Abstract
Amines have been used as sacrificial electron donors to reduce photoexcited Ru(II) or Ir(III) complexes, during which they are oxidized to nitrogen radical cations. Recently, the synthetic potential of these nitrogen radical cations have caught synthetic organic chemists’ attention. They have been exploited in various transformations yielding a number of elegant methods for amine synthesis. This article highlights recent developments on nitrogen radical cation chemistry under visible-light photocatalysis.
Keywords: amines, visible light, nitrogen radical cations, ruthenium, iridium
The ubiquity of amines in bioactive compounds and pharmaceuticals has turned methodology development for amine synthesis into a fertile research field.1 Although a number of methods are available for amine synthesis, sustainability for most of them still leave much to be desired. The ACS Green Chemistry Institute (GCI) pharmaceutical roundtable identified a wish list of 14 ‘more aspirational reactions,’ and five of them were related to amine synthesis.2 As such, there is a strong push from the organic community to use environmentally benign resources such as sunlight in methodology development. More importantly, the use of sunlight allows us to tap into the synthetic potential of underutilized reactive species, such as nitrogen radical cations in new ways. Herein we highlight some recent developments in photoredox-generated nitrogen radical cation chemistry, including our inspiration and current research efforts in this field.
Ruthenium and iridium polypyridyl complexes are two commonly used classes of photoredox catalysts that can be activated by visible light.3,4 The photoredox properties of ruthenium(II) polypyridyl complexes have been known for decades for its utility in water splitting5a and solar cells5b dating back to 1970. However, the acceptance of photoredox catalysts6 in the synthetic organic community has been rather muted until 2008 when MacMillan7 and Yoon8 groups published two seminal papers in the field. Subsequently, works from Stephenson,9 Akita,10 and others have expanded this field.11,12
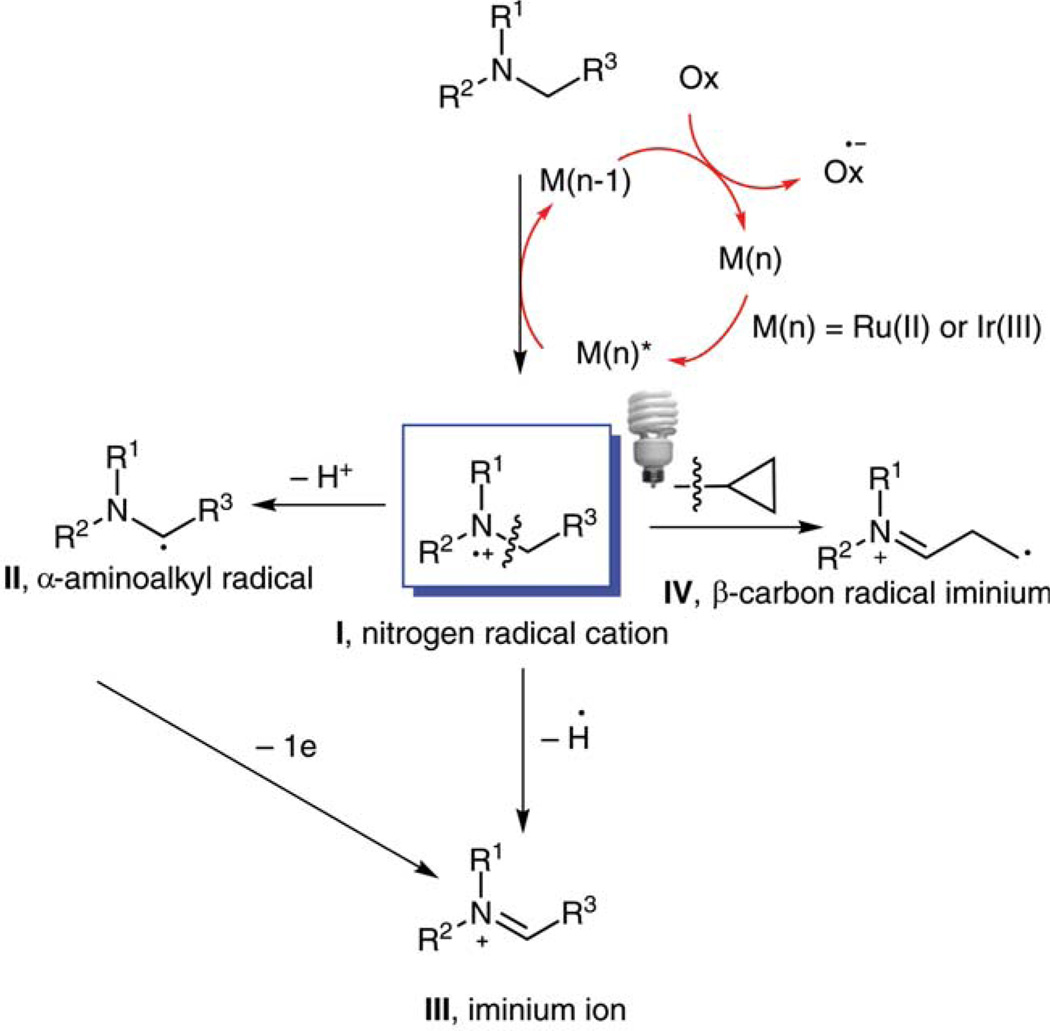
Amines often act as single-electron donors in photoredox processes and are subsequently oxidized to nitrogen radical cations I. Depending on the reaction conditions, the fate of these nitrogen radical cations varies (Scheme 1). It can lose a proton to form a nucleophilic α-aminoalkyl radical II. It can lose a hydrogen radical to produce iminium ion III. Radical II can be converted to iminium III by losing an electron. When the amines are substituted with a cyclopropane ring, the nitrogen radical cations undergo ring opening to generate a β-carbon radical iminium IV.
