Abstract
Galectin-3 is a β-galactoside–binding lectin widely expressed on epithelial and hematopoietic cells, and its expression is frequently associated with a poor prognosis in cancer. Because it has not been well-studied in human infectious disease, we examined galectin-3 expression in mycobacterial infection by studying leprosy, an intracellular infection caused by Mycobacterium leprae. Galectin-3 was highly expressed on macrophages in lesions of patients with the clinically progressive lepromatous form of leprosy; in contrast, galectin-3 was almost undetectable in self-limited tuberculoid lesions. We investigated the potential function of galectin-3 in cell-mediated immunity using peripheral blood monocytes. Galectin-3 enhanced monocyte interleukin 10 production to a TLR2/1 ligand, whereas interleukin 12p40 secretion was unaffected. Furthermore, galectin-3 diminished monocyte to dendritic cell differentiation and T-cell antigen presentation. These data demonstrate an association of galectin-3 with unfavorable host response in leprosy and a potential mechanism for impaired host defense in humans.
Keywords: human, bacterial infections, galectin-3, monocytes/macrophages, TLR2
Galectin-3 is expressed by numerous cell types, including leukocytes, and has been shown to influence human monocyte and macrophage function [1, 2]. Human peripheral blood monocytes express increasing levels of galectin-3 as they differentiate into macrophages [3]. In addition, galectin-3 is secreted by monocytes and macrophages [1, 3, 4] and can act in an autocrine or paracrine fashion to induce superoxide production [3]. Increasing evidence supports a role for galectin-3 in regulating adaptive immune responses, including both T-helper cell 1 (Th1) [5, 6] and T-helper cell 2 (Th2) responses [7, 8] depending on the context of the host immune response. Here we investigated the role of galectin-3 in human infectious disease using leprosy as a model.
Leprosy, caused by the intracellular bacterium Mycobacterium leprae, presents as a spectrum of clinical manifestations that correlate with the level of cell-mediated immunity against the bacteria [9]. At one end of the spectrum, patients with tuberculoid leprosy (T-lep) are able to restrict the pathogen; the number of skin lesions and bacteria are few, and patients mount a strong cell-mediated immune response against M. leprae. In contrast, patients with lepromatous leprosy (L-lep) are unable to contain the infection; the skin lesions are disseminated and contain numerous bacteria, reflecting a weak cell-mediated response against the pathogen. These clinical outcomes also reflect distinct T-helper cell cytokine patterns in the lesions. T–lep lesions have Th1 (interleukin 12 [IL-12] and interferon γ) cytokine patterns, whereas L-lep lesions have Th2 (interleukin 4 [IL-4], interleukin 5 [IL-5], and interleukin 10 [IL-10]) patterns [10–13]. In the context of M. leprae infection, the type 2 cytokines IL-4 and IL-10 have immunoregulatory roles [12, 14] suppressing T-cell responses to the pathogen [15]. We have previously used gene expression profiling to identify novel immunoregulatory pathways in microbial immunity [16, 17]. Reexamination of these microarray data (GSE17763) [18] revealed higher expression of LGALS3 in L-lep vs T-lep lesions, correlating with disseminated infection. To gain insight into galectin-3–mediated immune modulation, we examined its expression at the site of mycobacterial infection and its role on human monocytes in vitro.
METHODS
Patients and Clinical Specimens
The acquisition of all skin biopsy specimens from leprosy patients and peripheral blood from healthy human donors was reviewed and approved by the committees on investigations involving human subjects of the University of California, Los Angeles, and the John Wayne Cancer Institute. For all procedures, written informed consent was obtained. Clinical classification of patients with symptomatic M. leprae infection was performed according to the criteria of Ridley and Jopling [9].
Reagents and Antibodies
Extracts of Mycobacterium tuberculosis were prepared by probe sonication as previously described [19] and provided by Dr John Belisle (Colorado State University). Mycobacterial lipomannan was provided by Dr Larry Schlesinger (Ohio State University). These mycobacterial antigens were used in the antigen presentation experiments. To activate TLR2/1, a 19-kD lipopeptide of M. tuberculosis was purchased from EMC Microcollections [20]. Live M. leprae was prepared as previously described [21] and provided by Drs James Krahenbuhl and Ramanuj Lahiri (US National Hansen's Disease Program). Mycobacterium bovis TICE strain was purchased from Organon.
Recombinant human galectin-3 was prepared as previously described [22]. Galectin-3 was reconstituted in 10% glycerol in phosphate-buffered saline (PBS), which we used as vehicle control; the amount of lipopolysaccharide present in galectin-3 was below the limit of detection (<0.1 endotoxin unit/mL or 0.01 ng/mL), measured by Limulus amebocyte lysate assay (Lonza) according to the manufacturer's instructions. To demonstrate the lectin specificity of galectin-3, lactose (Sigma) was used as a specific inhibitor, and sucrose (Sigma) was used as a negative control because it does not bind to galectin-3. In the assays using lactose and sucrose, the sugars were added to the culture wells before the addition of galectin-3.
The following monoclonal antibodies were used: BCD1b3.1 (anti-CD1b [23]), 9C4 (anti-galectin-3, Abcam), B2C10 (anti-galectin-3 [24]), RPA-MI (anti-CD14, Zymed Laboratories), PG-M1 (anti-CD68, DakoCytomation), TU36 (anti-HLA-DR, BD Biosciences), and appropriate isotype controls (Caltag Laboratories, Sigma-Aldrich, and BD Biosciences). The following polyclonal antibodies were used: Alexa Fluor 568 goat antimouse IgG1 (Invitrogen), Alexa Fluor 488 goat antimouse IgG2b (Invitrogen), and Alexa Fluor 488 goat antimouse IgG3 (Invitrogen). Polyclonal rabbit anti-human galectin-3 [25] was used for immunoblotting.
