Abstract
Objective:
The aim of this 6-month prospective randomized clinical study was to compare the effectiveness of autogenous cortical bone (ACB) and bioactive glass (BG) grafting for the regenerative treatment of intraosseous periodontal defects.
Methods:
Via a split-mouth design, 15 chronic periodontitis patients (7 men, 8 women; mean age, 43.47 ± 1.45 years) who had probing pocket depths (PPDs) of ⩾6 mm following initial periodontal therapy were randomly assigned to receive 2 treatments in contralateral areas of the dentition: ACB grafting and BG grafting. The parameters compared in the patients were preoperative and 6-month postoperative PPDs, clinical attachment levels (CALs), and radiographic alveolar bone heights.
Results:
Both treatment modalities resulted in significant changes in postoperative measurements when compared to preoperative values (p < 0.01). PPDs were decreased, CALs were increased, and radiographic alveolar bone heights were increased by 5.00 ± 0.28, 4.60 ± 0.21, and 5.80 ± 0.43 mm in patients treated with ACB grafting and 5.13 ± 0.32, 4.67 ± 0.27, and 5.33 ± 0.36 mm in patients treated with BG grafting, respectively. Differences between the treatments were not statistically significant (P>.05).
Conclusions:
Within the limitations of this study, both ACB and BG grafting led to significant improvements in clinical and radiographic parameters 6 months postoperatively. These results suggest that either an ACB graft, which is completely safe with no associated concerns about disease transmission and immunogenic reactions, or a BG graft, which has an unlimited supply, can be selected for regenerative periodontal treatment.
Keywords: Bone graft, bioactive glass, periodontal defect, periodontal regeneration
INTRODUCTION
Periodontitis, an oral infectious disease, is characterized by clinical attachment loss, alveolar bone resorption, periodontal pocket formation, and gingival inflammation.1,2 One of the main objectives of periodontal therapy is regeneration of the tooth’s supporting periodontal tissues (i.e., the formation of new periodontal ligament, cementum with periodontal ligament fiber insertion, and bone) to their original levels, before they are lost due to periodontal disease.3
Although nonsurgical and conventional surgical periodontal therapy usually results in successful clinical outcomes, healing following these approaches is characterized by a long junctional epithelium.4 Bone grafting, widely used in reconstructive periodontal surgery, is a technique used to fill periodontal defects and enable regeneration of periodontal tissue.5,6 Bone grafting procedures with autogenous bone grafts, allografts, xenografts, and alloplasts are used to promote periodontal regeneration.7 Among the different graft materials available, autogenous bone remains the gold standard for osseous regeneration.7–9 Autogenous bone has osteogenic potential, as it contains cells that participate in osteogenesis.5,7 Rapid revascularization occurs around autogenous bone graft particles, and the graft can release growth and differentiation factors.7,10 Although autogenous bone grafts present some disadvantages, such as the need for secondary surgical sites and the resulting additional surgical morbidity, these can be minimized by using intraorally harvested bone.8,11 However, the use of this graft material is limited by the restricted number of donor sites in the oral cavity when carrying out extensive grafting.7,11
Bioactive glass (BG) is an alloplastic synthetic bone grafting material composed of calcium and sodium ions, phosphate, and silicon dioxide.12–16 BG is biocompatible and easy to use, with osteoconductive and osteostimulatory effects and an optimal pore size for vascularization.11,12,15,17 When BG comes into contact with tissue fluids, a series of chemical reactions occur, resulting in the formation of a hydroxycarbonate apatite (HCA) layer on the surface of the graft particles. Organic ground substance proteins such as glycosaminoglycans are incorporated into the HCA as it forms. Osteoblasts are attracted to the HCA layer and release organic constituents; this is followed by mineralization. Therefore, BG is an effective material for the treatment of periodontal bone defects.12,18–20 The use of BG for treating periodontal bone defects has produced satisfactory clinical and radiographic results.12,16,18,21,22 However, histologic evaluation of teeth treated with BG in humans has indicated epithelial downgrowth with minimal bone regeneration and no signs of new cementum or periodontal ligament formation.23,24 Immunohistochemical analysis in gingival epithelium has demonstrated increased epithelial cell proliferation after treatment with BG, when compared to treatment with bioabsorbable membrane. Overall, the literature indicates that these materials may provide osteoconduction but not necessarily periodontal regeneration.25
Data from both clinical and histological studies suggest that periodontal regeneration occurs following treatment with autogenous bone grafts.12,26–29 Interestingly, an autogenous cortical bone (ACB) graft sourced from the surgical site adjacent to the intraosseous defect is advantageous, as it prevents the need for a second surgical site while treating intraosseous periodontal defects. No clinical data are available on the comparison of ACB with BG in regenerative periodontal treatment. The aim of this 6-month prospective randomized clinical study was to compare the effectiveness of ACB and BG grafting for the regenerative treatment of intraosseous periodontal defects via a split-mouth design.