Scheme 1.
Reaction pathways of nitrogen radical cations under visible-light photocatalysis
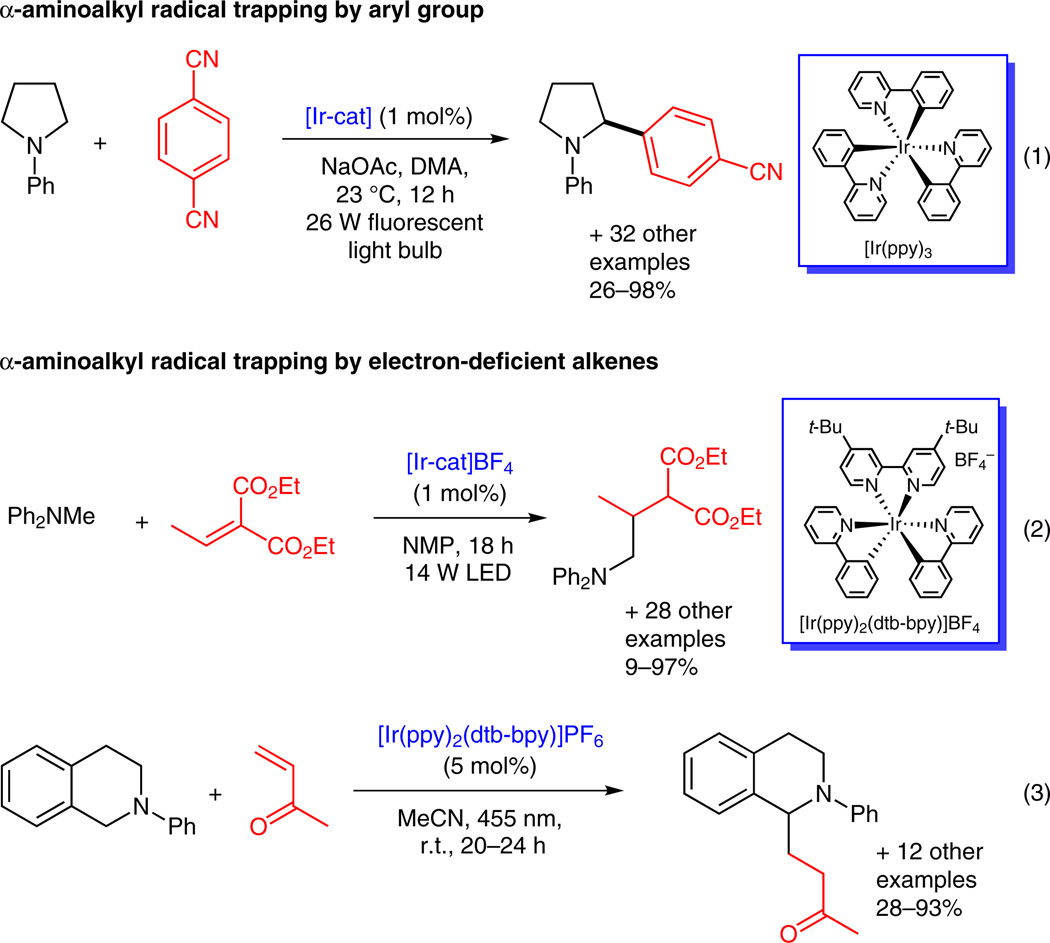
In 1977, Whitten’s group proposed that, in the presence of visible-light-activated ruthenium(II) complexes, triethylamine underwent the reactions from I to II and then to III.13 This hypothesis was supported by detection of acetaldehyde, presumably from hydrolysis of the iminium ion. However, the synthetic potential of the nucleophilic α-aminoalkyl radical II has not been explored until recently. MacMillan’s group reported the synthesis of benzylic and heterobenzylic amines via an α-amino C–H arylation reaction (Scheme 2, equation 1).14a The nitrogen radical cation generated from the one-electron oxidation of a tertiary amine was then deprotonated by NaOAc to provide the α-aminoalkyl radical. The radical was subsequently coupled with a 1,4-dicyanobenzene-derived arene radical anion to form a benzylic or heterobenzylic amine. Nishibayashi and co-workers demonstrated that α-aminoalkyl radicals can undergo 1,4-addition to α,β-unsaturated esters. They used α-aminoalkyl radicals to add intermolecularly to doubly activated alkenes, furnishing alkylated amines (Scheme 2, equation 2).14b Reiser’s and Pandey’s groups jointly reported that α-amino radicals can add to α,β-unsaturated enones, yielding 1,4-adducts (Scheme 2, equation 3).14c
Scheme 2.