Immunohistochemical Studies
Skin biopsy sections from paraffin-embedded tissue were deparaffinized by 4 xylene washes (5 minutes each) followed by 3 100% ethanol washes (3 minutes each). Endogenous peroxidases were blocked by incubation with 0.75% hydrogen peroxide in methanol (30 minutes) followed by a wash in 100% ethanol (3 minutes). Slides were then hydrated in 1× PBS (5 minutes). Antigen retrieval was accomplished by boiling the slides in a pressure cooker with sodium citrate buffer at a pH of 9.0 (Vector Laboratories H-3301). Immunoperoxidase labeling was performed as described [19] using the monoclonal antibodies (mAbs) against galectin-3 (B2C10) or appropriate isotypes.
Double-labeling immunofluorescence was performed by serial incubation of fresh-frozen tissue sections with mouse antihuman mAbs as described [19]. In brief, sections were serially incubated with a mouse mAb against galectin-3 (9C4) followed by goat antimouse IgG1 (Invitrogen) labeled with fluorochrome (Alexa 568). Sections were washed and incubated with antibodies for CD14 (RPA-MI, Zymed Laboratories) or CD68 (PG-M1, DakoCytomation) for 1 hour and then incubated with isotype-specific goat antimouse IgG Abs (Invitrogen) labeled with fluorochrome (Alexa 488) for 1 hour. Controls included staining with isotype-matched irrelevant Abs. Images were obtained using confocal laser microscopy (California NanoSystems Institute). Immunofluorescence was examined with a Leica TCS SP2 inverted confocal laser scanning microscope (Leica Microsystems).
TLR2/1 Activation
Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Hypaque gradient centrifugation (Ficoll-Paque; Pharmacia Biotech AB). Monocytes were adhered to plastic in Roswell Park Memorial Institute (RPMI) 1640 supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and 1% fetal bovine serum (FBS) for 2 hours (Omega). Nonadherent PBMCs were washed off and adherent monocytes were stimulated with recombinant human galectin-3 (60 μg/mL) or buffer (10% glycerol in PBS). Nineteen-kD lipopeptide (1 µg/mL, EMC Microcollections) was then added to the culture and cells were incubated in RPMI 1640 supplemented with penicillin, streptomycin, and 10% FBS for 2 days (Omega). Supernatants were collected and IL-10 (Invitrogen) and interleukin 12p40 (IL-12p40; Invitrogen) measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. The limits of detection for these assays were 5–78 pg/mL for IL-10, and 5–156 pg/mL for IL-12p40.
Monocyte Differentiation
Adherent monocytes (prepared as described above) were induced to differentiate into dendritic cells (DCs) with recombinant human granulocyte macrophage colony-stimulating factor (GM-CSF; 1 U/mL, Immunex) in the presence or absence of recombinant human galectin-3 (60 μg/mL) or buffer (10% glycerol in PBS). Cells were incubated in RPMI 1640 supplemented with penicillin, streptomycin, and 10% FBS for 2 days (Omega). Cells were then harvested, washed, and analyzed by flow cytometry and also used as antigen-presenting cells (APCs).
T-Cell Lines and Proliferation Assays
T-cell lines were derived from patients with mycobacterial infection as previously described [19]. For measurement of antigen-specific proliferation, T cells (1 × 104) were cultured with irradiated (5000 rads) heterologous CD1+ APCs (1 × 104) in culture medium (0.2 mL) in the presence or the absence of mycobacterial antigens for 3 days in triplicate 96-round bottom microtiter wells at 37°C in a 7% CO2 incubator. Cells were pulsed with 3H-thymidine (1 µCi/well; ICN Biomedicals) and harvested 4–6 hours later for liquid scintillation counting.
Monocyte Infection
Adherent monocytes (prepared as described above) were treated in the presence or absence of galectin-3 (60 μg/mL) or buffer (10% glycerol in PBS). To infect the cells, M. leprae or M. bovis was then added to the culture plates for 1 hour at 33°C in a 5% CO2 incubator [26]. To preserve the viability of the monocytes, the temperature was raised to 37°C after 1 hour and the cultures were incubated in RPMI 1640 supplemented with 10% FBS (Omega). Mycobacterium leprae cytokine induction experiments were performed at a multiplicity of infection (MOI) of 10, as MOI < 10 did not stimulate cytokines. Mycobacterium bovis was used at lower doses as MOI > 3 was cytotoxic.
Immunoblotting
Cells were lysed in RIPA buffer (Sigma) and a 1% protease inhibitor cocktail. Cell lysate protein samples were separated on Any kD Mini-PROTEAN TGX precast gel (Bio-Rad), transferred to nitrocellulose membranes, and immunoblotted with rabbit polyclonal antibodies against galectin-3 [25], and glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology).
