Abstract
Background. Dengue virus (DENV) causes hundreds of millions of infections annually. Four dengue serotypes exist, and previous infection with one serotype increases the likelihood of severe disease with a second, heterotypic DENV infection.
Methods. In a randomized, placebo-controlled study, the safety and immunogenicity of 4 different admixtures of a live attenuated tetravalent (LATV) dengue vaccine were evaluated in 113 flavivirus-naive adults. Serum neutralizing antibody levels to all 4 dengue viruses were measured on days 0, 28, 42, and 180.
Results. A single dose of each LATV admixture induced a trivalent or better neutralizing antibody response in 75%–90% of vaccinees. There was no significant difference in the incidence of adverse events between vaccinees and placebo-recipients other than rash. A trivalent or better response correlated with rash and with non-black race (P < .0001). Black race was significantly associated with a reduced incidence of vaccine viremia.
Conclusions. TV003 induced a trivalent or greater antibody response in 90% of flavivirus-naive vaccinees and is a promising candidate for the prevention of dengue. Race was identified as a factor influencing the infectivity of the LATV viruses, reflecting observations of the effect of race on disease severity in natural dengue infection.
Clinical Trials Registration NCT01072786.
Keywords: dengue vaccine, live attenuated tetravalent, clinical trial
Dengue virus (DENV) has become the most important arbovirus worldwide with estimates of as many as 500 million dengue infections occurring annually, resulting in more than 2 million cases of severe disease and 21 000 deaths [1]. There are 4 antigenically distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), each of which can cause the full spectrum of dengue illness, ranging from asymptomatic infection to life-threatening dengue shock syndrome. The geographical spread of the Aedes aegypti and Aedes albopictus mosquito vector and the 4 virus serotypes has led to an increased number of countries experiencing epidemic dengue fever [2]. Children bear the majority of dengue disease burden in hyperendemic areas in which all 4 serotypes circulate, whereas, in areas of low transmission or areas in transition to hyperendemicity, adult disease is frequently seen during periods of virus circulation [3–5].
Long-term homotypic immunity is induced by a single infection with DENV [6]; however, accompanying heterotypic protection is less durable [7]. Although severe disease can occur following primary infection, preexisting immunity to one DENV serotype has been identified as a risk factor for more severe disease upon secondary, heterotypic infection [8–11]. The potential immune enhancement resulting from prior infection may lead to increased virus replication, which has been shown to correlate with disease severity [4, 11]. The ideal DENV vaccine should protect against disease caused by all 4 serotypes; however, because the incidence of severe disease following a third or fourth DENV infection is low [12], it is possible that a trivalent response may be sufficiently protective.
To develop a live attenuated tetravalent (LATV) dengue vaccine that induces a balanced immune response to all 4 DENV serotypes, we previously evaluated 8 different monovalent dengue vaccine candidates in flavivirus-naive adults to determine their safety profile, replication kinetics, and immunogenicity prior to inclusion in a tetravalent formulation [13–20]. The candidates tested consisted of 1 DENV-1 candidate, 1 DENV-2 candidate, 3 DENV-3 candidates, and 3 DENV-4 candidates. One DENV-3 candidate was found to be overattenuated (rDEN3/4Δ30), and 1 DENV-4 candidate (rDEN4Δ30-4995) was not chosen for inclusion in a tetravalent admixture because it did not offer any advantage relative to the other 2 DENV-4 vaccine candidates. The monovalent vaccine candidates were prepared under current good manufacturing practice (cGMP) conditions and vialed individually at titers ranging from 6.8–7.8 log10 plaque-forming units (PFU)/mL; therefore, they could be mixed in various combinations to easily evaluate different tetravalent admixtures. Herein, we describe the safety and immunogenicity of a single subcutaneous dose of 4 different LATV admixtures administered to healthy flavivirus-naive adult subjects. Based on the results of this trial, a single LATV formulation was chosen for further evaluation in dengue-endemic regions.
MATERIALS AND METHODS
Ethics Statement
The study was performed under an investigational new drug application reviewed by the US Food and Drug Administration and received approval from the Western Institutional Review Board, the University of Vermont (UVM) Institutional Review Board, and the Institutional Biosafety Committees of Johns Hopkins University and UVM. Written informed consent was obtained from each volunteer in accordance with the Code of Federal Regulations (21 CFR 50) and International Conference on Harmonisation guidelines for Good Clinical Practice (ICH E6). The National Institute of Allergy and Infectious Diseases (NIAID) Data Safety Monitoring Board reviewed all safety data every 6 months.