Utilization of α-aminoalkyl radical in C–H functionalization
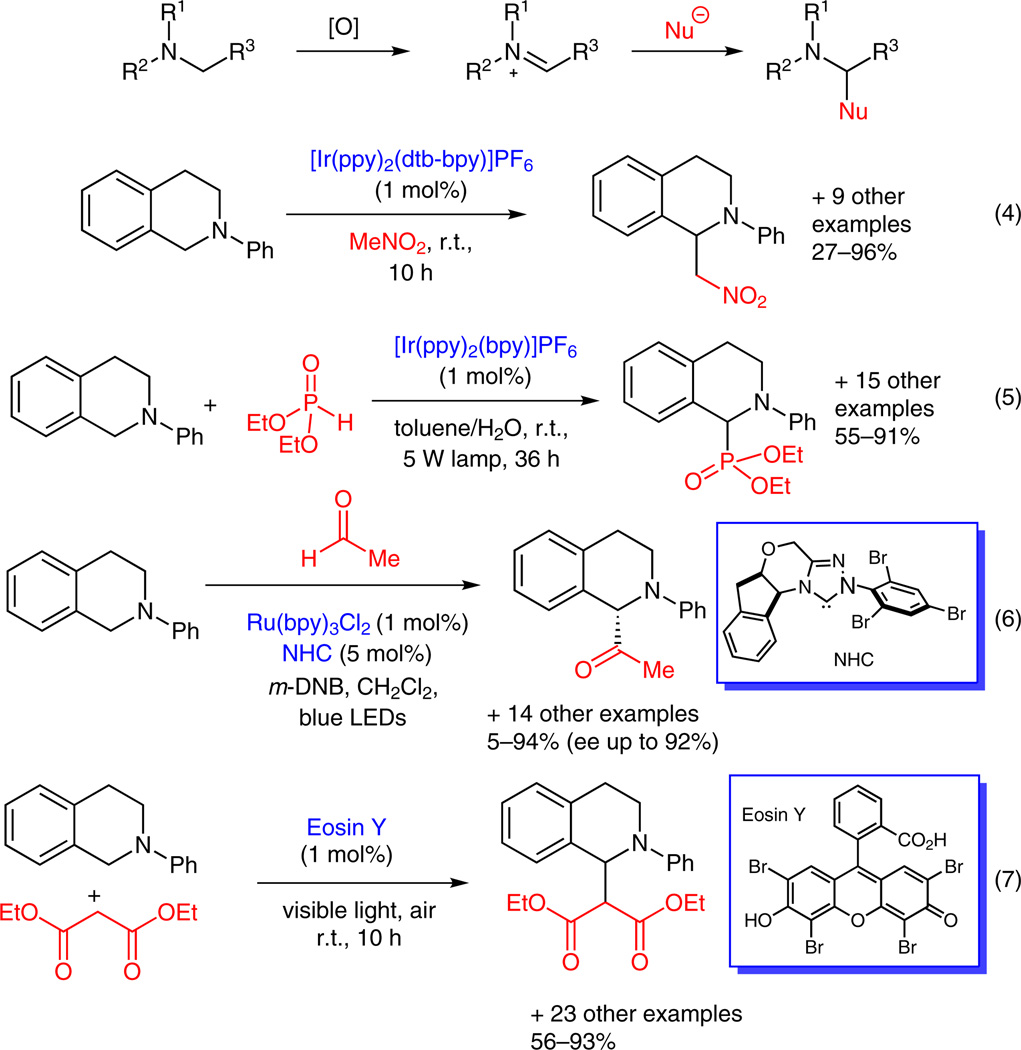
N-Aryltetrahydroisoquinolines are readily oxidized to iminium ions under photoredox conditions. Stephenson’s group has shown that iminium ions can be intercepted by the anion of MeNO2 (solvent), to provide aza-Henry products in modest to excellent yields (Scheme 3, equation 4).15a,b Rueping has extended this mild C–H functionalization strategy to an oxidative phosphonylation reaction, in which an sp3-benzylic carbon is activated via the formation of an iminium ion.15c Then diethylphosphite is added to the iminium ion to form a C–P bond (Scheme 3, equation 5). Rovis’ group has merged the photoredox catalysis with N-heterocyclic carbene (NHC) catalysis to develop a method for asymmetric α-acylation of tertiary amines (Scheme 3, equation 6).15d Acyl anions of aldehydes generated by NHC catalysis were added to iminium ions produced under photoredox conditions to afford α-amino ketones in good yield and high enantioselectivity.
Scheme 3.