RESULTS
Galectin-3 Expression in Leprosy
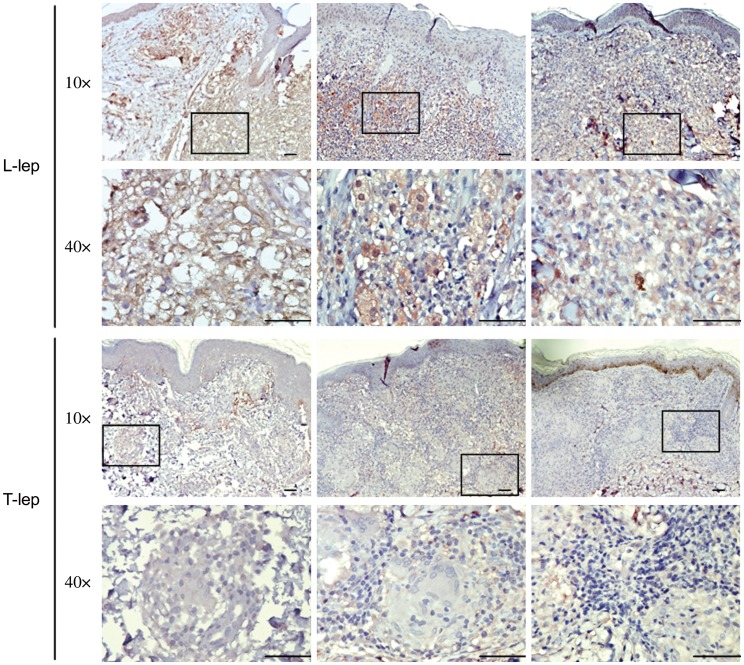
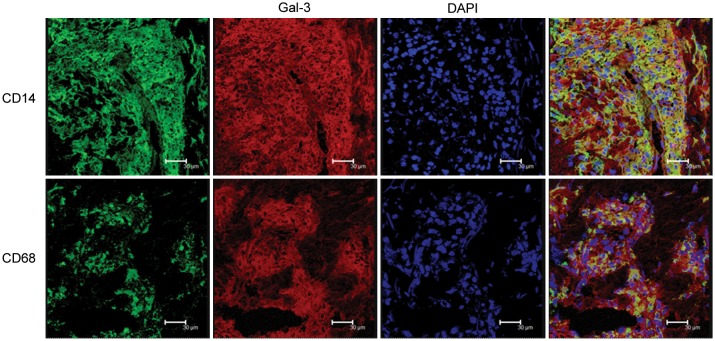
To confirm gene expression profiles obtained by microarray analysis in leprosy, we examined galectin-3 protein expression in leprosy skin lesions, by immunolabeling with a galectin-3 mAb. Galectin-3 was expressed in the granulomatous infiltrate of L-lep patients, with 80%–95% of the cells strongly labeled in contrast to T-lep lesions, in which approximately 10% of the cells were weakly galectin-3 positive (Figure 1). Galectin-3 was predominantly expressed on cells of the monocyte/macrophage lineage, as shown by its coexpression with CD14 and CD68 (Figure 2). These data confirm the gene expression profiles [18]. Although galectin-3 was expressed at higher levels in L-lep lesions compared to T-lep lesions, we found that live M. leprae infection (at MOIs ranging from 0.1 to 30) did not increase the expression either by galectin-3 messenger RNA (mRNA) or protein in human monocytes in 3 independent experiments (not shown and Supplementary Figure 1).
Figure 1.
Galectin-3 expression in leprosy lesions. Galectin-3 protein expression in the skin lesions of lepromatous leprosy (L-lep; n = 3) and tuberculoid leprosy (T-lep; n = 3) patients was investigated by immunohistochemical staining with monoclonal mouse anti-human galectin-3 (B2C10). Four-micrometer tissue sections of leprosy skin biopsy specimens were incubated with anti–galectin-3 and labeled with an immunoperoxidase method followed by hematoxylin for visualization of histology. Tissue sections labeled with isotype control antibodies were negative. Photographs were taken using ×10 and ×40 objective lenses. Scale bars = 50 μm.
Figure 2.
Coexpression of macrophage markers with galectin-3 (Gal-3) expression in lepromatous leprosy (L-lep) skin lesions. Skin lesions from L-lep patients were sectioned and immunolabeled with monoclonal antibodies as indicated and visualized by confocal laser microscopy. Images were photographed using a ×63 objective. Scale bars = 30 μm. CD14 and CD68 were visualized as green fluorescence, and Gal-3 (9C4) was visualized as red fluorescence. Nuclei were labeled with DAPI. Coexpression (yellow) revealed the relatively close proximity of Gal-3 to macrophage markers CD14 and CD68.