Trial Design and Study Setting
This Phase I trial was conducted as a randomized double-blind placebo-controlled study at the JHSPH and UVM. Study subjects were enrolled between July 2010 and February 2011 under study protocol CIR268, registered at ClinicalTrials.gov as Study NCT01072786. The objective of this phase 1 study was to evaluate the safety and immunogenicity of a single dose of different admixtures of the LATV dengue vaccine. Study outcomes included vaccine safety, vaccine viremia (characterized by mean peak titer, day of onset, and duration), and antibody response (characterized by geometric mean titer of neutralizing antibodies and the frequency and distribution of seropositivity). The serologic response was characterized as a 60% plaque-reduction neutralization titer (PRNT60) on study days 28 and 42 following vaccination.
Four cohorts of 28 subjects were evaluated. The cohorts differed in the admixture of vaccine received. Within each cohort of 28, subjects were block-randomized in groups of 7 such that 5 would receive vaccine and 2 would receive placebo. A sample size of 20 vaccinees and 8 placebo recipients in each cohort was chosen based on our previous phase I trials of monovalent vaccine components that made up each admixture [13–16, 18, 20]. The study pharmacist randomized subjects using a random number generator. All study staff involved in the clinical and laboratory assessment of the subjects remained blinded to the treatment assignment until all volunteers within a block of 7 reached study day 42 following vaccination.
Study Population
Healthy adult male and nonpregnant female volunteers 18–50 years of age were enrolled from Baltimore Maryland, Washington, DC, and Burlington, Vermont. Volunteers were enrolled if they met the following eligibility criteria: normal findings during physical examination; negative for serum antibodies to all DENV serotypes, yellow fever virus, West Nile virus, St. Louis encephalitis virus; negative for hepatitis B and C; negative for human immunodeficiency virus (HIV); normal blood hematology, and serum chemistry. Female volunteers were required to have a negative urine or serum pregnancy test at screening and on vaccination day and to agree to use contraception.
Vaccines
The 6 different monovalent dengue vaccines used in the preparation of each admixture are listed in Table 1. The safety, viral replication, and immunogenicity of each of these candidate vaccines were evaluated in previous clinical trials [13–20]. The vaccine viruses were stored at −80 ± 15°C until use. They were thawed, diluted, and combined into tetravalent admixtures immediately prior to vaccination to yield a potency of 3.3 log10 PFU/mL for each serotype. Qualified Leibovitz L-15 medium was used as diluent and placebo. The appearance of the vaccine and placebo was identical. Vaccine potency (virus titer) was confirmed for each admixture.
Table 1. LATV Admixtures Evaluated in Human Subjects
| Admixture | Administered Dose of Each Component (log10 PFU) | Monovalent Vaccine Component for Indicated Serotype |
|||
|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||
| TV001 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3-3′D4Δ30 | rDEN4Δ30 |
| TV002 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3-3′D4Δ30 | rDEN4Δ30-200,201 |
| TV003 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30/31 | rDEN4Δ30 |
| TV004’ | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30/31 | rDEN4Δ30-200,201 |
Abbreviations: DENV, dengue vaccine; PFU, plaque-forming units.
Clinical Procedures and Evaluation
On study day 0, subjects reported to the site of enrollment and were randomly assigned to receive either vaccine or placebo (vaccine diluent) given as a 0.5 mL subcutaneous injection. Subjects were monitored for immediate adverse reactions for at least 30 minutes after vaccination and given a digital thermometer and diary card to record oral temperature 3 times daily for 16 days. Clinical assessments and physical examinations were performed every other day through study day 16 and on study days 21, 28, 42, and 180 as described elsewhere [20].
Adverse Events
All adverse events were graded for intensity and relationship to vaccination. Intensity was defined as mild (easily tolerated, may have required 1 dose of medication), moderate (interfered with daily activity or required >1 dose of medication), or severe (prevented daily activity or required medical intervention). Abnormal hematology, coagulation, and serum chemistry findings were also graded as mild, moderate, or severe, using standardized toxicity tables. Dengue-like syndrome was defined as in previous studies [19]. Infection was defined as recovery of vaccine virus from the blood and/or seroconversion to DENV as measured by PRNT60 assay. Serious adverse events were defined in accordance with 21CFR312.32. Fever was determined by oral temperature recorded on 2 consecutive measurements ≥1 hour apart and was defined as grade 1 (100.4–101.4°F), grade 2 (101.5–102.4°F), or grade 3 (>102.4°F). Abnormal clinical laboratory findings were graded as mild, moderate, or severe using standardized toxicity tables. Serious adverse events were defined in accordance with 21CFR312.32.
