Abstract
Objective:
This in-vitro study was done to evaluate the effects of two different seal materials, Duraflor™ and Enamel Pro® Varnish, on enamel demineralization adjacent to orthodontic fixed appliances.
Methods:
Seventy-two extracted solid premolars were allocated to three groups as one control and two study groups after brackets were placed and bonded with Transbond™ XT. The control group received no topical fluoride application after bonding, whereas in the study groups two fluoride varnishes, Enamel Pro® Varnish and Duraflor™ were applied on the teeth adjacent to brackets. All specimens were then immersed separately in demineralization solution for 96 hours at constant temperature. Demineralization of the enamel surface was evaluated quantitatively by cross-sectional microhardness testing: indentations were made at the edge of the bracket base (0 μm) and at 100 and 200 μm distant from it. In all of these positions, 5 indentations were made at 10, 20, 40, 70 and 90 μm of depths from the external surface of the enamel.
Results:
The results revealed that, Enamel Pro® Varnish and Duraflor™ group values are higher than the values of control group at every depth. The differences between the depths showed that the microhardness values decreased significantly when the depth increased. In the control group, more demineralization occurred in every indentation compared to the study group.
Conclusion:
Duraflor™ and Enamel Pro® Varnish can be considered for use in clinic as an effective method to prevent or reduce demineralization during orthodontic treatment, especially in patients with poor oral hygiene.
Keywords: Duraflor™, Enamel Pro® Varnish, demineralization, fluoride varnish, seal material
INTRODUCTION
During orthodontic treatment, demineralization of the enamel adjacent to brackets is a frequent incidence as a consequence of poor oral hygiene. These demineralizations cause white spot lesions on the tooth surfaces of most orthodontic patients, which does not only result in an unaesthetic appearance but also endangers the success of the orthodontic treatment.1,2 The brackets and other fixed orthodontic appliances are pointed out as one of the main cause for these undesirable lesions since they facilitate the bacteria accumulation by providing retentive areas.3 Previous studies2,3 have shown that, in orthodontic patients enamel demineralization could develop only in just one month. At the onset of orthodontic treatment for the patients who lack proper oral hygiene, highly filled sealants can be used during bonding procedure in order to prevent or decrease demineralization.4–6 However, in the absence of good oral hygiene during orthodontic treatment, orthodontists should take additional protective measures to minimize formation of white spot lesions.
Previous studies proved the effectiveness of fluoride regimens like varnish, gel, rinse, and dentifrice,3,7,8 but among these methods the use of fluoride rinses that rely on patient compliance might be insufficient and provide only limited benefit in preventing demineralization.7,8 On the other hand, it has been shown that varnishes provide high concentration of fluoride to decrease enamel demineralization both in vitro and in clinical trials.9–14 They have the advantage of adhering to enamel surface longer than other topical fluoride products, which indicates that their ability to increase fluoride uptake in enamel is better.15 This will provide prevention against demineralization while allowing the clinician to use proven high bond strength composite resins for bonding. Also, the clinical application is easy and their thorough prophylaxis is not required. 16
Duraflor™ is a fluoride varnish that contains 5% sodium fluoride. Its contact with the enamel Allow the formation of calcium fluoride on the tooth surface that prevents demineralization. Besides forming calcium fluoride, it also provides fluoride reservoir on the enamel surface against cariogenic acid attacks over a longer period of time.9,17,18
Enamel Pro® Varnish is another 5% sodium fluoride varnish that additionally contains amorphous calcium phosphate (ACP) formula. Unlike Duraflor™, it delivers ACP to enamel to encourage the formation of hydroxyl-apatite to enhance remineralization and thus prevents the loss of enamel due to demineralization.19
It is likely that applying ACP containing varnishes onto enamel surface around brackets will reduce caries incidence. Sudjalim et al20 found that, using 9000 ppm NaF or caseine phosphopeptide - amorphous calcium phosphate on the bracket base together with Transbond™ XT diminishes enamel demineralization risk.
