Abstract
The clinical diagnosis of Parkinson disease (PD) is incorrect in 30% or more of subjects, particularly at the time of symptom onset. Because Lewy-type α-synucleinopathy (LTS) is present in the submandibular glands of PD patients, we assessed the feasibility of submandibular gland biopsy for diagnosing PD. We performed immunohistochemical staining for LTS in sections of large segments (simulating open biopsy) and needle cores of submandibular gland from 128 autopsied and neuropathologically classified subjects, including 28 PD, 5 incidental Lewy body disease, 5 progressive supranuclear palsy ([PSP] 3 with concurrent PD), 3 corticobasal degeneration, 2 multiple system atrophy, 22 Alzheimer disease with Lewy bodies (ADLB), 16 Alzheimer disease without Lewy bodies and 50 normal elderly. Immunoreactive nerve fibers were present in large submandibular gland sections of all 28 PD subjects (including 3 that also had PSP); 3 ADLB subjects were also positive, but none of the other subjects were positive. Cores from frozen submandibular glands taken with 18 gauge needles (total length 15–38 mm, between 10 and 118 sections per subject examined) were positive for LTS in 17 of 19 PD patients. These results suggest that biopsy of the submandibular gland may be a feasible means of improving PD clinical diagnostic accuracy. This would be particularly advantageous for subject selection in early-stage clinical trials, for invasive therapies or for verifying other biomarker studies.
Keywords: α-Synuclein, Biomarker, Clinical trial, Deep brain stimulation, Gene therapy, Lewy body, Parkinson disease, Surgery, Transplantation
INTRODUCTION
Estimates of the accuracy of the clinical diagnosis of Parkinson disease (PD), expressed as sensitivity or positive predictive value, range between 46% and 90% (1–8), with the higher accuracy figures being dependent on prolonged clinical observation and clinical response to levodopa. Because early treatment of PD would presumably be most beneficial, it is also evident that diagnostic inaccuracy is a critical impediment to clinical trials and especially clinical trials at early-stage disease. Additionally, inadequate clinical diagnostic accuracy has also very likely led to misleading biomarker studies because biomarkers that are assessed against the clinical diagnosis by definition cannot improve on clinical diagnostic accuracy. Even more importantly, for clinical trials and therapies that utilize invasive methods, such as deep brain stimulation, neural transplantation or gene therapy (9–17), misdiagnosis inevitably exposes considerable numbers of non-PD subjects to potentially damaging procedures without a therapeutic benefit. A more accurate clinical diagnosis could greatly reduce these undesirable situations.
Diseases of the CNS are often marked by characteristic histopathology in the peripheral nervous system (PNS), allowing a definitive diagnosis through skin, muscle, rectal or peripheral nerve biopsy (18). This approach has only recently been considered for PD or other movement disorders, with only a very few published studies based on colon and salivary gland biopsy (19–25). Involvement of the colon with Lewy-type synucleinopathy (LTS) appears to be too sparse to allow a high diagnostic sensitivity or positive predictive value, whereas a labial salivary gland study utilized only a few subjects and was inconclusive (24). To identify the most suitable site for peripheral biopsy in PD, we conducted an extensive survey for LTS in the PNS, assessing 41 different sites in 92 subjects (26). In agreement with previous, less extensive studies (27–29), we found that the gastrointestinal tract (GI) was particularly likely to contain LTS in PD subjects. Within the GI tract, there is a rostrocaudal gradient of LTS density, with the lower esophagus having the highest densities and the colon and rectum the lowest. Along with the lower esophagus, we found that the submandibular gland also had high densities of LTS. This has been confirmed by another group that found LTS in the submandibular glands of both PD subjects and subjects with incidental Lewy body disease (ILBD) (30); ILBD is the probable preclinical stage of PD and/or dementia with Lewy bodies (31–33). Because the submandibular gland is located subcutaneously, is easily accessible and is commonly biopsied for neoplasia (34), we hypothesized that it is the most promising biopsy site for PD. Therefore, we performed a feasibility study, using immunohistochemical staining for phosphorylated α-synuclein (25, 35, 36) to demonstrate LTS in both large blocks (simulated open biopsy) and needle cores of the submandibular glands of a set of 131 autopsied PD and non-PD subjects.