Galectin-3 Enhances IL-10 Production in Human Monocytes
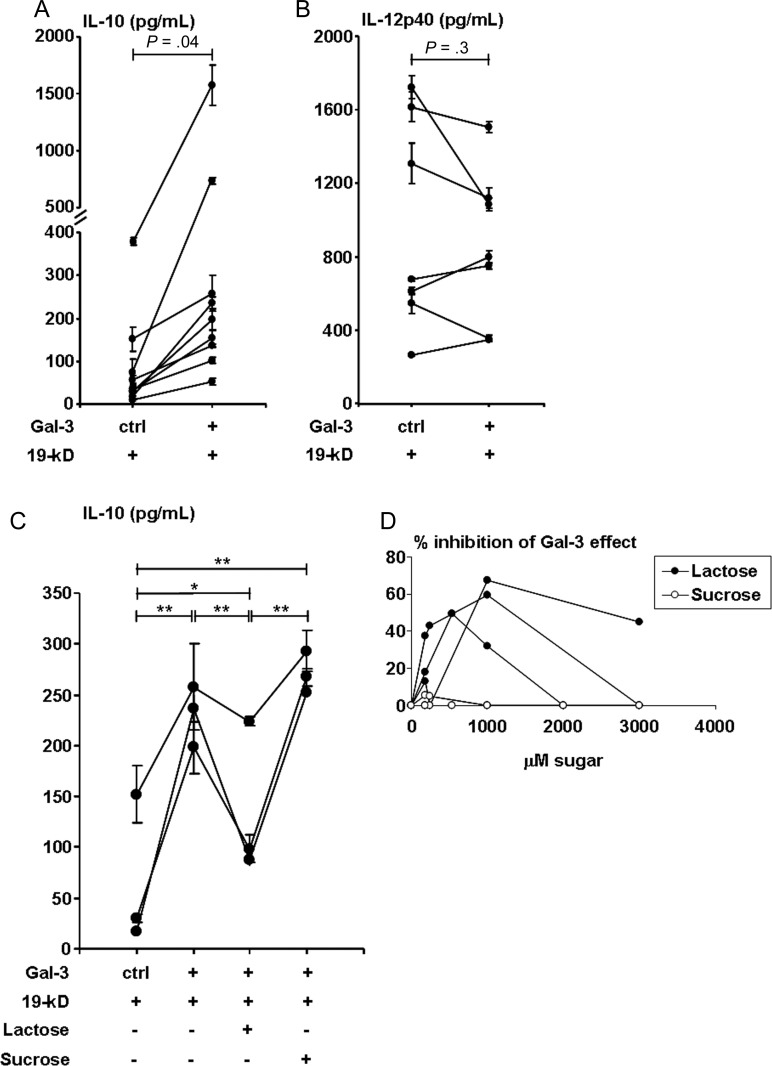
Given the association of galectin-3 with the progressive form of leprosy and the ability of galectin-3 to influence T-cell cytokine patterns [6–8], we investigated whether galectin-3 affected the innate immune cytokines known to influence Th1 vs Th2 responses. Differentiation of Th1 cells requires IL-12 [27], whereas IL-10 suppresses IL-12 production [28] and promotes Th2 differentiation. TLR2/1 activation of monocytes induces both IL-12 and IL-10 secretion [29]. Because TLR2/1 responses contribute to host defense in leprosy [30], we investigated whether galectin-3 influences TLR2/1-induced IL–12 and IL-10 release. Human monocytes were cultured with the TLR2/1 ligand, 19-kD lipopeptide, in the presence of galectin-3 or control buffer for 2 days. IL–10 and IL-12p40 levels were measured in cultured supernatants by ELISA. Galectin-3 upregulated IL–10 release by 5.7-fold (P = .04) as compared to TLR2/1 stimulation alone (Figure 3A), whereas TLR2/1-induced IL-12p40 release was unaffected (Figure 3B, P = .3). Galectin-3 alone did not induce IL-10 or IL-12p40 secretion (data not shown). The addition of galectin-3 increased the TLR2/1-induced IL-10/IL-12p40 ratio in monocytes, consistent with the cytokine pattern observed in L-lep lesions [11, 13, 31]. Interestingly, whereas monocytes cultured with live M. leprae or M. bovis in vitro produced IL-12p40, the presence of galectin-3 had no effect on IL-10 and had modest effect in decreasing only M. leprae induced IL-12p40 (Supplementary Figure 2C, P = .001).
Figure 3.
Effect of galectin-3 (Gal-3) on TLR2/1-induced cytokine profiles. Cytokine expression of (A) interleukin 10 (IL-10; n = 9 donors) and (B) interleukin 12p40 (IL-12p40; n = 7 donors) from monocytes treated with medium or 19-kD (a mycobacterial TLR2/1 ligand) in the presence of Gal-3 or control buffer (2 days) as measured by enzyme-linked immunosorbent assay. Values are expressed as the mean ± SEM of triplicates. Monocytes treated with medium alone secreted no IL-10, and IL-12p40 within a range from 0 to 132 pg/mL. Mixed-models statistical analysis was performed to compare Gal-3 with control buffer. In (A) compound symmetry variance–covariance structure was assumed, and in (B) unstructured variance–covariance structure was assumed. C, IL-10 (n = 3 donors) secretion from monocytes treated as in A and B, with the addition of lactose or sucrose (1 mM each). Each individual line represents 1 donor and the data points are the mean ± SEM of the triplicates for each experiment. Statistical analysis was performed using general linear model, and Tukey method was used for multiple comparison adjustment; *P = .001; **P < .0001. D, Dose titration of sugar inhibition of the effect of Gal-3 on IL-10 secretion. Results shown are from the same 3 donors as in (C). Values are reported as the percentage of decrease of 19-kD stimulated IL-10 secretion: [(pg/mL in the presence of Gal-3 – pg/mL in the presence of Gal-3 and sugar)/(pg/mL in the presence of Gal-3 – pg/mL in the absence of Gal-3)] × 100%. Statistical analysis was performed using mixed models assuming compound symmetry covariance matrix among repeated measures over several concentration levels.
To determine whether the increased IL-10 secretion induced by galectin-3 is dependent on its lectin properties, we tested the effect of saccharides on the ability of galectin-3 to influence IL-10 production. Galectin-3 binds lactose by its C-terminal lectin domain, whereas sucrose does not bind to galectin-3. Lactose inhibited the effect of galectin-3 on TLR2/1-induced IL-10 production (P < .0001, Figure 3C). In contrast, IL-10 secretion was unaffected in the presence of the control sugar sucrose (which does not bind galectin-3), indicating that the effect of galectin-3 was due to its lectin domain [32]. Inhibition by lactose was partial, as we performed dose titrations of each disaccharide and found that an appropriate dose of lactose was reached at 1 mM in comparison to sucrose (Figure 3D).
