Abstract
Upon the entry of the viral genome into the nucleus, herpes simplex virus type 1 (HSV-1) gene expression is rapidly repressed by constitutively expressed cellular proteins. This intrinsic antiviral defense is normally counteracted by ICP0, which allows virus infection to proceed efficiently. Replication of ICP0-null mutant HSV-1, however, is severely repressed by mechanisms that are conferred, at least in part, by nuclear domain 10 (ND10) components, including hDaxx, the promyelocytic leukemia (PML) protein, and Sp100. To investigate if these ND10 components repress viral gene expression in a cooperative manner, we simultaneously depleted host cells for hDaxx, PML, and Sp100 by multiple short hairpin RNA (shRNA) knockdown from a single lentivirus vector. We found that replication and gene expression of ICP0-null mutant HSV-1 were cooperatively repressed by hDaxx, PML, and Sp100 immediately upon infection, and all stages of virus replication were inhibited. Plaque-forming efficiency was enhanced at least 50-fold in the triple-depleted cells, a much larger increase than achieved by depletion of any single ND10 protein. Similar effects were also observed during infection of triple-depleted cells with human cytomegalovirus (HCMV). Moreover, using a cell culture model of quiescent infection, we found that triple depletion resulted in a much larger number of viral genomes escaping repression. However, triple depletion was unable to fully overcome the ICP0-null phenotype, implying the presence of additional repressive host factors, possibly components of the SUMO modification or DNA repair pathways. We conclude that several ND10 components cooperate in an additive manner to regulate HSV-1 and HCMV infection.
INTRODUCTION
Upon entry into the host cell, the herpes simplex virus 1 (HSV-1) capsid is released into the cytoplasm and then carried along microtubules to the nuclear envelope, where it docks onto a nuclear pore and ejects the viral genome into the nucleoplasm. There, the viral DNA is faced with intracellular antiviral defense mechanisms that have only recently been recognized, termed intrinsic resistance (1–3). Unlike the subsequent and complex innate and adaptive immunity pathways, intrinsic immunity does not require downstream synthesis of cellular effectors but is mediated directly by constitutively expressed and permanently active cellular restriction factors. Intrinsic resistance was first discovered in the context of retrovirus and lentivirus infections, with different stages of their replication cycles being targeted by diverse mechanisms (1). The HIV capsid, for example, is destabilized in the cytoplasm by the tripartite motif-containing protein TRIM5α, the fidelity of its coding potential is impaired by members of the APOBEC family, and the release of HIV particles is inhibited by BST-2/tetherin (4–6). More recently, there has been accumulating evidence that many DNA viruses, in particular herpesviruses, are also subject to intrinsic resistance by various mechanisms (2, 7–13).
One group of proteins mediating intrinsic immunity to HSV-1 is associated with nuclear compartments known as promyelocytic leukemia (PML) nuclear bodies, or nuclear domain 10 (ND10), which appear as discrete foci within the nucleoplasm. ND10 proteins are involved in many cellular pathways, such as the DNA damage response (DDR), chromatin modification, the stress response, senescence, and protein stability, and there are strong links between the functions of ND10 and protein modification with small ubiquitin-like modifier (SUMO) family members (9, 14, 15). During infection with a number of nuclear-replicating DNA viruses, parental viral genomes are frequently associated with ND10 proteins, with viral transcription and early DNA replication detectable in close proximity to these domains (16). On the other hand, a number of viral regulatory proteins cause the disruption of ND10, implying that an antiviral effect of ND10 or its components has to be overcome (2, 9).
During HSV-1 infection, ND10 components such as PML, Sp100, and hDaxx are rapidly recruited toward sites of parental viral genomes that have entered the nucleus, a process which at least in some instances is dependent on SUMO modification pathways and which subsequently has a repressive effect on viral gene expression (17, 18). PML is the key component required for ND10 integrity and is involved in numerous regulatory processes (14, 19–22). Another permanent component of ND10 is the nuclear autoantigen Sp100, for which a role in transcriptional repression has been suggested (23, 24). Human death domain-associated protein 6 (hDaxx) has also been shown to be involved in the repression of gene expression and chromatin modification (25, 26) and acts as a histone chaperone in complex with ATRX (27–30). All three of these major ND10 components are involved in intrinsic resistance to HSV-1 and human cytomegalovirus (HCMV) (10–12, 31–36). It is therefore tempting to predict that ND10 components might restrict viral gene expression by generating a repressive environment at the sites of incoming viral genomes.
This intrinsic repression of viral gene expression is, however, counteracted by a number of viral regulatory proteins through various mechanisms, including degradation or relocalization of ND10 components. In HCMV infections, for instance, PML and Sp100 are dispersed, and their SUMO-modified forms are degraded by the immediate-early (IE) protein IE1 (34, 37–41), while hDaxx is at least partially degraded through interactions with the viral tegument protein pp71 (pUL82), a process that is vital for the initiation of productive HCMV gene expression (12, 31, 32, 42). In HSV-1, the viral E3 ubiquitin ligase ICP0 plays an important role in the initiation of lytic replication and reactivation from latency. These activities correlate with its ability to disrupt ND10 by the proteasome-dependent degradation of PML and the SUMO-modified forms of both PML and Sp100 (40, 43, 44). HSV-1 mutants that fail to express ICP0 are unable to disrupt ND10 or degrade PML and display a profound defect in virus replication (45–47).
Depletion of PML, hDaxx, or Sp100 individually improves the replication of ICP0-null mutant HSV-1, but the effect is modest compared to the full extent of complementation possible (10, 11, 48). Although simultaneous depletion of PML and Sp100 increased this effect further, it could not substitute for the lack of ICP0 (10). Similarly, HCMV gene expression is increased by depletion of hDaxx, Sp100, or PML (34, 36, 38, 49), and simultaneous depletion of hDaxx and PML led to additional enhancements in replication efficacy compared to single-knockdown cells (36). Because PML is required for the assembly of ND10 in uninfected cells (50), it might be expected that depletion of PML would compromise the repressive activities of all ND10 components. Surprisingly, this is not the case. The restrictive effects of PML and hDaxx correlate with their recruitment to sites associated with HSV-1 genomes (17), and this spatial relocalization of individual ND10 components is not dependent on PML. For example, even in the absence of PML or Sp100, hDaxx is still recruited to parental HSV-1 genomes (10), and the absence of PML and hDaxx does not eliminate the targeting of Sp100 to HCMV nucleoprotein complexes (2). Therefore, the localization of these three ND10 components is independent of each other in virus-infected cells, implying that they might individually contribute to the repressive effect on viral genomes. The aim of this study was therefore to test the hypothesis that ND10 components repress viral genomes in a cooperative manner.
To achieve this, we describe a method to analyze HSV-1 and HCMV infection in cells depleted of three different ND10 components (PML, hDaxx, and Sp100), using the simultaneous expression of three different short hairpin RNAs (shRNAs) from a single lentivirus vector. We found that depletion of these three proteins increased the gene expression and plaque formation efficiencies of ICP0-null mutant HSV-1 and wild-type (wt) HCMV to a greater extent than the individual depletion of any one single protein or the simultaneous depletion of both hDaxx and PML. Triple depletion allowed an earlier onset and a faster progression of HSV-1 replication than in nondepleted cells. Furthermore, the establishment of quiescent HSV-1 infections was less efficient in triple-depleted cells, with many more cells escaping the repression that precedes quiescence. However, simultaneous depletion of PML, hDaxx, and Sp100 was not sufficient to complement completely the lack of ICP0, implying the presence of additional cellular repressors. These might include components of the SUMO or DDR pathways, because depletion of these three ND10 components did not compromise recruitment of SUMO conjugates or DDR proteins to the sites of incoming HSV-1 genomes.
MATERIALS AND METHODS
Viruses and cells.
The viruses used in this study include wt HSV-1 strain 17+ and its ICP0-null mutant derivative dl1403 (51). The viruses in1863 and dl1403/CMVlacZ are derivatives of the wt and ICP0-null mutant viruses which contain the lacZ gene under the control of the HCMV promoter/enhancer inserted into the tk gene (kindly provided by Chris Preston). The HSV-1 mutant tsK includes a temperature-sensitive lesion in ICP4, and mutant virus in1374 additionally contains a deletion of the ICP0 gene and a mutation within VP16 that inactivates its ability to stimulate IE gene expression (52, 53). Viruses were propagated in baby hamster kidney (BHK) cells and titrated in U2OS cells, in which ICP0 is not required for efficient replication of HSV-1. tsK and in1374 viruses were propagated at the permissive temperature of 31°C. All viruses were used at multiplicities of infection (MOIs) based on their PFU titers in U2OS cells, regardless of the cell type used (54). HCMV strain AD169 was propagated in and titrated on human diploid fibroblasts (HFs) (a gift from Thomas Stamminger), as described previously (55).
HFs and U2OS and HEK-293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, and BHK cells were grown in Glasgow modified Eagle's medium containing 10% newborn calf serum and 10% tryptose phosphate broth. HepaRG hepatocyte cells (56) were grown in William's medium E containing 10% fetal bovine serum (FBS) Gold (PAA Laboratories Ltd.), 2 mM glutamine, 5 μg/ml insulin, and 0.5 μM hydrocortisone. All cell growth media were supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. Lentivirus-transduced cells were maintained under continuous antibiotic selection, as appropriate.