Virus Quantitation and Serologic Assessment
Serum samples collected every other study day until day 16 were assayed for viable virus by amplification and direct titration on Vero cell monolayers as described elsewhere [14]. Titration was performed in a serotype-specific manner using the following monoclonal antibodies for detection: 1F1 (DENV-1), 3H5 (DENV-2), 8A1 (DENV-3), and 1H10 (DENV-4). The lower limit of virus detection was 3 PFU/mL. Neutralizing antibody responses were determined by PRNT60 assay as described previously [14], using DENV-1 (WP), DENV-2 (NGC), DENV-3 (Sleman/78), or DENV-4 (814669 Dominica 1981) as the target viruses. The initial serum dilution in this assay was 1:5, and seropositivity was defined as a PRNT60 ≥ 1:10 by study day 42 [21–23].
Data Analysis
Significant differences in the frequency of solicited adverse events and demographic characteristics were determined using χ2 or Student t test. Comparisons of mean peak virus titer, onset of viremia, and duration of viremia were performed using multivariate analysis with a post hoc Tukey-Kramer HSD test; mean values ± standard error (SE) are presented. Statistical analysis was performed using JMP software (version 9.0.2; SAS Institute).
Role of the Funding Source
The trial was funded by the NIAID Intramural Research Program, National Institutes of Health. The Regulatory Compliance Human Subjects Protection Branch (RCHSPB) of the National Institutes of Health acted as the Sponsor of the trial. RCHSPB was not involved in the study design; however, they did review the clinical protocol and consent form prior to submission of the Investigational New Drug Application to ensure compliance with the Code of Federal Regulations (21 CFR 50). RCHSPB was not involved in the data analysis or in generation of this paper.
RESULTS
Screening, Enrollment, Demographics
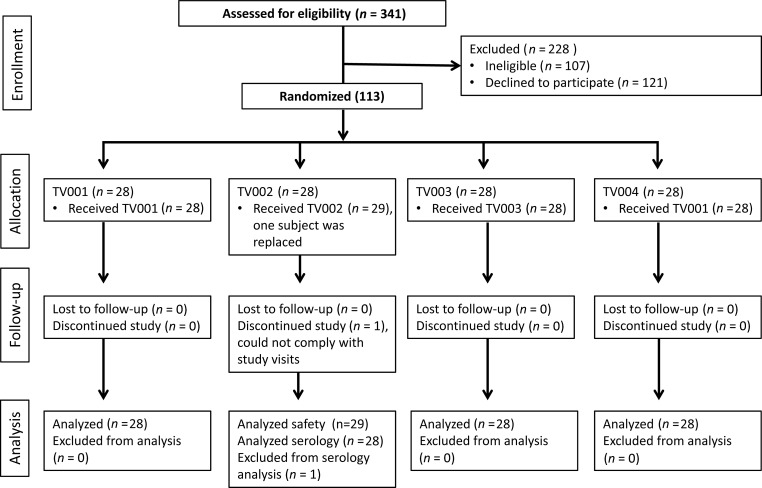
In total, 341 subjects were screened for study participation. Of those, 121 declined, 107 were ineligible, and 113 subjects were enrolled into the study (Figure 1). There was no significant difference in gender between vaccinees (52% women) and placebo recipients (41% women) or in age (29.8 years ± 9.5 standard deviations [SD], vaccinees; 31.8 years ± 9.6 SD, placebo-recipients). Forty-nine percent of vaccinees self-identified as white, 46% as black, 2% as multi-racial, 1% as Asian, and 1% Indian/Alaskan-native. This compares with 38% of placebo recipients who identified as white, 50% as black, 6% as multi-racial, 3% as Indian/Alaskan-native, and 3% as unknown. One subject withdrew from the study prior to study day 42 due to an inability to remain compliant with scheduled visits and was replaced. The safety data collected from this volunteer are included in the safety analysis.
Figure 1.
Flow diagram of the screening, enrollment, follow-up, and analysis of study participants.