Galectin-3 Interferes With GM-CSF–Induced DC Differentiation
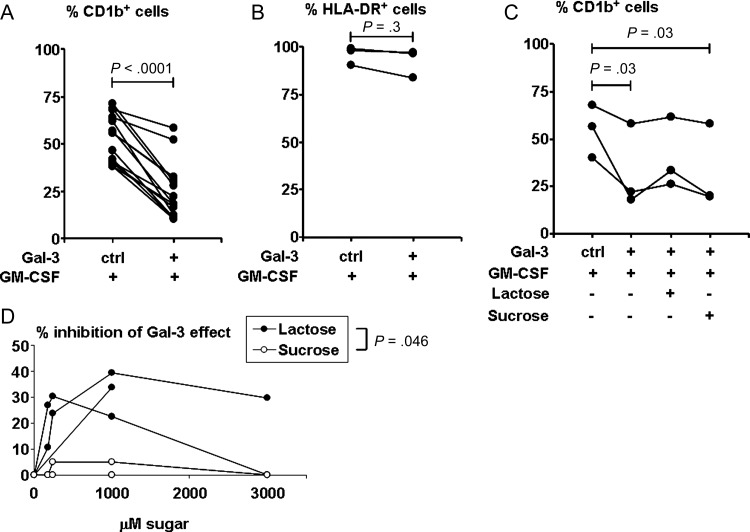
Dendritic cells are innate immune cells that influence T-cell responses. Because galectin-3 altered instructive cytokine profiles, we further queried its ability to influence DC differentiation. We hypothesized that galectin-3 may modulate the effect of GM-CSF–mediated DC differentiation from monocytes. Human monocytes were cultured with GM-CSF in the presence of galectin-3 or control buffer; then CD1b+ (Figure 4A) and HLA-DR+ (Figure 4B) expression were measured as markers for DCs [19, 20]. The presence of galectin-3 resulted in >50% reduction of GM-CSF–induced CD1b+ DC (P < .0001, Figure 4A), whereas major histocompatability complex (MHC) class II expression was unchanged (Figure 4B, P = .3). These data indicate that galectin-3 impairs GM-CSF–induced DC differentiation. To demonstrate whether the inhibition of GM-CSF–induced DC differentiation by galectin-3 is dependent on its lectin properties, we tested the effect of saccharides on the lectin's inhibition of CD1b expression. As with the effect on TLR2/1 stimulation of IL-10, lactose partially reversed the effect of galectin-3, whereas sucrose did not (Figure 4C and 4D).
Figure 4.
Effect of galectin-3 (Gal-3) on granulocyte macrophage colony-stimulating factor (GM-CSF)–treated monocytes. CD1b and HLA-DR expression on monocytes treated with medium or GM-CSF in the presence of Gal-3 or control buffer for 2 days as measured by flow cytometry is shown. Cell surface protein expression levels are expressed as the percentage positive for CD1b from 14 donors (A) and HLA-DR from 3 donors (B). Monocytes treated with medium alone expressed CD1b within a range of 0.3% to 14.5%, and HLA-DR within a range of 81.5% to 98.3%. Statistical analysis was performed using paired t test (A) and sign test (B), which is a nonparametric version of the paired t test. C, CD1b expression on monocytes treated as in (A) from 3 donors, with the addition of lactose or sucrose (1 mM each). Statistical analysis was performed with general linear model using Tukey method for multiple comparison adjustment. D, Dose titration of sugar inhibition of the effect of Gal-3 on CD1b expression. Results shown are from the same 3 donors as in (C). Values are reported as the percentage of decrease in the percentage of CD1b-positive cells induced by GM-CSF: [|(percent in the presence of Gal-3 minus percent in the presence of Gal-3 and sugar)|/|(percent in the presence of Gal-3 minus percent in the absence of Gal-3)|] ×100%. Statistical analysis was performed using mixed models assuming compound symmetry covariance matrix among repeated measures over several concentrations.
Galectin-3 Reduces Antigen-Presenting Capacity of Dendritic Cells
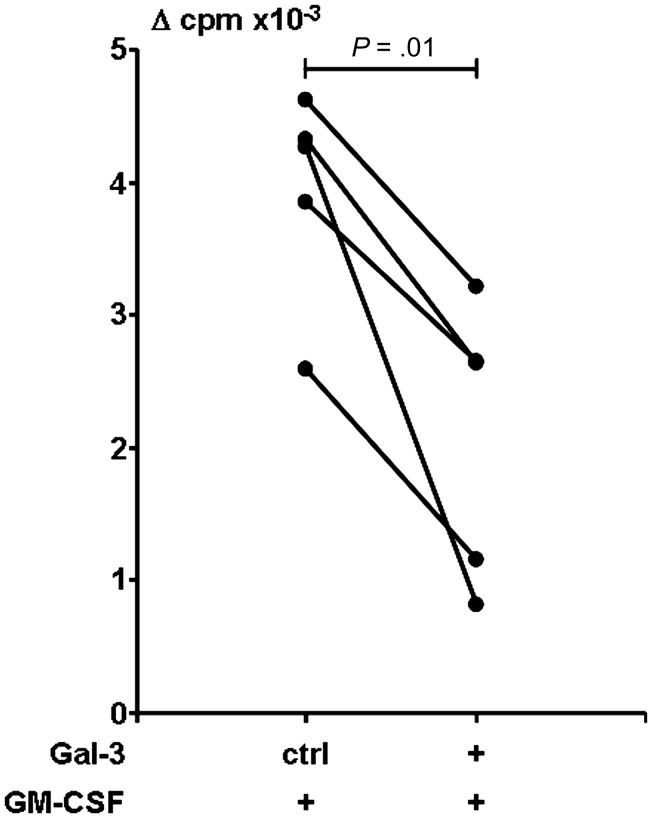
Because galectin-3 diminished GM-CSF–induced CD1b upregulation, we hypothesized that galectin-3 might affect the antigen-presenting function of GM-CSF–induced DCs. To determine whether the decrease of CD1b expression is functionally relevant, we used a mycobacterial-reactive CD1b-restricted T-cell line [33]. CD1 proteins are nonpolymorphic lipid antigen-presenting molecules; therefore, DCs derived from a donor with any MHC haplotype may be used as APCs for the T-cell line. Monocyte-derived DCs differentiated as in Figure 4 were tested for their potential to present antigen in the context of CD1b (Figure 5). In the presence of control buffer, GM-CSF–derived APCs stimulated CD1b-restricted T cells to proliferate in response to antigen (Δ counts per minute [cpm] mean = 3928, n = 5 experiments, 4 donors). In contrast, APCs derived with GM-CSF in the presence of galectin-3 weakly stimulated CD1b-restricted T-cell proliferation (Δ cpm mean = 2092), representing a 47% reduction (P = .01) in T-cell response. Thus, monocytes differentiated toward DCs in the presence of galectin-3 showed diminished antigen presentation. These data provide a potential regulatory pathway by which galectin-3 could contribute to the immune unresponsiveness observed in L-lep patients.