Lentivirus construction, transduction, and shRNA sequences.
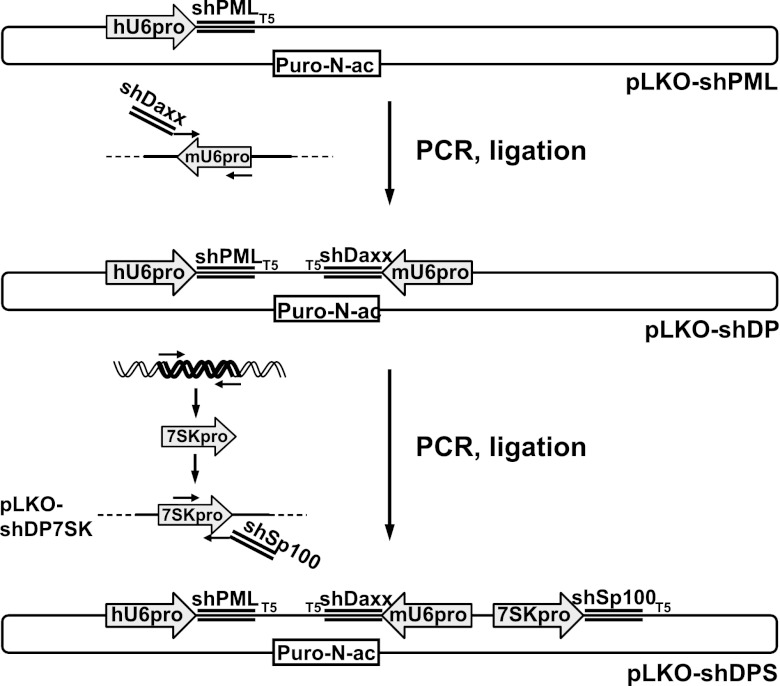
The backbone of the parental lentivirus vector plasmid expressing anti-PML and anti-hDaxx shRNAs (here named pLKO-shPML and pLKO-shD) were described previously (11, 48). For simultaneous depletion of PML and hDaxx, oligonucleotides dblsh.for1 and dblsh.rev1, containing the shDaxx-small interfering RNA (siRNA) sequence and priming in the mouse U6 promoter (mU6), were annealed in an initial PCR (see Table 1 for the sequences of all primers described here). A second PCR used this product and oligonucleotide dblsh.for2 as primers to amplify the mU6 promoter (from a plasmid kindly provided by Arvind Patel) to generate a fragment containing the mU6 promoter linked to the anti-hDaxx shRNA, which was inserted into pLKO-shPML using MfeI and EcoRI restriction sites, resulting in vector plasmid pLKO-shDP. For additional depletion of Sp100, the human 7SK promoter (p7SK) was amplified from genomic DNA isolated from HepaRG cells (using the Qiagen DNeasy blood and tissue kit), using oligonucleotides 7SKXma.for and 7SKXba.rev. The product was inserted into pLKO-shDP using XbaI and XmaI, giving pLKO-shDP7SK. Oligonucleotides shSp7SK.for and shSp7SK.rev, encoding the shSp100 shRNA sequence plus priming in p7SK, were annealed. A second PCR, using this product with oligonucleotide shSp7SK.for2 and template pLKO-shDP7SK, produced the p7SK-shSp100 fragment, which was inserted into pLKO-shDP using NotI and XbaI, resulting in plasmid pLKO-shDPS. The overall construction strategy is summarized in Fig. 1.
Table 1.
Oligonucleotides used in the construction of lentivirus vectors for double deletion (pLKO-shDP) and triple depletion (pLKO-shDPS)
| Primer | Sequence |
|---|---|
| dblsh.for1 | 5′-CCGCAATTGATCACCGGTTCGAAAAAGGAGTTGGATCTCTCAGAATCTCTTGAATTCTG-3′ |
| dblsh.rev1 | 5′-CTTGGAGAAAAGCCTTGTTTGGAGTTGGATCTCTCAGAATTCAAGAGATTCTGAGA-3′ |
| dblsh.for2 | 5′-GCCGACAATTGTCTAGACCCGGGATCCGACGCCGCCATCTCTA-3′ |
| 7SKXma.for | 5′-CCGGCCCCGGGCTGCAGTATTTAGCATGCCCCACCC-3′ |
| 7SKXba.rev | 5′-GGCCGTCTAGAGAGGTACCCAGGCGGCGCACAAGC-3′ |
| shSp7SK.for | 5′-CGCCGCCTGGGTACCTCGTGAGCCTGTGATCAATAATTCAAGAGATTATT-3′ |
| shSp7SK.rev | 5′-CCGTCTAGACGCGTAAAAAGTGAGCCTGTGATCAATAATCTCTTGAATT-3′ |
| shSp7SK.for2 | 5′-AGCAAGCGGCCGCTGATCTTCAGA-3′ |
Fig 1.
Construction of a single lentivirus vector for simultaneous depletion of hDaxx, PML, and Sp100. Shown is a diagrammatic representation of the triple-shRNA vector construction strategy. For generation of a lentivirus for simultaneous depletion of hDaxx and PML (pLKO-shDP), the mouse RNA polymerase III U6 promoter (mU6) was amplified using PCR primers encoding an shRNA against hDaxx (shDaxx) and inserted into a lentiviral vector encoding an shRNA against PML (pLKO-shPML) under the control of the human U6 promoter (hU6). For construction of a lentivirus for simultaneous depletion of hDaxx, PML, and Sp100 (pLKO-shDPS), the RNA polymerase III 7SK promoter (7SKpro) was amplified from human genomic DNA and isolated from a subsequent PCR using primers encoding an shRNA against Sp100 (shSp100). All lentivirus vectors used in this study express puromycin-N-acetyltransferase (Puro-N-ac).
Lentivirus supernatants were prepared by cotransfection of HEK-293T cells with the respective pLKO vector, pVSV-G (expressing the vesicular stomatitis virus [VSV] envelope protein), and pCMV.DR8.91 (expressing lentivirus helper functions), as described previously (48). HepaRG cells were transduced with lentiviruses expressing shRNAs directed against the respective cellular proteins or a scrambled control shRNA (shneg) with no specific target (pLKO-shneg [5′-GTTATCGCGCATATCACGCGT-3′]). Stable cell lines were selected using puromycin (initially 1 μg/ml and then reduced to 0.5 μg/ml during subsequent passages).
Infections, plaque assays, and establishment of quiescence.
Cells were seeded into 24-well dishes at 1 × 105 cells per well and infected the following day with wt HSV-1, in1863, dl1403/CMVlacZ, or HCMV AD169 using MOIs and protocols indicated in the relevant figure legends and text. For plaque assays, cells were seeded as described above and infected the following day with appropriate sequential 3-fold dilutions of in1863, dl1403/CMVlacZ, or HCMV AD169. After virus adsorption, the HSV-1-infected cells were overlaid with medium containing 1% human serum for 24 h and stained for β-galactosidase-positive plaques (57). HCMV titers were determined by a standard plaque assay on HFs.
For assaying the establishment of a quiescent state, cells in 24-well dishes were infected with in1374 at a multiplicity of infection (MOI) of 5 PFU per cell and at a nonpermissive temperature (NPT) (38.5°C) and then incubated at NPT for 24 h. Cells were stained for β-galactosidase activity the following day.
Western blot analysis.
Cells were seeded into 24-well dishes at 1 × 105 cells per well. The following day and after infection, as relevant, the cells were washed twice with phosphate-buffered saline (PBS) before harvesting in SDS-PAGE loading buffer. Proteins were resolved on 7.5% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes by Western blotting. HSV-1 proteins were detected using anti-ICP0 mouse monoclonal antibody (MAb) 11060, anti-ICP4 MAb 58S, anti-ICP8 MAb ab20194 (Abcam), anti-UL42 MAb Z1F11, and anti-VP5 MAb DM165, as previously described (26). HCMV IE1/2 proteins were detected by using MAb E13 (catalog number 2470-5604; AbD Serotec). PML was detected by using rabbit serum A301-167A (Bethyl Laboratories), hDaxx was detected by using rabbit serum D7810 (Sigma-Aldrich), and Sp100 was detected by using rabbit serum SpGH (58). The loading control was antiactin rabbit serum A5060 (Sigma-Aldrich).
Immunofluorescence and confocal microscopy.
Cells on 13-mm glass coverslips were infected with either wt or ICP0-null mutant HSV-1, or AD169, at the chosen multiplicity and harvested at the indicated time points. Cells were fixed with formaldehyde (5%, vol/vol) and treated with NP-40 (0.5%, vol/vol) before staining using the following antibodies: PML MAb 5E10 (59), Sp100 rabbit serum SpGH or rat serum r26 (18), hDaxx rabbit serum 07-471 (Upstate), SUMO-1 rabbit serum ab32058 (Abcam), SUMO-2/3 rabbit serum ab3742 (Abcam), 53BP1 rabbit serum ab21083 (Abcam), HCMV IE1 MAb 2470-5604 (AbD Serotec), β-galactosidase MAb Z378A (Promega), and ICP4 rabbit serum r74 (60). The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-rabbit and anti-mouse, Alexa Fluor 555 donkey anti-rabbit and anti-mouse, Alexa Fluor 647 donkey anti-mouse (catalog number A31571), Alexa Fluor 647 donkey anti-rabbit (catalog number A31573) (all from Invitrogen), or Cy3-labeled goat anti-rat IgG (Amersham) antibodies. The samples were examined by using a Zeiss LSM 510 confocal microscope with 488-nm, 543-nm, and 633-nm laser lines, scanning each channel separately under image capture conditions that eliminated channel overlap. The images were exported as TIF files and then processed by using Photoshop and Illustrator.