MATERIALS AND METHODS
Experimental Design
Two different approaches to the treatment of intraosseous periodontal defects were compared by using a split-mouth, randomized, controlled design. The same surgical procedures were performed in all patients: the application of either ACB or BG grafting materials was the only difference between the groups. Clinical and radiographic outcomes were measured on the day of surgery and 6 months postoperatively.
Study Population
Patients with chronic periodontitis exhibiting radiographic evidence of bone loss were recruited for the study. For inclusion, the subjects had to have similar interproximal osseous defects without furcation involvement in each of the contra-lateral quadrants, including the premolars and molars. The exclusion criteria were insufficient dental hygiene characterized by a plaque index,27 systemic diseases (i.e., diabetes mellitus, cancer, HIV, bone metabolic diseases, or disorders that compromise wound healing), chronic high-dose steroid therapy, radiation or immunosuppressive therapy, allergy or sensitivity to any drug, pregnancy, lactation, and smoking. The subjects had no history of drug therapy for at least 6 months before recruitment to the study.
The enrolled patients signed an informed consent form after receiving information about the study. The study protocol and consent forms were approved by the University Institutional Review Board.
Initial Periodontal Therapy
Initial periodontal therapy in all patients consisted of oral hygiene instruction, full-mouth scaling and root planing, and occlusal adjustments if necessary. Four to 6 weeks following the completion of this therapy, a periodontal reevaluation was performed to determine the patient’s response to the therapy and confirm the need for periodontal surgery. Furthermore, the following selection criteria had to be met: (1) probing pocket depth (PPD), ⩾6 mm; (2) radiographic and intrasurgical osseous defect depth, ⩾4 mm; (3) 2 or 3 osseous walls; and (4) no previous prosthetic restoration or endodontic treatment on the related tooth.
Via a split-mouth design, 15 paired interproximal intrabony defects were randomly treated with either ACB or BG grafting. Randomization was carried out in each case during surgical treatment and before allocation of the graft materials by a coin toss.
Clinical and Radiographic Measurements
The PPD and clinical attachment level (CAL) were measured and plaque index (PI)30 and gingival index (GI)31 scores were recorded immediately before surgery and 6 months postoperatively, by using a Florida Probe (Florida Probe Corp., Gainesville, FL, USA). PPD was measured as the distance from the gingival margin to the base of the periodontal pocket. CAL was recorded by combining the distance from the cemento-enamel junction (CEJ) to the gingival margin with probing depth. Measurements were made in 6 areas per tooth: mesiobuccal, distobuccal, midbuccal, mesiolingual, distolingual, and midlingual. Radiographic examinations were carried out prior to surgery and 6 months postoperatively. Standardized radiographs were obtained by using the parallel technique with a customized film-holder.32,33 The linear alveolar bone level, between the CEJ and the most apical alveolar bone, was determined by using millimeter-scale paper.34,35 All clinical and radiographic measurements were performed by the same investigator, who was blinded with respect to treatment modality. Prior to actual measurement, 10 subjects were randomly selected and used to calibrate the investigator. The investigator evaluated the subjects on 2 separate occasions, 48 hours apart. Calibration of the investigator was accepted if measurements at baseline and 48 hours were > 90% similar at the millimeter level.
Surgical Procedure
All surgical procedures were performed on an outpatient basis by 2 experienced periodontal clinicians, using aseptic conditions and under local anesthesia. The same clinician performed all surgical procedures and the other assisted during the procedures.
Following local anesthesia, buccal and lingual intracrevicular incisions were made, and full-thickness mucoperiosteal flaps were raised. All granulation tissues were removed from the defects, and the roots were thoroughly scaled and planed using hand and ultrasonic instruments. The surgical sites were then rinsed with sterile saline.
During surgery, the depth of the intrabony defect was determined as the distance from the alveolar bone crest to the bottom of the defect. This was the distance between the CEJ and the bottom of the osseous defect minus the distance between the CEJ and the most coronal extension of the alveolar bone crest.36
An adequate amount of particulate cortical bone was harvested from the buccal cortical plate adjacent to the intraosseous defect using a bone scraper and implanted into the intrabony defect. PerioGlas (US Biomaterials Corp., Alachua, FL, USA) was used as a BG graft material in the BG grafting group. PerioGlas was mixed with sterile saline to form a paste according to the manufacturer’s instructions and inserted into the intrabony defects. The flaps were repositioned and secured with 4-0 silk suture material by using the interrupted and vertical mattress suturing technique to achieve primary closure.
Postoperative Care
The patients were prescribed amoxicillin plus clavulanic acid (2 g/day for 7 days), flurbiprofen (200 mg/day for 3 days), and a 0.2% chlorhexidine gluconate mouth rinse (twice a day for 6 weeks). Mechanical tooth cleaning was not allowed in the surgical area for the first 6 weeks postoperatively. The sutures were removed 1 week after surgery. Recall appointments for supragingival professional tooth cleaning and oral hygiene reinforcement were scheduled every other week during the first 2 months after surgery, and once a month for the rest of the study period.