Safety and Reactogenicity
All admixtures of the vaccine were well tolerated. Vaccine-related serious adverse events were not reported, and none of the vaccinees developed a fever at any time during the 28-day safety follow-up period. A significant difference was not found in the incidence of any adverse event between the 4 cohorts (data not shown). The only adverse event that occurred with a significantly higher incidence in vaccinees was an asymptomatic rash; 52 vaccinees (64.2%) developed rash compared with 0 placebo recipients (Table 2). Rash was maculopapular in character and similar to what has been observed in vaccinees who received the monovalent dengue vaccines included in the tetravalent admixtures [14–20]. There were no significant differences in incidence of other solicited adverse events between vaccinees and placebo recipients including injection site reactogenicity, headache, myalgia, arthralgia, retro-orbital pain, neutropenia, or elevation in serum alanine aminotransferase (Table 2). Ninety-three percent of all local and solicited adverse events were mild in severity, 6% were moderate, and 1 adverse event (neutropenia) was severe. The neutropenia occurred in a subject whose baseline absolute neutrophil (ANC) count on study day 0 was 1975/mm3. The ANC nadir was 722/mm3 (severe) on study day 14, increased to 1247/mm3 (mild) at study day 16, and returned to within normal limits (≥1500/mm3) by study day 19.
Table 2. Percentage of LATV or Placebo Recipients Who Experienced Indicated Injection Site or Systemic Adverse Event
| Adverse Event | LATV (n = 81) (%) | Placebo (n = 32) (%) | P Value |
|---|---|---|---|
| Injection site | |||
| Erythema | 9.9 | 3.1 | .4415 |
| Pain | 0.0 | 3.1 | .2832 |
| Tenderness | 7.4 | 3.1 | .6709 |
| Induration | 2.5 | 3.1 | 1.0000 |
| Systemic | |||
| Fevera | 0.0 | 0.0 | n/a |
| Headache | 39.5 | 40.6 | 1.0000 |
| Rash | 64.2 | 0.0 | <.0001 |
| Neutropeniab | 25.9 | 15.6 | .3235 |
| Elevated ALTc | 2.5 | 3.1 | 1.0000 |
| Myalgia | 14.8 | 9.4 | .5501 |
| Arthralgia | 7.4 | 3.1 | .6709 |
| Retro-orbital pain | 9.9 | 6.2 | .7222 |
Abbreviations: ALT,alanine aminotransferase; LATV,live attenuated tetravalent vaccine.
a Oral temperature ≥100.4°F.
b Absolute neutrophil count <1500/mm3.
c Defined as >1.25 × the clinical laboratory upper limit of normal.
Low-level viremia occurred frequently; vaccine viruses of ≥1 serotypes were detected from 58 of 80 vaccinees (73%) over the 16-day postvaccination period (Table 3). DEN2/4Δ30 and DEN4Δ30-200,201 were detected less frequently than the other vaccine viruses. Although the majority of viremic subjects (37/58 [64%]) had only 1 serotype of vaccine virus detected in the blood, 17 of 58 (29%) had 2 viruses detected, and 4 of 58 (7%) vaccinees had 3 viruses detected. The mean peak titer of each serotype was very low, <10 PFU/mL (Table 3).
Table 3. Low-Level Viremia Is Observed for All 4 Dengue Virus (DENV) Serotypes Following Administration of the Indicated Admixture
| Admixture | Vaccine Components | % Subjects With Viremia | Mean Peak Titer ± SE (log10 PFU/mL) | Maximum Titer (log10 PFU/mL) | Mean Day of Onset (range) | Mean Duration in Days (range) |
|---|---|---|---|---|---|---|
| TV001 | DENΔ130 | 40 | 0.50 ± 0.14 | 0.5 | 12.1 (9–16) | 2.2 (1–5) |
| (N = 20) | DEN2/4Δ30 | 10 | 0.50 ± 0.00 | 0.5 | 11.0 (6–16) | 1.0 (all 1) |
| DEN3-3′D4Δ30 | 20 | 0.63 ± 0.12 | 1.0 | 11.0 (6–14) | 2.2 (1–4) | |
| DEN4Δ30 | 40 | 0.60 ± 0.09 | 1.2 | 9.6 (5–12) | 2.0 (1–5) | |
| Total % | 70 | |||||
| TV002 | DEN1Δ30 | 50 | 0.76 ± 0.14 | 1.6 | 11.3 (7–15) | 2.1 (1–6) |
| (N = 20) | DEN2/4Δ30 | 5 | 0.50 ± 0.00 | 0.5 | 9.0 | 1 |
| DEN3-3′D4Δ30 | 0 | … | … | … | … | |
| DEN4Δ30-200,201 | 5 | 0.50 ± 0.00 | 0.5 | 7.0 | 1 | |
| Total % | 60 | |||||
| TV003 | DEN1Δ30 | 30 | 0.73 ± 0.16 | 1.4 | 9.8 (8–12) | 2.3 (1–5) |
| (N = 20) | DEN2/4Δ30 | 10 | 0.50 ± 0.00 | 0.5 | 6.0 (all 6) | 1.0 (all 1) |
| DEN3-3Δ30/31-7164 | 40 | 0.59 ± 0.09 | 1.2 | 8.2 (5–14) | 2.6 (1–6) | |
| DEN4Δ30 | 25 | 0.50 ± 0.00 | 0.5 | 7.4 (6–9) | 1.4 (1–3) | |
| Total % | 75 | |||||
| TV004 | DEN1Δ30 | 40 | 0.55 ± 0.06 | 0.7 | 8.4 (2–12) | 2.8 (1–8) |
| (N = 20) | DEN2/4Δ30 | 35 | 0.50 ± 0.06 | 0.5 | 8.4 (6–12) | 2.3 (1–4) |
| DEN3Δ30/31-7164 | 75 | 0.59 ± 0.40 | 1.4 | 8.9 (5–14) | 2.8 (1–8) | |
| DEN4Δ30-200,201 | 0 | … | … | … | … | |
| Total % | 85 |
Abbreviations: PFU, plaque-forming units; SE, standard error.