Figure 5.
Effect of galectin-3 (Gal-3) on antigen-presenting cells (APCs) on the ability to present antigen to T cells from leprosy patients. T-cell proliferation using APCs previously treated with granulocyte macrophage colony-stimulating factor in the presence of Gal-3 or control buffer as in Figure 4 are shown. In triplicate, CD1b-restricted T cells and APCs (1:1) were cultured in the presence or absence of mycobacterial antigen (M. tuberculosis sonicate or mycobacterial lipomannan) for 3 days, and proliferation was measured by 3H thymidine incorporation. Proliferative response was reported as average of counts per minute (cpm) of wells in the presence of antigen minus the average of cpm of corresponding wells in medium alone (Δ cpm). T cells cultured with medium alone–derived APCs proliferated at a mean of 65 Δ cpm (range, 2–207 cpm). Shown are results from 5 experiments, 4 donors. Statistical analysis was performed with paired t test.
DISCUSSION
The extent of the cell-mediated immune response to M. leprae correlates with the host's ability to contain the infection. Here we show that across the clinical and immunological spectrum of leprosy, skin lesions from patients with weak cell-mediated immune responses to the mycobacteria (L-lep) express higher levels of galectin-3 protein compared to those in patients with strong cell-mediated immune responses (T-lep). Within L-lep lesions, CD14-positive and CD68-positive macrophages expressed galectin-3. Using monocytes as an in vitro surrogate for macrophages, we found that galectin-3 increased the IL-10/IL-12p40 ratio and impaired DC differentiation and antigen-presenting function. Together, these data provide a disease-relevant mechanism by which galectin-3 contributes to the immune unresponsiveness observed in human mycobacterial infection.
Although galectin-3 was expressed at higher levels in L-lep lesions known be infiltrated with numerous bacteria as compared to T-lep lesions, we found that live M. leprae infection (at MOIs ranging from 0.1 to 30) did not increase the expression either by galectin-3 mRNA or protein in human monocytes in 3 independent experiments (data not shown and Supplementary Figure 1). Monocytes and macrophages are known to express galectin-3 [1, 3, 4]; however, IFN-γ decreases galectin-3 expression in monocytes [3]. Therefore, we speculate that the overall cytokine milieu [11, 31] may contribute to the markedly diminished expression of galectin-3 in T-lep lesions. Galectin-3 has also been detected at higher levels in the lesions and sera of patients with malignant tumors [34, 35]. The overexpression of galectin-3 in cancer and its inhibitory role in intracellular microbial infections in humans (this study) and mice [7, 8] raises the question as to its physiological function. Galectin-3 deficient mice are viable and fertile, yet exhibit developmental [36], inflammatory [2, 37] and organ function deficiencies [38], indicating that galectin-3 mediates a wide range of cellular pathways. Because L-lep represents an immune deviation in response to M. leprae infection, we would speculate that galectin-3 induces a dysfunctional host response. Our in vitro studies, discussed below, would appear to support such a mechanism in leprosy, although we cannot exclude a role for other cellular pathways.
L-lep macrophages are laden with mycobacteria, indicating that they are capable of ingesting, but not killing, the pathogen. In the present study, we found macrophages in L–lep lesions expressing galectin-3, a cell population shown to express galectin-3 by other researchers [3]. We have previously established that galectin-3 is critical for macrophage phagocytosis [2]. Moreover, galectin-3 expressed by macrophages and epithelial cells can bind to microbial glycolipids [39, 40] and galectin-3 is present in phagosomal vesicles, including those containing mycobacteria [39]. Mycobacteria [41] and mycobacterial glycolipids [42] inhibit the microbicidal activity of macrophages. It is tempting to speculate from our findings, along with those described above, that galectin-3 could promote phagocytosis of mycobacteria and internalization of mycobacterial glycolipids, which may contribute to the intracellular survival of mycobacteria in leprosy [18].