Recently, some studies pointed out that the composition of caseine phosphopeptide with ACP has more effective anti-cariogenic effect since it stabilizes calcium and phosphate compounds.21,22 Caseine phosphopeptide-amorphous calcium phosphate compound increases the calcium and phosphate level in the saliva exceedingly and thus reduces the caries incidence and enhances the effect of topical fluoride.23,24
The aim of this in-vitro study was to compare the effects of 5% NaF containing Duraflor™ and 5% NaF - ACP containing Enamel Pro® Varnish on enamel demineralization around orthodontic brackets.
MATERIALS AND METHODS
Extracted seventy-two non-carious premolars for orthodontic reasons were used in this study. Their buccal surfaces were intact without any cracks or white spots. After the removal of any remaining soft tissue with a scaler, the teeth were stored in 0.1% thymol solution until use. Before experimental use the enamel surfaces were polished with a nonfluoridated pumice and water, rinsed with deionized water and dried with compressed air.
The buccal surfaces of the teeth were conditioned with 38% phosphoric acid (Etch-Rite; Pulpdent Corporation, Watertown, Massachusetts, USA) for 30 seconds followed by thorough washing and drying. Transbond™ XT (3M/Unitek, Monrovia, CA, USA) primer was applied on the etched enamel and polarized for 20 seconds, and brackets were placed on the middle third of the enamel parallel to the long axis with Transbond™ XT adhesive resin. After removing any residual adhesive around brackets with a dental scaler, the specimens were light-cured for 40 seconds with Mectron Starlight pS LED (Mectron s.p.a., Carasco, Italy). The teeth were then allocated to three groups of twenty four as one control and two study groups. The control group received no topical fluoride application after bonding. In the study groups two fluoride varnishes, Enamel Pro® Varnish (Premier Dental, PA, USA) and Duraflor™ (Medicom, Montreal, Canada), were applied on the teeth adjacent to brackets as recommended by their manufacturers and allowed to dry for 5 minutes.
All specimens were then immersed separately in 2 ml demineralization solution for 96 hours in an incubator at constant temperature of 37°C, with the solution changed every 4 hours. The immersion of the samples in the caries solution for 96 hours represents approximately 3 months of real time.20 The content of the solution used in this study is the same that was used by Gillgrass et al25 The pH of the solution was 4.4 and contained 2.2 mmol/L Ca2+, 2.2 mmol/L PO4-, 50 mmol/L acetic acid. After each caries challenge, in order to simulate mechanical wear of the varnish materials the teeth were brushed manually with a soft-bristled toothbrush (Oral B® ortho brush, Procter & Gamble, Cincinnati, Ohio) for 5 seconds. No further application of the varnishes was done after the initial application in the study groups.
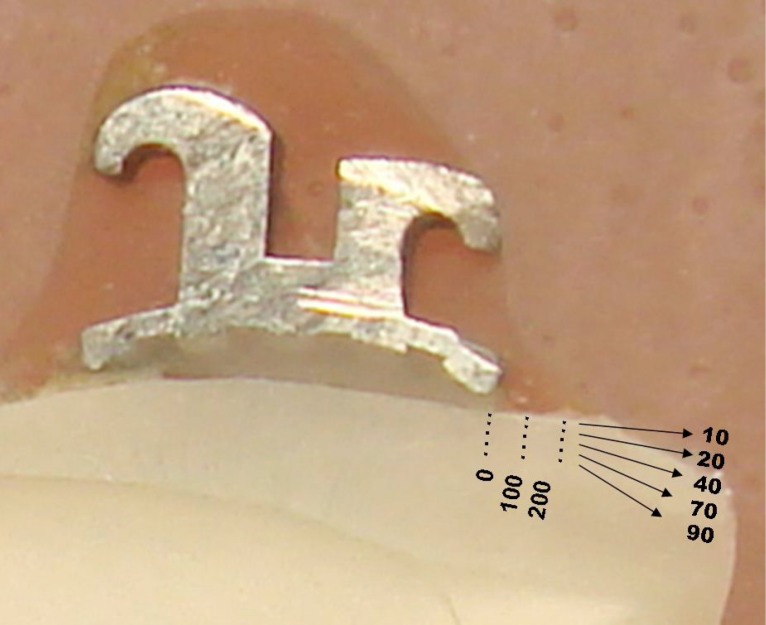
In order to assess the mineral loss on the enamel surface, microhardness test was done after the completion of 96 hours. The crowns were separated from the roots and hemi-sectioned vertically in the buccal-palatal direction, through the center of the bracket base with a 15 HC (large) wafering blade on an Isomet low-speed saw (Buehler®Lake Bluff, llliniois, USA). The half-crown sections were embedded in acrylic resin so that the cut surface was exposed according to the methods reported previously (Figure 1).25,26 Throughout the study, samples were kept in humid conditions to avoid drying.27,28
Figure 1.
