Abstract
Dynapenia (pronounced dahy-nuh-pē-nē-a, Greek translation for poverty of strength, power, or force) is the age-associated loss of muscle strength that is not caused by neurologic or muscular diseases. Dynapenia predisposes older adults to an increased risk for functional limitations and mortality. For the past several decades, the literature has largely focused on muscle size as the primary cause of dynapenia; however, recent findings have clearly demonstrated that muscle size plays a relatively minor role. Conversely, subclinical deficits in the structure and function of the nervous system and/or impairments in the intrinsic force-generating properties of skeletal muscle are potential antecedents to dynapenia. This review highlights in the contributors to dynapenia and the etiology and risk factors that predispose individuals to dynapenia. In addition, we address the role of nutrition in the muscular and neurologic systems for the preservation of muscle strength throughout the life span.
Keywords: Aging, Strength, Weakness, Function, Muscle, Disability, Sarcopenia, Dynapenia
What is dynapenia?
A staggering 16% to 18% of women and 8% to 10% of men in the United States older than 65 y cannot lift 10 lbs. or stoop/kneel down [1]. Physical functioning tasks of this nature are undoubtedly closely linked to physiologic capabilities, such as muscle strength and power production, and low muscle strength is well known to place older adults at an increased risk of mobility limitations [2-8] and mortality [9-12]. Accordingly, the preservation of muscle strength and power with advancing age is of high clinical significance. It was once thought that the well-characterized age-related atrophy of muscle was to blame for poor muscle strength. However, recent longitudinal and intervention-based studies have clearly demonstrated that muscle atrophy is a relatively small contributor to the loss of muscle strength [13-16]. Similarly, longitudinal studies delivering exogenous supplementation of androgens or growth factors have yielded an increase in muscle mass but only marginally improved muscle performance [17,18]. However, despite findings of this natured—and similar findings from more than 30 y ago [19]— the preponderance of scientific investigations have continued to focus primarily on determinates of skeletal muscle size. In 2008 we proposed the term dynapenia to define the age-related loss of muscle strength and power [20]. Dyna refers to “power, strength, or force” and penia refers to “poverty.” We should note that Morley et al. [21] recently used the term kratopenia to characterize the “loss of force” and dynapenia to characterize the “loss of power.” In the context of this review, we refer to dynapenia within the context of our original definition, which encompasses the broader aspects of skeletal muscle force performance and includes strength (i.e., maximal voluntary force) and/or mechanical power (a product of force times velocity), which are commonly measured using dynamometry equipment (e.g., isometric strength, isokinetic power, etc.). In the following paragraphs, we provide details on the causes and consequences of dynapenia.
What are the consequences of dynapenia?
The consequences of dynapenia are staggering; it increases the risk for physical disability [22,23], poor physical performance [2-8], and even death [9-12]. For example, we recently conducted an informal meta-analysis examining the relative risk between low levels of muscle strength and poor physical performance and/or physical disability. We found that in the vast majority of studies (90%), a significant association was noted, with the unweighted average of the relative risks being 2.2 (Fig. 1) [24]. As an aside, only 35% of the studies found significant associations with sarcopenia. We should note that there are limitations in presenting unweighted average relative risks [25]; however, the findings suggest that dynapenia is an important prognostic indicator of functional impairments in elders. With regard to the impact of dynapenia on mortality, Newman et al. [9] observed that grip and knee extensor muscle strengths were strongly related to mortality (even after accounting for muscle area and regional lean mass). Specifically for women, they observed crude hazard ratios of 1.84 for grip strength and 1.65 for knee extensor strength. For men, they observed crude hazard ratios of 1.36 for grip strength and 1.51 for knee extensor strength. More recently, Xue et al. [10] reported in a longitudinal study of 436 women that faster rates of decreases in grip and hip flexor strengths independently predicted mortality after accounting for potential confounders. The hazard ratios were 1.33 and 2.62 for the rates of decrease in grip and hip strengths, respectively. Collectively, these findings provide convincing data that dynapenia in older adults have serious negative consequences as it relates to physical disability, physical function, and mortality.
Fig. 1.
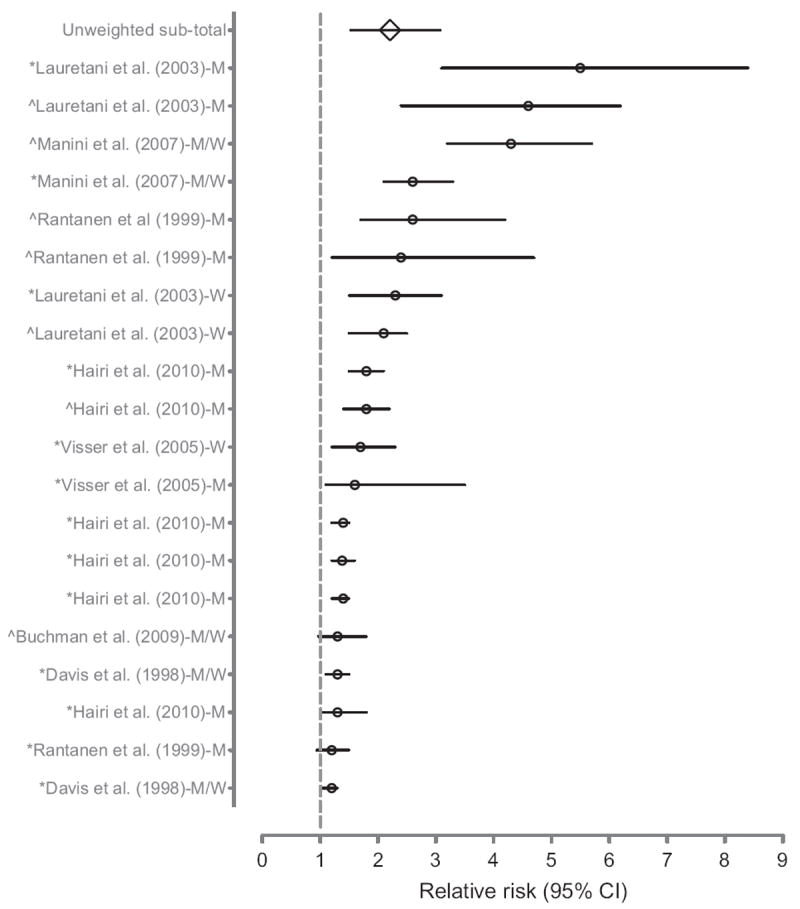
Relative risk of poor physical performance, functional limitation, or physical disability in older adults with dynapenia (low muscle strength). The counterfactuals are older adults with normal muscle strength or mass. Studies investigating multiple outcomes or expressing findings by sex are repeated. The author of each study is followed by whether the relative risk was estimated in men, women, or men and women and preceded by whether the outcome was a self-reported physical function/disability (*) or an observed physical performance (ˆ). Figure modified from Manini and Clark. CI, confidence interval; M, men; W, women.
