Abstract
STUDY QUESTION
How are ovarian steroid concentrations, gonadotrophins and menstrual cycle characteristics inter-related within normal menstrual cycles?
SUMMARY ANSWER
Within cycles, measures of estradiol production are highly related to one another, as are measures of progesterone production; however, the two hormones also show some independence from one another, and measures of cycle length and gonadotrophin concentrations show even greater independence, indicating minimal integration within cycles.
WHAT IS KNOWN ALREADY
The menstrual cycle is typically conceptualized as a cohesive unit, with hormone levels, follicular development and ovulation all closely inter-related within a single cycle. Empirical support for this idea is limited, however, and to our knowledge, no analysis has examined the relationships among all of these components simultaneously.
STUDY DESIGN, SIZE, DURATION
A total of 206 healthy, cycling Norwegian women participated in a prospective cohort study (EBBA-I) over the duration of a single menstrual cycle. Of these, 192 contributed hormonal and cycle data to the current analysis.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Subjects provided daily saliva samples throughout the menstrual cycle from which estradiol and progesterone concentrations were measured. FSH and LH concentrations were measured in serum samples from three points in the same menstrual cycle and cycle length characteristics were calculated based on hormonal data and menstrual records. A factor analysis was conducted to examine the underlying relationships among 22 variables derived from the hormonal data and menstrual cycle characteristics.
MAIN RESULTS AND THE ROLE OF CHANCE
Six rotated factors emerged, explaining 80% of the variance in the data. Of these, factors representing estradiol and progesterone concentrations accounted for 37 and 13% of the variance, respectively. There was some association between measures of estradiol and progesterone production within cycles; however, cycle length characteristics and gonadotrophin concentrations showed little association with any measure of ovarian hormone concentrations.
LIMITATIONS, REASONS FOR CAUTION
Our summary measures of ovarian hormones may be imprecise in women with extremely long or short cycles, which could affect the patterns emerging in the factor analysis. Given that we only had data from one cycle on each woman, we cannot address how cycle characteristics may covary within individual women across multiple cycles.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings are generalizable to other healthy populations with typical cycles, however, may not be applicable to cycles that are anovulatory, extreme in length or otherwise atypical. The results support previous findings that measures of estradiol production are highly correlated across the cycle, as are measures of progesterone production. Estradiol and progesterone concentrations are associated with one another, furthermore. However factor analysis also revealed more complex underlying patterns in the menstrual cycle, highlighting the fact that gonadotrophin concentrations and cycle length characteristics are virtually independent of ovarian hormones. These results suggest that despite integration of follicular and luteal ovarian steroid production across the cycle, cycle quality is a multi-faceted construct, rather than a single dimension.
STUDY FUNDING/COMPETING INTEREST(S)
The EBBA-I study was supported by a grant from the Norwegian Cancer Society (49 258, 05087); Foundation for the Norwegian Health and Rehabilitation Organizations (59010-2000/2001/2002); Aakre Foundation (5695-2000, 5754-2002) and Health Region East. The current analyses were completed under funding from the National Institutes of Health (K12 ES019852). No competing interests declared.
Keywords: menstrual cycle length, gonadotrophin, estradiol, progesterone, ovarian function
Introduction
The menstrual cycle is typically characterized as a single, cohesive unit in which hormone levels, follicular development and ovulation are all closely inter-related. From this perspective, high-quality cycles are not only ovulatory, but also have high estradiol (E2)and progesterone concentrations, characteristic mid-cycle LH and FSH peaks, and are ∼28 days in length. At the other end of the spectrum, low-quality cycles are not only anovulatory, but may also have low estradiol and progesterone concentrations, lack discernible LH and FSH peaks and be atypical in length. In other words, the classical view of the menstrual cycle implies that the quality of a given cycle is consistent across multiple measures, including follicular development, ovarian steroid and gonadotrophin concentrations, endometrial development and cycle length characteristics. This concept of consistent quality across the cycle is often implicitly accepted; however, few studies have directly examined this question. Certainly at the extreme end of the spectrum of impaired ovarian function, multiple aspects of hormone production and cycle characteristics can all be compromised, with possible cessation of menses and hormone cycling (Ellison, 1990). Within the range of typical, healthy cycling, however, the degree of cohesiveness or integration of cycle quality remains unclear. Empirically, if the quality of a cycle really is a single dimension, the various measureable components should show a high degree of covariance within cycles. The goal of the current analysis is to examine the patterns of association among component parts of the menstrual cycle (E2 concentrations, progesterone concentrations, gonadotrophin concentrations and cycle length variables) in order to better understand the normal menstrual cycle.
Theoretical support for close correlation of E2 and progesterone production comes from the fact that both ovarian steroids derive from the same underlying structures. That is, the very cells that together produce E2 in the follicular phase—the theca and granulosa cells of the pre-ovulatory follicle—are those that go on to comprise the progesterone-producing cells of the corpus luteum after ovulation (Strauss and Williams, 2004). Based purely on the underlying cellular physiology, therefore, we might predict consistency of ovarian steroid production across the menstrual cycle (e.g. robust follicular E2 production associated with robust luteal E2 and progesterone production). On the other hand, estrogen and progesterone play different physiological roles in reproduction, and epidemiological evidence indicates that they can vary independently of one another (Lipson and Ellison, 1996; Venners et al., 2006; Nunez-de la Mora et al., 2007; Nunez-De La Mora et al., 2008). For instance, one study measuring daily ovarian hormone profiles in premenopausal women found very low correlations between urinary estrogen and progesterone metabolite concentrations within a cycle (r = −0.003 to 0.13) (Windham et al., 2002). Thus, although a high degree of consistency between E2 and progesterone indices might be expected, empirical support for that prediction is mixed.
Much as we might expect consistency of ovarian steroid hormone production within a cycle, so might we predict that ovarian steroid production is closely associated with production of pituitary gonadotrophins. Ovarian steroid hormone production and release comes from coordination of ovarian theca and granulosa cell activity and is dependent upon gonadotrophin input from the pituitary gland, with LH stimulating theca cell function, while FSH influences E2 production by the granulosa cells (Strauss and Williams, 2004). Although some follicular development can proceed in the absence of FSH stimulation, suggesting there is limited ovarian hormone activity independent of pituitary input (Oktay et al., 1998), gonadotrophin stimulation is essential for advancing further to the steroid-producing antral phase (Irving-Rodgers et al., 2001). The complex feedback interactions between gonadotrophins and ovarian steroids continue throughout mid-cycle, when rising follicular E2 levels drive pre-ovulatory surges in FSH and LH (Richards et al., 2002). The apparent interdependence of gonadotrophin and ovarian steroid activity characteristic of normal ovarian function suggests that the two may be closely associated throughout the cycle, at least during certain periods. For instance, in one study of cycling women, there were weak correlations between E2 and FSH early in the follicular phase, but higher (inverse) correlations in the mid-follicular phase (Robertson et al., 2009) and other studies have found that E2 has inhibitory effects on FSH secretion in the luteal phase (Lasley et al., 1975; de Ziegler et al., 1992; Lahlou et al., 1999). In the luteal phase, moreover, both LH and FSH show weak-to-moderate negative correlations with progesterone concentrations, while LH and E2 concentrations show a weak positive correlation (Robertson et al., 2009). Thus, there is evidence to suggest some coordination of pituitary gonadotrophin and ovarian steroid production within the cycle, although the strength and direction of the relationship may vary at different points in the cycle.