Utilization of iminium ions in C–H functionalization
In parallel with the efforts towards developing new photoredox reactions catalyzed by ruthenium or iridium polypyridyl complexes, there has been a recent upsurge in the search for less expensive substitutes for the metal complexes. König reported that Eosin Y, an organic dye, can promote C–C and C–P bond formation from the sp3 C–H bonds of N-aryltetrahydroisoquinolines (Scheme 3, equation 7).15e Rose Bengal with graphene oxide (GO), TiO2 nanoparticles, and ZnO nanoparticles have also been shown to catalyze these visible-light-mediated transformations.15f,g Reiser recently revealed that Cu(dap)2Cl is as effective as ruthenium or iridium polypyridyl complexes to promote atom-transfer radical additions (ATRA) to olefins when exposed to LED green light. It also catalyzed allylation of α-halocarbonyl compounds, whereas ruthenium or iridium polypyridyl complexes have not been reported.15h
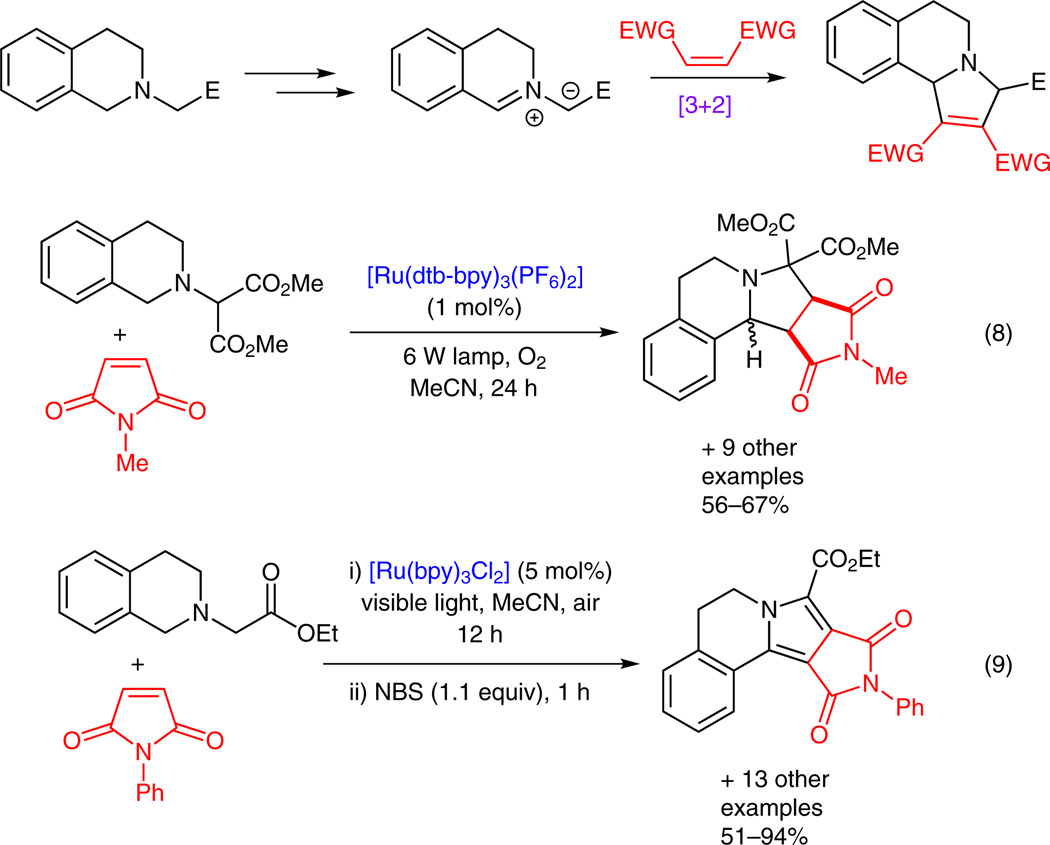
When a tetrahydroisoquinoline is substituted with an activated methylene group, an iminium ion generated in situ via photoredox catalysis can be converted to an azomethine ylide upon deprotonation (Scheme 4). Rueping and Xiao have independently demonstrated the feasibility of this new approach for generating azomethine ylides and subsequently applied them to [3+2] dipolar cycloaddition. Employing N-methylmaleimide as the dipolarophile, Rueping synthesized pyrrolo[2.1-a]isoquinolines (Scheme 4, equation 8).15i Xiao used N-phenylmaleimide as the dipolarophile in a [3+2] dipolar cycloaddition that was followed by NBS-promoted oxidation to generate dihydropyrrolo[2.1-a]isoquinolines (Scheme 4, equation 9).15j
Scheme 4.