What are the contributors to dynapenia?
It was originally thought that the loss of skeletal muscle mass (sarcopenia) largely explained the dynapenia commonly observed in older adults [26]; however, recent longitudinal data have suggested that other physiologic factors—independent of tissue size—play an important and likely larger role in determining who will develop muscle weakness [15]. Specifically, data from the Health, Aging and Body Composition study—a large prospective cohort of older adults—have indicated that the decrease in muscle strength is significantly more rapid than the concomitant loss of muscle mass, and that the change in quadriceps muscle area explains only ~6% to 8% of the between-subject variability in the change in knee extensor muscle strength [15]. Moreover, maintaining or gaining muscle mass does not prevent aging-related decreases in muscle strength [15] (Fig. 2). Accordingly, these findings indicate that the loss of muscle strength in older adults is weakly associated with the loss of lean body mass. Other findings using experimental disuse models of muscle weakness (e.g., bedrest) also have suggested that the relative contribution of muscle atrophy to weakness is modest [13,14,16]. For example, we have reported that the loss of muscle mass after 4 wk of leg muscle disuse (i.e., unilateral lower limb suspension, where one leg is unweighted by having young, healthy study participants ambulate on crutches) explains less than 10% of the associated loss of muscle strength [13,14]. Rather, our experimental disuse findings largely indicated that changes in neurologic function and/or the intrinsic force-generating properties of skeletal muscle contribute to muscle weakness and motor dysfunction [13,14,27-30]. Later we discuss the potential physiologic contributors to dynapenia.
Fig. 2.
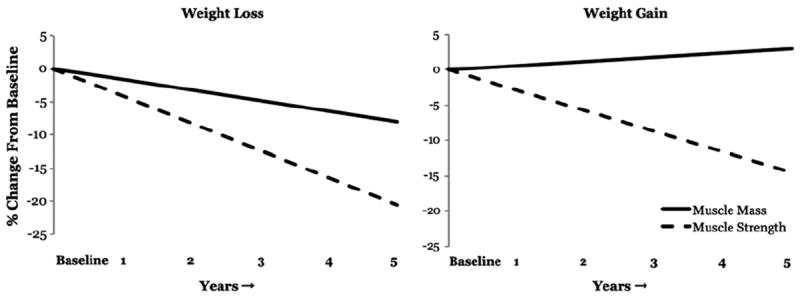
The age-related loss of muscle strength is weakly associated with the loss of muscle mass. These figures were adapted from published data obtained from the Health ABC Study to examine the relation between changes in knee extensor strength and quadriceps femoris cross-sectional area muscle (measured by computed tomography) in a 5-y longitudinal study of older adults [15]. These data represent the annualized rate of loss over a 5-y period in older adults who lost body weight (left; n = 309 men) and gained body weight (right; n = 143 men). Note that 1) muscle strength is lost at a substantially faster rate than muscle mass and 2) gaining muscle mass does not prevent the aging-related loss of muscle strength (right). Adapted from data presented by Delmonico et al. [15], with the created figure being approved by the corresponding author (M. J. Delmonico).
In brief, the contributors to dynapenia can be compartmentalized into two factors, i.e., 1) neurologic and 2) skeletal muscle properties, because it is well known that the output from these sources controls muscle force production [20,27,31]. For example, it is plausible that the nervous system’s ability to fully activate skeletal muscle voluntarily is impaired in dynapenic individuals, with this deficit in voluntary (central nervous system) activation caused by potential changes, such as a decreased excitatory drive, to the lower motor neurons and/or decreases in α-motor neuron excitability, which could result in suboptimal motor unit discharge rates [32]. In addition, dynapenic individuals could have fewer functioning motor units, which would theoretically affect muscle strength once a critical threshold is reached, particularly if collateral reinnervation does not occur or is incomplete. This is a logical pathway because it is clear that older adults possess fewer motor units compared with young adults [33,34]. Similarly, it is plausible that the muscle system’s ability to optimally produce force is impaired in dynapenic individuals, with this deficit in the intrinsic force-generating capacity of muscle (force/unit area) caused by potential changes in the excitation–contraction coupling process [35,36]. Figure 3 provides a conceptual model for how impairments in the nervous and muscle systems can contribute to dynapenia and subsequently lead to functional limitations, which subsequently place older adults at risk of physical disability. Later we describe the potential physiologic factors leading to dynapenia.
Fig. 3.