MATERIALS AND METHODS
Cases
The study was performed at Banner Sun Health Research Institute (BSHRI), which is part of Banner Health, a non-profit regional healthcare provider centered in Phoenix, Arizona. Banner Sun Health Research Institute and the Mayo Clinic Arizona are the principal members of the Arizona Parkinson's Disease Consortium (www.AZPD.org). Autopsies and neuropathological examinations were performed on elderly subjects who had volunteered for the BSHRI Brain and Body Donation Program; a general description is given in a previous publication (37) (BBDP; www.brainandbodydonationprogram.org). The operations of the BBDP have been approved by Institutional Review Boards. Specific neuropathological diagnostic criteria were used for Alzheimer disease (AD), PD (5), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA) (38). AD cases were given the diagnosis if they were classified as having dementia as well as an “intermediate” or “high” level of both neuritic plaques and neurofibrillary degeneration according to the National Institute on Aging – Reagan Institute criteria (39). AD cases with LTS but not meeting clinicopathological diagnostic criteria for PD or dementia with Lewy bodies (40) were designated as AD with Lewy bodies (ADLB). Brain LTS regional densities were used to classify subjects according to the Unified Staging System for Lewy Body Disorders (36).
Cases were chosen by searching the BBDP database for those with a neuropathological diagnosis of PD or ILBD that had died and had a full autopsy including the submandibular gland. Control groups with submandibular gland were also selected, consisting of cognitively normal elderly subjects and neuropathologically confirmed AD, PSP, CBD and MSA subjects.
Histological Methods
Sections (6-μm thick) were mounted on electrostatically charged glass slides (Fisher catalogue # 12-550-15 4951PLUS-600621), deparaffinized in 5 × 10 minute changes of Neo-Clear (EMD Chemicals, Billerica, MA) and brought to distilled water through 3 × 3 minute changes of 100% ethanol, 2 × 3 minute changes of 95% ethanol and 2 × 30 seconds changes of distilled water. Sections were then treated with 1:100 proteinase K (Enzo Life Sciences, Farmingdale, NY) dissolved in phosphate buffered saline, pH 7.0 (catalogue # 3024, Dako, Carpinteria, CA), on slide racks within a container immersed in a water bath maintained at 37° C for 20 minutes. Titration, using positive control slides, by time of digestion (e.g. between 15 minutes and 30 minutes) may be necessary because over-digested sections may fall off the slides or tissue structure may become degraded; staining sensitivity will be decreased if the sections are under-digested; proteinase K potency may vary from batch to batch. After washing 3 minutes in distilled water, endogenous peroxidase activity is suppressed by immersion for 30 minutes in 1% H2O2 in 0.1 M phosphate buffered saline with 0.3% Triton X-100, pH 7.4 (PBS-Tx). Sections were then washed 3 times in PBS-Tx as for all subsequent wash steps except where noted.
Sections were incubated at room temperature (RT) overnight in polyclonal antibody to α-synuclein phosphorylated at serine 129 (44) diluted 1:10,000. Because this antibody is not commercially available, it is suggested that those who wish to employ this method instead use either a monoclonal antibody (Wako, Richmond, VA) developed by the same research group and raised against the same phosphorylated epitope as the polyclonal antibody we used (44), or the LB509 monoclonal antibody (Zymed Laboratories, Invitrogen) (45) against non-modified α-synuclein, both diluted 1:1000 dilution in PBS-Tx.
Following primary antibody incubation and washing in PBS-Tx, sections were incubated for 2 hours at RT in biotinylated anti-rabbit (or anti-mouse for the alternative commercial antibodies) IgG diluted 1:1000. After washing, slides were treated for 30 minutes with an avidin-biotin-peroxidase complex ([ABC]; biotinylated secondary antibodies and ABC complex both obtained from Vector Laboratories, Burlingame, CA), with A and B components of the kit both diluted at 1:1000. After 2 washes in PBS-Tx and a last wash in 0.05 M Tris buffer at pH 7.6, slides were treated with 3,3'-diaminobenzidine (Sigma, St. Louis, MO; 3 mg/100 ml) in Tris buffer with added saturated nickel ammonium sulfate (Sigma; 2 ml/100 ml) and H2O2 (6 μl/100 ml of 1% H2O2) for 35 to 60 minutes in the dark. Appropriate staining intensity is determined by monitoring a standard positive control section. Sections were then washed 3 × 5 minutes in Tris buffer, 2 × 30 seconds in distilled water and counterstained with 1% Neutral Red for 2 minutes. Specific staining, including Lewy bodies and fibers, are black and all other nuclei and perikaryal neuronal cytoplasm are red. Because Neutral Red is removed easily by alcohols, slides must be taken very quickly through distilled water, 70% alcohol, 95% and into 100% with only 3 dips in each of the first 3 of these. After 2 × 3 minute changes of absolute alcohol, the slides were cleared with Neo-Clear and coverslipped.