Utilization of iminium ions in [3+2] cycloaddition reactions
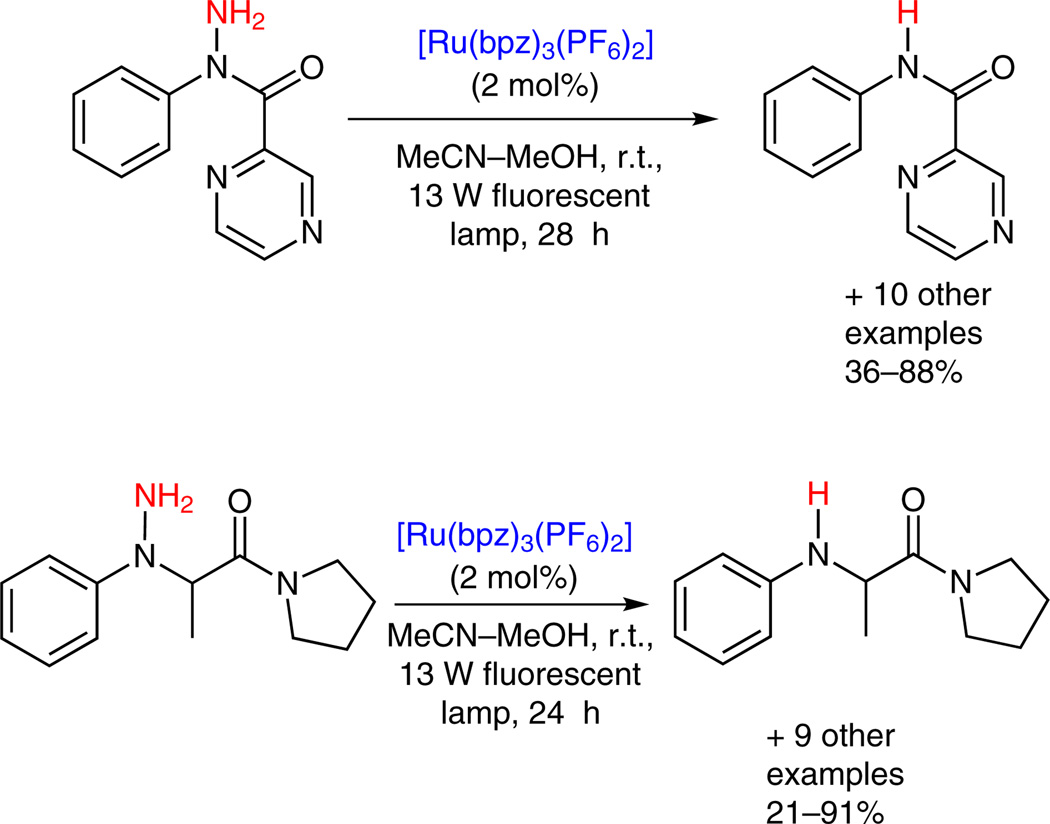
Our foray into visible-light photoredox catalysis began with the cleavage of N–N bonds of hydrazine and hydrazides (Scheme 5).11a These two classes of compounds were selected based on the results of a screening study on the reducing ability of amine derivatives upon exposure to light-activated Ru(bpz)3(PF6)2.16 N-Methyl-N-phenylhydrazine was among the strongest reductants screened and was photochemically converted to N-methylaniline via cleavage of the N–N bond. We subsequently examined this cleavage reaction in more depth by optimizing the catalytic system and investigating the substrate scope. Ru(bpz)3(PF6)2, developed by Lever in 1980,17 proved to be a more effective catalyst than Ru(bpy)32+ due to its higher redox potential [1.3 V vs. 0.78 V for Ru(bpy)32+*/Ru(bpy)31+]. A 13 W compact fluorescent lightbulb was sufficient to activate Ru(bpz)3(PF6)2. Air proved to be a better stoichiometric oxidant than pure oxygen because the latter yielded more side products. This catalytic system was found to be generally effective for N,N-disubstituted hydrazines and hydrazides when at least one of the two substituents was an aryl or heteroaryl group.
Scheme 5.
Photoinduced cleavage of N–N bonds of hydrazines and hydrazides
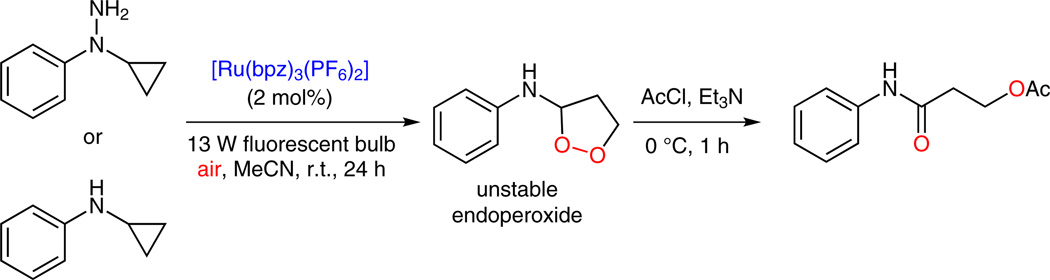
However, when N-cyclopropyl-N-phenylhydrazine was subjected to the optimized catalytic system, none of the desired cleavage product, N-cyclopropylaniline, was observed, but interestingly, a new and unstable product was produced (Scheme 6). Extensive studies18 of this reaction and a related report of aerobic opening of N-cyclopropylaniline19 revealed that the new product was an endoperoxide, whose structure was further confirmed by converting it to β-acetoxyamide by treatment with AcCl.
Scheme 6.
[3+2] Annulation with oxygen, formation of endoperoxide
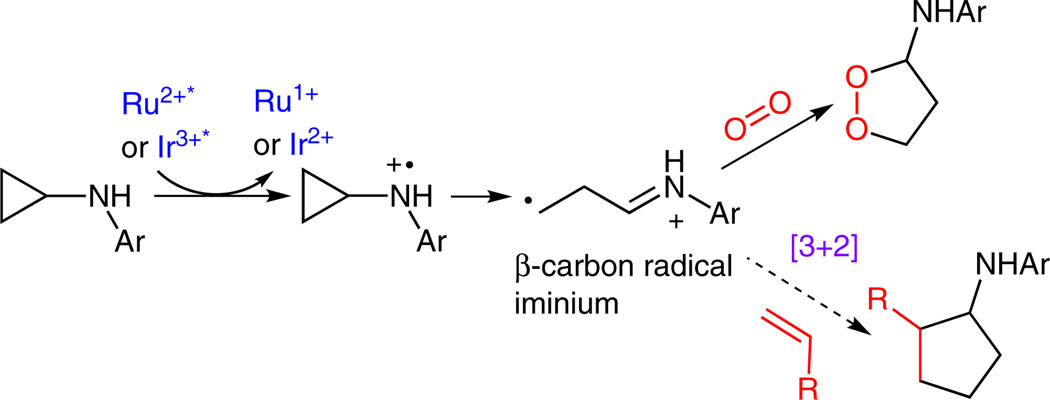
We hypothesized that the nitrogen radical cation derived from N-cyclopropylaniline promoted an irreversible ring opening of the cyclopropyl moiety, thereby generating a β-carbon radical iminium ion (Scheme 7). The radical iminium ion was then intercepted by O2 to form the endoperoxide product. Conceivably, if O2 were replaced by an olefin, a [3+2] annulation product would form to afford two new C–C bonds.
