previously described [8]. The Linear Array HPV test is an expanded version of an older generation Roche line blot assay used in our prior longitudinal analysis of the same cohort. Testing for Chlamydia trachomatis and Neisseria gonorrhoeae was performed using the Amplicor CT/NG PCR assay (Roche Diagnostics, Indianapolis, IN), and testing for Trichomonas vaginalis was performed using a modified Amplicor kit.
Analysis
Descriptive statistics were used to describe the sample characteristics. An HPV infection was defined as detection of a specific HPV type in ≥1 vaginal swab samples. Graphical tools were used to examine the association between age at first sex and age at first HPV detection. Bivariate analysis was performed to assess the relationship between a positive HPV test result and noncoital sexual behaviors before first reported coitus.
RESULTS
Thirty-six of 387 enrolled subjects reported no coitus at the time of enrollment. Of these 36, 14 were excluded for the following reasons: in later interviews, subjects reported the time of first vaginal sex to be before enrollment (n = 3); sampling for ≥2 consecutive specimens was inadequate (ie, specimens were β-globin negative), or >10% of all specimens were β-globin negative (n = 2); and vaginal nucleic acid amplification tests were positive for N. gonorrhoeae, C. trachomatis, or T. vaginalis before reported first coitus (n = 9). Thus, 22 subjects without a history of coitus at study enrollment were used for this analysis.
For these 22 subjects, the mean age at enrollment (±SD) was 14.7 ± 0.95 years; 20 (90.9%) were African American. The mean duration of follow-up after enrollment was 5.2 years (range, 4.5–8.2 years). All but 1 subject reported initial coitus during follow-up, with first sex occurring at a mean age (±SD) of 16.5 ± 1.2 years. The median time from enrollment to first coitus was 429 days. The 22 subjects had a mean number (±SD) of 6.7 ± 5.0 quarterly swab samples (range, 1–20 swab samples) collected before first coitus or the end of the follow-up period. All subjects had type-specific HPV detected before or after first coitus.
Detection of at least 1 HPV type occurred before first coitus for 10 subjects (45.2%). For 8 of these 10, ≥1 HR-HPV type was identified. Vaccine-related HPV types 6, 11, and 16 were detected in 3 of the 10 subjects.
A total of 29 type-specific infections were detected in these 10 subjects before first coitus. Three type-specific infections were vaccine-related HPV types, and 16 were HR-HPV types. The most frequently detected HR type-specific infections were HPV 66 (n = 3), followed by HPV 39 (n = 2) and HPV 51 (n = 2); by the IRAC definition, HPV 66 would have not been considered in this group [9]. HPV 16 was detected in 1 individual before first coitus; none had HPV 18 detected before first coitus. Thirteen LR-HPV type-specific infections were found; LR-HPV types 42 and 62 were detected in 3 participants, and HPV 6 was detected in 2 participants before first coitus.
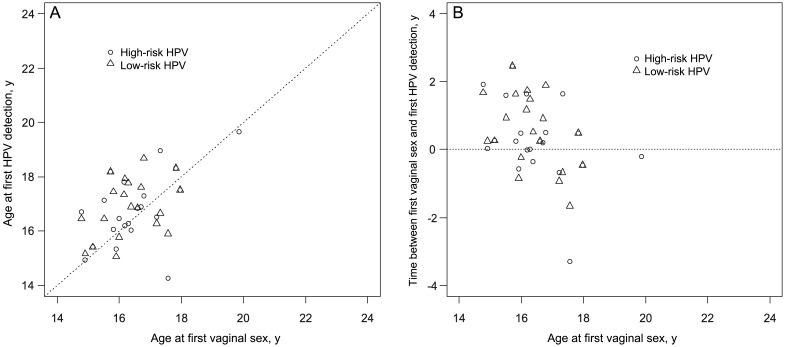
As stated previously, 29 (13.2%) of the type-specific HPV infections were detected before first vaginal sex. On recognition that most infections occurred after first coitus, we examined the ages at first coitus, first HPV detection, first HR-HPV and LR-HPV detection, and first vaccine-related HPV detection in these 22 participants (Figure 1). The mean age (±SD) at first coitus was 16.5 ± 1.2 years; the mean age (±SD) at first HPV detection was 16.7 ± 1.2 years. The mean age (±SD) at first HR-HPV detection was 16.9 ± 1.2 years, <4 months after initial coital experience. The mean time (±SD) from first sex to detection of first vaccine-related HPV type was 1.5 ± 1.2 years.
Figure 1.
Scatterplots of age at first vaginal sex and age at first detection of high-risk and low-risk human papillomavirus (HPV) types (A) and time between first vaginal sex and first detection of high-risk and low-risk HPV types (B) for 21 females. Data for 1 subject were excluded because first vaginal sex never occurred during the study.
In some cases, detection of HPV DNA in a single sample may have represented a transient deposition rather than true infection. When isolated samples were excluded and HPV type-specific detection was redefined by ≥2 positive samples (with at least 1 detection before first sex) for an individual, 19 type-specific HPV infections were identified before first coitus in 7 subjects (as opposed to 29 type-specific HPV infections in 10 subjects). For these 19 infections, the median interval between the first and second positive samples was 91 days (range, 70–1523 days), indicating that most of these infections were detected consecutively in at least 2 quarterly samples. The distribution of HPV infections as defined by a single positive sample or by ≥2 positive swab samples for specific HPV types is displayed in Table 1.