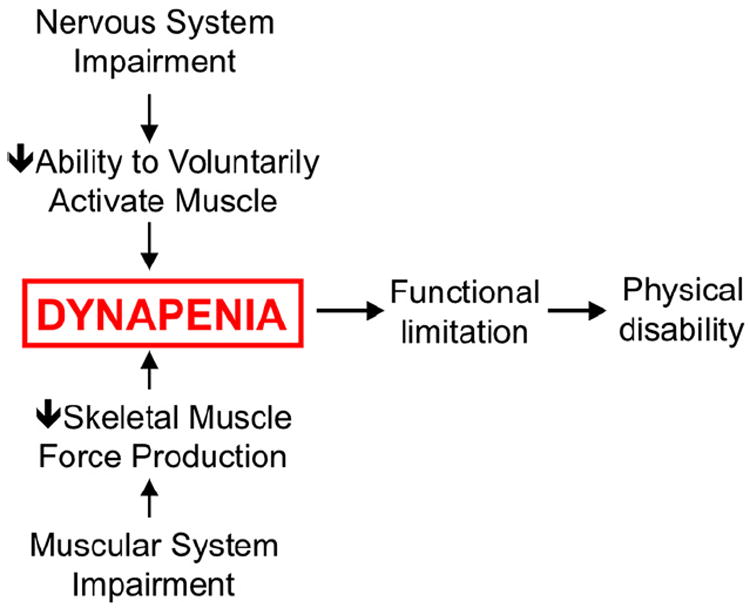
Conceptual model of how nervous and muscle system impairments lead to dynapenia.
Can older adults fully activate their skeletal muscles during a muscle contraction?
There is no question that impairments in the nervous system’s ability—or lack thereof—to fully activate skeletal muscle could, theoretically, be an explanatory contributor to dynapenia. Indeed, there is evidence to suggest that aging results in impaired agonist activation and/or increased antagonistic coactivation [37]; however, age-related differences in voluntary activation appear to vary between muscle groups and likely varies between subclasses of older adults (e.g., healthy versus physically disabled). Before more fully discussing the effects of aging on voluntary activation, we provide a brief overview of the assessment of voluntary activation [38,39]. A voluntary effort, or a voluntary contraction of a muscle, consists of the recruitment of motor neurons and, hence, muscle fibers by an increased descending drive. Hence, with an increased force of contraction, there is an increased activation of neurons in the primary motor cortex with increased firing of corticospinal neurons [40]. An increased descending drive recruits larger numbers of motor neurons in the spinal cord. Concomitantly, force summates more effectively because the central nervous system varies the rate of neuronal firing, thereby generating increased force at higher frequencies. This is known as rate modulation or rate coding. Nevertheless, each motor neuron innervates a number of muscle fibers that fire one-to-one with the motor neuron. Together, these comprise a motor unit. When a motor unit fires sufficiently fast, its muscle fibers produce a fused contraction. Although there are many influences on motor neurons during voluntary contractions, such as excitatory and inhibitory sensory feedback, and alterations in motor neuron properties that may make them more or less responsive to synaptic input [41], the descending drive from the motor cortex is the major determinant of the timing and strength of voluntary contractions.
Voluntary activation is commonly assessed using the interpolated twitch method or a derivative thereof (e.g., central activation ratio) [38,42]. Here, the motor nerve to the muscle, or the muscle itself, is electrically stimulated during a voluntary effort. During maximal voluntary efforts, any increment in force evoked by a stimulus indicates that voluntary activation is less than 100%. That is, some motor units are not recruited or are not firing fast enough to produce fused contractions [43]. The extra force evoked by stimulation during contraction can be quantified by comparison with the force produced by the whole muscle. Thus, voluntary activation represents the proportion of maximal possible muscle force that is produced during a voluntary contraction. Measurement of voluntary activation does not quantify the descending drive reaching the motor neurons or whether motor neuron firing rates are maximal, and it does not take into account the source of the drive to the motor neurons. However, mechanisms in the cortex, spinal cord, and muscle can influence voluntary activation [38].
There are equivocal reports in the literature on whether advancing age decreases voluntary activation capacity [44-63]. A synthesis of the literature, however, does provide some insight into potential explanations of these equivocal reports. Specifically, several studies examining the effect of age on voluntary isometric activation of the knee extensors [50,60] and the elbow flexors [44,51,52] have suggested that older adults, particularly those older than 70 to 75 y, exhibit a decrease in voluntary activation, whereas investigations on the age-related changes in the voluntary activation of the dorsiflexors yielded null findings [47,48,53,57,59,63]. Because of the functional differences between these muscles and the differences in their physiologic profiles (e.g., motor unit innervations and fiber type characteristics), these muscle-group specific effects are not overly surprising, especially when one considers that differences in the activation of different muscle groups have been reported in young subjects [42]. We should note there are several studies indicating that older adults have a meaningful level of impairment in voluntary activation. The impairments found in central activation are large enough to explain a large portion of observed muscle weakness in a given individual, such as inactivation on the level of ~ 15% or more [50,52,60]. One study that deserves particular attention is by Harridge et al. [50], which entails, to our knowledge, the oldest known cohort of individuals to date to undergo these types of assessments (n = 11, age range 85–97 y). In this study, all older adults required some degree of assistance with everyday activities, and—interestingly—all subjects showed evidence of incomplete voluntary activation during a maximal contraction, with activation ranging from 69% to 93% (mean 81 ± 7%). This finding suggests that deficits in voluntary activation can contribute to a significant portion of the muscle weakness observed in the very old.
What are the potential neurophysiologic mechanisms of dynapenia?
As stated previously, physiologic factors in the cortex, spinal cord, and muscle can influence voluntary activation. The neurons in the premotor and primary motor cortex form a complex network of glutamatergic interneurons, afferent projections, and pyramidal neurons that project to the striatum and spinal cord, among other areas of the central nervous system. Although it is often widely assumed that there is a progressive decay in the number of primary motor cortex (M1) neurons in normal aging, the available evidence suggests there is no such consequence with aging [64,65]. However, there are substantial morphometric changes in the motor cortex that do occur in with normal aging. For example, cadaveric dissections have suggested that individuals older than 65 y exhibit a staggering 43% volumetric decrease in the premotor cortex neuron cell body size compared with younger adults [64], and these observations have more recently been corroborated in living humans using high-resolution magnetic resonance imaging [66]. Furthermore, there is also evidence to suggest that age-related differences exist in the mass of white matter and of myelinated nerve fiber length, with individuals losing ~45% of the total length of the myelinated fibers, mostly in the smallest white matter nerve fibers [67]. Also, evidence from cross-sectional studies further has suggested that aging disrupts the integrity of the white matter [68]. Functionally, it appears that these changes from aging affect the connectivity of the cortex with itself and the rest of the central nervous system.