Serological Assessment
Each of the admixtures induced seropositivity rates of 50%–100% to each monovalent component following a single dose (Table 4). The DEN2/4Δ30 vaccine induced the lowest seropositivity rate in all of the admixtures (50%–65%). Admixture TV003 appeared to induce the most balanced antibody response, inducing seropositivity against DENV-1 and DENV-4 in 100% of vaccinees, against DENV-3 in 85% of vaccinees, and against DENV-2 in 50% of vaccinees. This admixture induced an antibody response to all 4 DENV serotypes (tetravalent response) in 45% of vaccinees and a trivalent response in an additional 45% of vaccinees for an overall trivalent or greater response in 90% of vaccinees after a single subcutaneous dose (Table 5). Additionally, the antibody titers against all 4 DENV serotypes were relatively balanced; there was <2-fold difference between the mean PRNT60 to each serotype (Table 4).
Table 4.
Robust Neutralizing Antibody Response to All 4 Dengue Virus (DENV) Serotypes Following a Single Dose of Live Attenuated Tetravalent Admixture
| Admixture | N | % Seropositivity (PRNT60 ≥ 10)a,c |
Mean Peak Titer (GMT) (PRNT60)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | Mean | DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||
| TV001 | 20 | 80 | 65 | 60 | 95 | 75 | 54 [B]b | 39 [AB] | 36 [B] | 154 [A] |
| TV002 | 20 | 80 | 60 | 75 | 90 | 76 | 118 [A] | 41 [AB] | 31 [B] | 32 [C] |
| TV003 | 20 | 100 | 50 | 85 | 100 | 84 | 62 [AB] | 44 [A] | 36 [B] | 65 [B] |
| TV004 | 20 | 75 | 50 | 85 | 85 | 74 | 36 [B] | 17 [B] | 124 [A] | 32 [C] |
Abbreviations: GMT,geometric mean titer; PRNT,plaque-reduction neutralization titer.
a Only those vaccinees who were seropositive by study day 42 were included in the analysis per the protocol. However, analysis of serum collected at study day 180 revealed a few late responders who became seropositive only after study day 42.
b Mean titers in each column not sharing the same letter are significantly different (α = 0.05).
c Recipients of placebo did not develop neutralizing antibody to any dengue serotype.
Table 5. A Majority of Vaccinees Generated a Trivalent or Greater Response to All Live Attenuated Tetravalent Vaccine Admixtures
| Admixture | N | % Subjects With Indicated Multivalent Response (Cumulative)a |
||||
|---|---|---|---|---|---|---|
| Tetra- | Tri- | Bi- | Mono- | None | ||
| TV001 | 20 | 30 | 50 (80) | 15 (95) | 0 (95) | 5 |
| TV002 | 20 | 30 | 45 (75) | 5 (100) | 0 (100) | 0 |
| TV003 | 20 | 45 | 45 (90) | 10 (100) | 0 (100) | 0 |
| TV004 | 20 | 35 | 40 (75) | 15 (90) | 5 (95) | 5 |
a Only those vaccinees who were seropositive by study day 42 were included in the analysis per the protocol. However, analysis of serum collected at study day 180 revealed a few late responders who became seropositive only after study day 42.