Submandibular Gland Tissue Processing and Analysis
Large (about 1.5 cm2) segments of submandibular gland from all study subjects were placed at autopsy into standard paraffin embedding cassettes and fixed in 10% neutral buffered formalin for 2 days at 4°C, followed by 2 × 60 minute changes in 50% ethanol, dehydration, paraffin infiltration and embedding. Sections were cut at 6 μm and stained for α-synuclein phosphorylated at serine 129 as described above. Initially, only 1 to 3 paraffin sections per subject were stained and examined; in a subset of subjects for whom the initial staining was negative, additional paraffin and/or free-floating 80-μm sections were cut, stained and examined. The densities of immunoreactive fibers within the submandibular glands were graded at sites of maximum density as mild, moderate, severe and very severe, according to the templates published by the Dementia with Lewy Bodies Consortium (40).
For a subset of 19 PD subjects (at the time, these were all of the PD subjects with autopsies including submandibular glands), needle core biopsy was simulated using submandibular glands that had been fresh-frozen at autopsy by placing on sheets of dry ice and then kept at −70°C to −80°C in a freezer. To obtain needle cores, frozen submandibular glands were taken out of the freezer and allowed to thaw slightly at RT until an 18-gauge spinal needle could be pushed completely through the gland with moderate effort. The tissue core within the needle was then pushed out, using the needle insert, onto a sponge inside a paraffin-embedding cassette. Three to 4 tissue cores were obtained from each gland with the sum length of cores varying between 15 and 38 mm. A second sponge was placed so as to sandwich the tissue cores between the sponges; the cassette lid was then attached and the cassette placed in 50 ml of 10% neutral buffered formalin overnight at 4°C (about 16–18 hours). The embedding procedure following this was the same as for the large gland segments. Serial paraffin sections were cut at 6 μm beginning with the first appearance of a core fragment on the faced block. Initially, every 5th section was collected (on the same electrostatically charged slides as used for the large block sections) but this was later changed to every 2nd section. Of these, every second section was immunostained for α-synuclein phosphorylated at serine 129, with the remaining unstained sections held in reserve.
RESULTS
Diagnostic Groups
Subjects with staining of large segments of submandibular gland included 28 with PD, 5 with ILBD, 5 with PSP (3 also had PD), 3 with CBD, 2 with MSA, 22 with ADLB, 16 with AD with no Lewy bodies and 50 non-demented normal elderly controls with no defined neurodegenerative clinicopathological disorder (Table 1). For a more detailed characterization of larger sample sizes of these subject groups, see (25). Ten cases had concurrent neuropathological diagnoses of PD and AD; 3 had neuropathological diagnoses of both PD and PSP; 3 had concurrent neuropathological diagnoses of PSP and AD; 1 had PSP/AD as well as dementia with Lewy bodies (DLB); 1 had PD/AD/PSP; 2 had CBD/AD and 1 had MSA/AD. The subjects ranged in age from 38 to 99, with mean age ranges for the diagnostic groups falling between 79.1 (PD) and 87.0 (ILBD). Mean Mini-Mental State Examination scores ranged from 12.0 for the ADLB group to 28.8 for the ILBD group. Mean motor Unified Parkinson's Disease Rating Scale (UPDRS) scores ranged from 9.4 for the normal subjects to 38.9 for the PD group. For the PD cases, UPDRS scores were performed with 10 subjects in the “ON” state and 16 subjects in the “OFF” state; this was not recorded for 2 subjects. Disease duration for PD cases ranged from 3.1 years to 45 years, with a mean of 14.7 ± 3.1 years (SD). The classification of cases with LTS according to the Unified Staging System is given in Table 2, along with the summary scores of brain regional LTS density. In general, PD subjects were almost all in Stage IV (Neocortical) and Stage III (Brainstem and Limbic), while ADLB subjects were mostly in Stage IIb (Limbic Predominant) or Stage III; ILBD subjects were distributed relatively evenly between Stages I (Olfactory Bulb Only), IIa (Brainstem Predominant), IIb and III, without any Stage IV cases. The sum of brain regional LTS scores was approximately 3-fold higher for PD as compared to either ILBD or ADLB.
Table 1.