That cycle length variables should be linked to hormone concentrations and follicular development is less obvious, but this prediction follows from the physiology nonetheless. The length of the follicular phase reflects the speed at which the antral follicle is recruited and develops, and thus by extension, follicular phase length should be related to gonadotrophin and ovarian steroid concentrations as well (Harlow et al., 2000; Cabral and de Medeiros, 2007). Experimental evidence in primates suggests that if the antral follicle is destroyed, the characteristic pre-ovulatory gonadotrophin surge is delayed, extending both the length of follicular phase and that of the total cycle (Goodman et al., 1977). In humans, a limited body of work suggests associations between ovarian steroid concentrations and cycle length parameters, including total cycle length, follicular phase length and luteal phase length, arguing further for consistency of cycle quality across multiple domains (Landgren et al., 1980; Harlow et al., 2000; Windham et al., 2002). In particular, short follicular phases and short cycles may be associated with relatively high estrogen and progesterone concentrations, whereas longer follicular phases may be characterized by lower average estrogen concentrations (Landgren et al., 1980; Harlow et al., 2000). Other studies have also observed positive correlations between progesterone levels and luteal phase length (Landgren et al., 1980; Windham et al., 2002).
To date, it has been difficult to study associations among different measures of ovarian function across the menstrual cycle because of the difficulty of obtaining repeated measures of hormonal variables across the entire cycle. Only a handful of studies have measured complete, daily E2 and progesterone profiles over the course of one or more cycles (de Souza et al., 1998; Windham et al., 2002; Liu et al., 2004; Santoro et al., 2004; Matthews et al., 2006). The convenience and non-invasiveness of saliva collection compared with blood or urine makes it an ideal medium for measuring daily ovarian steroid profiles. However, the extremely low concentrations of E2 in saliva made these analyses prohibitively difficult until relatively recently (O'Rourke and Ellison, 1993). In this analysis using data from healthy, cycling women participating in the Norwegian Energy Balance and Breast Cancer Aspects-I (EBBA-I) study, we adopt a factor analysis approach to examine whether the menstrual cycle truly is a cohesive unit. In particular, we focus on the relationships among four aspects of the menstrual cycle (E2 concentrations, progesterone concentrations, gonadotrophin concentrations, and measures of cycle length), looking at the strength of the associations between these components within cycles.
Methods
Subject population, participants and study design
Women were recruited for the EBBA-I study, based in Tromsø, Norway, between 2000 and 2002. The study's goal was to examine the role of energetics and other lifestyle variables on known breast cancer risk factors in healthy, premenopausal women. To participate, women had to be at the age of 25–35 with regular menstrual cycles and could not have been pregnant, lactated or used hormonal contraception in the previous 6 months. Women with known histories of infertility, gynecological disorders or chronic illnesses (such as type II diabetes) were excluded. In total, 206 women participated in EBBA-I, and the subject population, recruitment methods and study design have been described elsewhere in detail (Furberg et al., 2005). Subjects received 1000 Norwegian kroner (∼$160 US dollars at the time) to cover transportation and other expenses related to their participation.
Ethical approval
Participating women signed informed consent and the study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate as well as the human subjects review boards at all participating institutions.
Salivary steroid assay
As part of the study, subjects collected daily waking saliva samples over an entire menstrual cycle according to protocols developed by the Reproductive Ecology Laboratory at Harvard University (Lipson and Ellison, 1989). Free E2 concentrations were assayed in samples from 20 cycle days (reverse cycle Days −5 to −24), and progesterone concentrations were assayed for the last 14 days of each cycle (reverse cycle Days −1 to −14). Levels of both hormones were measured using 125I-based radioimmunoassay (RIA) kits (Diagnostics Systems Laboratory, Webster, TX, USA) using methods reported elsewhere (Furberg et al., 2005). The sensitivity of the estradiol assay was 4 pmol/l (1.1 pg/ml), the average intra-assay variability was 9%, and the interassay variability ranged from 23 to 13% for the low and high pools, respectively. The sensitivity of the progesterone assay was 13 pmol/l (4.1 pg/ml). Based on our assayed samples, the average intra-assay variability was 10%, and the inter-assay variability ranged from 19 to 12% for the low and high pools, respectively.
Once E2 assays had been completed, the daily concentrations across each cycle were examined in order to identify the day of the greatest mid-cycle drop in estradiol using methods described elsewhere (Lipson and Ellison, 1996). For each cycle, a mid-cycle E2 ‘drop day' was first determined. The drop day was defined as the second of the two consecutive days in a mid-cycle window during which the greatest decrease in E2 occurred. The mid-cycle window for identifying peak E2 was Days −18 to −12, thus the drop day was constrained to fall between Days −17 and −11. This E2 drop provides a good marker for the timing of ovulation, and the drop day was subsequently designated as Day ‘0’. Thus days in the follicular phase have negative prefixes (e.g. Day −1, Day −2), whereas days in the luteal phase have positive prefixes (e.g. Day +1, Day +2). A drop day could not be assigned for 14 subjects. Eight of the 14 had missing hormone data for at least 1 day during the interval between reverse cycle days −18 to −12. The remaining six subjects had no discernible rise or drop in E2 during the critical time window and their mid-cycle LH levels were low as well, suggesting that the cycles were anovulatory. Because determination of drop day is needed to calculate hormonal indices and separate cycles into follicular and luteal phases, these 14 women were excluded and only the 192 women with aligned cycles were included in analyses.
Creation of ovarian hormone indices
From the daily E2 and progesterone concentrations we were able to calculate a number of different indices of hormone levels, representing different components or periods of ovarian function. Each index was calculated as the mean hormone concentration across samples collected during a particular period of the cycle relative to ovulation (Day 0). Seven estradiol indices were calculated for each cycle: total E2 (mean E2 for all cycle days measured) reflects average E2 exposure across the cycle; follicular E2 (mean E2, Days −10 to −1) reflects average E2 prior to ovulation; mid-follicular E2 (mean E2, Days −10 to −6) reflects E2 production around the time of the emergence of the dominant follicle; late follicular E2 (mean E2, Days −5 to −1) reflects the secretory activity of the dominant follicle prior to ovulation; maximum follicular E2 (highest E2 concentration measured between Days −10 to −1), maximum E2 (highest E2 at any point in the cycle) and magnitude of the mid-cycle E2 drop (maximum E2 minus E2 on Day 0) reflect mid-cycle E2 secretion as well as the decrease in circulating E2 accompanying ovulation of the dominant follicle. If the follicular phase length was shorter than 10 days, follicular E2 and mid-follicular E2 calculations were adjusted accordingly (e.g. if the follicular phase was only 9 days, measurements were calculated as the mean of Days −9 through −1, rather than −10 through −1). Similarly, if data were missing (for instance, due to E2 concentrations below the sensitivity limit at the beginning of the cycle), indices were calculated as the mean across the days with measurable concentrations in that interval.