DISCUSSION
We sought to identify an optimal LATV dengue vaccine candidate by evaluating 4 different admixtures in flavivirus-naive adult subjects and found each of the admixtures to be well tolerated and immunogenic, inducing a trivalent or greater antibody response in 75%–90% of volunteers following a single dose. The most common vaccine-associated adverse event was a transient, asymptomatic rash in 64% of subjects. The incidence of rash was highly associated with ethnicity: 91% of white subjects developed rash, whereas only 35% of black volunteers developed rash (P < .0001, Table 6). Although this may be attributed in part to the difficulty of observing a faint rash on pigmented skin, it does not entirely explain the association as the presence of rash was also predictive of the immune response to the vaccine. The development of a trivalent or greater response was highly associated with the presence of rash (P ≤ .0001), and with race other than black (P ≤ .0001, Table 6). Ninety-eight percent of non-black subjects developed at least a trivalent antibody response compared with 59% of black subjects. In multivariate analysis, both race (non-black; P = .0075) and rash (P = .0256) were independent predictors of a trivalent or tetravalent antibody response. Additionally, black vaccinees were less likely to develop viremia than were non-black vaccinees (P = .0065). Black race has been identified as a protective factor against severe dengue disease in outbreaks in Cuba, Haiti, and Brazil [24–27]. From our study it is unclear whether the lower trivalent or tetravalent seropositivity rates in black vaccinees were due to an inherent resistance to infection with the LATV vaccine components, as evidenced by lower rates of viremia, or that their antibody response was, for some reason, less robust. Other clinical studies of dengue vaccines have not addressed racial difference in response to vaccination, probably due to the low number of black subjects enrolled or the lack of race-specific analysis [21, 28, 29]. However, studies that have evaluated racial differences in the response to vaccination with polysaccharide or subunit protein antigens for pathogens other than dengue showed either no effect of race on the antibody response or, in some cases, a greater neutralizing antibody response in black vaccinees [30–32]. Additional studies evaluating racial differences in infection and response rates to vaccination with LATV dengue vaccines are warranted as they may provide insight into innate protection against natural dengue illness.
Table 6. Differences in the Occurrence of Rash, Viremia, and Serologic Response to Live Attenuated Tetravalent Dengue Vaccine Admixtures in Black Subjects Compared With Non-Black Subjects
| Race | N | No. of Subjects With Indicated Response (%)a |
|||
|---|---|---|---|---|---|
| Rash | Viremia | ≥Trivalent | Tetravalent | ||
| Black | 37 | 13 (35) | 20 (57) | 22 (59) | 8 (22) |
| Non-blacka | 43 | 39 (91) | 36 (84) | 42 (98) | 20 (46) |
| P value (2-tail): | <.0001 | .0065 | <.0001 | .03 | |
a Non-black subjects include white (n = 39), multiple race (n = 2), Asian (n = 1), and Indian/Alaskan Native (n = 1).
A challenge to the development of a LATV dengue vaccine has been overcoming the presumed viral interference that may prevent the induction of a balanced immune response to all 4 serotypes [33, 34]. This interference may be related to the inherent infectivity and degree of attenuation of each vaccine component such that, when combined in a multivalent formulation, ≥1 serotypes can outcompete the others in a manner similar to that observed with live polio vaccine [35, 36]. To minimize any competition between the 4 replicating component viruses combined in a tetravalent admixture, we examined 6 candidate monovalent vaccines that were demonstrated to be both highly infectious and immunogenic [13–20]. Although the optimal monovalent candidates were carefully chosen, the composition of each tetravalent admixture may still have influenced the antibody response to the individual serotypes (Tables 4 and 5). These differences in antibody responses are likely related to the degree of attenuation and/or infectivity of each monovalent component. The 50% human infectious dose (HID50) of 4 of the candidates was determined to be ≤10 PFU; that of rDEN3-3′D4Δ30 and rDEN4Δ30-200, 201 was not determined. The monovalent candidate with the highest HID50 (∼10 PFU) was the chimeric rDEN2/4Δ30 vaccine, and interestingly, this virus appears to be the least infectious in the tetravalent formulations tested, inducing seropositivity rates ranging from 50% to 65%. Immunodominance of different DENV serotypes varied by admixture components: rDEN4Δ30-200,201 and rDEN3-3′D4Δ30 appeared to be more attenuated as monovalent vaccines than rDEN4Δ30 [13, 20] and rDEN3Δ30/31 (data not shown), respectively, and admixtures containing these vaccines (TV001, TV002, and TV004) demonstrated immunodominance of 1 of the other components of the admixture (Table 4). The most balanced antibody response was achieved when the more potent rDEN3Δ30/31 and rDEN4Δ30 candidates were mixed with rDEN1Δ30 and rDEN2/4Δ30 (TV003).