Scheme 7.
Design of the [3+2] annulation reaction
Cyclopropylamines are useful building blocks for synthesizing complex amines due to their proclivity toward ring opening upon oxidation to a nitrogen radical cation. Yet they have seen limited use in organic synthesis to date.20 For those reported applications, nitrogen radical cations were typically produced by UV light with a photosensitizer or a strong oxidant (e.g., CAN), which limits functional-group compatibility and the substrate scope of such methods.
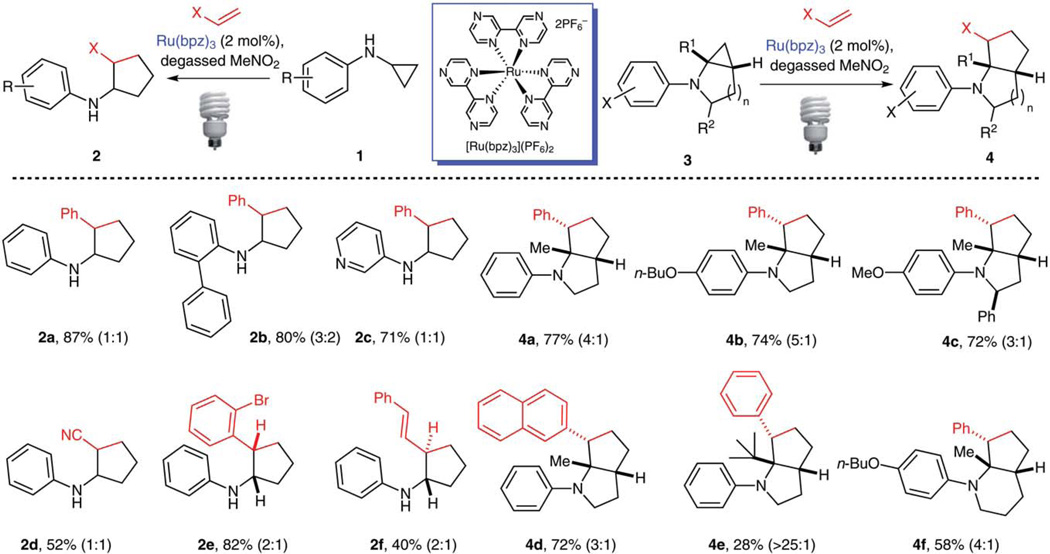
A variety of mono- and bicyclic cyclopropylamines were prepared by Buchwald–Hartwig cross-coupling reactions of arylbromides with cyclopropylamines and the Kulinkovich–de Meijere reaction of amides, respectively. Both classes of cyclopropylamines were found to be viable substrates for the [3+2] annulation reaction (Scheme 8).11b Some salient features of this reaction included 100% atom economy, no need for external oxidants, and facile initiation by 13 W compact fluorescent lightbulbs. For example, fused 5-5 bicyclic heterocycles 4a–d were obtained from [3.1.0]bicyclic cyclopropylamines with diastereoselectivities ranging from 3:1 to 5:1. Replacement of the angular methyl group by a bulky tert-butyl group dramatically increased the diastereoselectivity as a single diastereoisomer 4e was formed. Additionally, [4.1.0]bicyclic cyclopropylamine participated in the annulation reaction to afford fused 6-5 bicyclic heterocycle 4f. On the other hand, monocyclic cyclopropylamines showed little to no diastereoselectivity (2a–d). The conjugated diene preferentially reacted at the terminal double bond to provide trans-annulated cyclopentane 2f. Conversely, the annulation reaction of N-phenyl cyclopropane with 2-bromostyrene gave a cis-annulated cyclopentane 2d. Only modest diastereoselectivity (2:1) was achieved for both cases. Studies directed toward expanding the scope of the [3+2] annulation reaction to other classes of amines and carbon–carbon π bonds are under way.
Scheme 8.
Scope of the [3+2] annulation reaction
Society’s desire for sustainability has brought visible-light photocatalysis to the forefront of organic chemistry research. The use of visible light, operational simplicity and high atom economy of these processes embody the principles of sustainable chemistry. Ruthenium and iridium polypyridyl complex mediated SET reactions have opened new avenues for forming C–C and C–N bonds. We believe that the role of visible-light photocatalysis in method development has a bright future.
Acknowledgment
We thank the University of Arkansas, the Arkansas Bioscience Institute, and the NIH NCRR COBRE grant (P30 RR031154 and P30 GM103450-03) for generous support of this research. We also thank Professor Bill Durham for insightful discussions on photochemistry.