Aging also affects motor cortical properties at the systems level. Specifically, aging has been shown to result in decreased cortical excitability [69-72], increased activation in areas of sensorimotor processing and integration [73-75], and decreased cortical plasticity [76,77]. For example, using transcranial magnetic stimulation—a non-invasive technique that provides insight into human motor cortex excitability—we recently reported that older adults exhibit more intracortical inhibition and less intracortical facilitation compared with young adults [70], which is consistent with our observation of disuse-induced muscle weakness being associated with increases in intracortical inhibition [28,30]. In addition, the human motor cortex displays an age-dependent decrease in cortical plasticity [76,77], where the paired-associative electrical stimulation of the median nerve that increases the motor evoked potential amplitude in young and middle-aged adults is impaired in older adults [76]. Collectively, these findings suggest that aging results in decreased motor cortical excitability and cortical plasticity, which may contribute to age-related decreases in muscle performance.
In addition to these potential cortical mediators of dynapenia, age-related changes in spinal neurophysiologic properties could contribute to dynapenia because motor units demonstrate numerous age-related adaptations, including changes in morphology, behavior, and electrophysiology. With regard to changes in morphology, advancing age is thought to result in a smaller motor unit number and an increased number of fibers per motor unit (increased innervation ratio) because of the compensatory collateral sprouting by surviving neurons [34, 78-80]. Aging has also been shown to result in changes in spinal excitability. For example, Kido et al. [81] reported that the soleus H-reflex (a global measurement of spinal excitability) decreased gradually with age. Others have also observed that heteronymous facilitation [82] and oligosynaptic reflexes [83] decrease with age, which provides collective evidence that there is a general decrease in the excitability of spinal reflexes with age. The end result of the morphologic and physiologic adaptations in motor units with aging is alterations in the behavioral discharge properties of motor units. For instance, older adults have been reported to exhibit maximal motor unit firing rates that are ~35% to 40% lower than in young adults [84]. These lower firing rates appear to be largely inter-related to the longer twitch contraction durations in older muscle, which further illustrates the critical integrative control processes involved between the nervous and muscle systems as they relates to overall neuromuscular function. More recent evidence has suggested that more subtle age-related differences exist in motor unit behavior. Specifically, older adults have been reported to exhibit a greater variability in motor unit discharge rates that appears to largely influence their ability to maintain steady forces [85], and the occurrence of motor unit doublet discharges is lower in older adults [86]. Collectively, these changes in motor unit discharge properties likely contribute to the decreased functional properties of aged skeletal muscle.
What are the potential muscular mechanisms of dynapenia?
Muscle atrophy undoubtedly occurs with advancing age. More Specifically, recent longitudinal data have indicated that, on average, older men lose approximately 1% of their thigh muscle area per year and older women lose approximately 0.65% of their thigh muscle area per year [15]. With this stated—as illustrated in Figure 2—it should be noted that there is a large between-subject variability in the degree of atrophy observed with aging, and some older adults appear to exhibit no or nominal losses in muscle mass [15]. Several studies have indicated that the atrophy is primarily caused by a loss of fibers, with no predominant effect on any fiber type, and to a lesser extent by a decrease in fiber size—predominantly type II fibers [87,88]. There are many interacting factors leading to muscle wasting in older adults, and they often present themselves concurrently. Changes in muscle protein metabolism have been proposed as an explanatory factor in muscle wasting in older adults, because the balance between protein synthesis and degradation is largely responsible for the maintenance of lean mass. However, recent findings have suggested that basal muscle protein synthesis rates do not differ between young and old adults [89-94], and it is generally accepted that the difference in fasted rates of muscle protein synthesis or breakdown are not altered in healthy older adults [95]. Current hypotheses related to causative factors affecting muscle protein turnover surround the concept of older adults being resistant to anabolic stimuli, such as that associated with feeding, insulin, or physical activity. For example, although the ingestion or infusion of large quantities of amino acids/protein (~30–40 g) yield similar increases in muscle protein synthesis in young and older individuals [96-100], recent studies have indicated that older adults exhibit a decreased accretion of muscle proteins after the ingestion of smaller amounts of essential amino acids (6–15 g) [90,91]. Similarly, several recent studies have reported a blunted muscle protein synthesis response after an acute bout of resistance exercise in older subjects [101-103]. So, although muscle atrophy and changes in muscle protein metabolism undoubtedly occur with aging, as stated earlier, the relative contribution of the loss of muscle size associated with advancing age toward the observed muscle weakness is substantially less than originally assumed. In the following paragraphs, we summarize the other potential muscular mechanisms of dynapenia.
In addition to muscle size and anatomic structure, aged muscle appears to differ in other compositional manners. For instance, over the past decade, numerous studies have reported that aging increases the adipocyte content between muscle groups (intermuscular adipose tissue) and between muscle fascicles (intramuscular adipose tissue) [15,104,105]. The earliest of these studies suggested that greater muscle fat content is associated with decreased muscle strength [105], suggesting a potential mechanistic link between increases in fat infiltration in muscle and muscle weakness. Indeed, cytokine production from adipose tissue has been linked to decreased muscle force production [106,107], thus providing a theoretical basis to this assertion. However, more recent longitudinal data have failed to observe a direct relation between increased levels of intermuscular adipose tissue and strength loss with age [15]. Other compositional changes have also been speculated to potentially alter the intrinsic force-generating properties of aged skeletal muscle, such as changes in the ratio of myosin to actin [108], the functional interaction of actin and myosin proteins [109-111], the expression of the thin filament regulatory proteins troponin and tropomyosin [112], and/or the expression of cytoskeletal proteins [113]. However, currently little attention has been paid to these potential contributors as they relates to dynapenia, and further work is needed to better delineate their relative contributions.