Six indices of progesterone concentrations were calculated as well. Total progesterone (mean progesterone, Days 0 to +14) reflects average progesterone exposure during the luteal phase; early-mid luteal progesterone (mean progesterone, Days 0 to +9) represents the average circulating progesterone concentrations during the beginning and middle of the luteal phase; mid-luteal progesterone (mean progesterone, Days +5 to +9) reflects the level of progesterone secretion at the peak of the luteal phase; very early luteal progesterone (mean progesterone, Days 0 to +2) and early luteal progesterone (mean progesterone, Days +3 to +5) together reflect the early luteal progesterone rise, before any possible effects of hCG from a potential conceptus and late luteal progesterone (mean progesterone, Days +10 to +14) reflects post-peak secretion of progesterone during the regression of the corpus luteum prior to menstruation. If the luteal phase length was shorter than 14 days, total progesterone and late luteal progesterone calculations were adjusted accordingly (e.g. if the luteal phase was 12 days, total progesterone was calculated as the mean of Days 0 through +12, rather than 0 through +14). When progesterone values were missing for individual days, the hormone indices were calculated as the means of those days with data. Summary statistics for the hormonal variables are provided in Table II.
Table II.
Pearson's correlation matrix for estradiol and progesterone indices in the EBBA-I study (with mean and standard deviation).
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | M | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total E2 | 1.00 | 1.16 | 0.23 | ||||||||||||
| 2. Follicular E2 | 0.96 | 1.00 | 1.15 | 0.24 | |||||||||||
| 3. Mid-follicular E2 | 0.89 | 0.95 | 1.00 | 1.04 | 0.26 | ||||||||||
| 4. Late follicular E2 | 0.93 | 0.95 | 0.80 | 1.00 | 1.25 | 0.24 | |||||||||
| 5. Maximum E2 | 0.86 | 0.81 | 0.71 | 0.83 | 1.00 | 1.50 | 0.23 | ||||||||
| 6. Maximum follicular E2 | 0.85 | 0.85 | 0.72 | 0.89 | 0.92 | 1.00 | 1.46 | 0.17 | |||||||
| 7. Magnitude of E2 drop | 0.19 | 0.08 | 0.11 | 0.04 | 0.01 | 0.06 | 1.00 | 0.32 | 0.25 | ||||||
| 8. Luteal P | 0.60 | 0.56 | 0.49 | 0.58 | 0.63 | 0.45 | 0.12 | 1.00 | 2.01 | 0.25 | |||||
| 9. Early-mid luteal P | 0.56 | 0.54 | 0.46 | 0.56 | 0.01 | 0.45 | 0.09 | 0.97 | 1.00 | 2.04 | 0.26 | ||||
| 10. Very early luteal P | 0.56 | 0.57 | 0.53 | 0.55 | 0.01 | 0.41 | 0.02 | 0.81 | 0.82 | 1.00 | 1.90 | 0.31 | |||
| 11. Early luteal P | 0.49 | 0.48 | 0.39 | 0.53 | 0.40 | 0.43 | 0.07 | 0.88 | 0.92 | 0.70 | 1.00 | 2.06 | 0.31 | ||
| 12. Mid-luteal P | 0.49 | 0.45 | 0.38 | 0.47 | 0.39 | 0.39 | 0.13 | 0.90 | 0.93 | 0.60 | 0.83 | 1.00 | 2.11 | 0.26 | |
| 13. Late luteal P | 0.52 | 0.46 | 0.40 | 0.46 | 0.41 | 0.34 | 0.14 | 0.81 | 0.66 | 0.57 | 0.56 | 0.64 | 1.00 | 1.93 | 0.32 |
n = 192. Total E2 = mean E2, cycle days −13 to +12; follicular E2 = mean E2, cycle days −10 to −1; mid-follicular E2 = mean E2, cycle days −10 to −6; late follicular E2 = mean E2, cycle days −5 to −1; luteal E2 = mean E2, cycle days +5 to +9; maximum E2 = highest E2 between cycle days −10 to −1; magnitude of the E2 drop = maximum E2 minus E2 on Day 0; luteal P = mean P, cycle days 0 to +14; Early-mid-luteal P = mean P, cycle days 0 to +9; mid-luteal P = mean P, cycle days +5 to +9; very early luteal P = mean P, cycle days 0 to +2; early mid-luteal P = mean P, cycle days +3 to +5; and late luteal P = mean P, cycle days +10 to +14.
Calculation of menstrual cycle phase lengths
Three cycle length variables were measured using the hormone data and self-reported dates of menses. Overall cycle length was the number of days from menstrual onset to menstrual onset, as determined by self-reported menses. Follicular phase length was the number of days from menstrual onset to the mid-cycle estradiol drop day. Finally, the luteal phase length was the number of days from the day after the mid-cycle estradiol drop day to onset of subsequent self-reported menses.
Serum sample collection and gonadotrophin assay
At three points in the cycle fasting serum samples were taken by trained nurses at the University Hospital of Northern Norway, Tromsø. These collections were timed relative to the onset of menses to reflect the early follicular (1–2 days after the start of menses), pre-ovulatory (7–12 days after the start of menses) and luteal phases (21 days after the start of menses) of the cycle. LH and FSH were measured in serum samples from all three time points using Techicon Immuno1 immunometric assays (Bayer Corp, Tarrytown, NY). Both assays were standardized against the World Health Organization 2nd International Standard (for FSH: IRP 78/549 and for LH: IRP 68/40). The sensitivity of the FSH assay was 0.1 IU/l and the coefficient of variation was <7%. For LH, the assay sensitivity was 0.3 IU/l and the coefficient of variation was 5–10%.
Statistical analyses
All statistical analyses were carried out in SAS Enterprise 4.3 (SAS Corporation, Cary, NC). Because hormone values typically follow non-normal distributions, all hormone indices were first log transformed to normalize variances. We first examined bivariate correlations between the ovarian hormone indices in our analysis. We then conducted a factor analysis by principal components extraction, with and without orthogonal varimax rotation of axes on the correlation matrix of the study variables. The goal of factor analysis is to condense a large number of correlated variables into a smaller number of factors and in doing so, reveal underlying relationships among the variables. Orthogonal varimax rotation then rotates these factors so that they are uncorrelated with one another, creating factors for which one or more variables have high loadings, while loadings for the other variables are close to zero (Manly, 2005). Each factor has an eigenvalue, which indicates the amount of variance in all of the variables that is accounted for by that factor, and following the conventionally used Kaiser criterion, only factors with eigenvalues >1 (i.e. explaining >1% of the total variance) are retained in the analysis (Kaiser, 1958). Thus, factors with large eigenvalues explain a large amount of variance in the overall data, whereas factors with small eigenvalues explain little of the variance. A Scree plot (which helps to visually discriminate between those factors explaining a large fraction of the variance and those which are relatively unimportant) was made to confirm the number of factors that should be included in the analysis. For each factor with an eigenvalue >1, we examined the loading of each menstrual cycle variable, which is similar to a standardized regression coefficient when the factor is regressed on the variables (DeCoster, 1998). Loadings of ≥0.7 were considered strong loadings, while those <0.7, but ≥0.35 were considered moderate loadings. Loadings <0.35 were considered weak to negligible.