Statistical Analysis
Statistical analysis was performed using a commercially available software program (SPSS version 13.0, SPSS, Chicago, IL, USA). For the statistical analysis of clinical and radiographic data, only the recordings representing the deepest site in each defect were used. The Shapiro-Wilk test was used to investigate whether the data were normally distributed or not. The Wilcoxon signed-ranks test was used for intragroup and intergroup comparisons.
Power analysis indicated that 15 defects for each treatment modality would be sufficient to demonstrate statistical significance at the P<.05 level with a power of (at least) ⩾ 80%. The data are shown as mean ± standard deviation or median (range).
RESULTS
Fifteen patients (7 men and 8 women) aged 43.47 ± 1.45 years (37–55 years) with 30 intraosseous defects were treated. The radiographic defect angles were intermediate in both the ACB graft-treated group (30.00 ± 0.46 degrees) and the BG graft-treated group (29.83 ± 0.44 degrees). An analysis of the defect characteristics at baseline revealed no significant differences between the treatment modalities (P>.05), as summarized in Table 1.
Table 1.
Preoperative characteristics of intraosseous defects.
| ACB* | BG* | |
|---|---|---|
| Upper/lower teeth | 7/5 | 6/6 |
| Premolar/molar teeth | 6/6 | 5/7 |
| Defect wall component (2-wall/3-wall) | 9/3 | 9/3 |
| Depth of the intrabony defect (mm) | 5.20±0.22 | 5.07±0.23 |
| Defect angle (°) | 30.00±0.46 | 29.83±0.44 |
Depths of the intrabony defect and defect angles are expressed as the means ± standard error of means.
ACB: autogenous cortical bone BG: bioactive glass
No significant difference between the values of the groups (P>.05)
All the surgical sites healed uneventfully. Neither allergic reactions nor suppuration or abscess formation was observed at any surgical site. No teeth were extracted during the course of the study.
The PI and GI scores at baseline and 6 months are shown in Table 2. At 6 months postoperatively, GI scores were decreased significantly when compared to preoperative data in both groups (P<.01), but PI scores were not different from baseline values (P>.05). Intergroup comparisons of preoperative and postoperative data showed no significant differences between the groups (P>.05).
Table 2.
Plaque index and gingival index scores of intraosseous defects.
| Preoperative | Postoperative 6 months | Significance (P value) | |
|---|---|---|---|
| Plaque Index* | |||
| ACB | 0.52±0.02 | 0.49±0.02 | |
| 0.50 (0.40–0.60) | 0.50 (0.40–0.60) | >0,05 | |
| BG | 0.56±0.02 | 0.52±0.02 | |
| 0.60 (0.40–0.60) | 0.50 (0.40–0.60) | >0,05 | |
| Gingival Index* | |||
| ACB | 1.14±0.03 | 0.36±0.02 | |
| 1.10 (1.00–1.30) | 0.30 (0.20–0.50) | <0,01 | |
| BG | 1.19±0.03 | 0.31±0.03 | |
| 1,20 (1,00–1,30) | 0,30 (0,20–0,50) | <0,01 |