Depths and Region
After serially polishing the samples, demineralization lesions were assessed by microhardness profiles across the cut surface with a microhardness tester (Micromet 5114, Buehler®, Lake Bluff, llliniois, USA) fitted with a Vickers diamond. Analyses were done under a 200 g load for 5 seconds as reported by Moura et al26 Fifteen indentations were made in each half crown. Three regions were selected as; edge of the bracket base (0 μm) and at 100 and 200 μm distant to (occlusal side) For per region 5 indentations were made at 10, 20, 40, 70 and 90 μm from the external surface of the enamel (Figure 1).
Statistical calculations were performed with NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA) program for Windows. In each thickness group (10–90 μ) repeated measures of Friedman test was used, Kruskal Wallis test was used in the comparison of groups, post Hoc Dunn’s multiple comparison test was utilized in the comparison of subgroups, Statistical significance level was established at P<.05.
RESULTS
In our study, the statistical differences of microhardness tests that were applied on the buccolingual surfaces of the teeth are given in Table 1. The results show that statistically significant differences were observed between depths in each group except for the control group.
Table 1.
Statistical differences of microhardness tests.
| DuraflorTM | Enamel Pro® Varnish | Control (Transbond™ XT) | |||
|---|---|---|---|---|---|
|
| |||||
| Depth | Median (IQR) | Median (IQR) | Median (IQR) | p | |
| Region 200 μm | 10μ | 373.25 (354.8 – 384.88)*‡ | 361.7 (352.43 – 380.9)* ‡ | 63.2 (56.3 – 70.6) | 0.0001 |
| 20μ | 375.15 (360.35 – 389.85)* ‡ | 337.95 (309.23 – 366.8)* ‡ | 62.6 (57.13 – 70.98) | 0.0001 | |
| 40μ | 346.1 (338.18 – 362.98)* ‡ | 314.8 (296.95 – 356.33)* ‡ | 61.35 (43.08 – 68.1) | 0.0001 | |
| 70μ | 330.4 (325.2 – 348.55)* ‡ | 328.1 (301.1 – 368.58) *‡ | 60.5 (42.85 – 64.3) | 0.0001 | |
| 90μ | 312.1 (311.4 – 323.23)* ‡† | 319.45 (280.73 – 349.85) *‡ | 61.65 (51.1 – 65.1) | 0.0001 | |
| p | 0.0001 | 0.008 | 0.15 | ||
|
| |||||
| Region 100 μm | 10μ | 369.65 (355.88 – 383.68)* ‡ | 352.25 (337.05 – 371.8)* ‡ | 61.1 (40.03 – 67.73) | 0.0001 |
| 20μ | 375.35 (360.68 – 385.63)* ‡ | 330.45 (290.85 – 360.1)* ‡ | 60.45 (51.43 – 68.68) | 0.0001 | |
| 40μ | 357.8 (338.85 – 367.25)* ‡ | 312.45 (281.98 – 340.68)* ‡ | 55.85 (40.75 – 70.7) | 0.0001 | |
| 70μ | 333.1 (327.15 – 350.53)* ‡ | 340.5 (322.35 – 352.5)* ‡ | 59.8 (39.55 – 70.72) | 0.0001 | |
| 90μ | 308.6 (301.8 – 316.43)* ‡† | 339.45 (317.7 – 349.6)* ‡ | 60.8 (37.43 – 70.33) | 0.0001 | |
| p | 0.0001 | 0.024 | 0.708 | ||
|
| |||||
| Region 0 μm (Bracket edges) | 10μ | 390.15 (358.45 – 395.3)* ‡ | 366.9 (343.9 – 384.48)* ‡ | 62.7 (46 – 75.1) | 0.0001 |