One of the useful aspects of principal component extraction is the collapsing of highly correlated variables into a smaller number of axes representing linear functions of those correlated variables. Here, for instance, although high correlations might be expected among the different indices of each steroid (particularly those that overlap), it is not necessarily the case that seemingly related indices would all cluster on the same rotated axes resulting from factor analysis. Luteal and follicular E2 secretion, for example, might be governed by different patterns of gonadotrophin secretion and hence manifest significant independence. Similarly mid-follicular E2 might reflect the combined secretory activity of a recruited cohort of follicles under FSH stimulation, whereas late follicular and maximum follicular E2 presumably reflect secretion by the dominant follicle alone. The degree to which these aspects of E2 production are independent will affect the degree to which they individually correlate with average E2 levels over the entire follicular phase or the entire cycle as well. Thus, the current analyses allow us to examine the relationships among the specified variables without making any a priori assumptions about the independence (or multicollinearity) of different indices of ovarian steroid levels and other cycle characteristics. Instead, factor analysis allows us to identify those clusters of variables that are highly redundant and thus reduce the number of indices studied.
Results
General characteristics of the study subjects are provided in Table I. The study population was predominantly Caucasian and highly educated with a mean age of 31 years. Sixty two percent of subjects were married and half had at least one child. The average cycle length was 28 days (range: 20–47), of which 15 were spent in the follicular phase and 13 in the luteal phase. Bivariate analyses indicated moderate-to-strong positive correlations between the E2 and progesterone indices and are presented in Table II. Most of the E2 indices have correlation coefficients with each other in the range 0.7–0.95. The exception is the magnitude of the mid-cycle E2 drop, which correlates very weakly, and typically negatively, with the other E2 variables. Similarly, the progesterone indices have correlation coefficients with each other in the range of 0.57–0.97. Except for the magnitude of the E2 drop, the correlations between the E2 and progesterone indices are moderate, ranging from 0.38 to 0.60.
Table I.
Means (SD) and proportions of selected characteristics in the EBBA-I subject population (n = 192).
| Mean (SD) | |
|---|---|
| Demographics and anthropometrics | |
| Age (years) | 30.7 (3.1) |
| Years of schooling | 16.1 (3.0) |
| Height (cm) | 167.0 (6.5) |
| Weight (kg) | 68.1 (11.7) |
| BMI (kg/m2) | 24.4 (3.8) |
| Reproductive characteristics | |
| Married, % | 61.5 |
| Parous, % | 49.5 |
| Age at menarche (years) | 13.1 (1.4) |
| Previous use of hormonal contraception, % | 80.6 |
| Cycle length (days) | 28.4 (3.4) |
| Follicular phase length (days) | 15.0 (3.8) |
| Luteal phase length (days) | 13.4 (1.7) |
| Mean follicular estradiol (pmol/l): cycle days −10 to −1 | 18.0 (9.6) |
| Mean progesterone (pmol/l): cycle days 0 to +14 | 143.4 (74.1) |
| Lifestyle characteristics | |
| Energy intake (kJ/day) | 8097 (1891) |
| Alcohol use (units/week) | 3.5 (3.4) |
| Current smokers, % | 22.3 |
The unrotated factor matrix (not shown) generated six factors with eigenvalues >1. Typically, the first unrotated factor represents the single vector that captures the greatest amount of the multivariate variance and in this case, all of the E2 and progesterone measures (aside from the magnitude of the E2 drop) had loadings of 0.70 or greater on Factor 1. Of the remaining variables, seven had loadings between 0.05 and 0.16, with the remaining three having loadings <0.05. Factor 1 of the unrotated matrix accounted for only 38% of the total multivariate variance in the sample, however, indicating that the majority of the multivariate variance could not be captured by a single axis.
Subsequent orthogonal varimax rotation of the axes obtained from the factor analysis maximized the separation of the factor loadings of the original variables onto different axes and generated six rotated factors with eigenvalues >1, which together explained 80% of the variation in the data. Factor loadings for the six rotated factors are presented in Table III. The varimax rotation largely succeeded in separating the original variables onto different axes, each of which was orthogonal to, or independent of, the others. All variables loaded on at least one factor, but no variable had a strong loading (≥0.7) on more than one factor. Two variables, mid-cycle LH and mid-cycle FSH, showed split moderate loadings on more than one factor. Mid-cycle LH loaded strongly on Factor 5 and moderately (and negatively) on Factor 3, while mid-cycle FSH showed a moderate, negative loading on Factor 3, and moderate, positive loadings on Factors 4 and 5. Only two original variables, mid-cycle FSH and magnitude of the mid-cycle E2 drop, did not have a strong loading on any of the six rotated factors. Mid-cycle FSH instead had moderate loadings on three factors, while the magnitude of the mid-cycle E2 drop had a moderate loading on one factor.
Table III.
Rotated factor structure of ovarian function measures in the EBBA-I study.
| Standard factor loadings |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Cycle length | 0.096 | 0.026 | 0.900 | 0.262 | 0.011 | 0.112 |
| Follicular length | 0.096 | 0.014 | 0.878 | 0.247 | −0.020 | −0.302 |
| Luteal length | −0.013 | 0.023 | −0.077 | −0.003 | 0.074 | 0.966 |
| Mean total E2 | 0.908 | 0.344 | 0.042 | −0.020 | −0.078 | 0.020 |
| Mean follicular E2 | 0.921 | 0.310 | 0.082 | 0.023 | −0.016 | −0.056 |
| Mean mid-follicular E2 | 0.851 | 0.267 | 0.130 | 0.052 | −0.035 | −0.074 |
| Mean late follicular E2 | 0.901 | 0.325 | 0.037 | −0.001 | 0.003 | −0.081 |
| Maximum E2 | 0.891 | 0.220 | −0.025 | 0.018 | −0.030 | 0.083 |
| Maximum follicular E2 | 0.910 | 0.195 | −0.001 | −0.006 | 0.028 | 0.040 |
| Magnitude of E2 drop | −0.035 | −0.088 | 0.020 | 0.408 | 0.290 | 0.123 |
| Mean luteal P | 0.302 | 0.944 | 0.016 | −0.047 | −0.037 | −0.006 |
| Mean early-mid-luteal P | 0.281 | 0.941 | 0.031 | −0.073 | 0.023 | 0.020 |
| Mean very early luteal P | 0.331 | 0.755 | 0.119 | −0.011 | 0.039 | −0.086 |
| Mean early luteal P | 0.226 | 0.888 | 0.022 | −0.086 | 0.033 | −0.060 |
| Mean mid-luteal P | 0.221 | 0.891 | −0.010 | −0.102 | −0.002 | 0.068 |
| Mean late luteal P | 0.265 | 0.734 | −0.028 | 0.052 | −0.130 | 0.079 |
| Early follicular LH | 0.070 | −0.039 | 0.133 | 0.015 | 0.729 | 0.163 |
| Early follicular FSH | 0.013 | 0.112 | 0.041 | −0.136 | 0.667 | −0.061 |
| Mid-cycle LH | 0.020 | −0.098 | −0.368 | 0.151 | 0.761 | −0.010 |
| Mid-cycle FSH | 0.043 | −0.156 | −0.468 | 0.487 | 0.500 | −0.128 |
| Luteal LH | 0.039 | 0.008 | 0.304 | 0.797 | −0.060 | 0.001 |
| Luteal FSH | 0.025 | −0.065 | 0.137 | 0.849 | −0.106 | −0.084 |
| Percent of total variance explained | 37 | 13 | 11 | 9 | 5 | 5 |
E2, estradiol; P, progesterone; LH, luteinizing hormone; FSH, follicle stimulating hormone.