Several noncoital behaviors were reported before first coitus and first HPV detection. Most subjects (n = 18; 81.8%) reported deep kissing; over half (n = 12; 54.6%) reported that they had either touched a partner's genitals or had been touched on the genitals by a partner before first coitus. Four subjects (18.2%) reported having given oral-genital contact, and 5 (22.7%) reported having received oral-genital contact. No subject reported penile-anal intercourse before first coitus. Three subjects reported none of the noncoital behaviors before first coitus but were HPV positive before first coitus. Moreover, 7 subjects reported at least 1 noncoital sexual behavior before first HPV detection and first coitus. No specific noncoital behavior reported was more likely to be associated with detection of HPV.
DISCUSSION
Detection of HPV types was seen in nearly half of this closely followed cohort of 22 subjects before first vaginal sex. Ten subjects (45.5%) had HPV detected before first coitus; 7 of these 10 subjects had the same HPV type detected a second time. Other recent studies have addressed HPV detection before the initiation of vaginal sex. In a larger longitudinal cohort of college women, HPV was detected in 9% of the cohort before first coitus [4]. In another study of young adults, the authors found no HPV in two consecutive tests performed two years apart [10]. In part, the results reported here may differ from those of both studies because of the frequent sampling that occurred and the use of methods that permitted detection of a greater number of HPV types. By using similar HPV detection methods, Widdice et al detected HPV in 11.6% of young women (8 of 69) who, at the time of first HPV vaccination, denied ever having vaginal sex [11].
Noncoital behaviors may explain some of the HPV detected before first coitus. Like other studies that documented vaginal HPV before coitarche, all 22 subjects in our study denied sexual abuse at enrollment or during the follow-up [3, 4]. The reporting of other sexual behaviors before onset of first vaginal sex is well documented [12]. Studies of HPV transmission in young adult heterosexual couples supports hand-genital and oral-genital transmission [13, 14]. Hand-genital or mouth-genital contact may occur with several different partners before first coitus and allows for a plausible explanation of HPV detection before first coitus [15]. However, there was no assessment of noncoital intercourse (ie, genital-genital contact called “frottage”), which could also have transmitted HPV. Many of the HPV types in this reported study were redetected in the individual within a short period of 3 months, suggesting that these were true infections rather than transient depositions. However, reexposure could also explain these findings. Finally, reporting accuracy may have also influenced our results, but our data have some internal consistency that supports the accuracy of self-reports.
Additional studies are needed to further assess factors for transmission of HPV before vaginal sex, and further evaluation is needed to understand whether these early infections are associated with HPV-related outcomes, including cancer. In summary, these findings confirm the need for HPV vaccination in early adolescence and before first sex. Given these findings that sexual contact other than vaginal intercourse places a woman at risk for HPV infection, the need for HPV vaccination during early adolescence becomes absolute.
Notes
Financial support. This work was supported by the National Institutes of Health (grant R01 AI072020-01A2 to D. R. B.).
Potential conflicts of interest. M. L. S. is an investigator for HPV vaccine trials sponsored by Merck. D. R. B. received lecture fees, advisory board fees, and intellectual property fees from Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Manhart LE, Holmes KK, Koutsky LA, et al. Human papillomavirus infection among sexually active young women in the United States: Implications for developing a vaccination strategy. Sex Transm Dis. 2006;33:502–8. doi: 10.1097/01.olq.0000204545.89516.0a. [DOI] [PubMed] [Google Scholar]
- 3.Doerfler D, Bernhaus A, Kottmel A, Sam C, Koelle D, Joura EA. Human papilloma virus infection prior to coitarche. Am J Obstet Gynecol. 2009;200:487 e1–5. doi: 10.1016/j.ajog.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Winer RL, Hughes JP, Feng Q, et al. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2010;20:699–707. doi: 10.1158/1055-9965.EPI-10-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cason J, Mant CA. High-risk mucosal human papillomavirus infections during infancy & childhood. J Clin Virol. 2005;32(Suppl 1):S52–8. doi: 10.1016/j.jcv.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Katz BP, Fortenberry JD, Tu W, Harezlak J, Orr DP. Sexual behavior among adolescent women at high risk for sexually transmitted infections. Sex Transm Dis. 2001;28:247. doi: 10.1097/00007435-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001;10:101–6. [PubMed] [Google Scholar]
- 11.Widdice L, Brown D, Bernstein D, et al. Prevalence of HPV in sexually experienced and inexperienced females initiating HPV vaccination. J Adolesc Health. 2011;48 S2–S. [Google Scholar]
- 12.Halpern-Felsher BL, Cornell JL, Kropp RY, Tschann JM. Oral versus vaginal sex among adolescents: perceptions, attitudes, and behavior. Pediatrics. 2005;115:845. doi: 10.1542/peds.2004-2108. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez BY, Wilkens LR, Xuemei Z, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer RL, Hughes JP, Feng Q, et al. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiol Biomarkers Prev. 2010;19:1682–5. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Sullivan LF, Cheng MM, Harris KM, Brooks-Gunn J. I wanna hold your hand: the progression of social, romantic and sexual events in adolescent relationships. Perspect Sex Reprod Health. 2007;39:100–7. doi: 10.1363/3910007. [DOI] [PubMed] [Google Scholar]