| 20μ | 365.25 (346.23 – 381.18) *‡ | 356.45 (301.55 – 385.5)* ‡ | 58.1 (51.85 – 65.98) | 0.0001 | |
| 40μ | 351.45 (320.15 – 371.48)* ‡ | 318.95 (306.5 – 362.98)* ‡ | 58.45 (36.88 – 65.2) | 0.0001 | |
| 70μ | 342.9 (315.08 – 357.6)* ‡ | 327 (286.05 – 341.98)* ‡ | 51.6 (37.73 – 66.55) | 0.0001 | |
| 90μ | 276.3 (261.68 – 283.93)* ‡† | 324.05 (303.3 – 351.85)* ‡ | 49.95 (37.68 – 60.73) | 0.0001 | |
| p | 0.0001 | 0.002 | 0.071 | ||
|
| |||||
| 10μ Depth 200/100/0 R | p | 0.173 | 0.397 | 0.615 | |
| 20μ Depth 200/100/0 R | p | 0.308 | 0.477 | 0.604 | |
| 40μ Depth 200/100/0 R | p | 0.72 | 0.403 | 0.949 | |
| 70μ Depth 200/100/0 R | p | 0.729 | 0.372 | 0.689 | |
| 90μ Depth 200/100/0 R | p | 0.0001 | 0.55 | 0.245 | |
Dunn’s Multiple Test * Control < D&P P<.0001
Dunn’s Multiple Test ‡ D group 10μ> 20,40,70,90μ 20μ> 20,40,70μ P<.05 group 10μ> 20,40,70,90 P<.05
Dunn’s Multiple Test † K0 < K100-K200 P<.0001
In the Enamel Pro® Varnish, Duraflor™ and Control groups at the bracket edges (0 μm) and the regions 100 and 200 μm, statistically significant differences were observed at 10, 20, 40, 70 and 90 μm depths. (P=.0001). Microhardness values of the control group were significantly lower than Enamel Pro® Varnish and DuraflorTM groups (P=.0001).
In the Control group at the bracket edges (0 μm) and the regions 100 and 200 μm, there were no statistically significant differences observed at 10, 20, 40, 70 and 90 μm depths. (P=.150). Enamel Pro® Varnish and Duraflor™ group values were higher than the values of Control (Transbond™ XT) group at every depth.
In the Enamel Pro® Varnish group at the bracket edges (0 μm) and the regions 100 and 200 μm, statistically significant differences were observed between 10, 20, 40, 70 and 90 μm depths. (P=.008). Microhardness values of 10μm depth were significantly higher than 20μm, 40μm, 70μm, 90μm depths (P=.045, P=.01), at the 200 μm region 70μm values were significantly higher than 90μm depth (P=.012). There were no significant differences between other groups (P>.05).
In the Duraflor™ group at the bracket edges (0 μm) and the regions 100 and 200 μm, statistically significant differences were observed between 10, 20, 40, 70 and 90 μm depths. (P=.0001). Microhardness values of 10μm depth were significantly higher than 20μm, 40μm, 70μm, 90μm depths at region 0 μm (P=.041, P=.002), Microhardness values of 20μm depth were significantly higher than 40μm, 70μm, 90μm depths; (P=.002, P=.004) whereas, 40μm values were significantly higher than 70μm and 90μm depths (P=.034, P=.002). At the 90μm depth microhardness values of the bracket edge (0 μm) were found lower than the 100 and 200 μm regions. There were no significant differences among rest of the groups (P>.05).