Factor loadings ≥0.70 appear in boldface, whereas those ≥0.35 but <0.70 appear in italics. Rotation sums of squared loadings for the 6-factor model equal 79.6%.
The first rotated factor explained 37% of the variance and included all of the measures of E2 except for the magnitude of the mid-cycle E2 drop. The second rotated factor included the six progesterone indices and explained 13% of the variance. The third rotated factor accounted for 11% of the variance and included cycle length and follicular phase length with minor loadings on mid-cycle gonadotrophin concentrations. The fourth rotated factor explained 9% of the variance and included luteal gonadotrophin concentrations, with a minor loading on mid-cycle FSH concentrations and the magnitude of the E2 drop. The fifth rotated factor, explaining 5% of the variance, had major loadings on early follicular and mid-cycle LH concentrations and minor loadings on early follicular and mid-cycle FSH loadings. Finally, only the luteal phase length was included in the sixth rotated factor, which accounted for 5% of the variance in the data set. Sensitivity analyses (not shown) using only subjects with complete daily hormone data did not change the basic relationships among variables and factors.
Discussion
The purpose of this analysis is to examine the extent to which the menstrual cycle is a cohesive unit in healthy, reproductive-age women, as measured by the strength of the relationships among hormonal measurements and cycle characteristics. Or, phrased as a question, to what extent is any one measure of menstrual function predictive of or independent of others within the same cycle? Of particular interest is the extent to which there is coordination of ovarian steroid production across the follicular and luteal phases of the cycle. In our study population, the relationship between follicular phase E2 and luteal phase progesterone is significantly positive, as reflected in the bivariate correlations. These correlations are much higher than reported in at least one other study in cycling women, in which correlations between urinary E2 and progesterone metabolite concentrations were 0.13 or lower (Windham et al., 2002). Because urinary assays measure conjugated metabolite concentrations and are thus one or more steps removed from circulating free hormone concentrations, such assays may introduce additional noise related to inter-individual metabolic variation (Gann et al., 2001). For that reason, the stronger correlations found in the current study based on free (bioactive) salivary steroid concentrations may be a more accurate reflection of the true relationship between E2 and progesterone concentrations in healthy, cycling women.
In the current study, the positive association between follicular and luteal steroid profiles is further illustrated by sorting the study subjects into quartiles on the basis of the indices of one steroid and comparing the full daily profiles of the other. Sorting the subjects into quartiles by mean follicular E2 concentrations shows that women with high mean follicular E2 concentrations also tend to have high progesterone concentrations throughout the luteal phase (Fig. 1). Similarly, when subjects are sorted by mean luteal progesterone concentrations, those with the highest quartile of luteal progesterone concentrations tend to have high follicular E2 concentrations as well (Fig. 2). In both cases, the quartiles are clearly distinct from one another. Thus crude analyses suggest that, across women, within a cycle, levels of one of these hormones are indicative of levels of the other.
Figure 1.
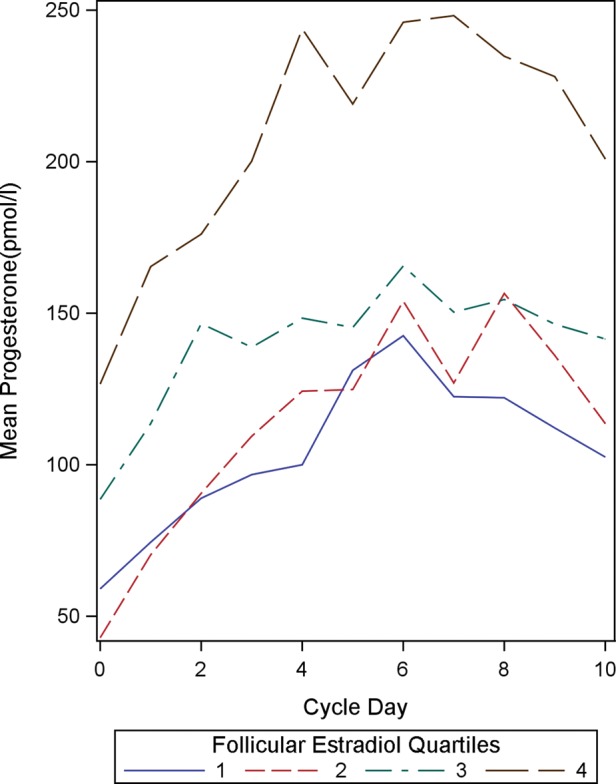
Mean luteal progesterone concentrations (in pmol/l) by mean follicular estradiol quartiles (n = 192). Cycle day 0 represents the day of ovulation.
Figure 2.
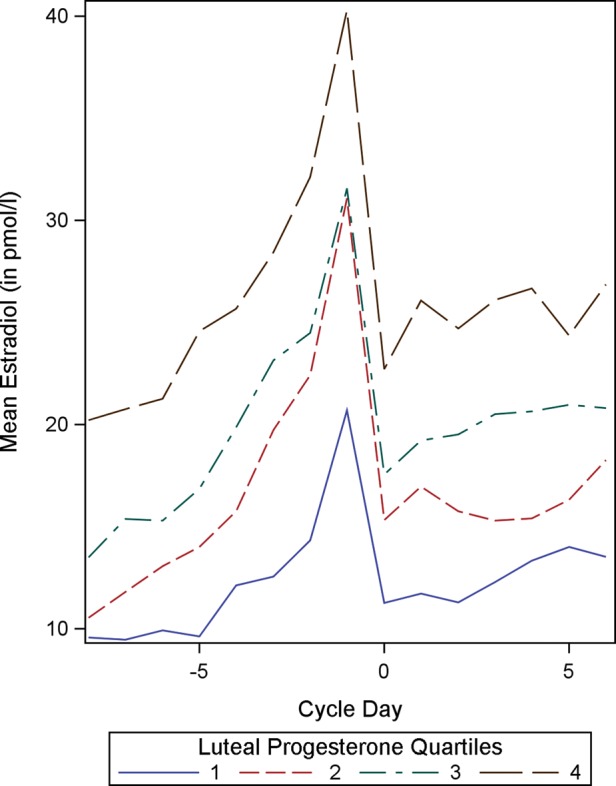
Mean follicular estradiol concentrations (in pmol/l) by mean luteal progesterone quartiles (n = 192). Cycle day 0 represents the day of ovulation.
The subsequent factor analysis allowed simultaneous examination of the relationships between the ovarian steroid concentrations and other measures of cycle quality to identify more complex underlying patterns. The factors obtained after varimax rotation represent the ‘sorting' of variables into groups that are highly correlated among the group while being orthogonal, or independent, of the groups represented by other factors. Factor 1 has very strong loadings (0.85 or greater) for all the E2 indices except the magnitude of the E2 drop. It reflects the high consistency of E2 production across the ovarian cycle and supports previous work finding high correlations between E2 measures at multiple points across the cycle (Windham et al., 2002). The progesterone indices load weakly on this factor (0.20–0.30), with loadings being highest for early luteal progesterone measures and lower for indices capturing the later part of the luteal phase. This suggests that E2 and progesterone production cannot be fully disentangled, particularly in the early luteal phase. Factor 2 has very high loadings (0.73 or greater) for all the progesterone indices, suggesting that progesterone production is highly consistent across the luteal phase. In Factor 2 there are weak loadings (0.2–0.34) for most of the E2 indices, again indicating that there is some aspect of the relationship between progesterone and E2 production that cannot be disarticulated, as suggested in the crude analyses.