Each of the tetravalent admixtures was immunogenic, inducing a trivalent or greater seropositive response in up to 90% (TV003) of vaccinees. A single subcutaneous dose of TV003 induced a seropositivity response in flavivirus-naive subjects that is comparable to 3 doses of the leading candidate LATV dengue vaccine (CYD) given over a 12–15-month schedule [28, 37]. Although neutralizing antibody has long been thought to mediate protection against DENV, the recently published phase 2b efficacy trial reported that CYD failed to protect against DENV-2, despite its ability to induce measurable neutralizing antibody to DENV-2 [38]. The reasons for this failure are unclear and under investigation but may include an antigenic mismatch between the vaccine virus and the circulating DENV-2 responsible for the observed illness, induction of only heterotypic or low-quality antibody to DENV-2, lack of T-cell epitopes for DENV owing to the presence of yellow fever virus nonstructural proteins from the chimeric background, or a difference in the infectivity or virion structure specific to the DENV-2 component. Any of these possible mechanisms give all dengue vaccine researchers reasons for concern. However, there are several notable differences between the CYD candidate vaccine and the admixtures we have described. For the tetravalent vaccine components described here, we have previously confirmed the levels of infectivity for each monovalent vaccine candidate and shown the 50% human infectious dose to be 10 PFU or less. The infectivity of the CYD vaccine components has not been described. Unlike the CYD vaccine, all nonstructural proteins expressed by our tetravalent admixtures are encoded by DENV and should present relevant T-cell epitopes important for cell-mediated immunity. Finally, vaccine viremia was previously observed in recipients of CYD following the second and third dose, indicating incomplete immunity against the attenuated vaccine virus strains [22, 28, 37]. In contrast, preliminary results from our ongoing assessment of the effect of a challenge dose of vaccine given 6 months after the first dose in 46 vaccinees indicates a difference compared to CYD. Following the second dose of any of our LATV admixtures, virus was not detected on any day from any volunteer, and there was very little boosting in antibody titer, indicating the vaccine induced sterile immunity (data not shown).
Based on the safety and immunogenicity profile of the 4 different admixtures we evaluated, TV003 was chosen as the LATV admixture for further development and evaluation. Several factors make this admixture an attractive LATV candidate. TV003 induced a trivalent or greater neutralizing antibody response in 90% of flavivirus-naive adult vaccinees following a single dose. This response rate is comparable to 3 doses of the CYD dengue vaccine candidate and to 2 doses of a second LATV dengue vaccine candidate given 3 months apart [28, 37, 39]. In addition, it is clear that no single vaccine component is replicating to a level that outcompetes the remaining serotypes, enabling TV003 to elicit a well-balanced neutralizing antibody response that is not dominated by a single serotype. However, it remains to be seen if this response will be adequate to provide protection. Finally, because of the relatively low dose administered, the vaccine is very economical to produce, with production cost estimates of $0.20/dose for a multidose presentation and $0.70/dose for a single-dose presentation in Brazil [40]. Based on the success of these initial studies, we have initiated an expanded safety evaluation of TV003 in healthy flavivirus-naive adults; an evaluation of the safety and immunogenicity of TV003 in flavivirus-experienced adults; and an age de-escalation trial in a DENV-endemic area. Additionally, manufacturers in Brazil, India, and Vietnam have licensed the vaccine for in-country production and use. The Instituto Butantan of Brazil has initiated production of TV003 and is expected to begin phase 2 clinical trials in 2012. We have presented the first report of a well-tolerated LATV dengue vaccine that elicits a balanced, trivalent or greater antibody response in 90% of vaccinated flavivirus-naive subjects following a single dose. Should future studies of this vaccine prove it to be efficacious, the vaccine could be a cost-effective means of controlling dengue in endemic areas and an important public health asset.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) Intramural Research Program, National Institutes of Health (contract HHSN272200900010C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Beatty ME, Stone A, Fitzsimons DW, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis. 2010;4:e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB, Suaya JA, Shepard DS. The burden of dengue infection. Lancet. 2007;369:1410–1. doi: 10.1016/S0140-6736(07)60645-X. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Research. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 4.Fox A, Le NM, Simmons CP, et al. Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. PLoS Negl Trop Dis. 2011;5:e967. doi: 10.1371/journal.pntd.0000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 6.Imrie A, Meeks J, Gurary A, et al. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 2007;20:672–5. doi: 10.1089/vim.2007.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabin A. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Papaevangelou G, Halstead SB. Infections with two dengue viruses in Greece in the 20th century. Did dengue hemorrhagic fever occur in the 1928 epidemic? Am J Trop Med Hyg. 1977;80:46–51. [PubMed] [Google Scholar]