DISCUSSION
The main causes of enamel demineralization during orthodontic treatment are the mineral content of the enamel, bacterial plaque accumulation and diet of the patient.25 Demineralization may be prevented or reduced by decreasing the effects of these causes. Although the preventive methods like toothpastes and mouth rinses are effective, they had not been entirely successful since they depend on patient compliance.4,29 Therefore, during the last years studies are being made to develop methods that do not need patient compliance. The studies concerning the effects of fluoride varnishes showed that they are much more effective in preventing acid attacks not only due to their high fluoride concentration, but also they have the property of adhering to the enamel surface longer than other topical fluoride products.30 For these fluoride varnishes to be effective, they should be applied by the clinician regularly not only because the high fluoride is enough for preventing decalcification, but also there should be a constant fluoride reservoir in the mouth.9,10,31
Duraflor™ forms calcium fluoride on enamel surface and this supplies a fluoride reserve against acid attacks in the mouth. Thus, it is effective in inhibiting demineralization on enamel surface. According to our results, Duraflor™ group showed less demineralization than control (Trans-bond™ XT) group in all of the depths.
Gorton and Featherstone,2 Sudjalim et al20 Banks and Richmond32 and Schmit et al33 found Transbond™ XT is ineffective in preventing demineralization similar to our findings.
Likewise our study Todd et al9 applied Duraflor™ onto the enamel around orthodontic brackets which were bonded to extracted human teeth and found similar results. It was concluded that the teeth applied with Duraflor™ exhibited 50% less demineralization. Enamel Pro® Varnish, which is another 5% NaF containing varnish, deposits not only fluoride but also ACP (amorphous calcium phosphate) onto the enamel surface. Unlike Duraflor™, it inhibits demineralization by making “amorphous calcium phosphate crystals” and forming “apatite” on enamel surface.
In a study, Schumacher et al34 exhibited that a biologically active material containing ACP might inhibit demineralization by the way of releasing cavity fighting components including calcium and phosphate similar to our findings.
Skrtic et al19 reported that 71% of mineral content of demineralized teeth might be recovered by the use of ACP-filled composite resins, which is similar to our finding for Enamel Pro® Varnish group showed higher microhardness values between all the regions and at all depths when compared to the control group.
Skrtic et al19,35 and Antonucci et al36,37 found out that the ACP-filled polymers were very suitable to release saturated levels of calcium and phosphate ions for the formation of hydroxyapatite crystals, which is very effective both in inhibiting demineralization and enhancing remineralization.
The higher affinity of ACP to fluoride makes it capable of releasing nearly four times as much fluoride to oral environment when compared to the conventional transparent varnishes. Because ACP stabilizes the calcium and phosphate ions in the saliva, it inhibits demineralization of enamel and even dentin.21,22
In our study, no statistically significant difference was found between Enamel Pro® Varnish and Duraflor™ groups (P>.05), whereas, these two study groups showed significantly higher microhardness levels than Control (Transbond™ XT) at all regions and depths. Enamel Pro® Varnish and Duraflor™ had similar effects in inhibiting and preventing demineralization of enamel.
Similar to our findings, Dunn38 concluded that ACP containing materials are very effective in preventing demineralization due to inadequate oral hygiene and microleakage and also in accelerating the remineralization of the decalcified teeth.
Also, Uysal et al39 found that Aegis-Ortho material that contains ACP is effective in preventing demineralization resulting from inadequate oral hygiene and microleakage. However, its bonding strength was found less than Transbond™ XT.
The findings of the present study suggest that both ACP-containing fluoride varnishes and traditional fluoride varnishes are effective in preventing demineralization of the teeth due to poor oral hygiene. Future in vivo studies, examining the efficacy of these varnishes in preventing enamel demineralization, appear warranted.
CONCLUSIONS
With the limitation of this in-vitro study, It is concluded that fluoride-containing varnishes are very effective in both preventing and inhibiting demineralization since they have high fluoride concentration. Duraflor™ (5% NaF) and Enamel Pro® Varnish (5% NaF + ACP) had similar effects for inhibiting and preventing demineralization of enamel.