To determine a potential mechanism for galectin-3–mediated immune deviation in leprosy, we established an in vitro system using monocytes and soluble galectin-3. Galectin-3 modulated monocyte cytokine production by increasing the TLR2/1-mediated production of IL-10, an anti-inflammatory cytokine present at elevated levels in the lesions of L-lep patients [12]. IL-10 acts directly on monocytes; it reduces MHC expression [43] and inhibits IL-12 production [13, 28], thereby inhibiting Th1 responses and favoring Th2 cytokine production, the pattern found in L-lep lesions [11, 31]. The role of galectin-3 in T helper–cell cytokine patterns has also been addressed in murine models of infection. The absence of galectin-3 in vivo leads to enhanced Th1 immunity in both Toxoplasma gondii and Schistosoma mansoni infectious models [7, 8], whereas in Paracoccidioides brasiliensis infection an unfavorable Th2 response is observed [6]. Therefore, the role of galectin-3 in Th1 vs Th2 development is context dependent. Our findings in leprosy may reflect on the outcome of infection with M. leprae since T-cell responsiveness in L-lep patients is weak [44] and Th2 cytokine production is a signature of L-lep lesions [11, 31].
We found that galectin-3 influenced monocyte function. The comparatively higher levels of galectin-3 expressed in L-lep skin lesions may not only affect the recruitment of macrophages, but also may promote the differentiation of monocytes to favor macrophages rather than DCs. To more thoroughly examine the potential effect of galectin-3 on DC differentiation and T-cell responsiveness in leprosy, we investigated the ability of monocytes to differentiate into DCs in the presence of galectin-3. Galectin-3 prevented GM-CSF–mediated upregulation of CD1-expressing DCs and T-cell antigen presentation. Reduced CD1 expression has been reported in L-lep lesions [45], suggesting that DC differentiation might be prevented by galectin-3 in leprosy. Furthermore, T-cell responses in L-lep patients are weak [44], and CD1-restricted T cells are reduced relative to T-lep patients [46], a potential outcome of the deficient DC differentiation caused by galectin-3.
To investigate the mechanism of the effect of galectin-3 on monocytes, lactose competitive inhibition studies showed a dose–response effect that inhibited up to 67% of the effect of galectin-3, indicating that the effect of galectin-3 on monocytes is mediated at least in part through the carbohydrate recognition domain (CRD). This partial inhibition by lactose may be explained by the multiple binding domains of galectin-3. Lactose binds to the CRD contained in the C-terminal region of galectin-3 and inhibits CRD-mediated stimuli. In addition, galectin-3 contains other domains that may mediate potential functions not involving the CRD [47]. Furthermore, lactose is a disaccharide with a relatively low association constant when compared to endogenous glycan ligands, which are polylactosamines and branched high-affinity glycans recognized by galectin-3. Differences in association constants are several orders in magnitude; for example, glycoprotein IgE has 1000 times higher binding compared to lactose [25]. Therefore, the fact that some inhibition with lactose was observed, and none with the sucrose control, suggests that at least some of the effect of galectin-3 is mediated through the CRD. This may be through lectin activity (glycan-peptide), but peptide-peptide interactions via the CRD cannot be excluded without more work [48].
In the present study we found that galectin-3 regulated cytokine secretion profiles induced by mycobacterial ligands, reduced CD1b expression, and decreased antigen presentation by monocyte-derived DCs. Whereas live M. leprae or M. bovis infection of human monocytes stimulated IL-12p40, the addition of galectin-3 decreased M. leprae–induced IL-12p40 by 30.4% (Supplementary Figure 2C). This suggests that live mycobacterial infection in vitro may be dependent on the activation of pattern recognition receptors other than through TLR2/1. Given the polymorphisms in TLR2 and TLR1 genes, which may contribute to the TLR2/1 response to lipopeptides and to the pathogenesis of leprosy [49], we hypothesize that activation of TLR2/1 may be relevant in leprosy when the mycobacteria are released or secreted. This may not have occurred within the time frame of our in vitro experiment. Interestingly, the effect of galectin-3 on TLR2/1 activation enhanced IL-10, whereas galectin-3 decreased IL-12p40 in live M. leprae infection; both of which could promote Th2 responses in leprosy. Our findings, together with in vivo expression of galectin-3 at the site of L-lep skin lesions, suggest a role for galectin-3 in a less favorable response to cutaneous mycobacterial infection. We envision a scenario whereby galectin-3 expressed in L-lep lesions promotes the differentiation of infiltrating monocytes into macrophages rather than DCs, producing IL-10, which promotes a Th2 response that favors permissive infection by M. leprae. The mechanisms regulating the expression of galectin-3 in mycobacterial infection require further study and may provide a potential target for intervention to promote host defense in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Myung-Shin Sim, Director of Department of Biostatistics at the John Wayne Cancer Institute, for providing her expertise in statistical analysis; Dr John Belisle of the Department of Microbiology, Immunology and Pathology at Colorado State University; Dr Larry Schlesinger of the Center for Microbial Interface Biology of Ohio State University for providing mycobacterial antigens; and Drs James Krahenbuhl and Ramanuj Lahiri of the US National Hansen's Disease Program for providing helpful discussion and live Mycobacterium leprae through the generous support of the American Leprosy Missions and Society of St Lazarus. We also thank Dr Matthew Schibler of the Advanced/Microscopy/Spectroscopy Laboratory Macro-Scale Imaging Laboratory California NanoSystems Institute UCLA.