RESULTS
Construction of a lentiviral vector for simultaneous depletion of three different transcripts.
We investigated whether intrinsic resistance to HSV-1 is mediated through the cooperative action of several different ND10 proteins by depleting cells of hDaxx, PML, and Sp100 simultaneously. Previous studies have shown that single depletion of hDaxx, PML, or Sp100 enhanced ICP0-null mutant HSV-1 gene expression and replication efficiency and increased the proportion of cells that escaped repression during the establishment of quiescent infections. Moreover, simultaneous depletion of PML and Sp100 led to a further increase in these effects (10, 11, 48). These studies imply that intrinsic resistance might be mediated through several cellular proteins acting cooperatively. However, there are practical limitations to the methods used previously for the isolation of cells depleted of several proteins. Consecutive lentivirus-based knockdown experiments that generate stable cell lines entail multiple cell passages, which may not be feasible when using limited-passage primary cells. Increased toxicity due to multiple selection drugs in the medium as well as mixed cell populations with variable degrees of depletion of the proteins of interest are further restrictions to the consecutive-knockdown approach.
To overcome these limitations, we constructed a single lentivirus vector that expresses three different shRNAs simultaneously, using an approach adapted from that described in a previous study (61). To avoid the problem of recombination in a lentiviral vector containing multiple identical sequences, we used three different RNA polymerase III promoters (mouse U6, human U6, and 7SK) to drive shRNA expression (Fig. 1) (for full details of the construction, see Materials and Methods). The shRNA sequences used here against hDaxx, PML, and Sp100 were shown to be efficient in previous studies, and puromycin provides a potent but well-tolerated selection for the generation of stable cell lines (10, 11, 48). We designed the vector in a way that allows the straightforward exchange of the selection marker as well as the shRNA sequences, potentially facilitating a wide range of depletion-based studies in different contexts.
Simultaneous depletion of hDaxx, PML, and Sp100 in HepaRG cells.
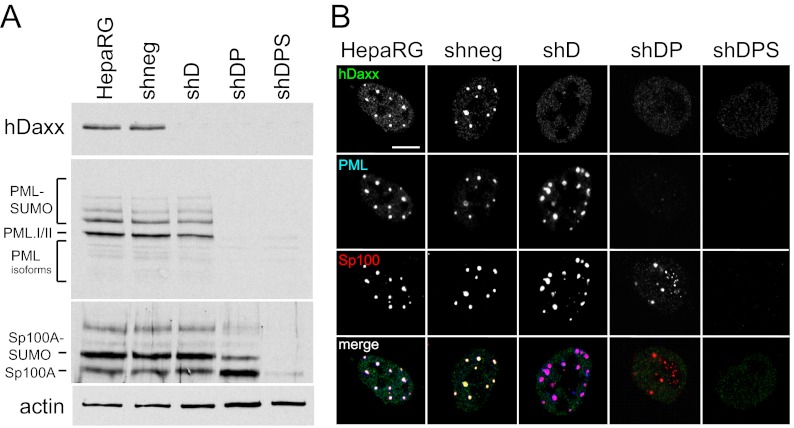
Human HepaRG hepatocytes have been used successfully to study the role of ND10 proteins in HSV-1 infection (10, 11, 48). Therefore, we initially tested the efficiency of the double- and triple-protein-depletion vectors in these cells. As shown previously, we found an efficient knockdown of hDaxx after transduction with lentiviruses derived from pLKO-shD (11). Cells isolated after transduction with lentiviruses made from pLKO-shDP and pLKO-shDPS exhibited an efficient knockdown of both hDaxx and PML and also of Sp100 in the case of the triple-shRNA vector. Consistent with previous results (48), in shDP cells, the SUMO-modified forms of Sp100 are present in reduced amounts, because their formation is enhanced by PML. Cells that were transduced with the shDPS vector and that were depleted of PML were consistently depleted of hDaxx and Sp100 as well, and knockdown was similarly efficient for each of the three proteins. This demonstrates that when a cell is transduced with these lentiviruses, it expresses both (or all three) shRNAs at levels that are sufficient to achieve a robust knockdown of the targeted proteins, verifying the stability of the vector. After puromycin selection, nearly 100% of the cells in the resulting populations displayed a high level of depletion of the relevant proteins, as visualized by immunofluorescence (Fig. 2 and data not shown).
Fig 2.
Triple depletion of hDaxx, PML, and Sp100 in HepaRG cells. HepaRG cells were transduced with lentiviruses expressing shRNAs against hDaxx (shD); hDaxx and PML (shDP); or hDaxx, PML, and Sp100 (shDPS) or a scrambled shRNA with no cellular target (shneg). (A) Immunoblot analysis of extracts from the generated cell lines demonstrating depletion of the respective proteins, compared to parental untransduced cells (HepaRG). Antibodies used for detection were anti-hDaxx serum D7810, anti-PML serum A301-167A, and anti-Sp100 serum SpGH. The loading control was antiactin serum A5060. (B) Verification of depletion by immunofluorescence using the same cell lines. Cells were simultaneously stained with rabbit anti-hDaxx 07-471 antibody, mouse anti-PML MAb 5E10, and rat anti-Sp100 r26 antibody. Secondary antibodies were Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 633 donkey anti-mouse, and goat anti-rat Cy3 IgG antibodies. Bar, 10 μm.
ICP0-null HSV-1 replication and gene expression are cooperatively repressed by hDaxx, PML, and Sp100.
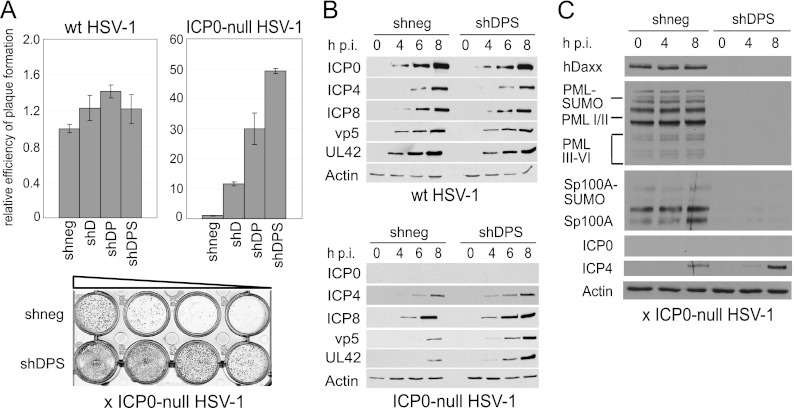
We used the set of singly, doubly, and triply depleted cells to analyze the consequences of multiple depletion on the efficiency of ICP0-null mutant HSV-1 replication. We found that simultaneous depletion of hDaxx and PML caused an increase in the efficiency of viral plaque formation in HepaRG cells compared to the single knockdown of hDaxx (Fig. 3A). This is consistent with an analogous study on the effect of double depletion of PML and hDaxx on the efficiency of HCMV infection (36). Strikingly, a triple depletion of hDaxx, PML, and Sp100 caused an even greater increase in ICP0-null mutant HSV-1 plaque formation, with virus replication being at least 50-fold more efficient than that in nondepleted cells (Fig. 3A). In previous studies, we calculated increases in plaque formation by comparing numbers of plaques at a given dose of virus (10, 48). This approach was not possible in this instance because dilutions giving a reliable number of plaques in the control cells gave too many plaques for accurate counting in the depleted cells. Therefore, we calculated apparent titers in PFU/ml, which tends to underestimate the increase in the probability of plaque formation at low multiplicities because ICP0-null mutant plaque numbers in restrictive cells decrease more rapidly with dilution than expected on the basis of a linear relationship. Therefore, the 50-fold increase found here is a minimal estimate compared to the increases in probability of plaque formation in depleted cells reported in our previous studies.
Fig 3.
Consequences of triple depletion on ICP0-null HSV-1 replication in HepaRG cells. (A) Relative efficiency of plaque formation of HSV-1 in depleted and nondepleted cells. Cells were infected with HSV-1 strain in1863 and ICP0-null HSV-1 strain dl1403/CMVlacZ at sequential dilutions. At 24 h postinfection, cells were fixed and stained for β-galactosidase expression. Bars represent mean relative plaque-forming titers in depleted compared to nondepleted (shneg) control cells in three independent experiments. A typical example of a stained plaque dilution series is shown at the bottom, with plaques shown as dark spots. (B) Kinetics of viral protein expression in depleted and nondepleted cells. Cells were infected with wt or ICP0-null HSV-1 at an MOI of 2, and cell lysates were sampled at 0, 4, 6, and 8 h postinfection (h p.i.). Samples were resolved on a 7.5% polyacrylamide gel, and membranes were probed using anti-ICP0 (11060), anti-ICP4 (58S), anti-ICP8 (ab20194), anti-UL42 (Z1F11), and anti-VP5 (DM165) antibodies. The loading control was antiactin serum A5060. (C) The shRNAs in triple-depleted cells are able to overcome any interferon-induced increased expression of PML, Sp100, and hDaxx that might occur during ICP0-null mutant HSV-1 infection. Control (shneg) and triple-depleted (shDPS) cells were infected with ICP0-null mutant HSV-1 at an MOI of 2, and samples were then harvested at the indicated time points and analyzed by Western blotting, as described in the legend of Fig. 2A.