A likely muscular contributor to dynapenia is impairment in the excitation–contraction coupling processes, which are series of biophysical events involved in converting the electrical signal for muscle activation into contractile force. Theoretically speaking, the disruption of any of the events in the excitation–contraction coupling process could result in the suboptimal activation of muscle, thus decreasing muscle quality (force per unit tissue area), and contribute to dynapenia. In particular, impairments in calcium (Ca2+) release from the sarcoplasmic reticulum have been suggested to explain the deficits of muscle quality (the intrinsic force-generating capacity of skeletal muscle relative to its tissue size) in aged muscle [113-123]. The effects of aging on the principal proteins involved in voltage-induced Ca2+ release (e.g., ryanodine Y receptors and dihydropyridine receptors) have received the most scientific attention. These studies have indicated that older mammalian skeletal muscle exhibits decreases in the dihydropyridine receptor, namely the α-1s subunit, thus disrupting the voltage-induced Ca2+ release process [118-120]. We should also note that it has recently been suggested that sarcoplasmic reticulum Ca2+ release impairment can occur independent of dihydropyridine receptor function [113]. Specifically, Russ et al. [113] observed a decreased protein–protein interaction between the ryanodine Y receptor and FKBP older rat muscle, which they suggested may result in decreased muscle quality. It should be noted that the role for sarcoplasmic reticulum-related proteins that are not directly involved in Ca2+ release has begun to be investigated recently (e.g., junctophilin and mitsugumin 29). Some of these proteins, which are associated with the triadic junctions between the t-tubules and terminal cisternae, appear to decrease with age [122,123] and may negatively affect the structure–function relation of the sarcoplasmic reticulum and t-tubules.
What are future directions for research in dynapenia?
The obvious long-term goal of scientific investigations within this area is to develop effective interventions to prevent and treat dynapenia, which ultimately should lower the rates of physical limitations in eldery adults. However, to achieve this goal, we believe that several critical issues need to be urgently addressed. First, an objective definition of dynapenia that is agreed on by a consensus panel is needed. A definition would expand clinical research by setting a universal criterion that can be compared across clinical centers. Such consensus definitions are immensely helpful in providing clear goals for experimental interventions (nutritional or otherwise) and success in combating disease. There have been some attempts to generate a definition for sarcopenia (not dynapenia). In one attempt, the European Working Group on Sarcopenia in Older People treated sarcopenia as a syndrome by including gait speed and grip strength in the operational definition. According to their decision algorithm, all adults older than 65 y should be assessed for gait speed. It is then recommended that individuals with a gait speed slower than 0.80 m/s be tested for appendicular or total muscle mass that is used to diagnose sarcopenia. Older adults without gait speed impairments (>0.80 m/s) would perform a grip strength assessment. Individuals with low grip strength are then referred for measurement of appendicular or total muscle mass to diagnose sarcopenia. The algorithm certainly has some strong points—namely feasibility, because gait speed and grip strength testing could easily be conducted at small clinics. However, the cutpoints proposed for low muscle mass have not resulted in consistently significant associations with health outcomes [124, 125], misclassifies obese older adults [125], and has a marginal discriminative ability in identifying older adults at risk of disability (area under the receiver operator curve ~0.70) [126]. The sarcopenic syndrome approach opens a difficult pathway for treatment because each component has a complex etiology that likely requires different intervention and treatment strategies. Such complexity also makes it difficult to target “at-risk” individuals. A new approach proposed by Morley et al. [21] defines sarcopenia as “low muscle mass with limited mobility.” The definition uses sarcopenia as it was originally intended (>2 SD loss in muscle mass compared with 20- to 30-y-olds) but adds that individuals with a gait speed slower than 1.0 m/s are those who should be targeted for clinical trials. This approach does not include muscular strength as a component and as such stays true to the original definition of sarcopenia. A consensus operational definition for dynapenia has not been developed, but we previously suggested a decision algorithm to initiate discussions along these lines [24]. Specifically, we proposed that adults older than 60 y undergo a brief screening for major disease risk factors. Those with low risk factors conduct a grip strength test, which is known to have a high specificity for identifying those not at risk of dynapenia. Those who screen for a large number of risk factors or test positive for low grip strength then undergo a knee extension strength test that is used to determine if an individual is dynapenic. Unfortunately, the data to test the validity of the detection algorithm are not yet available, although the data to test other algorithms are not available either. Today, we are at a crossroads on how to best define and characterize sarcopenia and dynapenia, which has resulted in some confusion in the literature and among experts in the field. Although this was not intended per se, we believe these definitions and characterizations need to be simplified to promote the identification of target populations and the ability to conduct trials to determine if a given treatment strategy (e.g., therapeutic compound) is effective. One approach that we recently proposed surrounds developing a risk profile for low physical performance, which should likely include sarcopenia (as simply defined as the loss of, or low, muscle mass), dynapenia, and other potential contributors such as poor balance, depression, etc. The development of this risk profile would be similar to that seen in cardiovascular disease, which includes hypercholesterolemia, hypertension, and hyperglycemia as independent risk factors. Each factor has its own disease etiology and definition that can be targeted for interventions through changes in behavior, nutrition, and addition of pharmaceuticals. Such an approach could be developed for the loss in physical function—a multifactorial condition that includes sarcopenia and dynapenia and other risk factors yet to be fully determined.