General Characteristics of the Study Subjects
| Diagnosis (N) | Age (y) | Gender (% Male) | Mean MMSE (SD)1 | Mean UPDRS (SD)2 | Mean PMI (h) (SD) | Mean Braak Neurofibrillary Stage (SD) | Mean CERAD NP Density (SD) |
|---|---|---|---|---|---|---|---|
| Normal (50) | 82.5 (13.6) | 56.0 | 27.4 (2.3) | 9.4 (8.8) | 4.1 (3.0) | 3.1 (1.2) | 1.1 (1.4) |
| ILBD (5) | 87.0 (7.1) | 80.0 | 28.8 (1.3) | 19.2 (10.9) | 2.8 (0.9) | 3.4 (0.5) | 0.6 (1.3) |
| PD3 (28) | 79.1 (6.5) | 78.6 | 22.5 (6.2) | 38.9 (19.5) | 4.4 (3.3) | 3.5 (0.8) | 1.2 (1.4) |
| PSP3 (5) | 83.4 (1.5) | 60.0 | 23.4 (2.9) | 43 (23.7) | 3.2 (1.2) | 4.6 (1.1) | 2.2 (1.3) |
| CBD (4) | 77.0 (6.7) | 25.0 | 19.7 (7.8) | 47 (38.1) | 3.5 (1.3) | 4.7 (0.6) | 1.0 (1.7) |
| MSA (2) | 79.5 (6.4) | 50.0 | 27.5 (0.7) | 11 | 2.7 (0.2) | 3.5 (2.1) | 1.5 (2.1) |
| ADLB (22) | 83.0 (7.2) | 59.0 | 12.0 (8.0) | 22.1 (21.3) | 4.4 (5.6) | 5.4 (0.7) | 2.9 (0.3) |
| ADNLB (16) | 84.2 (6.1) | 62.5 | 12.9 (8.3) | 16.4 (16.2) | 6.0 (10.5) | 4.5 (1.3) | 2.8 (0.4) |
ILBD, incidental Lewy body disease; PD, Parkinson disease; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; MSA, multiple system atrophy; ADLB, Alzheimer disease with Lewy bodies; ADNLB, Alzheimer disease with no Lewy bodies; MMSE, Mini-Mental State Examination score, UPDRS, Unified Parkinson's Disease Rating Scale; PMI, postmortem interval (hours), CERAD, Consortium to Establish a Registry for Alzheimer's Disease, NP, neuritis plaque.
MMSE scores were not available for 4 PD, 2 ADLB, 1 ADNLB and 11 Normal patients.
UPDRS scores were not available for 2 PD, 1 PSP, 1 CBD, 1 MSA, 11 ADLB, 5 ADNLB and 11 Normal patients.
There were 3 PSP cases that also had PD; therefore the PD and PSP groups have overlapping members.
Table 2.
Classification of Subjects According to Phosphorylated α-Synuclein Histopathology and the Unified Staging System for Lewy Body Disorders
| Diagnosis | Olfactory Bulb | Brainstem Predominant | Limbic Predominant | Brainstem and Limbic | Neocortical | Summary Brain Score |
|---|---|---|---|---|---|---|
| Only (I) | (IIa) | (IIb) | (III) | (IV) | ||
| ILBD | 1 | 1 | 2 | 1 | 0 | 7.8 (3.3) |
| PD1 | 0 | 1 | 0 | 11 | 15 | 28.4 (6.7) |
| ADLB | 2 | 0 | 15 | 5 | 0 | 10.0 (5.8) |
ILBD, incidental Lewy body disease; PD, Parkinson disease; ADLB, Alzheimer disease with Lewy bodies. Number and percentage of subjects in each stage are given, along with the mean (SD) summary score of synucleinopathy density score for all 10 standard brain regions.
One PD subject was not classifiable due to missing brain regions needed for staging; 2 PD subjects do not have summary brain scores due to missing regions; 1 ADLB subject was not classifiable due to missing brain regions; 4 ADLB subjects do not have summary brain scores due to missing regions.
Phosphorylated α-Synuclein LTS in Large Submandibular Gland Blocks
Immunoreactive nerve fibers were present within sections of large submandibular gland specimen blocks of all 28 PD subjects, including the 3 that also met neuropathological diagnostic criteria for PSP. Additionally, 3 ADLB subjects were positive for LTS but none of the normal control subjects or any other subjects were positive, including the 2 other PSP cases, the 2 CBD cases and the 2 MSA cases. In most cases, positive staining was present in the initial 1 to 3 sections examined but in 3 PD cases that were initially negative as well as in a subset of ADLB cases and all of the ILBD cases, additional sections were immunostained: Of the 3 PD cases, 1 of these had 2 80-μm-thick free-floating sections stained and examined while for the other 2 cases, 4 additional paraffin sections were stained and examined for each case; all were positive on at least 1 of the additional sections. Nine ADLB cases had additional sections stained and examined. Of these, 5 had 1 to 5 additional 80-μm-thick stained sections examined and 7 had an additional 4 stained paraffin sections examined (4 cases had extra sections of both types stained and examined); of these, 2 cases were positive on at least 1 of the additional sections. One ILBD case had an additional 20 paraffin sections stained and examined whereas another 4 ILBD cases each had 10 additional paraffin sections stained and examined; none of the additional sections were positive.