Viral protein expression was also significantly enhanced in the triply depleted cells, with immediate-early (ICP4), early (UL42 and ICP8), and late (VP5) proteins all being detected at earlier times and in higher abundances than in the control cells (Fig. 3B). In agreement with previous studies, neither single, double, nor triple depletion affected the plaque formation efficiency of wt HSV-1, and simultaneous depletion of hDaxx, PML, and Sp100 had no influence on viral gene expression during wt HSV-1 infection (Fig. 3A and B, left). Because both PML and Sp100 are interferon-inducible proteins, we also tested whether any interferon induction during infection of triple-depleted cells could overcome the effects of the shRNAs. We found that this was not the case, in that none of the depleted proteins increased in abundance during infection with ICP0-null mutant HSV-1 (Fig. 3C).
Simultaneous depletion of hDaxx, PML, and Sp100 in human diploid fibroblasts.
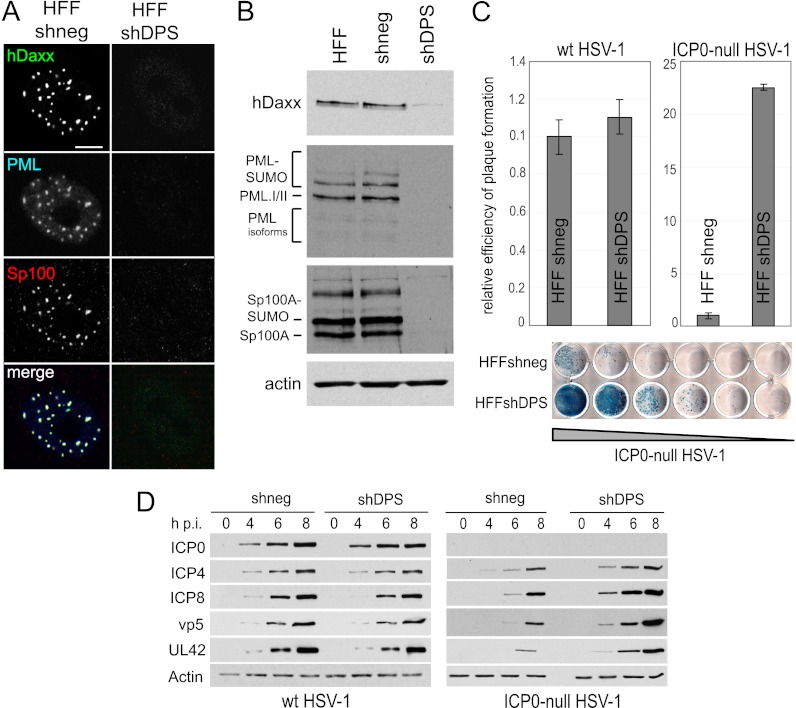
Limited-passage human diploid fibroblasts (HFs) are highly restrictive for ICP0-null mutant HSV-1 infection, with the mutant exhibiting a 1,000-fold defect in plaque formation compared to permissive cell lines such as U2OS (54, 62). Since HFs are not transformed and are capable of only limited passage after isolation from primary tissue, they provide a model of infection that more closely resembles physiological conditions in peripheral tissues. However, consecutive depletion of several cellular proteins necessitates a high number of cell passages, which may exceed the life span of this type of cell. Simultaneous knockdown using a single lentivirus would overcome this constraint. We therefore tested whether HFs could be depleted of hDaxx, PML, and Sp100 simultaneously using the pLKO-shDPS vector. Each targeted protein was thoroughly depleted in all transduced cells (Fig. 4A and B), and transduction efficiency after selection was again close to 100% throughout the cell monolayer (data not shown). Analysis of the effect of triple knockdown in these cells on ICP0-null mutant HSV-1 infection indicated a striking increase in the efficiency of viral plaque formation compared to that in control HFs (Fig. 4C), consistent with the results using the HepaRG cell background. There was also an equally increased rate of all stages of viral protein expression in the absence of the cellular repressors (Fig. 4D). As expected, triple depletion had no influence on the plaque formation efficiency or viral gene expression of wt HSV-1 infection in HFs (Fig. 4C and D).
Fig 4.
Triple depletion in HFs improves replication and gene expression of ICP0-null mutant HSV-1. HFs were transduced with a lentivirus expressing shRNA with no cellular target (shneg) or targeting hDaxx, PML, and Sp100 (shDPS). (A) Verification of depletion by immunofluorescence. Cells were simultaneously stained with rabbit anti-hDaxx 07-471 antibody, mouse anti-PML MAb 5E10, and rat anti-Sp100 r26 antibody. Secondary antibodies are described in the legend of Fig. 2. Bar, 10 μm. (B) Immunoblot analysis of extracts from the generated cell lines demonstrating depletion of the respective proteins. Antibodies used for detection are described in the legend of Fig. 2. (C) Relative efficiency of plaque formation in depleted and nondepleted HFs. Cells were infected with HSV-1 strain in1863 and ICP0-null HSV-1 strain dl1403/CMV/lacZ at sequential dilutions. At 24 h postinfection, cells were fixed and stained for β-galactosidase expression. Bars represent mean relative efficiencies of plaque formation determined by calculating virus titers in depleted compared to nondepleted (shneg) cells in three independent experiments. A typical example of a stained plaque dilution series is shown at the bottom, with plaques stained in blue. (D) Kinetics of viral protein expression in depleted and nondepleted cells. Cells were infected with wt or ICP0-null HSV-1 at an MOI of 2, and cell lysates were sampled at 0, 4, 6, and 8 h postinfection (h p.i.). The samples were analyzed as described in the legend of Fig. 3.
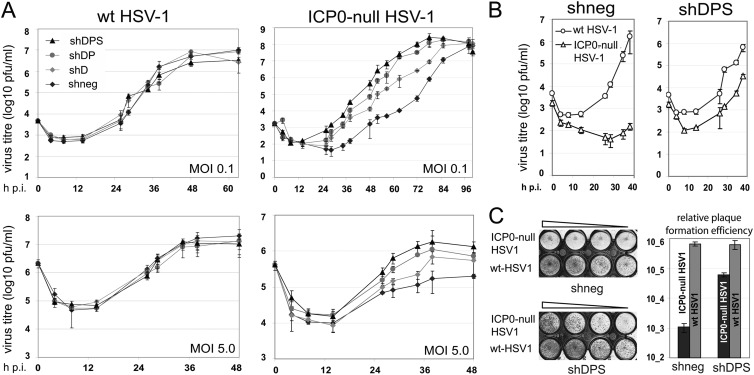
Growth curve analysis of ICP0-null mutant HSV-1 in cells depleted of hDaxx, PML, and Sp100.
We next performed a kinetic analysis of wt and ICP0-null mutant HSV-1 replication in control and triple-depleted HepaRG cells. As expected, there were no differences in the growth kinetics of wt HSV-1 in the two cell lines, in either multistep low-MOI (0.1) or single-step high-MOI (5.0) experiments (Fig. 5A, left). The growth of ICP0-null HSV-1 in triple-depleted cells, however, differed significantly from that in nondepleted cells. Progeny virus yield and the peak in virus titer were both reached earlier in the triple-depleted than in the nondepleted cells, with growth curves showing earlier entry into log phase, a steeper gradient, and overall more rapid replication in the triple-depleted cells than in the controls. Therefore, ICP0-null mutant HSV-1 replication not only started earlier but also progressed at a higher rate in terms of the production of progeny virions in the depleted cell populations (Fig. 5A, right). These effects were dose dependent, being more pronounced at low than at high MOIs (Fig. 5A, right). Interestingly, ICP0-null mutant HSV-1 also replicated more rapidly and efficiently, albeit to a lesser degree, in cells depleted of hDaxx alone, or of hDaxx and PML together, than in the controls (Fig. 5A, right). Therefore, replication appears to become more efficient with the number of host proteins depleted, arguing for a cooperative effect of hDaxx, PML, and Sp100 in the repression of HSV-1 replication in the absence of ICP0.
Fig 5.
