Abstract
The mammalian Target of Rapamycin (mTOR) defines a crucial link between nutrient sensing and immune function. In CD4+ T cells, mTOR has been shown to play a critical role in regulating effector and regulatory T cell differentiation as well as the decision between full activation versus the induction of anergy. In this chapter, we describe how our group has employed the Cre-lox technology to genetically delete components of the mTOR signaling complex in T cells. This has enabled us to specifically interrogate mTOR function in T cells both in vitro and in vivo. We also describe techniques used to assay immune function and signaling in mTOR-deficient T cells at the single-cell level.
Keywords: T cells, CD4, mTOR
1. Introduction
The initiation of an adaptive immune response requires the integration of many varied signals. The mammalian Target of Rapamycin (mTOR), an evolutionarily conserved serine–threonine protein kinase, is a nutrient sensor which interprets environmental cues (1). T cells utilize mTOR to integrate many immunologic signals and promote T helper cell differentiation (2). In CD8+ T cells, mTOR has been shown to play a role in regulating the generation of memory cells (13, 4). Inhibition of mTOR with the macrolide compound rapamycin or genetic deletion of the mTOR kinase results in failed T helper cell effector differentiation and alternate regulatory T cell generation (2, 5–7).
mTOR signals via two nutrient-sensitive protein complexes: mTORC1 and mTORC2. mTORC1 is characterized by the adaptor protein raptor and the small GTPase Rheb, and is read out by the phosphorylation of the ribosomal S6 kinase (S6K1) and 4E-BP1. mTORC1 has been implicated in the initiation of translation, inhibition of apoptosis, and initiation of mitochondrial metabolism (8). mTORC2 is characterized by the adaptor protein rictor and the mSIN1 proteins and is read out by the phosphorylation of Akt on its hydrophobic motif, serine 473 (9). While less is known about mTORC2 signaling and function, it has been implicated in actin reorganization as well as cell survival.
Here, we describe T cell-specific deletion of the mTOR kinase resulting in the ablation of total mTOR signaling. We discuss an in vivo model of Th1 differentiation (viral infection) and the interrogation of mTOR activity at the single-cell level using flow cytometry.
2. Materials
2.1. Generation and Genotyping of CD4-Cre × Floxed Mice
Tail snips from pups.
Tail lysis buffer: 100 mM Tris–HCl, pH 8.0, 5 mM EDTA, 0.2% SDS, 200 mM NaCl.
Proteinase K (Qiagen).
ddH 2O.
Platinum PCR Supermix (Invitrogen).
Forward and reverse primers (see Table 1).
2% TAE-agarose gels.
Table 1.
Primers and product sizes for genotyping mTOR-defi cient mice
| Gene | Direction | Sequence | Product length |
|---|---|---|---|
| Cre | FOR | CGA TGC AAC GAG TGA GG | ~300 |
| REV | GCA TTG CTG TCA CTT GGT CGT | ||
| Mtor | FOR | CCC AGC ACT TGG GAA TCA GAC AG | ~550 flox |
| REV | CAG GAC TCA GGA CAC AAC TAG CCC | ~350 wt |
2.2. Purification of CD4 T Cells
Ack lysing buffer (Quality Biological).
MACS CD4 isolation kit (negative selection, Miltenyi-Biotec).
LS columns (Miltenyi-biotec).
Midi MACS magnet (Miltenyi-biotec).
Phosphate-buffered Saline (PBS), pH 7.4 (Quality Biological).
Magnetic sorting buffer: PBS supplemented with 2 mM EDTA and 0.5% BSA.
2.3. Vaccinia-OVA Infection and OT-II Adoptive Transfer
Vaccinia -OVA (kept at stock solution of PBS at 2 × 107 PFU/mL).
C57/BL6 host mice (Jackson Laboratories).
OT-II wild-type or mutant cells (bred from stock at Jackson Laboratories).
Mouse immobilizer (Braintree Scientific).
28 G 1/2 insulin syringes (Becton Dickinson).
Ceramic heat lamp.
2.4. Direct Ex Vivo Interrogation of T Cells
Culture medium: 45% RPMI 1640 medium, 45% EHAA (Click’s) medium, 10% fetal bovine serum (FBS) supplemented with L-glutamine, Gentamicin reagent (Quality Biologicals), Ciprofloxacin (Sigma), and antibiotic/mycotic (Mediatech) (10).
Anti-CD3 (clone 2 C11) and anti-CD28 (clone 37.51).
OVA 323-339 (class II-restricted) peptide (AnaSpec), reconstituted in water at 10 mg/mL.