Biography

Dr. Soumitra Maity (right), originally from West Bengal (India), received his BSc in Chemistry from Ramakrishna Mission Residential College, Narendrapur in 2002 and MSc in 2004 from Calcutta University. His PhD research was conducted at the Indian Association for the Cultivation of Science (Kolkata, India) and was focused on the synthesis of natural products with Professor Subrata Ghosh. After successfully defending his PhD thesis, he moved to USA in September 2010 to work with Professor Zheng at the University of Arkansas to develop new methods for the formation of C–C and C–N bonds by visible-light photocatalysis. Professor Nan Zheng (left) was born in China and did his undergraduate studies at the University of Science and Technology of China (USTC). He earned his PhD (2005) under the tutorship of Professor William R. Roush at the University of Michigan, Ann Arbor (USA). Then he took up a NIH postdoctoral position in Professor Stephen L. Buchwald’s group at MIT (USA). Since July 2008, he has been an Assistant Professor at the University of Arkansas, Fayetteville (USA). His independent research is focused on visible-light photocatalysis.
References
- 1.(a) Joule JA, Mills K. Heterocyclic Chemistry. 5th ed. Wiley: Chichester; 2010. [Google Scholar]; (b) Mack DJ, Weinrich ML, Vitaku E, Njarðarson JT. Top 200 Brand Name Drugs by US Retail Sales in 2010. http://cbc.arizona.edu/njardarson/group/top-pharmaceuticals-poster. [Google Scholar]
- 2.Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JL, Jr, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY. Green. Chem. 2007;9:411. [Google Scholar]
- 3.(a) Inagaki A, Akita M. Coord. Chem. Rev. 2010;254:1220. [Google Scholar]; (b) Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, Zelewsky AV. Coord. Chem. Rev. 1988;84:85. [Google Scholar]; (c) Kalyanasundaram K, Grätzel M. Coord. Chem. Rev. 1998;177:347. [Google Scholar]
- 4.(a) Lowry MS, Bernhard S. Chem.–Eur. J. 2006;12:7970. doi: 10.1002/chem.200600618. [DOI] [PubMed] [Google Scholar]; (b) Flamigni L, Barbieri A, Sabatini C, Ventura B, Barigelletti F. Top. Curr. Chem. 2007;281:143. [Google Scholar]
- 5.(a) Sprintschnik G, Sprintschnik HW, Kirsch PP, Whitten DG. J. Am. Chem. Soc. 1976;98:2337. [Google Scholar]; (b) O’Regan B, Grätzel M. Nature (London) 1991;353:737. [Google Scholar]
- 6.For recent reviews on photoredox catalysis, see: Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617. doi: 10.1021/jo202538x. Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. Teplý F. Collect. Czech. Chem. Commun. 2011;76:859. Yoon TP, Ischay MA, Du J. Nat. Chem. 2010;2:527. doi: 10.1038/nchem.687. Zeitler K. Angew. Chem. Int.. Ed. 2009;48:9785. doi: 10.1002/anie.200904056.
- 7.(a) Nagib DA, MacMillan DWC. Nature (London) 2011;480:224. doi: 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pham PV, Nagib DA, MacMillan DWC. Angew. Chem. Int. Ed. 2011;50:6119. doi: 10.1002/anie.201101861. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Lin S, Ischay MA, Fry CG, Yoon TP. J. Am. Chem. Soc. 2011;133:19350. doi: 10.1021/ja2093579. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu Z, Shen M, Yoon TP. J. Am. Chem. Soc. 2011;133:1162. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ischay MA, Lu Z, Yoon TP. J. Am. Chem. Soc. 2010;132:8572. doi: 10.1021/ja103934y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Wallentin C-J, Nguyen JD, Finkbeiner P, Stephenson CRJ. J. Am. Chem. Soc. 2012;134:8875. doi: 10.1021/ja300798k. [DOI] [PubMed] [Google Scholar]; (b) Furst L, Narayanam JMR, Stephenson CRJ. Angew. Chem. Int. Ed. 2011;50:9655. doi: 10.1002/anie.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dai C, Narayanam JMR, Stephenson CRJ. Nat. Chem. 2011;3:140. doi: 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]; (d) Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- 10.(a) Koike T, Akita M. Chem. Lett. 2009;38:166. [Google Scholar]; (b) Yasu Y, Koike T, Akita M. Chem. Commun. 2012;48:5355. doi: 10.1039/c2cc31748f. [DOI] [PubMed] [Google Scholar]
- 11.(a) Zhu M, Zheng N. Synthesis. 2011:2223. doi: 10.1055/s-0030-1260082. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Maity S, Zhu M, Shinabery RS, Zheng N. Angew. Chem. Int. Ed. 2012;51:222. doi: 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Some recent examples of photocatalytic reactions: Hari DP, Schroll P, König B. J. Am. Chem. Soc. 2012;134:2958. doi: 10.1021/ja212099r. Zou Y-Q, Chen J-R, Liu X-P, Lu L-Q, Davis RL, Jørgensen KA, Xiao W-J. Angew. Chem. Int. Ed. 2012;51:784. doi: 10.1002/anie.201107028. Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J. Am. Chem. Soc. 2011;133:18566. doi: 10.1021/ja208068w. Larraufie M-H, Pellet R, Fensterbank L, Goddard J-P, Lacote E, Malacria M, Ollivier C. Angew. Chem. Int. Ed. 2011;50:4463. doi: 10.1002/anie.201007571. Neumann M, Fuldner S, König B, Zeitler K. Angew. Chem. Int. Ed. 2011;50:951. doi: 10.1002/anie.201002992. Andrews RS, Becker JJ, Gagné MR. Org. Lett. 2011;13:2406. doi: 10.1021/ol200644w. Su Y, Zhang L, Jiao N. Org. Lett. 2011;13:2168. doi: 10.1021/ol2002013. Zlotorzynska M, Sammis GM. Org. Lett. 2011;13:6264. doi: 10.1021/ol202740w. Maji T, Karmakar A, Reiser O. J. Org. Chem. 2011;76:736. doi: 10.1021/jo102239x. Chen Y, Kamlet AS, Steinman JB, Liu DR. Nat. Chem. 2011;3:146. doi: 10.1038/nchem.932. Liu Q, Li Y-N, Zhang H-H, Chen B, Tung C-H, Wu L-Z. J. Org. Chem. 2011;76:1444. doi: 10.1021/jo102062u. Zhou L, Yang H, Wang P. J. Org. Chem. 2011;76:5873. doi: 10.1021/jo200692c. Andrews RS, Becker JJ, Gagné MR. Angew. Chem. Int. Ed. 2010;49:7274. doi: 10.1002/anie.201004311. Borak JB, Falvey DE. J. Org. Chem. 2009;74:3894. doi: 10.1021/jo900182x.