Data are expressed as the means ± standard error of means and medians (minimum-maximum).
ACB: autogenous cortical bone BG: bioactive glass
No significant difference between the values of the groups (P>.05)
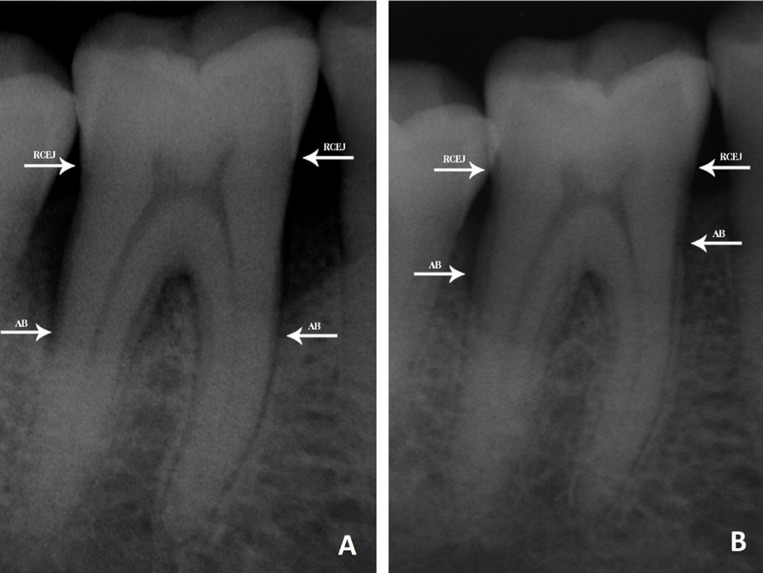
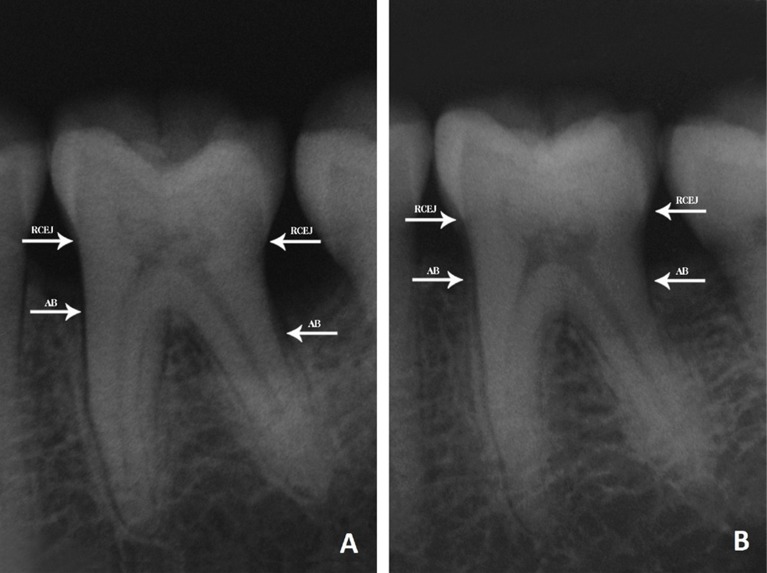
Clinical and radiological findings for the ACB and BG grafting groups at baseline and 6 months are shown in Table 3. Intragroup comparisons showed that there was a significant decrease in the PPDs, and a gain in the CALs and radiographic alveolar bone heights at 6 months postoperatively in both groups, when compared to preoperative findings (P<.01). The changes in PPD were 5.00 ± 0.28 and 5.13 ± 0.32 mm in the ACB and BG grafting groups, respectively. The increases in CAL were 4.60 ± 0.21 mm for the ACB grafting group and 4.67 ± 0.27 mm for the BG grafting group. The preoperative radiographic alveolar bone height was found to be improved by an average of 5.80 ± 0.43 and 5.33 ± 0.36 mm in the ACB and BG grafting groups, respectively (Figures 1 and 2). No statistically significant difference in any of the clinical parameters was observed between the groups (P>.05).
Table 3.
Clinical and radiological findings of intraosseous defects (mm).
| Preoperative | Postoperative 6 months | Significance(P value) | Difference | |
|---|---|---|---|---|
| PPD* | ||||
| ACB | 8.07±0.28 | 3.07±0.18 | 5.00±0.28 | |
| 8.0 (6.0–10.0) | 3.0 (2.0–4.0) | <0.01 | 5.0 (3.0–7.0) | |
| BG | 8.33±0.37 | 3.13±0.17 | 5.13±0.32 | |
| 9.0 (6.0–10.0) | 3.0 (2.0–4.0) | <0.01 | 5.0 (3.0–7.0) | |
| CAL* | ||||
| ACB | 8.80±0.22 | 4.20±0.17 | 4.60±0.21 | |
| 9.0 (8.0–10.0) | 4.0 (3.0–5.0) | <0.01 | 5.0 (3.0–6.0) | |
| BG | 8.93±0.37 | 4.27±0.21 | 4.67±0.27 | |
| 9.0 (6.0–11.0) | 4.0 (3.0–5.0) | <0.01 | 5.0 (3.0–6.0) | |
| REC* | ||||
| ACB | 0.73±0.18 | 1.13±0.19 | −0.40±0.13 | |
| 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | <0.05 | 0.0 (−1.0–0.0) | |
| BG | 0.60±0.19 | 1.13±0.19 | −0.53±0.17 | |
| 0.0 (0.0–2.0) | 1.0 (0.0–2.0) | <0.05 | 0.0 (−2.0–0.0) | |
| RABH* | ||||
| ACB | 9.93±0.63 | 4.13±0.27 | 5.80±0.43 | |
| 10.0 (6.0–15.0) | 4.0 (2.0–6.0) | <0.01 | 6.0 (3.0–9.0 | |
| BG | 9.13±0.45 | 3.80±0.20 | 5.33±0.36 | |
| 9.0 (6.0–12.0) | 4.0 (3.0–5.0) | <0.01 | 5.0 (3.0–8.0) |
Data are expressed as the means ± standard error of means and medians (minimum-maximum).
ACB: Autogenous cortical bone BG: Bioactive glass
PPD: probing pocket depth CAL: clinical attachment level REC: gingival recession RABL: radiological alveolar bone height
No significant difference between the values of the groups (P>.05)
Figure 1.
Radiological appearances of the intraosseous defect.
A. Before treatment of ACB B. After treatment of ACB (RCEJ: Radiographic cemento-enamel junction, AB: Alveolar bone)
Figure 2.
Radiological appearances of the intraosseous defect.