REFERENCES
- 1.Chang HS, Walsh LJ, Freer T. J. Enamel decalcification during orthodontic treatment - aetiology and prevention. Aust Dent J. 1997;42:322–327. doi: 10.1111/j.1834-7819.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorton J, Featherstone JDB. In vivo inhibition of demineralization around orthodontic brackets. Am J Orthod Dentofac. 2003;123:10–14. doi: 10.1067/mod.2003.47. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly MM, Featherstone JDB. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am J Orthod Dentofac. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Featherstone JDB. Prevention of enamel demineralization: An in vitro study using light-cured filled sealant. Am J Orthod Dentofac. 2005;128:592–600. doi: 10.1016/j.ajodo.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Paschos E, Kleinschrodt T, Luedemann TC, Huth KC, Hickel R, Kunzelmann KH, Janson IR. Effect of different bonding agents on prevention of enamel demineraliation around orthodontic brackets. Am J Orthod Dentofac. 2009;135:603–612. doi: 10.1016/j.ajodo.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T, Dunn WJ, Taloumis LJ. Effect o fluoride varnish on the in vitro bond strength of orthodontic brackets using a self-etching primer system. Am J Orthod Dentofac. 2004;125:351–356. doi: 10.1016/j.ajodo.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Geiger AM, Gerolick L, Gwinnet AJ, Benson BJ. Reducing white spot lesions in orthodontic populations with fluoride rinsing. Am J Orthod Dentofac. 1992;101:403–407. doi: 10.1016/0889-5406(92)70112-N. [DOI] [PubMed] [Google Scholar]
- 8.Geiger AM, Gerolick L, Gwinnet AJ, Griswold PG. The effect of a fluoride program on white spot formation during orthodontic treatment. Am J Orthod Dentofac. 1988;93:29–37. doi: 10.1016/0889-5406(88)90190-4. [DOI] [PubMed] [Google Scholar]
- 9.Todd MA, Staley RN, Kanellis MJ, Donly KJ, Wefel JS. Effect of a fluoride varnish on demineralization adjacent to orthodontic brackets. Am J Orthod Dentofac. 1999;116:159–167. doi: 10.1016/s0889-5406(99)70213-1. [DOI] [PubMed] [Google Scholar]
- 10.Koch G, Petersson LG. Caries preventive effect of a fluoride-containing varnish (Duraphat) after 1 year’s study. Community Dent Oral. 1975;3:262–266. doi: 10.1111/j.1600-0528.1975.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Bruyn H, Arends J. Fluoride varnishes: a review. J Biol Buccale. 1987;15:71–82. [PubMed] [Google Scholar]
- 12.Helfenstein U, Steiner M. Fluoride varnishes (Duraphat): a meta-analysis. Community Dent Oral. 1994;22:1–5. doi: 10.1111/j.1600-0528.1994.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 13.Seppa L, Leppanen T, Hausen H. Fluoride varnish versus acidulated phosphate fluoride gel: a 3-year clinical trial. Caries Res. 1995;29:327–330. doi: 10.1159/000262086. [DOI] [PubMed] [Google Scholar]
- 14.Holm AK. Effect of a fluoride varnish (Duraphat) in pre-school children. Community Dent Oral. 1979;7:241–245. doi: 10.1111/j.1600-0528.1979.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 15.Arends J, Lodding A, Petersson LG. Fluoride uptake in enamel: in vitro comparison of topical agents. Caries Res. 1980;14:403–413. doi: 10.1159/000260483. [DOI] [PubMed] [Google Scholar]
- 16.Seppa L. Effect of dental plaque on fluoride uptake by enamel from a sodium fluoride varnish in vivo. Caries Res. 1983;17:71–75. doi: 10.1159/000260651. [DOI] [PubMed] [Google Scholar]
- 17.Brudevold F, McCann HG, Nilsson R, Richardson B, Coklica V. The chemistry of caries inhibition problems and challenges in topical treatment. J Dent Res. 1967;46:37–34. doi: 10.1177/00220345670460013801. [DOI] [PubMed] [Google Scholar]
- 18.Retief DH, Sorvas PG, Bradley EL, Taylor RE, Walker AR. In vitro fluoride uptake, distribution and retention by human enamel after 1- and 24-hour application of various topical fluoride agents. J Dent Res. 1980;59:573–582. doi: 10.1177/00220345800590030401. [DOI] [PubMed] [Google Scholar]