- 13.DeLaive PJ, Lee JT, Sprintschnik HW, Abruña H, Meyer TJ, Whitten DG. J. Am. Chem. Soc. 1977;99:7094. [Google Scholar]
- 14.For utilization of α-amino alkyl radicals in organic reactions, see: MacNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. Miyake Y, Nakajima K, Nishibayashi Y. J. Am. Chem. Soc. 2012;134:3338. doi: 10.1021/ja211770y. Kohls P, Jadhav D, Pandey G, Reiser O. Org. Lett. 2012;14:672. doi: 10.1021/ol202857t.
- 15.For utilization of iminium ions in organic reactions, see: Freeman DB, Furst L, Condie AG, Stephenson CRJ. Org. Lett. 2012;14:94. doi: 10.1021/ol202883v. Condie AG, González-Gómez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464. doi: 10.1021/ja909145y. Rueping M, Leonori D, Poisson T. Chem. Commun. 2011;47:9615. doi: 10.1039/c1cc13660g. DiRocco DA, Rovis T. J. Am. Chem. Soc. 2012;134:8094. doi: 10.1021/ja3030164. Hari DP, König B. Org. Lett. 2011;13:3852. doi: 10.1021/ol201376v. Pan Y, Wang S, Kee CW, Dubuisson E, Yang Y, Loh KP, Tan C-H. Green. Chem. 2011;13:3341. Wang C, Xie Z, deKrafft KE, Lin W. J. Am. Chem. Soc. 2011;133:13445. doi: 10.1021/ja203564w. Pirtsch M, Paria S, Matsuno T, Isobe H, Reiser O. Chem.–Eur. J. 2012;18:7336. doi: 10.1002/chem.201200967. Rueping M, Zhu S, Koenigs RM. Chem. Commun. 2011;47:12709. doi: 10.1039/c1cc15643h. Zou Y-Q, Lu L-Q, Fu L, Chang N-J, Rong J, Chen J-R, Xiao W-J. Angew. Chem. Int. Ed. 2011;50:7171. doi: 10.1002/anie.201102306.
- 16.Zhu M, Durham B, Zheng N. unpublished results. [Google Scholar]
- 17.Crutchley RJ, Lever ABP. J. Am. Chem. Soc. 1980;102:7129. [Google Scholar]
- 18.Shinabery RS, Zheng N. unpublished results. [Google Scholar]
- 19.Wimalasena K, Wickman HB, Mahindaratne MPD. Eur. J. Org. Chem. 2001:3811. [Google Scholar]
- 20.(a) Madelaine C, Six Y, Buriez O. Angew. Chem. Int. Ed. 2007;46:8046. doi: 10.1002/anie.200702903. [DOI] [PubMed] [Google Scholar]; (b) Lee HB, Sung MJ, Blackstock SC, Cha JK. J. Am. Chem. Soc. 2001;123:11322. doi: 10.1021/ja017043f. [DOI] [PubMed] [Google Scholar]; (c) Takemoto Y, Yamagata S, Furuse S, Hayase H, Echigo T, Iwata C. Chem. Commun. 1998:651. [Google Scholar]; (d) Lee JUJS, J S, Blackstock SC, Cha JK. J. Am. Chem. Soc. 1997;119:10241. [Google Scholar]; (e) Larquetoux L, Kowalska JA, Six Y. Eur. J. Org. Chem. 2004:3517. [Google Scholar]; (f) Larquetoux L, Ouhamou N, Chiaroni A, Six Y. Eur. J. Org. Chem. 2005:4654. [Google Scholar]; (g) Lee J, Berritt S, Prier CK, Joullié MM. Org. Lett. 2011;13:1083. doi: 10.1021/ol103105q. [DOI] [PubMed] [Google Scholar]