Nevertheless, the degree to which indices of the two ovarian steroids separate onto different axes in the factor analysis reflects the degree to which they are actually independent of each other. It is noteworthy that no other variables have loadings on the first two rotated factors, which we therefore regard as the E2 and progesterone factors, respectively. In particular, the loadings for both the gonadotrophin variables and the cycle length measures are extremely low. This suggests that a woman's circulating E2 and progesterone concentrations are not a clear function of her circulating gonadotrophin concentrations, nor are they closely related to her cycle length. Rather, other factors including gonadotrophin receptor densities or sub-types, co-gonadotrophins such as insulin and IGF-1, or other unknown genetic, developmental or constitutional components may explain inter-individual variance in ovarian steroid concentrations. It remains possible that differences in gonadotrophin concentrations may account for more of the documented within-individual variance in ovarian steroid concentrations (between multiple cycles in the same woman, for instance) (Lipson and Ellison 1996; Venners et al., 2006).
Factor 3 has very high loadings (0.88 or greater) for total cycle length and follicular phase length, which confirms the close association between follicular phase length and overall cycle length that has been noted elsewhere (Waller et al., 1998; Fehring et al., 2006). The moderate negative loadings of the mid-cycle concentrations of LH and FSH on this factor are more surprising, suggesting that factors associated with slow follicular growth (resulting in a longer follicular phase and longer total cycle length) may later result in poor steroid response in the luteal phase. Because this factor is independent of steroid concentrations themselves (Factors 1 and 2), even in the luteal phase, it may indicate that higher gonadotrophin levels are required to stimulate a given amount of steroid production in cycles with longer follicular phases than in those with shorter follicular phases. This may again be consistent with variation in the ovarian responsiveness to gonadotrophin stimulation rather than the level of that stimulation itself, an effect that might be moderated at the receptor level. Further study is needed to understand these unexpected relationships.
Factor 4 has strong loadings (0.80 or greater) for luteal FSH and LH concentrations. It also has moderate loadings for mid-cycle FSH (0.49) and the magnitude of the mid-cycle estradiol drop (0.41). It is noteworthy that the magnitude of the mid-cycle E2 drop only clusters with luteal gonadotrophins (albeit moderately) and not with any of the ovarian steroid measures. In fact, the magnitude of the mid-cycle E2 drop is the only steroid index that correlates significantly with levels of gonadotrophin stimulation, although it is somewhat surprising that it clusters with luteal, rather than mid-cycle gonadotrophin concentrations. Baseline gonadotrophin concentrations tend to be very low across the entire luteal phase and pulsatile release of gonadotrophins occurs at low frequency (Johnson and Everitt, 2000; Hall, 2004), and so it is unclear why they should be associated with the magnitude of the E2 drop. Additional research is needed to confirm and better understand this unexpected observation.
Factor 5 has high loadings (>0.70) for early follicular and mid-cycle LH, with moderate loadings (0.5–0.7) for early follicular and mid-cycle FSH. This indicates that gonadotrophin concentrations in the first half of the cycle are closely associated even though the two have distinct functional differences, with FSH stimulating further development of the antral follicle, while LH promotes ovarian steroid production and eventually, ovulation (Hall, 2004; Strauss and Williams, 2004). Nevertheless, given that both are produced and secreted by a common source (the pituitary gland) and that both are responsive to fluctuating ovarian steroid concentrations (through negative feedback), it is not surprising that FSH and LH concentrations would load on the same factor. More surprising, perhaps, is how weak the ovarian steroid loadings are on this factor, which may indicate that across women, there is little relationship between early follicular and mid-cycle gonadotrophins and ovarian steroid concentrations. Once again, this suggests that it may be sensitivity to gonadotrophin stimulation (for instance through receptor densities or the effect of co-gonadotrophins), and not absolute gonadotrophin concentrations that are most important for regulating ovarian steroid production.
Finally, only the luteal phase length loads on Factor 6 (0.97), indicating that it is virtually independent of the other hormone and cycle characteristics considered in this analysis. It is not surprising that the luteal phase length did not cluster with the other cycle length variables given that the literature suggests that while total cycle length and follicular phase length are tightly correlated, the luteal phase length tends to be less variable and show only moderate correlations with both (Waller et al., 1998; Fehring et al., 2006). In fact, one study found that only 3% of the variance in the total cycle length was attributable to variation in the luteal phase length, whereas follicular phase variation explained over 84% (Waller et al., 1998). At least one study has identified differences in urinary ovarian steroid concentrations in relation to luteal phase length; however, those differences were in comparisons of cycles with short (≤10 days), average (11–14 days) and long (≥15 days) luteal phases and did not consider luteal phase lengths continuously within the normal range (Windham et al., 2002). In general, little is known about predictors or determinants of luteal phase length and additional research is needed to understand the existing variation and how it is related to other cycle indicators of ovarian function.
Overall, the results of this factor analysis suggest that although there is some consistency of menstrual function across domains, the particular cycle measures considered here also show considerable independence from one another across women. Perhaps of greatest interest are the associations between E2 and progesterone indices, which clustered onto two distinct factors, but also showed minor loadings on each other's primary factors, suggesting some inter-dependence between the two. This finding is interesting in light of previous work suggesting that follicular E2 concentrations are higher in conception cycles than non-conception cycles (Lipson and Ellison, 1996). One explanation is that high E2 concentrations better stimulate the developing oocyte and prime the endometrium for proliferation, thus increasing the odds of a successful conception. Our results suggest a second explanation and should be considered as well, namely that there may also be correlated luteal phase effects, including endometrial secretions and support for implantation that is necessary for successful conception. The associations between E2 measures (which are primarily follicular) and progesterone measures (which are luteal) is clinically important; moreover, in that it further supports the idea that luteal phase defects are actually a product of problems with follicular development earlier in the cycle (DiZerega and Hodgen, 1981).
At the same time, E2 and progesterone measures also showed a degree of independence from one another, and the fact that ovarian steroid concentrations were not associated with variation in gonadotrophin concentrations or in cycle and phase lengths, moreover, suggests that it may be mediated by tissue sensitivity, perhaps reflecting differences in receptor expression or variation, or other physiological, genetic, developmental or constitutional factors. Such a mechanism would be consistent with findings of a study of adult Bangladeshi migrants to the UK, that indicated that progesterone, but not E2, was related to individual developmental history (Nunez-de la Mora et al., 2007; Nunez-De La Mora et al., 2008). Indeed, at least one study found that progesterone levels tend to be predictable within individuals over intervals of as much as one1 year, whereas E2 levels may vary dramatically within individuals over the same time period (Chatterton et al., 2005). The dissociation of E2 and progesterone profiles observed in such studies suggests that developmental history may exert long-lasting influence on some aspects of ovarian steroid production, whereas other aspects are more responsive to acute cues in the immediate environment.