A. Before treatment of BG B. After treatment of BG (RCEJ: Radiographic cemento-enamel junction, AB: Alveolar bone)
DISCUSSION
The objective of this 6-month prospective randomized clinical study was to compare clinical and radiological results after the grafting of ACB and BG in the regenerative treatment of intraosseous periodontal defects, via a split-mouth design. The findings of this study showed that both ACB and BG grafts were significantly effective in the treatment of periodontal defects and provided similar improvements in clinical and radiographic parameters. No statistically significant differences were observed in clinical and radiological parameters between the treatments. In addition, throughout the study, no infection or adverse complications occurred in the ACB and BG treatment groups. New bone formation has been demonstrated histologically in human extraction defects treated with BG after 6 months.37 There is also evidence that almost entirely osteoconductive bone growth occurs after 6 months of BG implantation in dogs.38 Data suggest that the transformation of BG particles and infiltration of bone tissue start at 4 months, and all BG particles disappear via resorption at 16 months following the grafting procedure in humans.39
A reduction in PPD and a gain in CAL are the most important clinical outcomes of regenerative therapy.40 It is well documented that a gain in the CAL after any type of regenerative and conventional periodontal treatment is dependent on the initial pocket depth; that is, the deeper the initial PPD, the greater the PPD reduction and clinical attachment gain.41 There were no differences between the treatment groups in terms of the initial PPD and intrabony defect depth. Gain in the CAL might have resulted from periodontal regeneration via new attachment, or healing characterized by the formation of long junctional epithelium between the new regenerated tissues and the root surface.42 In addition to observing clinical and radiographic results, histological analysis of regenerative periodontal therapy is important. As has been reported, both radiographic interpretations and changes in CAL measurements over time are reliable ways to assess the outcome of intrabony defect treatments.43 In other words, the use of CAL and radiographic evaluation are equally indicative of the outcome of periodontal therapies. When interpreting the findings of the present study, it should be noted that the changes in CAL observed are in agreement with the gain in radiographic alveolar bone height. Moreover, our data indicating a greater significant change in radiographic alveolar bone level than improvement in CAL were in accordance with the literature.44–46 An explanation for this fact might be that measurement of CAL is performed clinically and the alveolar bone level is measured radiographically; smaller changes in CAL are related to the presence of junctional epithelium, which is not seen in radiographs. Radiographic changes in the alveolar bone height may also be used when a reentry procedure is not performed. In the present study, reentry surgery was not performed for ethical reasons and the probability of further alveolar bone loss.32 Instead, a split-mouth design was used in the same patient to ensure that the defects were comparable and had the same healing potential.
Considering the findings of the present study in conjunction with those of previous clinical reports, changes in PPD and CAL were 4.1 ± 1.8 and 3.0 ± 1.447; 4.1 ± 0.2 and 3.2 ± 0.214; and 5.1 ± 0.3 and 4.7 ± 0.3 mm (in the present study), respectively, following BG treatment, and changes in PPD and CAL were 2.8 ± 0.9 and 2.0 ± 0.945; 4.9 ± 1.0 and 4.5 ± 0.844; and 5.0 ± 0.3 and 4.6 ± 0.2 mm (in the present study), respectively, following ACB graft treatment. The results obtained in previous studies may have been influenced by defect characteristics and center and/or operator effects, which depend on differences in the enrolled patients, technical ability, clinical organization, and experience of the clinicians, or a combination of this factors.48
Although root resorption and ankylosis have been reported after the use of autogenous iliac bone grafts in periodontal defects,49 an experimental study has indicated that no significant root resorption and ankylosis were observed after the treatment of periodontal defects with autogenous bone grafts from intraoral sources.6 Similarly, in the present study, neither inflammatory reactions nor root resorption and ankylosis were observed in either treatment group.
Despite the increased number of clinical and experimental studies using ACB grafts for periodontal regenerative therapy in recent years,9,50,51 ACB grafts are reported to be osteoconductive but not osteogenic, since only a few cells survive.9,52 In an experimental study using a dog model with surgically created Class II furcation defects, periodontal healing was similar irrespective of treatment with surgical debridement alone, ACB grafting, or ACB grafting with a calcium sulfate barrier.9 It is important to note that using an ACB graft minimizes additional surgical morbidity, as there is no secondary surgical site.
BG has been demonstrated to be biocompatible, make direct contact with bone, and have an ability to enhance regenerative healing.19,53 Some clinical studies have shown better clinical results with BG compared to the open flap debridement procedure in the treatment of intraosseous defects.32,47 As well as observing clinical and radiological results, histological analysis is necessary to evaluate the type of healing which occurs after treatment. In a histological study, it has been reported that BG grafting has both osteoconductive properties and an osteostimulatory effect.38 Histological analysis of 5 human intrabony defects that were treated with BG confirmed new formation of root cementum and connective tissue attachment at only 1 tooth.23 Although data suggests there is no histological evidence in humans that BG improves periodontal regeneration treatment outcomes54, BG was selected from the available alloplastic synthetic bone grafting materials to treat intraosseous periodontal defects in the current study, due to the results of histological studies and various clinical reports.23,32,38,47
CONCLUSION
Within the limitations of this study, both ACB and BG grafting led to similar improvements in clinical and radiographic parameters 6 months after the treatment of intraosseous periodontal defects. Autogenous bone grafts, a rich source of bone and marrow cells, have been accepted as the gold standard for bone grafting procedures. Autogenous bone is frequently harvested from intra-oral sites, often from the surgical site adjacent to the intraosseous defects. The use of an ACB graft does not require a second surgery site. However, harvesting of intraoral bone is restricted to donor sites that yield comparatively limited graft volume. Thus, in order to overcome this important limitation, autogenous bone can be combined with other types of graft material.