Initial stages of HSV-1 replication are cooperatively repressed by hDaxx, PML, and Sp100. (A) Growth of wt and ICP0-null mutant HSV-1 in nondepleted HepaRG cells (shneg) and HepaRG cells depleted of hDaxx (shD); hDaxx and PML (shDP); and hDaxx, PML, and Sp100 (shDPS). Cells were infected at an MOI of 0.1 or 5.0, supernatant samples were harvested at the indicated time points postinfection, and viral titers in U2OS cells were determined by plaque assay. Data points represent mean values determined by three independent experiments. (B) Direct comparison of growth efficiency of wt and ICP0-null HSV-1 in shneg and shDPS HepaRG cells, respectively. (C) Plaque formation efficiency in direct comparisons of wt and ICP0-null HSV-1 in shneg and shDPS HepaRG cells. Cells were infected with HSV-1 strains in1863 and dl1403/CMVlacZ at sequential MOIs from 0.1 to 0.003. At 24 h postinfection, the cells were fixed and stained for β-galactosidase expression. Bars represent mean relative efficiencies of plaque formation determined by calculating virus titers in three independent experiments.
When comparing the growth characteristics of wt and ICP0-null HSV-1 in each HepaRG-derived cell line directly, it appears that depletion of hDaxx, PML, and Sp100 reduced the plaque formation defect of the ICP0 deletion mutant compared to the wt virus from around 600-fold to 10-fold (Fig. 5C). Also, in the triple-depleted cells, ICP0-null mutant HSV-1 growth kinetics resembled that of the wt virus more closely than in the nondepleted cells, with the ICP0 deletion mutant showing a delay in initiation of replication but replicating at an overall comparable rate to that of the wt virus (Fig. 5B). However, triple depletion was unable to restore completely either the growth kinetics or plaque formation efficiency of ICP0-null mutant HSV-1 to wt levels, implying the presence of additional host factors that restrict virus replication.
Components of the SUMO and DNA repair pathways are recruited to parental HSV-1 genomes in the absence of hDaxx, PML, and Sp100.
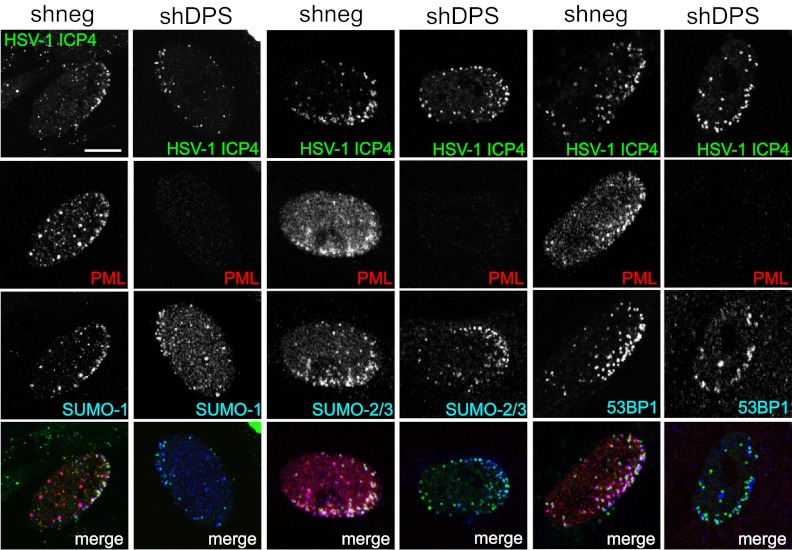
One hallmark of intrinsic resistance to HSV-1 is the recruitment of restrictive factors, including ND10 components, to nuclear sites associated with parental viral genomes and early replication compartments (18). Because triple depletion of PML, hDaxx, and Sp100 did not completely restore the growth of ICP0-null mutant virus to wt levels, we considered whether recruitment of other restrictive host factors remains unimpaired in triply depleted cells. ICP0 induces the widespread degradation of SUMO-conjugated proteins during HSV-1 infection, and the SUMO interaction motifs of PML, hDaxx, and Sp100 are required for their recruitment to the HSV-1-induced foci (17, 63). This implies an important role of the SUMO modification pathway in intrinsic antiviral defense, and therefore, we tested whether recruitment of SUMO conjugates (17) still occurs in triple-depleted cells. We found that SUMO-2/3 and, to a lesser extent, SUMO-1 are found in close proximity to ICP0-null mutant HSV-1 genomes in the absence of hDaxx, PML, and Sp100 (Fig. 6). These observations support the concept that SUMO modification pathways play a role in intrinsic resistance and indicate that as-yet-unidentified SUMO-modified host proteins, or components of the SUMO modification pathway itself, might contribute to the repression of HSV-1 replication in the absence of ICP0.
Fig 6.
Components of the SUMO and DNA repair pathways are recruited to sites associated with HSV-1 genomes in triple-depleted cells. HFs were transduced with either a lentivirus expressing shRNAs against hDaxx, PML, and Sp100 (shDPS) or a scrambled shRNA with no cellular target (shneg). Cells were infected with ICP0-null HSV-1 expressing enhanced yellow fluorescent protein-tagged ICP4 at an MOI of 0.1, fixed at 24 h postinfection, and then simultaneously stained with mouse anti-PML MAb 5E10 and SUMO-1 rabbit serum ab32058, SUMO-2/3 rabbit serum ab3742, or 53BP1 rabbit serum ab21083. Secondary antibodies were Alexa Fluor 555 donkey anti-mouse and Alexa Fluor 647 donkey anti-rabbit antibodies. PML served as a marker for depletion. Bar, 10 μm.
Another group of proteins that are recruited to sites associated with parental HSV-1 genomes at the early stages of infection is involved in the DNA damage response (64, 65). The pathway of assembly of DNA repair proteins at sites of damaged DNA, and in the vicinity of HSV-1 genomes, is a complex process that is impeded by ICP0 because it induces the degradation of two essential enzymes in the DDR pathway, RNF8 and RNF168 (64–66). The 53BP1 protein provides a convenient marker for the assembly of DNA repair foci, and because it lies downstream of RNF8 and RNF168 in the pathway, its recruitment to these foci is inhibited by ICP0. Replication of ICP0-null mutant HSV-1 is increased in cells that are deficient of RNF168, and therefore, it appears that the DDR contributes to intrinsic resistance to HSV-1 (64–66). We found that the relocalization of 53BP1 to sites close to the viral genomes was equally distinct in control and triple-depleted HFs (Fig. 6), demonstrating that recruitment of 53BP1 to ICP0-null HSV-1 genomes is independent of hDaxx, PML, and Sp100 and indicating that the component of intrinsic resistance that might be imparted by the DDR pathway remains intact in the triple-depleted cells.
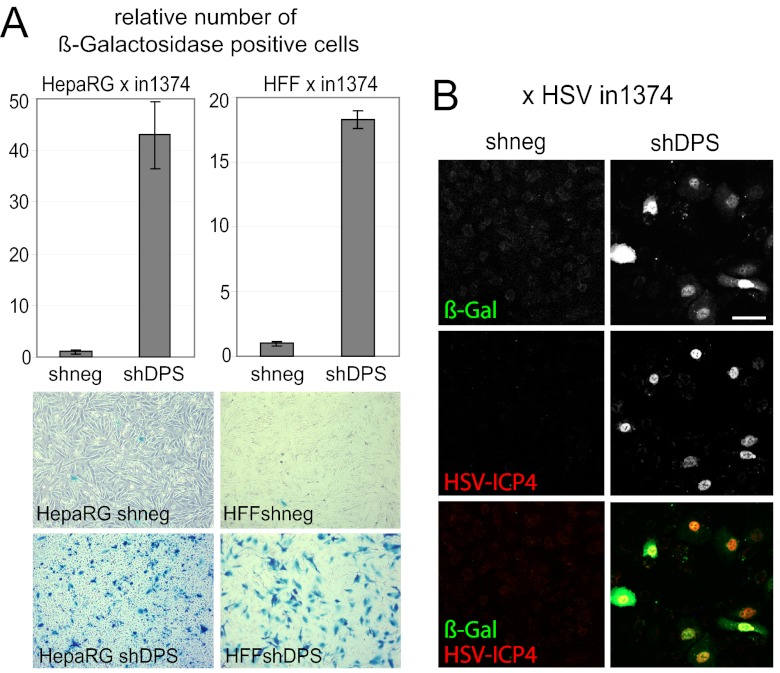
Decreased ability to establish quiescent HSV-1 infections in cells depleted of hDaxx, PML, and Sp100.
Because herpesvirus genomes are maintained in the host in a latent state as part of the virus life cycle, it is tempting to hypothesize that during coevolution with their hosts, herpesviruses might have adapted to intrinsic resistance in a way that allows them to utilize the repression of their genomes as a means to facilitate the initiation of latency. If repressive host proteins are part of the mechanism to establish and/or maintain latency, then depletion of these repressors might decrease the establishment of quiescent infection. We addressed this hypothesis with a cell culture model of quiescence that uses in1374, an HSV-1 mutant carrying mutations in VP16, ICP0, and ICP4, which is rapidly repressed even in high-MOI infections, allowing the generation of cultures in which a high proportion of cells harbor in1374 genomes in a repressed state (67). Establishment of quiescent genomes can be deduced by the absence of expression of the HCMV promoter-driven lacZ marker gene present in in1374. The proportion of cells infected with in1374 can be determined by coinfection with HSV-1 tsK, which expresses ICP0 and thus leads to expression of the marker gene (68). This control confirmed that the majority of cells had been infected with in1374 in our experiments (data not shown). Compared to the shneg control cells, we found that simultaneous depletion of hDaxx, PML, and Sp100 led to substantial increases in the proportions of cells infected with in1374 that expressed the marker gene at 24 h after infection, indicating a failure to establish quiescence. This occurred in both HepaRG and HF cell backgrounds (Fig. 7A). Furthermore, all the cells expressing the β-galactosidase marker protein also expressed ICP4 (Fig. 7B), indicating that both the HCMV promoter/enhancer linked to the marker gene and also an authentic HSV-1 IE promoter were able to escape repression in the triply depleted cells. Therefore, depletion of these ND10 components not only allows increased replication of ICP0-null mutant HSV-1 but also decreases the efficiency of the establishment of quiescence.