Financial support. This work was supported by the National Institutes of Health (grant number AR59126-3 to D. J. L., grant number AI22553 to R. L. M., and grant numbers AI20958 and AR056343 to F. T. L.); the Joseph B. Gould Foundation (to A. W. C., P. A. S., and D. J. L.); and the Swiss National Science Foundation (grant number PASMP3–123256 to M. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–30. [PubMed] [Google Scholar]
- 2.Sano H, Hsu DK, Apgar JR, et al. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–97. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu FT, Hsu DK, Zuberi RI, et al. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Cherayil BJ, Weiner SJ, Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med. 1989;170:1959–72. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortegano I, del Pozo V, Cardaba B, et al. Galectin-3 down-regulates IL-5 gene expression on different cell types. J Immunol. 1998;161:385–9. [PubMed] [Google Scholar]
- 6.Ruas LP, Bernardes ES, Fermino ML, et al. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS One. 2009;4:e4519. doi: 10.1371/journal.pone.0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breuilh L, Vanhoutte F, Fontaine J, et al. Galectin-3 modulates immune and inflammatory responses during helminthic infection: impact of galectin-3 deficiency on the functions of dendritic cells. Infect Immun. 2007;75:5148–57. doi: 10.1128/IAI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardes ES, Silva NM, Ruas LP, et al. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am J Pathol. 2006;168:1910–20. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 10.Cooper CL, Mueller C, Sinchaisri T-A, et al. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989;169:1565–81. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 12.Sieling PA, Abrams JS, Yamamura M, et al. Immunosuppressive roles for interleukin-10 and interleukin-4 in human infection: in vitro modulation of T cell responses in leprosy. J Immunol. 1993;150:5501–10. [PubMed] [Google Scholar]
- 13.Sieling PA, Wang X-H, Gately MK, et al. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–47. [PubMed] [Google Scholar]
- 14.Libraty DH, Airan LE, Uyemura K, et al. Interferon-gamma differentially regulates interleukin-12 and interleukin-10 production in leprosy. J Clin Invest. 1997;99:336–41. doi: 10.1172/JCI119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modlin RL, Kato H, Mehra V, et al. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. Nature. 1986;322:459–61. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- 16.Bleharski JR, Li H, Meinken C, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–30. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 17.Lee DJ, Li H, Ochoa MT, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis. 2010;201:558–69. doi: 10.1086/650318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoya D, Cruz D, Teles RM, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–53. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DJ, Sieling PA, Ochoa MT, et al. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J Immunol. 2007;179:8128–36. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahiri R, Randhawa B, Krahenbuhl JL. Effects of purification and fluorescent staining on viability of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 2005;73:194–202. [PubMed] [Google Scholar]
- 22.Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–73. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- 23.Behar SM, Porcelli SA, Beckman EM, et al. A pathway of costimulation that prevents anergy in CD28- T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–18. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu FT, Hsu DK, Zuberi RI, et al. Modulation of functional properties of galectin-3 by monoclonal antibodies binding to the non-lectin domains. Biochemistry. 1996;35:6073–9. doi: 10.1021/bi952716q. [DOI] [PubMed] [Google Scholar]
- 25.Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167–74. [PubMed] [Google Scholar]
- 26.Lahiri R, Randhawa B, Krahenbuhl JL. Infection of mouse macrophages with viable Mycobacterium leprae does not induce apoptosis. J Infect Dis. 2010;201:1736–42. doi: 10.1086/652499. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh C-S, Macatonia SE, Tripp CS, et al. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 28.D'Andrea A, Aste-Amezaga M, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, et al. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12 but not IL-10. J Immunol. 2000;165:3804–10. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 30.Krutzik SR, Ochoa MT, Sieling PA, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–32. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 31.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 32.Sano H, Hsu DK, Yu L, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–64. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- 33.Torrelles JB, Sieling PA, Arcos J, et al. Structural differences in lipomannans from pathogenic and non-pathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011;286:35438–46. doi: 10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iurisci I, Tinari N, Natoli C, et al. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93. [PubMed] [Google Scholar]
- 35.Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995;147:815–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Colnot C, Sidhu SS, Balmain N, et al. Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev Biol. 2001;229:203–14. doi: 10.1006/dbio.2000.9933. [DOI] [PubMed] [Google Scholar]
- 37.Hsu DK, Yang RY, Pan Z, et al. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–83. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bichara M, Attmane-Elakeb A, Brown D, et al. Exploring the role of galectin 3 in kidney function: a genetic approach. Glycobiology. 2006;16:36–45. doi: 10.1093/glycob/cwj035. [DOI] [PubMed] [Google Scholar]
- 39.Beatty WL, Rhoades ER, Hsu DK, et al. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002;4:167–76. doi: 10.1046/j.1462-5822.2002.00183.x. [DOI] [PubMed] [Google Scholar]
- 40.Fowler M, Thomas RJ, Atherton J, et al. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006;8:44–54. doi: 10.1111/j.1462-5822.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 41.Brown CA, Draper P, Hart PD. Mycobacteria and lysosomes: a paradox. Nature. 1969;221:658–60. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- 42.Sibley LD, Hunter SW, Brennan PJ, et al. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988;56:1232–6. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Waal-Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godal T, Myklestad B, Samuel DR, et al. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971;9:821–31. [PMC free article] [PubMed] [Google Scholar]
- 45.Sieling PA, Jullien D, Dahlem M, et al. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol. 1999;162:1851–8. [PubMed] [Google Scholar]
- 46.Sieling PA, Torrelles JB, Stenger S, et al. The human CD1-restricted T cell repertoire is limited to cross-reactive antigens: implications for host responses against immunologically related pathogens. J Immunol. 2005;174:2637–44. doi: 10.4049/jimmunol.174.5.2637. [DOI] [PubMed] [Google Scholar]
- 47.Lepur A, Carlsson MC, Novak R, et al. Galectin-3 endocytosis by carbohydrate independent and dependent pathways in different macrophage like cell types. Biochim Biophys Acta. 2012;1820:804–18. doi: 10.1016/j.bbagen.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modlin RL. The innate immune response in leprosy. Curr Opin Immunol. 2010;22:48–54. doi: 10.1016/j.coi.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.