Second, we need to identify biological contributors to dynapenia. That is, we need to better understand what is causing muscle weakness in older adults, and whether these specific contributors vary from person to person or clinical population to clinical population. Because studying the mechanisms of volitional force and movement control is difficult in animal studies—and because drawing cause-and-effect conclusions from human studies using a cross-sectional design is difficult—prospective, longitudinal cohort studies are urgently needed or, at a minimum, case–control studies that evaluate the potential biological contributors to dynapenia. Knowledge of this nature is needed to identify new therapeutic targets to alleviate dynapenia.
What role does nutrition play in dynapenia?
There is a substantial amount of literature examining the effects of dietary nutrients on the overall health of the nervous and muscular systems [127-129]; however, considerably less is known about the specific role of micronutrients on muscle strength. The emerging literature that does exist has largely focused on the effects of vitamins D and E, selenium, and carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene). These findings have suggested that low levels of vitamin E [130-132], carotenoids [130,133], and selenium (that below a level that limits the synthesis of selenoproteins) [134,135] are associated with lower muscle strength. The mechanisms that dictate these associations are not completely understood, but are likely related to the control of oxidative stress that damages DNA, proteins, and lipids with aging. Interestingly, there is some evidence that carotenoid and selenium plasma concentrations are associated with inflammation (i.e., interleukin-6) [136], which is associated with lower levels of muscle strength [137-139]. Additional studies are needed to determine whether supplementation of carotenoids and selenium decrease inflammation to an extent that would affect muscle strength in elders.
In addition to the micronutrients mentioned earlier, there has been growing interest over the past 5 y on the impact of vitamin D on muscle (for review, please see Annweiler et al. [140]). Vitamin D receptors on muscle initiate the nuclear response leading to de novo protein synthesis and the activity of these receptors decreases with aging [141]. In addition, there is evidence that vitamin D receptors located on the hippocampus have a role in neuromuscular function [142]. However, the literature is conflicting on the association of vitamin D levels with muscle strength. For example, a recent review found that five observational studies demonstrated a relation between vitamin D and physical performance, whereas three studies found no such association [140]. In clinical trials, the evidence is even more contentious, with four studies finding a significant effect of vitamin D supplementation on muscle strength and three studies finding no effect [140]. More recently, Janssen et al. [143] found that supplementing the diet with cholecalciferol (400 IU/d + calcium 500 mg/d) in women with low 25-hydroxyvitamin D levels did not change muscle strength compared with a group of individuals taking a placebo. This finding is consistent with a more recent study indicating that 150 000 IU of oral cholecalciferol delivered every 3 mo does not alter muscle strength [144]. Although more recent trials have not established a benefit of vitamin D supplementation on muscle strength, there remains controversy about the dosage, participant selection, level of plasma 25-hydroxyvitamin D needed to intervene, and the potential role of the parathyroid hormone [145-147]. As such, the effect of vitamin D on muscle strength requires further investigation.
Summary
Dynapenia is the age-associated loss of muscle strength. The biologic contributors to dynapenia are likely multifactorial and includes the nervous and muscle systems. However, the complex nature of dynapenia should not deter efforts that offer clear-cut definitions and knowledge about risk factors. We could learn much from other disciplines, where the complex etiology of the disease has not prevented an agreed-on consensus definition and clear risk factors that predispose individuals (e.g., hypertension, hypercholesterolemia, diabetes etc.). The clinical consequences of dynapenia are significant, because it increases the risk for functional limitations, disability, and mortality. Future work originating from many scientific disciplines, e.g., epidemiology and physiology, is required to provide the fundamental knowledge needed to eventually develop effective interventions to prevent and treat dynapenia.
Acknowledgments
This work was supported in part by grant R15HD065552 from the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development to B. C. Clark. T. M. Manini was supported by grants R21AG031974 and P30AG028740 from the National Institutes of Health’s National Institute on Aging.
References
- 1.CDC. Statistics FIFoA-R edition. Washington, DC: US Government Printing Office; 2008. Older Americans 2008: key indicators of well-being. [Google Scholar]
- 2.Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–7. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Harris TB, Fox KM, Hawkes W, Hebel JR, Yahiro JY, et al. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci. 2000;55:M434–40. doi: 10.1093/gerona/55.8.m434. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–6. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasselgren L, Olsson LL, Nyberg L. Is leg muscle strength correlated with functional balance and mobility among inpatients in geriatric rehabilitation? Arch Gerontol Geriatr. 2011;52:e220–5. doi: 10.1016/j.archger.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–52. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Xue QL, Beamer BA, Chaves PH, Guralnik JM, Fried LP. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: the women’s health and aging study II. J Am Geriatr Soc. 2010;58:2076–84. doi: 10.1111/j.1532-5415.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takata Y, Ansai T, Soh I, Awano S, Yoshitake Y, Kimura Y, et al. Physical fitness and 6.5-year mortality in an 85-year-old community-dwelling population. Arch Gerontol Geriatr. 2011;54:28–33. doi: 10.1016/j.archger.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Artero EG, Lee DC, Ruiz JR, Sui X, Ortega FB, Church TS, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol. 2011;57:1831–7. doi: 10.1016/j.jacc.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark BC, Fernhall B, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and applied ischemia efficacy. J Appl Physiol. 2006;101:256–63. doi: 10.1152/japplphysiol.01402.2005. [DOI] [PubMed] [Google Scholar]
- 14.Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting: II. Neurological properties and motor imagery efficacy. J Appl Physiol. 2006;101:264–72. doi: 10.1152/japplphysiol.01404.2005. [DOI] [PubMed] [Google Scholar]
- 15.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- 17.Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–16. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–7. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan WJ, Hall MR, Timothy JI, Robinson M. Is weakness in old age due to muscle wasting? Age Ageing. 1980;9:188–92. doi: 10.1093/ageing/9.3.188. [DOI] [PubMed] [Google Scholar]
- 20.Clark BC, Manini TM. Sarcopenia=/=dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 23.Xue QL, Walston JD, Fried LP, Beamer BA. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the women’s health and aging study. Arch Intern Med. 2011;171:1119–21. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 24.Manini TM, Clark BC. Dynapenia and Aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81:107–15. doi: 10.1093/jnci/81.2.107. [DOI] [PubMed] [Google Scholar]