Fig 7.
Establishment of quiescent infection in triple-depleted HFs and HepaRG cells. (A) Cells were infected with HSV-1 strain in1374 and incubated at the restrictive temperature of 38.5°C, and at 24 h postinfection, they were then stained for β-galactosidase activity. (Top) Relative cell numbers of β-galactosidase-expressing cells in HepaRG cells and HFs transduced with a lentivirus expressing shRNAs with no cellular target (shneg) or targeting hDaxx, PML, and Sp100 (shDPS). Bars represent mean values obtained from three independent experiments. (Bottom) Images of the respective stained cell monolayers at 24 h postinfection. (B) Triple-depleted HepaRG cells were infected with in1374 as described above for panel A and then fixed at 24 h postinfection and stained for immunofluorescence analysis with anti-β-galactosidase and anti-ICP4 (r74) antibodies. Bar, 100 μm.
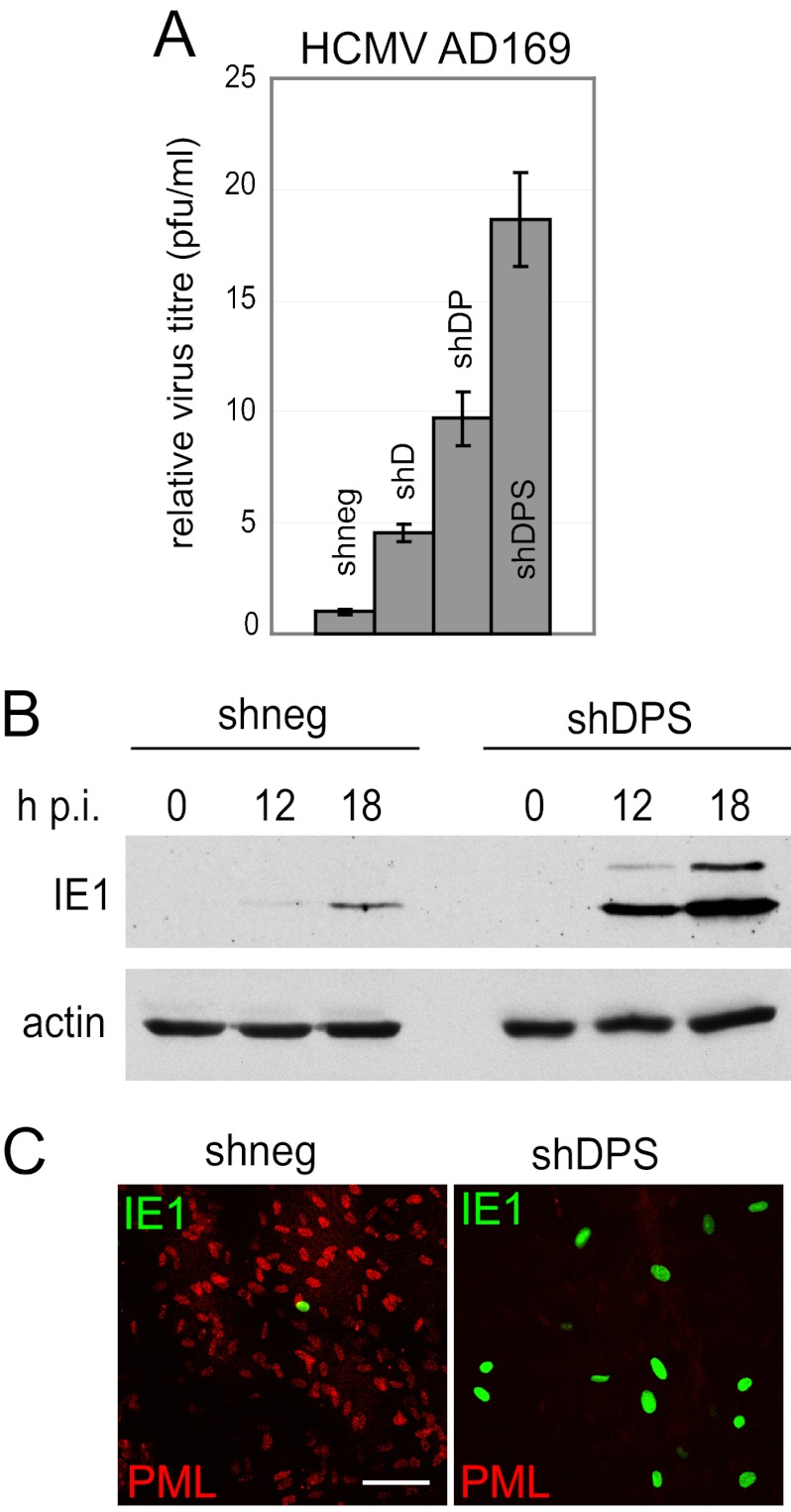
Replication and gene expression of wt HCMV are enhanced by simultaneous depletion of hDaxx, PML, and Sp100.
In previous studies, single depletion of hDaxx, Sp100, or PML led to increased HCMV gene expression and replication efficiency (34, 36, 38, 49), which could be further enhanced by simultaneous depletion of hDaxx and PML (36). Analogous to the observation that simultaneous depletion of PML and Sp100 did not impede the targeting of hDaxx to nuclear sites of incoming HSV-1 genomes, the absence of PML and hDaxx did not inhibit recruitment of Sp100 to HCMV nucleoprotein complexes (2). This implies that ND10 components might also restrict HCMV infection in a cooperative manner. Infection of triple-depleted HFs with wt HCMV gave results similar to those for HSV-1 infections, namely, increased viral gene expression and growth kinetics (Fig. 8A and B) and larger numbers of cells with active viral gene expression than in nondepleted cells (Fig. 8C). These findings strongly support the hypothesis that ND10-mediated intrinsic resistance is a conserved cellular function, targeting at least two different human herpesviruses. Although the targeting of ND10 proteins by HSV-1 and HCMV is mediated by very different viral proteins that share little or no sequence homology (namely, ICP0, IE1, and pp71), and through mechanisms that differ considerably, it leads to the same consequence, a reduction in repression of virus replication.
Fig 8.
Triple depletion of HFs increases wt HCMV replication and gene expression. (A) HCMV AD169 replication in depleted and nondepleted HFs. Cells were infected with HCMV AD169 at an MOI of 0.1, supernatants were harvested at 9 days postinfection, and relative virus titers were determined by standard plaque assay on HFs. (B) Kinetics of viral IE protein expression in depleted and nondepleted cells. Cells were infected with HCMV AD169 at an MOI of 0.5, and cell lysates were sampled at the indicated time points. Samples were resolved on a 7.5% polyacrylamide gel, and membranes were probed using anti-IE1/2 MAb E13. The loading control was antiactin A5060 antibody (Sigma-Aldrich). (C) Immunofluorescence analysis of the proportions of cells expressing IE1. Cells were infected with HCMV AD169 at an MOI of 0.01, fixed at 48 h postinfection, and simultaneously stained with mouse anti-IE1/2 MAb E13 and rabbit anti-PML antibodies. Secondary antibodies were Alexa Fluor 488 donkey anti-mouse and Alexa Fluor 647 goat anti-rabbit antibodies. PML served as a marker for depletion. Bar, 100 μm.
DISCUSSION
Intrinsic defense against virus infection is a recently described phenomenon, and the mechanisms by which this response is triggered, implemented, and regulated are actively under investigation. Studies on the role of ND10 proteins in intrinsic resistance to herpesvirus infections have shown that hDaxx, PML, and Sp100 all act repressively on both HSV-1 and HCMV. Although PML is required for the assembly of ND10 in uninfected cells, it is not required for the recruitment of either Sp100 or hDaxx to sites associated with parental HSV-1 genomes, and it appears that the latter two proteins are still able to repress viral infections even in the absence of PML. This suggests that different ND10 components can exert their repressive functions independently. Moreover, codepletion of PML and Sp100 enhanced ICP0-null mutant HSV-1 replication more than the depletion of either single protein (10), just as the codepletion of hDaxx and PML increased replication of HCMV to higher levels than in singly depleted cells (36). The hypothesis arises, therefore, that components of ND10 confer intrinsic resistance in a cooperative manner, so that their individual activities combine to maximize the antiviral effect.