The current study suggests that either an ACB graft, which is completely safe with no concerns associated with disease transmission and immunogenic reactions, or a BG graft, which has an unlimited supply, can be selected for regenerative periodontal treatment.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no financial relationships related to any products involved in this study.
REFERENCES
- 1.Socransky SS, Haffajee AD. The nature of periodontal disease. Ann Periodontol. 1997;2:3–10. doi: 10.1902/annals.1997.2.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Flemmig TF. Periodontitis. Ann Periodontol. 1999;4:32–38. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 3.Polimeni G, Xiropaidis AV, Wikesjö UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 2006;41:30–47. doi: 10.1111/j.1600-0757.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 4.Caton JG, Greenstein G. Factors related to periodontal regeneration. Periodontol 2000. 1993;1:9–15. [PubMed] [Google Scholar]
- 5.Carranza FA, Takei HH, Cochran DL. Reconstructive periodontal surgery. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Clinical periodontology. Vol. 10. St Louis, Missouri, USA: Saunders; 2006. pp. 968–990. [Google Scholar]
- 6.Brunsvold MA, Mellonig JT. Bone grafts and periodontal regeneration. Periodontol 2000. 1993;1:80–91. [PubMed] [Google Scholar]
- 7.Kim CS, Choi SH, Cho KS, Chai JK, Wikesjö UM, Kim CK. Periodontal healing in one-wall intra-bony defects in dogs following implantation of autogenous bone or a coral-derived biomaterial. J Clin Periodontol. 2005;32:583–589. doi: 10.1111/j.1600-051X.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 8.MacNeill SR, Cobb CM, Rapley JW, Glaros AG, Spencer P. In vivo comparison of synthetic osseous graft materials. A preliminary study. J Clin Periodontol. 1999;26:239–245. doi: 10.1034/j.1600-051x.1999.260407.x. [DOI] [PubMed] [Google Scholar]
- 9.Deliberador TM, Nagata MJ, Furlaneto FA, Melo LG, Okamoto T, Sundefeld ML, Fucini SE. Autogenous bone graft with or without a calcium sulfate barrier in the treatment of Class II furcation defects: a histologic and histometric study in dogs. J Periodontol. 2006;77:780–789. doi: 10.1902/jop.2006.050209. [DOI] [PubMed] [Google Scholar]
- 10.Marx RE. Clinical application of bone biology to mandibular and maxillary reconstruction. Clin Plast Surg. 1994;21:377–392. [PubMed] [Google Scholar]
- 11.Chan C, Thompson I, Robinson P, Wilson J, Hench L. Evaluation of Bioglass/dextran composite as a bone graft substitute. Int J Oral Maxillofac Surg. 2002;31:73–77. doi: 10.1054/ijom.2001.0143. [DOI] [PubMed] [Google Scholar]
- 12.Cancian DC, Hochuli-Vieira E, Marcantonio RA, Garcia Júnior IR. Utilization of autogenous bone, bioactive glasses, and calcium phosphate cement in surgical mandibular bone defects in Cebus apella monkeys. Int J Oral Maxillofac Implants. 2004;19:73–79. [PubMed] [Google Scholar]
- 13.Ducheyne P. Bioceramics: Material characteristics versus in vivo behavior. J Biomed Mater Res. 1987;21:219–236. [PubMed] [Google Scholar]
- 14.Froum SJ, Weinberg MA, Tarnow D. Comparison of bioactive glass synthetic bone graft particles and open debridement in the treatment of human periodontal defects. A clinical study. J Periodontol. 1998;69:698–709. doi: 10.1902/jop.1998.69.6.698. [DOI] [PubMed] [Google Scholar]
- 15.Keles GC, Cetinkaya BO, Albayrak D, Koprulu H, Acikgoz G. Comparison of platelet pellet and bioactive glass in periodontal regenerative therapy. Acta Odontol Scand. 2006;64:327–333. doi: 10.1080/00016350600758651. [DOI] [PubMed] [Google Scholar]
- 16.Lovelace TB, Mellonig JT, Meffert RM, Jones AA, Nummikoski PV, Cochran DL. Clinical evaluation of bioactive glass in the treatment of periodontal osseous defects in humans. J Periodontol. 1998;69:1027–1035. doi: 10.1902/jop.1998.69.9.1027. [DOI] [PubMed] [Google Scholar]
- 17.Wilson J, Low SB. Bioactive ceramics for periodontal treatment: comparative studies in the Patus monkey. J Appl Biomater. 1992;3:123–129. doi: 10.1002/jab.770030208. [DOI] [PubMed] [Google Scholar]
- 18.Anderegg CR, Alexander DC, Freidman M. A bioactive glass particulate in the treatment of molar furcation invasions. J Periodontol. 1999;70:384–387. doi: 10.1902/jop.1999.70.4.384. [DOI] [PubMed] [Google Scholar]