There are some limitations to the interpretation of results from this study. First, the current study assessed only inter-individual effects, finding, for instance, that women who have high E2 levels tend to have high progesterone levels and vice versa. Because hormone levels were only measured for the duration of a single cycle in this study, we are unable to examine whether the same trend holds between cycles within individual women. Additional research following women longitudinally over time is needed to determine whether, within a given woman, high E2 cycles are likely to also feature high progesterone levels, while low E2 cycles tend to have low progesterone levels. Similarly, our results do not address whether other cycle characteristics tend to covary within individual women across multiple cycles.
Because we measured E2 concentrations only in samples collected on reverse cycle days −5 to −24 (i.e. from 5 to 24 days before the start of the next menstrual bleeding), our E2 indices may be inaccurate for any women with extremely long cycles. Although the recruitment criteria generally excluded women with atypical cycles, in practice, ∼5% of subjects had cycles longer than 35 days. In a 35-day cycle, for instance, by assaying E2 concentrations only in Days −5 to −24 of the cycle, our calculated E2 indices are artificially truncated, omitting concentrations in the early follicular phase. In contrast, in women with shorter cycles, nearly the entire follicular phase would be captured in our E2 indices. Similarly, given that progesterone concentrations were only assayed in samples from reverse cycle days −1 to −14 (i.e. from 1 to 14 days before the start of the next menstrual bleeding), in women with extremely long luteal phases, the progesterone indices might not capture the earliest days of the luteal phase. For several reasons, however, we believe that this potential error is unlikely to affect our results. First, we conducted a sensitivity analysis (not shown) restricting the analyses to subjects with cycle lengths ranging from 24 to 34 days and found that although there were slight differences in the exact factor loadings, the patterns and relationships that emerged were unchanged from those found using the whole cohort. Secondly, the fact that ovarian hormones and cycle phase lengths load on different factors in our analysis suggests the two are largely independent of one another. If there were significant confounding of these variables, there would have been strong loadings of ovarian hormone and cycle length characteristics on the same factors, which there was not. Ultimately, if there were bias due to improper calculation of hormone indices in long cycles, it would be for cycles with longer follicular phases to have higher average E2 levels (since it would be the early follicular levels, which are typically low, that were omitted from the calculated indices), and we do not see any evidence of that. Any bias in progesterone levels due to cycle length would be similar, but there is strong evidence from many studies, including these data, that variation in the luteal phase length is minimal (Matsumoto et al., 1962; Vollman, 1977). Thus, we suggest that any bias in this regard is negligible.
Another limitation is our subject population, which was specifically recruited to be ages 25–35 and self-identifying as having regular cycles. Our population's cycle length and cycle phase lengths were typical of healthy women in this age range; however, we cannot necessarily extrapolate our findings to address this question in other groups of women (Treloar et al., 1967; Chiazze et al., 1968; Vollman, 1977). In particular, women with less typical cycles (who would have been excluded from participation in the current study) might show different patterns of cycle hormones and characteristics, as might the 14 subjects whose hormonal profiles did not allow us to readily identify an E2 drop day (and hence were excluded from analysis). Whether these results also apply to younger and older women (whose cycles may tend to be more erratic and have lower hormone levels) remains unknown (Treloar et al., 1967; Chiazze et al., 1968; Lipson and Ellison, 1992). Further research is also needed to determine whether these patterns hold true in non-western populations in which the level of ovarian function (as evidenced by E2 and progesterone concentrations) is typically lower (Ellison et al., 1993). Given the results of migrant studies (Nunez-de la Mora et al., 2007; Nunez-De La Mora et al., 2008), it may be of particular interest to examine women whose current environment differs radically from the environment in which they were born and raised. Perhaps under such conditions, there will be even weaker relationships across domains, with different cycle components reflecting developmental and current conditions.
Finally, our findings on the relative independence of gonadotrophin concentrations from other measures of cycle quality should be interpreted with caution. As discussed, one possibility is that although serum gonadotrophin concentrations may not be directly associated with ovarian steroid concentrations or cycle length characteristics, other indicators of gonadotrophin activity (such as ovarian receptor densities) may be. It is also possible, however, that because gonadotrophins are released in approximately hourly pulses (Kazer et al., 1987; Moret et al., 2009), our measurement techniques (based on single serum samples at three points in the cycle) may have been too imprecise to capture circulating concentrations and have resulted in additional ‘noise' in our data. Gonadotropin concentrations would be better quantified by repeated blood sampling at short intervals (approximately 5 min) during an extended time period followed by pulse detection analysis (Moret et al., 2009). Even with that improved methodology, however, we would not be able to address whether the serum gonadotropin concentrations reflected the concentrations in the follicle, which are ultimately of greatest relevance and interest.
In conclusion, this study is the first to directly address the extent to which multiple components of the menstrual cycle and ovarian function are inter-related in healthy, cycling, western women. We have found that there is a significant degree of coordination of ovarian steroid production across the cycle; however, E2 and progesterone production also show considerable independence from one another. We have determined, furthermore, that across women, circulating gonadotropin concentrations and cycle length characteristics are almost entirely unrelated to ovarian steroid concentrations, suggesting that these aspects of cycle quality are independent of one another. Contrary to the textbook depiction of the menstrual cycle, cycle quality is not uniform across measures. Even in healthy, cycling women, different components of the cycle (ovarian steroids, gonadotropins and cycle phase lengths) do not necessarily covary in a straightforward, predictable manner. Future research may look at not only how these measures of ovarian function are related to (or independent of) one another within women, but also how additional aspects of ovarian function, such as follicular development, follicular gonadotropin levels or endometrial proliferation fit into this complex system.
Authors' roles
E.B. analyzed and interpreted the data and drafted the manuscript. P.E. directed hormone analyses in the study and helped with data interpretations and manuscript drafting. S.L. conducted the hormone analyses in the study and edited the manuscript for intellectual content. A.F. helped to design the study, implement it and critically reviewed the manuscript. I.T. designed the study, directed implementation and data collection and critically reviewed the manuscript.
Funding
The EBBA-I study was supported by a grant from the Norwegian Cancer Society (49 258, 05087); Foundation for the Norwegian Health and Rehabilitation Organizations (59010-2000/2001/2002); Aakre Foundation (5695-2000, 5754-2002), and Health Region East. The current analyses were completed under funding from the National Institutes of Health (K12 ES019852). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
None declared.
Acknowledgements
We thank the Norwegian EBBA-I study subjects, as well as our research staff, Gunn Knudsen, Anna Kirsti Jenssen and Sissel Andersen. In addition, we thank Lauren Parlett for her assistance in preparing graphics.
References
- Cabral ZA, de Medeiros SF. Follicular growth pattern in normal-cycling Brazilian adolescents. Fertil Steril. 2007;88:1625–1631. doi: 10.1016/j.fertnstert.2007.01.127. doi:10.1016/j.fertnstert.2007.01.127. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. J Endocrinol. 2005;186:77–84. doi: 10.1677/joe.1.06025. doi:10.1677/joe.1.06025. [DOI] [PubMed] [Google Scholar]
- Chiazze L, Jr, Brayer FT, Macisco JJ, Jr, Parker MP, Duffy BJ. The length and variability of the human menstrual cycle. JAMA. 1968;203:377–380. doi:10.1001/jama.1968.03140060001001. [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. doi:10.1210/jc.83.12.4220. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Bergeron C, Cornel C, Medalie DA, Massai MR, Milgrom E, Frydman R, Bouchard P. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74:322–331. doi: 10.1210/jcem.74.2.1730810. doi:10.1210/jc.74.2.322. [DOI] [PubMed] [Google Scholar]
- DeCoster J. Overview of factor analysis. 1998. http://www.stat-help.com/notes.html .