- 19.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 20.Sudjalim TR, Woods MG, Manton DJ, Reynolds EC. Prevention of demineralization around orthodontic brackets: in vitro. Am J Orthod Dentofac. 2007;131:705,e1–705e9. doi: 10.1016/j.ajodo.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–1595. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, Reynolds C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–348. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 24.Rose RK. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 2000;45:569–575. doi: 10.1016/s0003-9969(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 25.Gillgrass TJ, Creanor SL, Foye RH, Millett DT. Varnish or polymeric coating for the prevention of demineralization? An ex vivo study. J Orthod. 2000;28:291–295. doi: 10.1093/ortho/28.4.291. [DOI] [PubMed] [Google Scholar]
- 26.Moura MS, de Melo Simplício AH, Cury JA. In-vivo effects of fluoridated antiplaque dentifrice and bonding material on enamel demineralization adjacent to orthodontic appliances. Am J Orthod Dentofacial Orthop. 2006;130:357–363. doi: 10.1016/j.ajodo.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 27.White JM, Featherstone JDB. A longitidunal microhardness analysis of fluoride dentifrice effects on lesion progression in vitro. Caries Res. 1987;21:502–512. doi: 10.1159/000261059. [DOI] [PubMed] [Google Scholar]
- 28.Featherstone JDB, Ten Cate JM, Shariati M, Arends J. Comparison of artificial caries like lesions by quantitive microrasiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 29.Grobler SR, Du Toit IJ, Basson NJ. The effect of honey on human tooth enamel in vitro observed by electron microscopy and microhardness measurements. Arch Oral Biol. 1994;39:147–153. doi: 10.1016/0003-9969(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 30.Herkströter FM, Witjes M, Ruben J, Arends J. Time dependency of microhardness indentations in human and bovine dentine compared with human enamel. Caries Res. 1989;23:342–344. doi: 10.1159/000261203. [DOI] [PubMed] [Google Scholar]
- 31.Staudt CB, Lussi A, Jacquet J, Kiliaridis S. White spot lesions around brackets: in vitro detection by laser fluorescence. Eur J Oral Sci. 2004;112:237–243. doi: 10.1111/j.1600-0722.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 32.Banks PA, Richmond S. Enamel sealants: a clinical evaluation of their value during fixed appliance therapy. Eur J Orthod. 1994;16:19–25. doi: 10.1093/ejo/16.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Schmit JL, Staley RN, Wefwl JS, Kanellis M, Jakobsen JR, Keenan PJ. Effect of fluoride varnish on demineralization on adjacent to brackets bonded with RMGI cement. Am J Orthod Dentofac. 2002;122:125–134. doi: 10.1067/mod.2002.126595. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher GE, Antonucci JM, O’Donnell JNR, Skrtic D. The use of amorphous calcium phosphate composites as bioactive basing materials. Their effect on the strength of the composite/adhesive/dentin bond. J Am Dent Assoc. 2007;138:1476–1484. doi: 10.14219/jada.archive.2007.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials. 2004;25:1141–1150. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Antonucci JM, Skrtic D, Eanes ED. Remineralizing dental composites based on amorphous calcium phosphate. Polym Prepr. 1995;36:779–780. [Google Scholar]
- 37.Antonucci JM, Skrtic D, Eanes ED. Bioactive polymeric dental materials based on amorphous calcium phosphate. Polym Prepr. 1994;35:460–461. [Google Scholar]
- 38.Dunn WJ. Shear bond strength of an amorphous calcium-phosphatecontaining orthodontic resin cement. Am J Orthod Dentofac. 2007;131:243–247. doi: 10.1016/j.ajodo.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Uysal T, Ustdal A, Nur M, Catalbaş B. Bond strength of ceramic brackets bonded to enamel with amorphous calcium phosphate-containing orthodontic composite. Eur J Orthod. 2010;32:281–284. doi: 10.1093/ejo/cjp115. [DOI] [PubMed] [Google Scholar]