Depletion of single cellular proteins can be done straightforwardly by using lentivirus-based transduction of shRNAs. Depletion of several proteins is more challenging, especially in limited-passage HFs, the cell line of choice for productive HCMV infections. A lentivirus that allows the simultaneous expression of up to three shRNAs overcomes many experimental limitations. The vector that we describe allows efficient depletion of all three targeted proteins in a stable manner in a very high proportion of cells in the resultant cell lines. Potentially, this vector could also be used in combination with other lentiviruses with different selection markers to reintroduce mutant variants of the depleted proteins into the same cells, allowing interaction studies in the absence of the endogenous wt proteins. Furthermore, the system could also be employed to express three different shRNAs against the same target to maximize depletion of proteins for which only partial knockdown has so far been achieved.
Here, we have used this tool to investigate the repressive effects of hDaxx, PML, and Sp100. The results from assays of gene expression, plaque-forming efficiency, and establishment of quiescence all point to the conclusion that these proteins act in a cooperative or additive manner. The plaque-forming efficiency of ICP0-null mutant HSV-1 was enhanced by at least 50-fold in the triple-depleted cells, a much larger increase than that achieved by the depletion of any single ND10 protein. Viral protein expression and virus replication were detected at earlier times, and the virus replication cycle reached its peak sooner than in nondepleted cells. Given that analogous effects were also observed in HCMV-infected cells, it would be interesting to examine the effect of a triple depletion on the replication of other viruses or their mutants that are subject to ND10-mediated intrinsic resistance.
Individual herpesviruses have evolved different mechanisms to counteract ND10-mediated intrinsic defense. For example, ICP0 of HSV-1 induces the degradation of PML and SUMO-modified Sp100 and also the dispersal of hDaxx and ATRX (11, 43, 44). HCMV takes a two-step approach: the tegument protein pp71 first targets hDaxx and ATRX (12, 31–33, 69), and the immediate-early protein IE1 then disperses PML and Sp100 and induces the loss of their SUMO-modified forms (8, 37–41, 70, 71). Epstein-Barr virus (EBV) disrupts ND10 and the ATRX-hDaxx complex through the actions of BZLF1 and BNRF1, respectively (8, 72), and causes a dispersal of Sp100 and hDaxx from ND10 during lytic replication (73). Along the same lines, the murine gammaherpesvirus 68 tegument protein orf75c induces degradation of PML (74), while the related protein ORF3 of herpesvirus saimiri specifically degrades Sp100 but affects neither PML nor hDaxx (75). Although via different mechanisms and mediated by viral regulatory proteins that in most cases have little or no sequence similarity, all of these viruses appear to target the same group of cellular proteins to counteract the initial repression of viral gene expression and promote virus replication.
Our data demonstrate the cooperative repressive effects of three ND10 proteins, but ICP0-null mutant HSV-1 still replicated about 10-fold less efficiently than the wt virus in triple-depleted cells. Therefore, there must be additional repressive cellular factors whose activities can be overcome by ICP0. We found that SUMO-2/3 and, to a lesser extent, SUMO-1 were still recruited to sites associated with ICP0-null mutant HSV-1 genomes in cells depleted of hDaxx, PML, and Sp100. The decrease in SUMO-1 recruitment in triple-depleted cells was also observed for cells singly depleted of PML (63), implying that a significant proportion of the SUMO-1 that is recruited may be conjugated to PML. However, the nature of the SUMO-2/3 conjugates that are recruited in triply depleted cells remains to be defined, and it is possible that some of these could be involved in repression of viral gene expression.
SUMO modification appears to be an important factor in intrinsic resistance to herpesvirus infections. For example, the SUMO-conjugating enzyme Ubc9 is required for fully efficient intrinsic resistance to HSV-1 (63), and ICP0 induces the degradation of not only PML and its SUMO-modified forms but also the SUMO-modified forms of Sp100 and SUMO conjugates more generally (40, 63, 76, 77), while mutant forms of PML and hDaxx that are unable to interact with SUMO are no longer recruited to sites of incoming viral genomes and are unable to repress replication of ICP0-null mutant virus (17). Several alphaherpesvirus orthologues of ICP0 also destabilize SUMO-conjugated proteins in general (63). IE1 of HCMV induces the loss of SUMO modification of PML and Sp100 via an unknown mechanism that does not involve degradation, leading to their displacement from ND10 (34, 37, 38, 71). SUMO-modified PML is also the target of BZLF1, the principal inducer of EBV lytic gene expression. BZLF1 itself is modified by SUMO-1 and, when overexpressed, competes with PML for the limited amounts of SUMO-1 in the nucleus, leading to a disruption of ND10 (72). SUMO modification is also involved in varicella-zoster virus (VZV) replication, illustrated by the requirement for the SUMO interaction motifs of orf61 for its ability to associate with and disrupt ND10 and for normal pathogenesis of the virus in the skin (78).
Whether the mechanisms of ND10-related intrinsic defense are involved in the establishment and maintenance of latency remains to be elucidated. Repressive environments created by recruitment of ND10 proteins might physically restrict access to viral genomes of factors required for transcription, thereby enhancing the potential for the establishment of quiescence. This concept is supported by the observations that viral genomes are enclosed in a cage of PML in quiescently infected cells and latently infected mouse neurons (79, 80). Depletion of the three major ND10 components would reduce this sequestration and might enable greater access of positively acting factors during both initial replication and escape from quiescence. Evolution has evidently led to a fine balance between repression and replication of viral genomes, allowing for persistence and dissemination of the virus on the one hand and maintaining host cell integrity on the other. If this balance is tipped in favor of the virus, there may be severe consequences for the host. An understanding of the mechanism of intrinsic cellular resistance upon nuclear entry of viral genomes might help explain increased susceptibility to virus infections, especially herpesviruses, when not caused by defects in the innate or adaptive immune system (81). Moreover, it might provide clues to mechanisms involved in the reactivation of herpesviruses from latency, which continues to be a serious threat to immunocompromised individuals, transplant recipients, and neonates (82–85) and which, despite ongoing extensive research, remains poorly understood.
ACKNOWLEDGMENTS
We thank Roel van Driel for anti-PML antibody 5E10, Hans Will for anti-Sp100 antibody SpGH, Arvind Patel for the mouse U6 promoter plasmid, and Chris Preston for the viruses HCMV AD169, in1863, in1374, and dl1403/CMVlacZ.
This study was funded by the Medical Research Council and by an EC FP7 Marie Curie fellowship awarded to Mandy Glass (PIEF-GA-2009-251948).
Footnotes
Published ahead of print 5 December 2012
REFERENCES
- 1. Bieniasz PD. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109–1115 [DOI] [PubMed] [Google Scholar]
- 2. Tavalai N, Stamminger T. 2008. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim. Biophys. Acta 1783:2207–2221 [DOI] [PubMed] [Google Scholar]
- 3. Tavalai N, Stamminger T. 2009. Interplay between herpesvirus infection and host defense by PML nuclear bodies. Viruses 1:1240–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99–103 [DOI] [PubMed] [Google Scholar]
- 5. Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 6. Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853 [DOI] [PubMed] [Google Scholar]
- 7. Reichelt M, Wang L, Sommer M, Perrino J, Nour AM, Sen N, Baiker A, Zerboni L, Arvin AM. 2011. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus. PLoS Pathog. 7:e1001266 doi:10.1371/journal.ppat.1001266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. 2011. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog. 7:e1002376 doi:10.1371/journal.ppat.1002376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Everett RD, Chelbi-Alix MK. 2007. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89:819–830 [DOI] [PubMed] [Google Scholar]
- 10. Everett RD, Parada C, Gripon P, Sirma H, Orr A. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 82:2661–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lukashchuk V, Everett RD. 2010. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 84:4026–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saffert RT, Kalejta RF. 2007. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J. Virol. 81:9109–9120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schreiner S, Martinez R, Groitl P, Rayne F, Vaillant R, Wimmer P, Bossis G, Sternsdorf T, Marcinowski L, Ruzsics Z, Dobner T, Wodrich H. 2012. Transcriptional activation of the adenoviral genome is mediated by capsid protein VI. PLoS Pathog. 8:e1002549 doi:10.1371/journal.ppat.1002549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernardi R, Pandolfi PP. 2007. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 8:1006–1016 [DOI] [PubMed] [Google Scholar]
- 15. Van Damme E, Laukens K, Dang TH, Van Ostade X. 2010. A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics. Int. J. Biol. Sci. 6:51–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maul GG. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660–667 [DOI] [PubMed] [Google Scholar]
- 17. Cuchet-Lourenco D, Boutell C, Lukashchuk V, Grant K, Sykes A, Murray J, Orr A, Everett RD. 2011. SUMO pathway dependent recruitment of cellular repressors to herpes simplex virus type 1 genomes. PLoS Pathog. 7:e1002123 doi:10.1371/journal.ppat.1002123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everett RD, Murray J. 2005. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 79:5078–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dellaire G, Bazett-Jones DP. 2004. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26:963–977 [DOI] [PubMed] [Google Scholar]
- 20. Kumar PP, Bischof O, Purbey PK, Notani D, Urlaub H, Dejean A, Galande S. 2007. Functional interaction between PML and SATB1 regulates chromatin-loop architecture and transcription of the MHC class I locus. Nat. Cell Biol. 9:45–56 [DOI] [PubMed] [Google Scholar]
- 21. Xu ZX, Timanova-Atanasova A, Zhao RX, Chang KS. 2003. PML colocalizes with and stabilizes the DNA damage response protein TopBP1. Mol. Cell. Biol. 23:4247–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong S, Salomoni P, Pandolfi PP. 2000. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2:E85–E90 doi:10.1038/35010583 [DOI] [PubMed] [Google Scholar]