- DiZerega GS, Hodgen GD. Luteal phase dysfunction infertility: a sequel to aberrant folliculogenesis. Fertil Steril. 1981;35:489–499. doi: 10.1016/s0015-0282(16)45488-9. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Human ovarian function and reproductive ecology: new hypotheses. Am Anthropol. 1990;92:933–952. doi:10.1525/aa.1990.92.4.02a00050. [Google Scholar]
- Ellison PT, Panter-Brick C, Lipson SF, O'Rourke MT. The ecological context of human ovarian function. Hum Reprod. 1993;8:2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]
- Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35:376–384. doi: 10.1111/j.1552-6909.2006.00051.x. doi:10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, Ellison PT, Thune I. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol Biomarkers Prev. 2005;14:33–40. [PubMed] [Google Scholar]
- Gann PH, Giovanazzi S, Van Horn L, Branning A, Chatterton RT., Jr Saliva as a medium for investigating intra- and interindividual differences in sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10:59–64. [PubMed] [Google Scholar]
- Goodman AL, Nixon WE, Johnson DK, Hodgen GD. Regulation of folliculogenesis in the cycling rhesus monkey: selection of the dominant follicle. Endocrinology. 1977;100:155–161. doi: 10.1210/endo-100-1-155. doi:10.1210/endo-100-1-155. [DOI] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology. Philadelphia, PA: Elsevier Saunders; 2004. pp. 195–211. [Google Scholar]
- Harlow SD, Baird DD, Weinberg CR, Wilcox AJ. Urinary oestrogen patterns in long follicular phases. Hum Reprod. 2000;15:11–16. doi: 10.1093/humrep/15.1.11. doi:10.1093/humrep/15.1.11. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, van Wezel IL, Mussard ML, Kinder JE, Rodgers RJ. Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction. 2001;122:761–775. doi:10.1530/rep.0.1220761. [PubMed] [Google Scholar]
- Johnson MH, Everitt BJ. Essential Reproduction. 5th edn. Oxford, UK: Blackwell Science; 2000. [Google Scholar]
- Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23:187–200. doi:10.1007/BF02289233. [Google Scholar]
- Kazer RR, Kessel B, Yen SS. Circulating luteinizing hormone pulse frequency in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1987;65:233–236. doi: 10.1210/jcem-65-2-233. doi:10.1210/jcem-65-2-233. [DOI] [PubMed] [Google Scholar]
- Lahlou N, Chabbert-Buffet N, Christin-Maitre S, Le Nestour E, Roger M, Bouchard P. Main inhibitor of follicle stimulating hormone in the luteal-follicular transition: inhibin A, oestradiol, or inhibin B? Hum Reprod. 1999;14:1190–1193. doi: 10.1093/humrep/14.5.1190. doi:10.1093/humrep/14.5.1190. [DOI] [PubMed] [Google Scholar]
- Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Wang CF, Yen SS. The effects of estrogen and progesterone on the functional capacity of the gonadotrophs. J Clin Endocrinol Metab. 1975;41:820–826. doi: 10.1210/jcem-41-5-820. doi:10.1210/jcem-41-5-820. [DOI] [PubMed] [Google Scholar]
- Lipson SF, Ellison PT. Development of protocols for the application of salivary steroid analyses to field conditions. Am J Hum Biol. 1989:249–255. doi: 10.1002/ajhb.1310010304. doi:10.1002/ajhb.1310010304. [DOI] [PubMed] [Google Scholar]
- Lipson SF, Ellison PT. Normative study of age variation in salivary progesterone profiles. J Biosoc Sci. 1992;24:233–244. doi: 10.1017/s0021932000019751. [DOI] [PubMed] [Google Scholar]
- Lipson SF, Ellison PT. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod. 1996;11:2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. doi:10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160:131–140. doi: 10.1093/aje/kwh188. doi:10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Multivariate Statistical Methods: A Primer. 3rd edn. Boca Raton, FL: Chapman and Hall/CRC; 2005. [Google Scholar]
- Matsumoto S, Nogami Y, Ohkuri S. Statistical studies on menstruation: a criticism on the definition of normal menstruation. Gunma J Med Sci. 1962;11:294–318. [Google Scholar]
- Matthews KA, Santoro N, Lasley B, Chang Y, Crawford S, Pasternak RC, Sutton-Tyrrell K, Sowers M. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789–1795. doi: 10.1210/jc.2005-1057. doi:10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- Moret M, Stettler R, Rodieux F, Gaillard RC, Waeber G, Wirthner D, Giusti V, Tappy L, Pralong FP. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology. 2009;89:131–139. doi: 10.1159/000160911. doi:10.1159/000160911. [DOI] [PubMed] [Google Scholar]
- Nunez-de la Mora A, Chatterton RT, Choudhury OA, Napolitano DA, Bentley GR. Childhood conditions influence adult progesterone levels. PLoS Med. 2007;4:e167. doi: 10.1371/journal.pmed.0040167. doi:10.1371/journal.pmed.0040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-De La Mora A, Bentley GR, Choudhury OA, Napolitano DA, Chatterton RT. The impact of developmental conditions on adult salivary estradiol levels: why this differs from progesterone? Am J Hum Biol. 2008;20:2–14. doi: 10.1002/ajhb.20698. doi:10.1002/ajhb.20698. [DOI] [PubMed] [Google Scholar]
- O'Rourke MT, Ellison PT. Salivary estradiol levels decrease with age in healthy, regularly-cycling women. Endocr J. 1993;1:487–494. [Google Scholar]
- Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. doi:10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the Inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. doi:10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Hale GE, Jolley D, Fraser IS, Hughes CL, Burger HG. Interrelationships between ovarian and pituitary hormones in ovulatory menstrual cycles across reproductive age. J Clin Endocrinol Metab. 2009;94:138–144. doi: 10.1210/jc.2008-1684. doi:10.1210/jc.2008-1684. [DOI] [PubMed] [Google Scholar]
- Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. doi:10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- Strauss JF, Williams CJ. The ovarian life cycle. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia, PA: Elsevier Saunders; 2004. pp. 213–254. [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- Venners SA, Liu X, Perry MJ, Korrick SA, Li Z, Yang F, Yang J, Lasley BL, Xu X, Wang X. Urinary estrogen and progesterone metabolite concentrations in menstrual cycles of fertile women with non-conception, early pregnancy loss or clinical pregnancy. Hum Reprod. 2006;21:2272–2280. doi: 10.1093/humrep/del187. doi:10.1093/humrep/del187. [DOI] [PubMed] [Google Scholar]
- Vollman RF. The Menstrual Cycle. Philadelphia: Saunders; 1977. [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147:1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. doi:10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- Windham GC, Elkin E, Fenster L, Waller K, Anderson M, Mitchell PR, Lasley B, Swan SH. Ovarian hormones in premenopausal women: variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology. 2002;13:675–684. doi: 10.1097/00001648-200211000-00012. doi:10.1097/00001648-200211000-00012. [DOI] [PubMed] [Google Scholar]