- 26.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 27.Clark BC. In vivo alterations in skeletal muscle form and function after disuse atrophy. Med Sci Sports Exerc. 2009;42:363–72. doi: 10.1249/MSS.0b013e3181a645a6. [DOI] [PubMed] [Google Scholar]
- 28.Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol. 2008;105:868–78. doi: 10.1152/japplphysiol.90530.2008. [DOI] [PubMed] [Google Scholar]
- 29.Clark BC, Pierce JR, Manini TM, Ploutz-Snyder LL. Effect of prolonged unweighting of human skeletal muscle on neuromotor force control. Eur J Appl Physiol. 2007;100:53–62. doi: 10.1007/s00421-007-0399-6. [DOI] [PubMed] [Google Scholar]
- 30.Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve. 2011;42:363–72. doi: 10.1002/mus.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchateau J, Enoka RM. Neural adaptations with chronic activity patterns in able-bodied humans. Am J Phys Med Rehabil. 2002;81(11 Suppl):S17–27. doi: 10.1097/00002060-200211001-00004. [DOI] [PubMed] [Google Scholar]
- 32.Clark BC, Taylor JL. Age-Related Changes in Motor Cortical Properties and Voluntary Activation of Skeletal Muscle. Curr Aging Sci. 2011;4:192–9. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc. 2010;42:1644–50. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- 34.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–7. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 35.Delbono O. Expression and Regulation of Excitation-Contraction Coupling Proteins in Aging Skeletal Muscle. Curr Aging Sci. 2011;4:248–59. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russ DW, Grandy JS, Toma K, Ward CW. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol (Oxf) 2011;201:391–403. doi: 10.1111/j.1748-1716.2010.02191.x. [DOI] [PubMed] [Google Scholar]
- 37.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–51. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 38.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 39.Taylor JL, de Haan A, Gerrits KH, de Ruiter CJ. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107:354–7. doi: 10.1152/japplphysiol.91220.2008. [DOI] [PubMed] [Google Scholar]
- 40.Ashe J. Force and the motor cortex. Behav Brain Res. 1997;87:255–69. doi: 10.1016/s0166-4328(97)00752-3. [DOI] [PubMed] [Google Scholar]
- 41.Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behm DG, Whittle J, Button D, Power K. Intermuscle differences in activation. Muscle Nerve. 2002;25:236–43. doi: 10.1002/mus.10008. [DOI] [PubMed] [Google Scholar]
- 43.Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–64. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24:98–106. doi: 10.1002/1097-4598(200101)24:1<98::aid-mus11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 45.Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon J, Kay D, Tarpenning KM, Marino FE. Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct Imaging. 2007;27:91–100. doi: 10.1111/j.1475-097X.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 47.Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol. 2007;103:1628–35. doi: 10.1152/japplphysiol.00320.2007. [DOI] [PubMed] [Google Scholar]
- 48.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–52. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- 49.De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–91. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- 50.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–9. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol. 2008;105:1199–209. doi: 10.1152/japplphysiol.01246.2007. [DOI] [PubMed] [Google Scholar]
- 52.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–62. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- 53.Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–9. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- 54.Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol. 2005;99:31–8. doi: 10.1152/japplphysiol.01426.2004. [DOI] [PubMed] [Google Scholar]
- 55.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91:1341–9. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- 56.Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11:405–12. doi: 10.1016/s1050-6411(01)00023-2. [DOI] [PubMed] [Google Scholar]
- 57.Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–75. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- 58.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 59.Simoneau E, Martin A, Van Hoecke J. Muscular performances at the ankle joint in young and elderly men. J Gerontol A Biol Sci Med Sci. 2005;60:439–47. doi: 10.1093/gerona/60.4.439. [DOI] [PubMed] [Google Scholar]
- 60.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- 61.Wilder MR, Cannon J. Effect of age on muscle activation and twitch properties during static and dynamic actions. Muscle Nerve. 2009;39:683–91. doi: 10.1002/mus.21233. [DOI] [PubMed] [Google Scholar]
- 62.Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999;54:M249–53. doi: 10.1093/gerona/54.5.m249. [DOI] [PubMed] [Google Scholar]
- 63.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–46. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 64.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging. 1991;12:336–8. doi: 10.1016/0197-4580(91)90013-a. [DOI] [PubMed] [Google Scholar]
- 65.Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–54. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 67.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- 68.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–81. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Kossev AR, Schrader C, Dauper J, Dengler R, Rollnik JD. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333:83–6. doi: 10.1016/s0304-3940(02)00986-2. [DOI] [PubMed] [Google Scholar]
- 70.McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–8. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99:1483–93. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- 72.Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- 73.Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–96. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–6. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32:747–60. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 76.Fathi D, Ueki Y, Mima T, Koganemaru S, Nagamine T, Tawfik A, Fukuyama H. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol. 2009;121:90–3. doi: 10.1016/j.clinph.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 77.Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53:521–4. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- 78.Siu PM, Alway SE. Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front Biosci. 2009;14:432–52. doi: 10.2741/3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 80.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–93. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–48. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 82.Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res. 1995;104:167–70. doi: 10.1007/BF00229867. [DOI] [PubMed] [Google Scholar]
- 83.Brooke JD, Singh R, Wilson MK, Yoon P, McIlroy WE. Aging of human segmental oligosynaptic reflexes for control of leg movement. Neurobiol Aging. 1989;10:721–5. doi: 10.1016/0197-4580(89)90009-2. [DOI] [PubMed] [Google Scholar]