Only staining that was morphologically consistent with nerve fibers was considered to be positive; these were most frequently found in nerve fascicles running in the connective tissue stroma (Fig. 1A, B). Occasionally, these were closely applied to the peripheral surface of arterioles (Fig. 1C) or adjacent to ducts (Fig. 1D). Fibers were frequently seen within gland parenchyma, interweaving amongst serous gland cells (Fig. 1E, F). Immunoreactive nerve fibers were most often normal in appearance but occasional enlarged and distorted fibers were present (Fig. 1A). The mean of density scores for immunoreactive nerve fibers in the PD group was 1.7 ± 0.9 (SD); the 3 positive ADLB cases all had density scores of 1. Immunoreactive ganglion cell bodies were not observed in any of the sections. Density scores within the submandibular gland did not significantly correlate with density scores within the brains of PD subjects, their Unified Stage or with the UPDRS scores of PD subjects. There were significant correlations between submandibular density scores and brain scores or UPDRS scores when all subjects were included but these correlations were considered to be only a result of differential membership within the PD group.
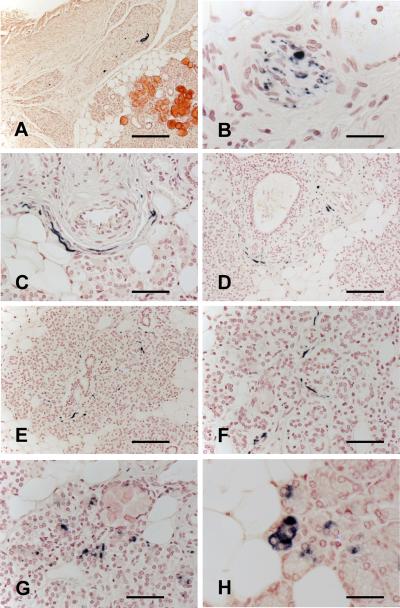
Figure 1.
Phosphorylated α-synuclein-immunoreactive nerve fibers in large blocks (1–2 cm2) of submandibular gland from autopsied Parkinson disease (PD) patients. (A–F) Immunoreactive nerve fibers, some enlarged and distorted, within fascicles in the connective tissue stroma (A, B), closely applied to the smooth muscle layer of an arteriole (C), adjacent to a salivary ductule (D) and interweaving among serous gland cells (E, F). (G, H) Staining of gland cell cytoplasm was considered to be non-specific because it was seen in some subjects from all diagnostic categories; it is seen here in a PD case (G) and in a normal control (H). Scale bars: A, 250 μm; B, H, 35 μm; C, 60 μm; D, E, 150 μm; F, 75 μm; G, 40 μm.
The cytoplasm of some serous gland cells was stained in glands from some subjects in all diagnostic categories, (Fig. 1G, H); rarely, secretory material within ductules was also stained (not shown). Because it was not related to diagnosis, this staining was considered negative and non-specific.
Phosphorylated α-Synuclein LTS Staining in Needle Cores from PD Subjects
For each case, the number and percentage of sections with a positive LTS structure was recorded. Initially, with the examination of between 10 and 45 slides per case, 15 cases were positive with a median section positive percentage of 66%. Three cases were negative and one case was a technical failure because all sections came off the slides after proteinase K pretreatment; electrostatically charged slides were inadvertently not used. Additional sections were stained and examined on these 4 cases and between 56 and 89 stained slides per case were examined; this resulted in 2 more positive cases (median section positive percentage of 6%), while 2 cases remained negative despite examination of a total of 80 and 118 sections.
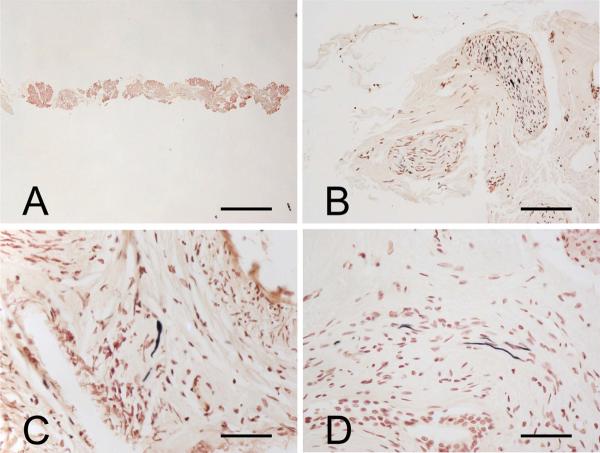
The appearance of one of the tissue cores at low magnification is shown in Figure 2A. The specifically stained nerve elements within tissue cores were similar to those seen with the larger tissue blocks (Fig. 2B–D), with nerve fibers seen most often and in the highest densities within nerve fascicles running in the connective tissue stroma (Fig. 2B).
Figure 2.