- 9.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–84. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 10.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–3. [PubMed] [Google Scholar]
- 13.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29:7242–50. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–13. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 15.Durbin AP, McArthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2:167–73. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 16.Durbin AP, McArthur JH, Marron JA, et al. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum Vaccin. 2006;2:255–60. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 17.Durbin AP, Schmidt A, Elwood D, et al. Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis. 2011;203:327–34. doi: 10.1093/infdis/jiq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durbin AP, Whitehead SS, McArthur J, et al. rDEN4 Delta 30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191:710–8. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 19.Durbin AP, Whitehead SS, Shaffer D, et al. A single dose of the DENV-1 candidate vaccine rDEN1Δ30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis. 2011;5:e1267. doi: 10.1371/journal.pntd.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McArthur JH, Durbin AP, Marron JA, et al. Phase I clinical evaluation of rDEN4Delta30-200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg. 2008;79:678–84. [PMC free article] [PubMed] [Google Scholar]
- 21.Edelman R, Wasserman SS, Bodison SA, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69:48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 22.Capeding RZ, Luna IA, Bomasang E, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine. 2011;29:3863–72. doi: 10.1016/j.vaccine.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 23.Lang J. Recent progress on Sanofi Pasteur's dengue vaccine candidate. J Clin Virol. 2009;46(Suppl 2):S20–4. doi: 10.1016/S1386-6532(09)70291-4. [DOI] [PubMed] [Google Scholar]
- 24.Kouri GP, Guzman MG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull World Health Organ. 1989;67:375–80. [PMC free article] [PubMed] [Google Scholar]
- 25.Blanton RE, Silva LK, Morato VG, et al. Genetic ancestry and income are associated with dengue hemorrhagic fever in a highly admixed population. Eur J Hum Genet. 2008;16:762–5. doi: 10.1038/ejhg.2008.4. [DOI] [PubMed] [Google Scholar]
- 26.Halstead SB, Streit TG, Lafontant JG, et al. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–3. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 27.Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81:816–20. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 28.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis. 2010;201:370–7. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- 29.Sun W, Cunningham D, Wasserman SS, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naive adults. Hum Vaccin. 2009;5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 30.Crum-Cianflone NF, Roediger M, Huppler Hullsiek K, et al. The association of ethnicity with antibody responses to pneumococcal vaccination among adults with HIV infection. Vaccine. 2010;28:7583–8. doi: 10.1016/j.vaccine.2010.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granoff DM, Shackelford PG, Pandey JP, Boies EG. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986;154:257–64. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- 32.Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;190:1962–9. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 33.Guy B, Barban V, Mantel N, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80:302–11. [PubMed] [Google Scholar]
- 34.Anderson KB, Gibbons RV, Edelman R, et al. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J Infect Dis. 2011;204:442–50. doi: 10.1093/infdis/jir279. [DOI] [PubMed] [Google Scholar]
- 35.Payne AM. Oral immunization against poliomyelitis. Bull World Health Organ. 1960;23:695–703. [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado YA, Pena-Cruz V, de la Luz Sanchez M, et al. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. J Infect Dis. 1997;175:545–53. doi: 10.1093/infdis/175.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated tetravalent dengue vaccine in dengue-naive children, adolescents, and adults in Mexico City: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J. 2011;30:e9–17. doi: 10.1097/INF.0b013e3181fe05af. [DOI] [PubMed] [Google Scholar]
- 38.Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–67. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 39.Santangelo JD. DENVax: A live attenuated tetravalent dengue vaccine for dengue fever. 2012 World Vaccine Manufacturing Congress. Washington DC. [Google Scholar]
- 40.Mahoney RT, Francis DP, Frazatti-Gallina NM, et al. Cost of production of live attenuated dengue vaccines: A case study of the Instituto Butantan, Sao Paulo, Brazil. Vaccine. 2012;30:4892–6. doi: 10.1016/j.vaccine.2012.02.064. [DOI] [PubMed] [Google Scholar]