- 19.Wilson J, Pigott GH, Schoen FJ, Hench LL. Toxicology and biocompatibility of bioglasses. J Biomed Mater Res. 1981;15:805–817. doi: 10.1002/jbm.820150605. [DOI] [PubMed] [Google Scholar]
- 20.Williams DF. The biocompatibility and clinical uses of calcium phosphate ceramics. In: Williams DF, editor. Biocompatibility of Tissue Analogs. Vol. 2. Boca Raton, FL: CRC Press; 1985. pp. 44–65. [Google Scholar]
- 21.Cancian DCJ, Hochuli-Vieira E, Marcantonio RAC, Marcantonio E., Jr Use of BioGran and Calcitite in bone defects. Histologic study in monkeys (Cebus apella) Int J Oral Maxillofac Implants. 1999;14:859–864. [PubMed] [Google Scholar]
- 22.Ong MMA. Evaluation of a bioactive glass alloplastic in treating periodontal intrabony defects. J Periodontol. 1998;69:1346–1354. doi: 10.1902/jop.1998.69.12.1346. [DOI] [PubMed] [Google Scholar]
- 23.Nevins ML, Camelo M, Nevins M, King CJ, Oringer RJ, Schenk RK, Fiorellini JP. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20:458–467. [PubMed] [Google Scholar]
- 24.Sculean A, Windisch P, Keglevich T, Gera I. Clinical and histologic evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. Int J Periodontics Restorative Dent. 2005;25:139–147. [PubMed] [Google Scholar]
- 25.Cetinkaya BO, Keles GC, Ayas B, Aydin O, Kirtiloglu T, Acikgoz G. Comparison of the proliferative activity in gingival epithelium after surgical treatments of intrabony defects with bioactive glass and bioabsorbable membrane. Clin Oral Investig. 2007;11:61–68. doi: 10.1007/s00784-006-0087-7. [DOI] [PubMed] [Google Scholar]
- 26.Block MS, Kent JN. Long-term radiographic evaluation of hydroxylapatite-augmented mandibular alveolar ridges. J Oral Maxillofac Surg. 1984;42:793–796. doi: 10.1016/0278-2391(84)90347-1. [DOI] [PubMed] [Google Scholar]
- 27.Boyne PJ. Transplantation, implantation and grafts. Dent Clin North Am. 1971;15:433–53. [PubMed] [Google Scholar]
- 28.Brown KLB, Cruess RL. Bone and cartilage transplantation in orthopedic surgery. J Bone Joint Surg. 1982;64:270–279. [PubMed] [Google Scholar]
- 29.Marx RE. Philosophy and particulars of autogenous bone grafting. Oral Maxillofac Surg Clin North Am. 1993;5:599–605. [Google Scholar]
- 30.Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 31.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 32.Zamet JS, Darbar UR, Griffiths GS, Bulman JS, Brägger U, Bürgin W, Newman HN. Particulate bioglass as a grafting material in the treatment of periodontal intrabony defects. J Clin Periodontol. 1997;24:410–418. doi: 10.1111/j.1600-051x.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths GS, Coulthurst SK, Gillett IR, Johnson NW. A film and cassette holder for simultaneous xeroradiography and conventional radiography in longitudinal studies. Br Dent J. 1988;164:365–367. doi: 10.1038/sj.bdj.4806456. [DOI] [PubMed] [Google Scholar]
- 34.Von Wowern N, Westergaard J, Kollerup G. Bone mineral content and bone metabolism in young adults with severe periodontitis. J Clin Periodontol. 2001;28:583–588. doi: 10.1034/j.1600-051x.2001.028006583.x. [DOI] [PubMed] [Google Scholar]
- 35.Oates TW, Graves DT, Cochran DL. Clinical, radiographic and biochemical assessment of IL-1/TNF-a antagonist inhibition of bone loss in experimental periodontitis. J Clin Periodontol. 2002;29:137–143. doi: 10.1034/j.1600-051x.2002.290208.x. [DOI] [PubMed] [Google Scholar]
- 36.Sculean A, Chiantella GC, Windisch P, Arweiler NB, Brecx M, Gera I. Healing of intra-bony defects following treatment with a composite bovine-derived xenograft (Bio-Oss Collagen) in combination with a collagen membrane (Bio-Gide PERIO) J Clin Periodontol. 2005;32:720–724. doi: 10.1111/j.1600-051X.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 37.Norton MR, Wilson J. Dental implants placed in extraction sites implanted with bioactive glass: human histology and clinical outcome. Int J Oral Maxillofac Implants. 2002 Mar-Apr;:249–257. [PubMed] [Google Scholar]
- 38.Schepers EJ, Ducheyne P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: a 1–24 month experiment with several materials and particle sizes and size ranges. J Oral Rehabil. 1997;24:171–181. [PubMed] [Google Scholar]