- 84.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–13. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- 85.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 86.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–95. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- 87.Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clinical orthopaedics and related research. 2006;450:231–7. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- 88.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences. 1988;84:275–94. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 89.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 90.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 91.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 92.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 94.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fry CS, Rasmussen BB. Skeletal Muscle Protein Balance and Metabolism in the Elderly. Curr Aging Sci. 2011;4:260–8. doi: 10.2174/1874609811104030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 97.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 98.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–62. [PMC free article] [PubMed] [Google Scholar]
- 99.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107:1655–62. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288:E922–9. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 104.Song MY, Ruts E, Kim J, Janumala I, Heymsfueld S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–80. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 105.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 106.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–84. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 107.Wilcox P, Osborne S, Bressler B. Monocyte inflammatory mediators impair in vitro hamster diaphragm contractility. Am Rev Respir Dis. 1992;146:462–6. doi: 10.1164/ajrccm/146.2.462. [DOI] [PubMed] [Google Scholar]
- 108.Thompson LV, Durand D, Fugere NA, Ferrington DA. Myosin and actin expression and oxidation in aging muscle. J Appl Physiol. 2006;101:1581–7. doi: 10.1152/japplphysiol.00426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Höök P, Li X, Sleep J, Hughes S, Larsson L. In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J Physiol. 1999;520(Pt 2):463–71. doi: 10.1111/j.1469-7793.1999.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–49. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 111.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–7. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 112.Donoghue P, Staunton L, Mullen E, Manning G, Ohlendieck K. DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J Proteomics. 2010;73:1441–53. doi: 10.1016/j.jprot.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 113.Russ DW, Grandy JS. Increased desmin expression in hindlimb muscles of aging rats. J Cachexia Sarcopenia Muscle. 2011;2:175–80. doi: 10.1007/s13539-011-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boncompagni S, d’Amelio L, Fulle S, Fano G, Protasi F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance. J Gerontol A Biol Sci Med Sci. 2006;61:995–1008. doi: 10.1093/gerona/61.10.995. [DOI] [PubMed] [Google Scholar]
- 115.Delbono O. Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle. Biogerontology. 2002;3:265–70. doi: 10.1023/a:1020189627325. [DOI] [PubMed] [Google Scholar]
- 116.Delbono O, Renganathan M, Messi ML. Excitation-Ca2+release-contraction coupling in single aged human skeletal muscle fiber. Muscle Nerve Suppl. 1997;5:S88–92. doi: 10.1002/(sici)1097-4598(1997)5+<88::aid-mus21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol. 2000;178:175–83. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- 118.Renganathan M, Delbono O. Caloric restriction prevents age-related decline in skeletal muscle dihydropyridine receptor and ryanodine receptor expression. FEBS Lett. 1998;434:346–50. doi: 10.1016/s0014-5793(98)01009-6. [DOI] [PubMed] [Google Scholar]
- 119.Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, et al. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J Cell Biol. 2006;174:639–45. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weisleder N, Ma JJ. Ca2+ sparks as a plastic signal for skeletal muscle health, aging, and dystrophy. Acta Pharmacol Sin. 2006;27:791–8. doi: 10.1111/j.1745-7254.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 121.Moreno RJ, Messi ML, Zheng Z, Wang ZM, Ye P, D’Ercole JA, Delbono O. Role of sustained overexpression of central nervous system IGF-I in the age-dependent decline of mouse excitation-contraction coupling. J Membr Biol. 2006;212:147–61. doi: 10.1007/s00232-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 122.Wang ZM, Messi ML, Delbono O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78:1947–54. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas MM, Vigna C, Betik AC, Tupling AR, Hepple RT. Initiating treadmill training in late middle age offers modest adaptations in Ca2+ handling but enhances oxidative damage in senescent rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1269–78. doi: 10.1152/ajpregu.00663.2009. [DOI] [PubMed] [Google Scholar]
- 124.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 125.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 126.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 127.Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutri Biochem. 2010;21:1–13. doi: 10.1016/j.jnutbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 128.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391–6. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kurpad AV, Vaz M. Protein and amino acid requirements in the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S131–42. doi: 10.1038/sj.ejcn.1601035. [DOI] [PubMed] [Google Scholar]
- 130.Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61:594–9. doi: 10.1093/gerona/61.6.594. [DOI] [PubMed] [Google Scholar]
- 131.Bartali B, Frongillo EA, Guralnik JM, Stipanuk MH, Allore HG, Cherubini A, et al. Serum micronutrient concentrations and decline in physical function among older persons. JAMA. 2008;299:308–15. doi: 10.1001/jama.299.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ble A, Cherubini A, Volpato S, Bartali B, Walston JD, Windham BG, et al. Lower plasma vitamin E levels are associated with the frailty syndrome: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:278–83. doi: 10.1093/gerona/61.3.278. [DOI] [PubMed] [Google Scholar]
- 133.Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Giacomini V, Corsi AM, et al. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63:376–83. doi: 10.1093/gerona/63.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lauretani F, Semba RD, Bandinelli S, Ray AL, Guralnik JM, Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: the InCHIANTI Study. Am J Clin Nutr. 2007;86:347–52. doi: 10.1093/ajcn/86.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beck J, Ferrucci L, Sun K, Walston J, Fried LP, Varadhan R, et al. Low serum selenium concentrations are associated with poor grip strength among older women living in the community. Biofactors. 2007;29:37–44. doi: 10.1002/biof.5520290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 137.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526–9. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 139.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 140.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–8. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 141.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Min Res. 2004;19:265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 142.Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104:49–56. doi: 10.1016/s0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 143.Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clin Exp Res. 2010;22:78–84. doi: 10.1007/BF03324819. [DOI] [PubMed] [Google Scholar]
- 144.Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility and muscle strength in older postmenopausal women: a randomised controlled trial. J Bone Min Res. 2011 doi: 10.1002/jbmr.524. [DOI] [PubMed] [Google Scholar]
- 145.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 146.Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, III, Amin S. Is vitamin D a determinant of muscle mass and strength? J Bone Min Res. 2011 doi: 10.1002/jbmr.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]