Phosphorylated α-synuclein-immunoreactive nerve fibers in needle cores taken from frozen submandibular glands of autopsied Parkinson disease patients. (A) Low-magnification image of a typical tissue core. (B–D) Immunoreactive nerve fibers are most frequently seen within the connective tissue stroma. Panel (C) shows an immunoreactive nerve fiber near the peripheral surface of an arteriole. Scale bars: A, 1 mm; B, 100 μm; C, 50 μm; D, 150 μm.
DISCUSSION
For neurodegenerative diseases, brain biopsy would provide the most accurate diagnosis but the cost-benefit ratio has been considered too high to make this a possibility. Many diseases of the CNS are diagnosed with PNS biopsies but this has only been very recently considered for PD. We conducted an extensive survey for LTS in the PNS of subjects with PD (25) and concluded that because they have the highest LTS prevalence and densities, the lower esophagus and submandibular gland are the most promising biopsy sites. The submandibular gland is located subcutaneously, making it easily accessible. It is commonly biopsied for neoplasia (33, 34), and the complication rate is low. The results of the present investigation show that submandibular gland biopsy has promise as a diagnostic procedure for PD and, if utilized, would likely increase PD diagnostic accuracy.
We found nerve elements immunoreactive for α-synuclein phosphorylated at serine 129 in 100% of the larger tissue blocks taken from 28 autopsied PD subjects and in 89% of PD subjects sampled with needle cores. Another group has reported positive labial salivary gland biopsies in 2 of 3 PD subjects (24).
Studies from 2 research groups have previously advanced colonic biopsies for diagnosing PD. Data from these groups, however, have been surprising and conflicting. One group found a relatively low prevalence and density of colonic LTS per biopsy site (including a low prevalence in MSA cases) (20–24), while the other group found high densities and prevalence (18, 19). Based on our own experience (25), and that of others (26, 46), there is a rostrocaudal gradient of LTS in the GI tract, with the lower esophagus having the highest densities, rectum the lowest, with generally successively lower densities in each intervening GI subdivision. Also, we caution that false positive results are a particular problem when using immunohistochemistry (IHC) to assess whether α-synuclein staining is normal or pathological. Artifactual staining of collagen, polymorphonuclear leukocytes and gland cell cytoplasm is common when IHC protocols developed for CNS tissue are applied in the peripheral tissues. Because α-synuclein is one of the most abundant proteins in neural tissue, another common problem is the staining of normal, rather than pathological α-synuclein. We use 2 approaches to minimize this. We use a primary antibody against α-synuclein phosphorylated at serine 129 (44), which is a biochemical hallmark of PD and other Lewy body disorders but is present at extremely low levels in normal neural tissue (47), and is not detectable with IHC (36). Additionally, we pretreated the tissue sections with proteinase K, which not only serves as a superior epitope exposure method (35, 48), but also preferentially digests normal α-synuclein as compared to the aggregated, pathological form. Detection of normal, non-aggregated α-synuclein has been recognized as a confounding factor in some recent studies. One group reported that more than half of autopsy colon samples, and a large percentage of bladder and prostatic specimens, from normal middle-aged and elderly subjects had positive α-synuclein staining in neural tissue elements (42, 43); another group found that 44% of subjects without a CNS Lewy body disorder had positive α-synuclein immunoreactivity in the spinal cord (41); this non-specific α-synuclein immunoreactivity increased with subject age, increasing its likelihood of confounding results for the PD age group.
Although the colon is routinely biopsied in many countries as a screening procedure for colon cancer and is known to have a relatively low complication rate, these biopsies are purposely limited to mucosa and submucosa. We and others have found that the intermyenteric plexus has much higher densities of LTS than submucosa (25, 46, 49), but because of the risk of viscus perforation and subsequent peritonitis, a biopsy including the intermyenteric plexus would have a much higher risk-to-benefit ratio than mucosal-submucosal biopsy and hence would probably never be part of routine colon cancer screening. Therefore biopsies for routine colon cancer screening will probably not be well suited to screening for LTS.
We suggest that biopsy of the submandibular gland would be particularly useful in the selection of subjects for invasive therapies, even at early stages of disease. Pharmacologic therapy of PD is initiated empirically as the risks of therapy are low and therapy can be discontinued if it is ineffective or causes adverse effects. Surgical treatment of PD, which in the past has included pallidotomy, thalamotomy, deep brain stimulation and neural transplantation, however, carries significant risk of serious complications, up to 40% overall (9–17). These therapies are thought to be less effective or ineffective for non-PD causes of parkinsonism and, therefore, accurate diagnosis of PD is considered essential for patient selection. Five years of observation with a favorable response to levodopa has been recommended for surgical PD candidates (13), restricting such therapies to later stage disease.