- 39.Tadjoedin ES, de Lange GL, Holzmann PJ, Kulper L, Burger EH. Histological observations on biopsies harvested following sinus floor elevation using a bioactive glass material of narrow size range. Clin Oral Implants Res. 2000;11:334–44. doi: 10.1034/j.1600-0501.2000.011004334.x. [DOI] [PubMed] [Google Scholar]
- 40.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Aleksic Z, Kenney EB. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J Clin Periodontol. 2003;30:746–751. doi: 10.1034/j.1600-051x.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 41.Cortellini P, Carnevale G, Sanz M, Tonetti MS. Treatment of deep and shallow intrabony defects. A multicenter randomized controlled clinical trial. J Clin Periodontol. 1998;25:981–987. doi: 10.1111/j.1600-051x.1998.tb02402.x. [DOI] [PubMed] [Google Scholar]
- 42.Listgarten MA, Rosenberg MM. Histological study of repair following new attachment procedures in human periodontal lesions. J Periodontol. 1979;50:333–344. doi: 10.1902/jop.1979.50.7.333. [DOI] [PubMed] [Google Scholar]
- 43.Zybutz M, Rapoport D, Laurell L, Persson GR. Comparisons of clinical and radiographic measurements of inter-proximal vertical defects before and 1 year after surgical treatments. J Clin Periodontol. 2000;27:179–186. doi: 10.1034/j.1600-051x.2000.027003179.x. [DOI] [PubMed] [Google Scholar]
- 44.Keles GC, Sumer M, Cetinkaya BO, Tutkun F, Simsek SB. Effect of autogenous cortical bone grafting in conjunction with guided tissue regeneration in the treatment of intraosseous periodontal defects. Eur J Dent. 2010;4:403–411. [PMC free article] [PubMed] [Google Scholar]
- 45.Shirmohammadi A, Chitsazi MT, Lafzi A. A clinical comparison of autogenous bone graft with and without autogenous periodontal ligament graft in the treatment of periodontal intrabony defects. Clin Oral Investig. 2009;13:279–286. doi: 10.1007/s00784-008-0235-3. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–265. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 47.Park JS, Suh JJ, Choi SH, Moon IS, Cho KS, Kim CK, Chai JK. Effects of pretreatment clinical parameters on bioactive glass implantation in intrabony periodontal defects. J Periodontol. 2001;72:730–740. doi: 10.1902/jop.2001.72.6.730. [DOI] [PubMed] [Google Scholar]
- 48.Sanz M, Tonetti MS, Zabalegui I, Sicilia A, Blanco J, Rebelo H, Rasperini G, Merli M, Cortellini P, Suvan JE. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: results from a multicenter practice-based clinical trial. J Periodontol. 2004;75:726–733. doi: 10.1902/jop.2004.75.5.726. [DOI] [PubMed] [Google Scholar]
- 49.Levin MP, Getter L, Cutright DE. A comparison of iliac marrow and biodegradable ceramic in periodontal defects. J Biomed Mater Res. 1975;9:183–195. doi: 10.1002/jbm.820090207. [DOI] [PubMed] [Google Scholar]
- 50.Trombelli L, Annunziata M, Belardo S, Farina R, Scabbia A, Guida L. Autogenous bone graft in conjunction with enamel matrix derivative in the treatment of deep periodontal intra-osseous defects: a report of 13 consecutively treated patients. J Clin Periodontol. 2006;33:69–75. doi: 10.1111/j.1600-051X.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- 51.Guida L, Annunziata M, Belardo S, Farina R, Scabbia A, Trombelli L. Effect of autogenous cortical bone particulate in conjunction with enamel matrix derivative in the treatment of periodontal intraosseous defects. J Periodontol. 2007;78:231–238. doi: 10.1902/jop.2007.060142. [DOI] [PubMed] [Google Scholar]
- 52.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 53.Sculean A, Chiantella GC, Windisch P, Gera I, Reich E. Clinical evaluation of an enamel matrix protein derivative (Emdogain) combined with a bovine-derived xenograft (Bio-Oss) for the treatment of intrabony periodontal defects in humans. Int J Periodontics Restorative Dent. 2002;22:259–267. [PubMed] [Google Scholar]
- 54.Karring T, Lindhe J, Cortellini P. Regenerative periodontal therapy. In: Lindhe J, Karring T, Lang NP, editors. Clinical periodontology and implant dentistry. Vol. 4. Oxford, UK: Wiley-Blackwell Publishing Ltd; 2003. pp. 650–704. [Google Scholar]