- 23. Isaac A, Wilcox KW, Taylor JL. 2006. SP100B, a repressor of gene expression preferentially binds to DNA with unmethylated CpGs. J. Cell. Biochem. 98:1106–1122 [DOI] [PubMed] [Google Scholar]
- 24. Seeler JS, Marchio A, Sitterlin D, Transy C, Dejean A. 1998. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc. Natl. Acad. Sci. U. S. A. 95:7316–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Leo C, Zhu J, Wu X, O'Neil J, Park EJ, Chen JD. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaelson JS, Leder P. 2003. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J. Cell Sci. 116:345–352 [DOI] [PubMed] [Google Scholar]
- 27. Elsaesser SJ, Allis CD. 2010. HIRA and Daxx constitute two independent histone H3.3-containing predeposition complexes. Cold Spring Harb. Symp. Quant. Biol. 75:27–34 [DOI] [PubMed] [Google Scholar]
- 28. Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319–3330 [DOI] [PubMed] [Google Scholar]
- 29. Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. 2010. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. U. S. A. 107:14075–14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, Qin J, Zhou S, Higgs D, Wang W. 2003. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. U. S. A. 100:10635–10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cantrell SR, Bresnahan WA. 2006. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J. Virol. 80:6188–6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Preston CM, Nicholl MJ. 2006. Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 87:1113–1121 [DOI] [PubMed] [Google Scholar]
- 33. Woodhall DL, Groves IJ, Reeves MB, Wilkinson G, Sinclair JH. 2006. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J. Biol. Chem. 281:37652–37660 [DOI] [PubMed] [Google Scholar]
- 34. Tavalai N, Adler M, Scherer M, Riedl Y, Stamminger T. 2011. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J. Virol. 85:9447–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tavalai N, Papior P, Rechter S, Leis M, Stamminger T. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 80:8006–8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tavalai N, Papior P, Rechter S, Stamminger T. 2008. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 82:126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang H, Kim ET, Lee HR, Park JJ, Go YY, Choi CY, Ahn JH. 2006. Inhibition of SUMO-independent PML oligomerization by the human cytomegalovirus IE1 protein. J. Gen. Virol. 87:2181–2190 [DOI] [PubMed] [Google Scholar]
- 38. Kim YE, Lee JH, Kim ET, Shin HJ, Gu SY, Seol HS, Ling PD, Lee CH, Ahn JH. 2011. Human cytomegalovirus infection causes degradation of Sp100 proteins that suppress viral gene expression. J. Virol. 85:11928–11937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Korioth F, Maul GG, Plachter B, Stamminger T, Frey J. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229:155–158 [DOI] [PubMed] [Google Scholar]
- 40. Muller S, Dejean A. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkinson GW, Kelly C, Sinclair JH, Rickards C. 1998. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J. Gen. Virol. 79:1233–1245 [DOI] [PubMed] [Google Scholar]
- 42. Cantrell SR, Bresnahan WA. 2005. Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication. J. Virol. 79:7792–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chelbi-Alix MK, de The H. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935–941 [DOI] [PubMed] [Google Scholar]
- 44. Everett RD, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Everett RD. 2011. The role of ICP0 in counteracting intrinsic cellular resistance to virus infection, p 39–50 In Weller SK. (ed), Alphaherpesviruses: molecular virology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 46. Everett RD, Parsy ML, Orr A. 2009. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 83:4963–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hagglund R, Roizman B. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Everett RD, Rechter S, Papior P, Tavalai N, Stamminger T, Orr A. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995–8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adler M, Tavalai N, Muller R, Stamminger T. 2011. Human cytomegalovirus immediate-early gene expression is restricted by the nuclear domain 10 component Sp100. J. Gen. Virol. 92:1532–1538 [DOI] [PubMed] [Google Scholar]
- 50. Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, Yeh ET, Strauss JF, III, Maul GG. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stow ND, Stow EC. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571–2585 [DOI] [PubMed] [Google Scholar]
- 52. Preston CM. 1979. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J. Virol. 29:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Preston CM, Nicholl MJ. 1997. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J. Virol. 71:7807–7813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Everett RD, Boutell C, Orr A. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McFarlane S, Nicholl MJ, Sutherland JS, Preston CM. 2011. Interaction of the human cytomegalovirus particle with the host cell induces hypoxia-inducible factor 1 alpha. Virology 414:83–90 [DOI] [PubMed] [Google Scholar]
- 56. Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. U. S. A. 99:15655–15660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jamieson DR, Robinson LH, Daksis JI, Nicholl MJ, Preston CM. 1995. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus type 1 Vmw65 mutants. J. Gen. Virol. 76:1417–1431 [DOI] [PubMed] [Google Scholar]
- 58. Guldner HH, Szostecki C, Schroder P, Matschl U, Jensen K, Luders C, Will H, Sternsdorf T. 1999. Splice variants of the nuclear dot-associated Sp100 protein contain homologies to HMG-1 and a human nuclear phosphoprotein-box motif. J. Cell Sci. 112:733–747 [DOI] [PubMed] [Google Scholar]
- 59. Stuurman N, de Graaf A, Floore A, Josso A, Humbel B, de Jong L, van Driel R. 1992. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101:773–784 [DOI] [PubMed] [Google Scholar]
- 60. Everett RD, Sourvinos G, Leiper C, Clements JB, Orr A. 2004. Formation of nuclear foci of the herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes. J. Virol. 78:1903–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gou D, Weng T, Wang Y, Wang Z, Zhang H, Gao L, Chen Z, Wang P, Liu L. 2007. A novel approach for the construction of multiple shRNA expression vectors. J. Gene Med. 9:751–763 [DOI] [PubMed] [Google Scholar]
- 62. Yao F, Schaffer PA. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boutell C, Cuchet-Lourenco D, Vanni E, Orr A, Glass M, McFarlane S, Everett RD. 2011. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 7:e1002245 doi:10.1371/journal.ppat.1002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chaurushiya MS, Lilley CE, Aslanian A, Meisenhelder J, Scott DC, Landry S, Ticau S, Boutell C, Yates JR, III, Schulman BA, Hunter T, Weitzman MD. 2012. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol. Cell 46:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lilley CE, Chaurushiya MS, Boutell C, Everett RD, Weitzman MD. 2011. The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog. 7:e1002084 doi:10.1371/journal.ppat.1002084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. 2010. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 29:943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Preston CM. 2007. Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein ICP0. J. Virol. 81:11781–11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Preston CM, Nicholl MJ. 2005. Human cytomegalovirus tegument protein pp71 directs long-term gene expression from quiescent herpes simplex virus genomes. J. Virol. 79:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lukashchuk V, McFarlane S, Everett RD, Preston CM. 2008. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J. Virol. 82:12543–12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ahn JH, Hayward GS. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee HR, Kim DJ, Lee JM, Choi CY, Ahn BY, Hayward GS, Ahn JH. 2004. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78:6527–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adamson AL, Kenney S. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bell P, Lieberman PM, Maul GG. 2000. Lytic but not latent replication of Epstein-Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J. Virol. 74:11800–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ling PD, Tan J, Sewatanon J, Peng R. 2008. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J. Virol. 82:8000–8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Full F, Reuter N, Zielke K, Stamminger T, Ensser A. 2012. Herpesvirus saimiri antagonizes nuclear domain 10-instituted intrinsic immunity via an ORF3-mediated selective degradation of cellular protein Sp100. J. Virol. 86:3541–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cuchet-Lourenco D, Vanni E, Glass M, Orr A, Everett RD. 2012. Herpes simplex virus 1 ubiquitin ligase ICP0 interacts with PML isoform I and induces its SUMO-independent degradation. J. Virol. 86:11209–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Everett RD, Orr A, Preston CM. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang L, Oliver SL, Sommer M, Rajamani J, Reichelt M, Arvin AM. 2011. Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin. PLoS Pathog. 7:e1002157 doi:10.1371/journal.ppat.1002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Everett RD, Murray J, Orr A, Preston CM. 2007. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 81:10991–11004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Catez F, Picard C, Held K, Gross S, Rousseau A, Theil D, Sawtell N, Labetoulle M, Lomonte P. 2012. HSV-1 genome subnuclear positioning and associations with host-cell PML-NBs and centromeres regulate LAT locus transcription during latency in neurons. PLoS Pathog. 8:e1002852 doi:10.1371/journal.ppat.1002852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dropulic LK, Cohen JI. 2011. Severe viral infections and primary immunodeficiencies. Clin. Infect. Dis. 53:897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ariza-Heredia EJ, Razonable RR. 2011. Human herpes virus 8 in solid organ transplantation. Transplantation 92:837–844 [DOI] [PubMed] [Google Scholar]
- 83. Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245–256 [DOI] [PubMed] [Google Scholar]
- 84. Griffiths A. 2011. Slipping and sliding: frameshift mutations in herpes simplex virus thymidine kinase and drug-resistance. Drug Resist. Updat. 14:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mori T, Kato J. 2010. Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int. J. Hematol. 91:588–595 [DOI] [PubMed] [Google Scholar]