Additionally, submandibular gland biopsy could be used to help select subjects for clinical trials of new pharmacologic agents or to validate other diagnostic modalities. Dopaminergic imaging of the striatum has been put forward as a diagnostic procedure but does not distinguish PD from PSP, MSA or ILBD, all of which have significant striatal dopamine deficits. Base on autopsy biochemical studies, incidental Lewy body disease has an approximately 50% reduction in striatal dopaminergic markers (30–32), and it is present in about 25% of normal elderly subjects (36). The prevalence of PSP may be much higher than previously thought (50, 51), i.e. 50% or more of PSP subjects do not have the characteristic vertical gaze palsy and/or present with dementia rather than a movement disorder (52–54). Although imaging-to-autopsy studies have recently been successful in assessing the accuracy of amyloid imaging for Alzheimer disease (55), there have as yet been no autopsy-based estimates of the sensitivity and specificity of dopaminergic imaging for the diagnosis of PD. Submandibular gland biopsy could be used to assess the true accuracy of dopaminergic imaging as well as other PD blood and cerebrospinal fluid-based candidate biomarkers.
Further investigation of submandibular gland biopsy should include clinical trials to assess the complication rate and to estimate the numbers of clinically diagnosed PD subjects with confirmatory submandibular gland LTS. For open biopsies of the submandibular gland that obtain at least 1 cc of glandular tissue, 10 paraffin sections should be sufficient. For needle biopsies that obtain similar submandibular gland tissue volumes as we used here, it would be advisable to stain and examine 100 sections. This is not beyond what has been used for diagnostic biopsies of some other selected diseases. For example, for Hirschsprung disease, more than 100 stained sections are commonly examined (56), and these are often stained with multiple methods. We did not find that free-floating 80-μm sections were necessary or clearly superior to paraffin sections to any degree over and above that which their extra thickness would give.
We suggest that submandibular biopsy initially be performed on a research basis, until the procedure has been performed and assessed in living subjects, and in at least a small series of living subjects that subsequently come to autopsy so that a final neuropathological correlation may be done. If these studies indicate a sufficiently high sensitivity and specificity, the procedure should then become useful for many types of research studies, for validation of other potential biomarkers and for clinical trial subject selection. Ultimately, submandibular gland biopsy might be useful for directing therapy in clinical practice should PD-specific disease-modifying therapeutic interventions become available.
Acknowledgments
Funding: The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson's Research.
Footnotes
Competing Interests: All authors have declared that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Caslake R, Moore JN, Gordon JC, et al. Changes in diagnosis with follow-up in an incident cohort of patients with parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79:1202–7. doi: 10.1136/jnnp.2008.144501. [DOI] [PubMed] [Google Scholar]
- 2.Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism--a prospective study. Can J Neurol Sci. 1991;18:275–8. doi: 10.1017/s0317167100031814. [DOI] [PubMed] [Google Scholar]
- 3.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57:1497–9. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Cornford ME, Chang L, Miller BL. The neuropathology of parkinsonism: an overview. Brain Cogn. 1995;28:321–41. doi: 10.1006/brcg.1995.1261. [DOI] [PubMed] [Google Scholar]
- 7.Litvan I, MacIntyre A, Goetz CG, et al. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998;55:969–78. doi: 10.1001/archneur.55.7.969. [DOI] [PubMed] [Google Scholar]
- 8.Gibb WR. Neuropathology of Parkinson's disease and related syndromes. Neurol Clin. 1992;10:361–76. [PubMed] [Google Scholar]
- 9.Obeso JA, Rodriguez MC, Gorospe A, et al. Surgical treatment of Parkinson's disease. Baillieres Clin Neurol. 1997;6:125–45. [PubMed] [Google Scholar]
- 10.Subramanian T. Cell transplantation for the treatment of Parkinson's disease. Semin Neurol. 2001;21:103–15. doi: 10.1055/s-2001-13125. [DOI] [PubMed] [Google Scholar]
- 11.Master Z, McLeod M, Mendez I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson's disease. J Med Ethics. 2007;33:169–73. doi: 10.1136/jme.2005.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paluzzi A, Belli A, Bain P, et al. Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg. 2006;20:290–5. doi: 10.1080/02688690601012175. [DOI] [PubMed] [Google Scholar]
- 13.Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(Suppl 14):S171–96. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- 14.Tir M, Devos D, Blond S, et al. Exhaustive, one-year followup of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients. Neurosurgery. 2007;61:297–304. doi: 10.1227/01.NEU.0000285347.50028.B9. [DOI] [PubMed] [Google Scholar]
- 15.Gill CE, Konrad PE, Davis TL, et al. Deep brain stimulation for Parkinson's disease: the Vanderbilt University Medical Center experience, 1998-2004. Tenn Med. 2007;100:45–7. [PubMed] [Google Scholar]
- 16.Seijo FJ, Alvarez-Vega MA, Gutierrez JC, et al. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson's disease. Review of 272 procedures. Acta Neurochir (Wien ) 2007;149:867–75. doi: 10.1007/s00701-007-1267-1. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez RL, Fernandez HH, Haq I, et al. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13:253–60. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- 18.Ceuterick-de Groote C, Martin JJ. Extracerebral biopsy in lysosomal and peroxisomal disorders. Ultrastructural findings. Brain Pathol. 1998;8:121–32. doi: 10.1111/j.1750-3639.1998.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shannon KM, Keshavarzian A, Dodiya HB, et al. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's Disease? Evidence from 3 cases. Mov Disord. 2012;27:716–9. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 20.Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov Disord. 2012;27:709–15. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 21.Lebouvier T, Neunlist M, Bruley d V, et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebouvier T, Chaumette T, Damier P, et al. Pathological lesions in colonic biopsies during Parkinson's disease. Gut. 2008;57:1741–3. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- 23.Pouclet H, Lebouvier T, Coron E, et al. Analysis of colonic alpha-synuclein pathology in multiple system atrophy. Parkinsonism Relat Disord. 2012;18:893–5. doi: 10.1016/j.parkreldis.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Pouclet H, Lebouvier T, Coron E, et al. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson's disease. Neurogastroenterol Motil. 2012;24:e202–e205. doi: 10.1111/j.1365-2982.2012.01887.x. [DOI] [PubMed] [Google Scholar]
- 25.Cersosimo MG, Perandones C, Micheli FE, et al. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson's disease patients. Mov Disord. 2011;26:188–90. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- 26.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi K, Takahashi H, Takeda S, et al. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 1988;76:217–21. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi K, Takahashi H, Takeda S, et al. Lewy bodies in the enteric nervous system in Parkinson's disease. Arch Histol Cytol. 1989;52(Suppl):191–4. doi: 10.1679/aohc.52.suppl_191. [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi K, Takahashi H, Ohama E, et al. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–3. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 30.Del Tredici K, Hawkes CH, Ghebremedhin E, et al. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 2010;119:703–13. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 31.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–51. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson DW, Fujishiro H, Delledonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–44. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 33.Delledonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–80. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 34.Lussier C, Klijanienko J, Vielh P. Fine-needle aspiration of metastatic nonlymphomatous tumors to the major salivary glands: a clinicopathologic study of 40 cases cytologically diagnosed and histologically correlated. Cancer. 2000;90:350–6. [PubMed] [Google Scholar]
- 35.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol (Berl) 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 39.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 40.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 41.Oinas M, Paetau A, Myllykangas L, et al. alpha-Synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol. 2010;119:715–22. doi: 10.1007/s00401-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 42.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, et al. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology. 2007;68:2012–8. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 43.Munoz DG, Gold A. Expression of alpha-synuclein (AS) in autonomic neurons is increased in Parkinson's disease (PD) J Neuropathol Exp Neurol (Abstract) 2008;67:489. [Google Scholar]
- 44.Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 45.Jakes R, Crowther RA, Lee VM, et al. Epitope mapping of LB509, a monoclonal antibody directed against human alpha-synuclein. Neurosci Lett. 1999;269:13–6. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- 46.Annerino DM, Arshad S, Taylor GM, et al. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–80. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lue LF, Walker DG, Adler CH, et al. Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol. 2012;22:745–56. doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hladik CL, White CL. Comparison of digestive enzyme and formic acid pretreatment for optimal immunohistochemical demonstration of alpha-synuclein-immunoreactive cerebral cortical Lewy neurites. J Neuropathol Exp Neurol. 2003;62:237–47. [Google Scholar]
- 49.Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Evidente VG, Adler CH, Sabbagh MN, et al. Neuropathological findings of PSP in the elderly without clinical PSP: possible incidental PSP? Parkinsonism Relat Disord. 2011;17:365–71. doi: 10.1016/j.parkreldis.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain. 2001;124:1438–49. doi: 10.1093/brain/124.7.1438. [DOI] [PubMed] [Google Scholar]
- 52.Davis PH, Bergeron C, McLachlan DR. Atypical presentation of progressive supranuclear palsy. Ann Neurol. 1985;17:337–43. doi: 10.1002/ana.410170406. [DOI] [PubMed] [Google Scholar]
- 53.Gearing M, Olson DA, Watts RL, et al. Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology. 1994;44:1015–24. doi: 10.1212/wnl.44.6.1015. [DOI] [PubMed] [Google Scholar]
- 54.Birdi S, Rajput AH, Fenton M, et al. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord. 2002;17:1255–64. doi: 10.1002/mds.10211. [DOI] [PubMed] [Google Scholar]
- 55.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–83. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knowles CH, De GR, Kapur RP, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]