GolgiPlug (brefeldin A) (BD Biosciences).
2.5. Intracellular Cytokine Staining
PBS, pH 7.4 (Quality Biological).
Surface staining buffer: PBS supplemented with 2% FBS and 0.2% sodium azide.
PerCP-conjugated antibody to CD4 (L3T4, BD Biosciences).
BD Cytofix/Cytoperm (BD Biosciences).
BD Permwash (BD Biosciences).
FITC-conjugated anti-IFN-γ (XMG1.2).
APC-conjugated anti-IL-4 (BD Biosciences).
2.6. Multiparameter Phospho-FACS
PBS, pH 7.4 (Quality Biological).
Surface staining buffer: PBS supplemented with 2% FBS and 0.2% sodium azide.
Biotin-anti-CD4 (L3T4) (BD Biosciences).
Fixation buffer (Formalin diluted to 4% in PBS) (Sigma).
Ice cold 90% methanol (Sigma).
Blocking buffer: PBS supplemented with 10% FBS, and 500-fold dilution of FcBlock (BD Biosciences).
Intracellular staining buffer: PBS supplemented with 1% FBS.
Monoclonal mouse antibody to pS6K1 (T389) (Cell Signaling Technology).
Monoclonal rabbit antibody to pAkt (S473, clone D9E) (Cell Signal Technology).
DyLight 649-conjugated anti-rabbit IgG secondary (Jackson ImmunoResearch).
Oregon Green 488-anti-mouse IgG secondary (Invitrogen).
Strepdavidin-conjugated-PE (BD Biosciences).
3. Methods
The use of the macrolide antibiotic rapamycin has greatly facilitated the discovery and elucidation of mTOR function (11). While it was originally thought that rapamycin only inhibited the mTORC1 signaling pathway, it is clear that rapamycin can affect mTORC2 as well (12). We find that mTORC2 in lymphocytes is exquisitely sensitive to inhibition by rapamycin even at concentrations as low as 20 nM. Further, it is clear that rapamycin has a wide variety of diverse effects on many cells regulating immune responses (13). In order to study the specific role of mTOR function in T cells, we have taken a genetic approach. First, we have taken advantage of the expertise and generosity of other investigators by breeding previously generated floxed mice with CD4-Cre. Since CD4 is expressed at the double-positive stage of T cell development, breeding CD4-Cre mice with mTOR-floxed mice leads to the efficient deletion of mTOR in both CD4 and CD8 T cells. Further, because CD4 comes up relatively late in T cell development, the ultimate elimination of mTOR protein which is even later in development does not appear to significantly affect the generation of single-positive T cells. By breeding CD4-Cre, mTOR-floxed mice to TCR transgenic mice, we can greatly enhance our ability to specifically activate the genetically altered cell of interest. Further, back-crossing the mice to a congenic marker allows for the ability to track antigen-specific, genetically altered T cell in vivo in a wild-type host. Proper genotyping and husbandry are absolutely critical to the success of these assays.
To assess the role of mTOR in T cells in regulating CD4+ T cell function in response to infection, we routinely adoptively transfer the genetically altered T cells into a host prior to infection. Vaccinia, a potent inducer of an antiviral Th1 response, can be engineered to express a number of model antigens. Here, we report the use of Vaccinia -OVA to induce Th1 differentiation of OT-II (OVA specific) CD4+ T cells. These adoptively transferred cells are marked with the congenic marker Thy1.1 and thus are readily distinguished from host T cells by FACS. CD4+ T cells adoptively transferred into vaccinated hosts become IFN-gamma producing Th1 cells that do not express IL-4, a Th2 cytokine. However, T cells deficient in total mTOR signaling fail to differentiate into Th1 or Th2 cells (2). In as much as the frequency of the antigen-specific T cells is relatively low in vivo, we have employed FACS as a means of both interrogating cells for cytokine production as well as multiparameter phospho-FACS to detect mTORC1 and mTORC2 activation in T cells at the single-cell level.
3.1. Generation and Genotyping of CD4-Cre × Floxed Mice
CD4-Cre mice on a B6 background should be bred to mice homozygous for floxed mTOR (sometimes, known as Frap1 or Mtor).
The F1 generation should be bred back to homozygous floxed founders such that some progenies have a CD4-Cre transgene and be homozygous floxed at the locus of choice.
These mice should also be bred to a Thy1.1 (or other congenic marker) and, ideally, to a TCR-transgenic background (this chapter uses OT-II) (see Note 1).
At 3 weeks of age, separate pups from dams and sterilely snip 1–2 mm of tail for genotyping.
Incubate in 100 μL tail lysis buffer containing 2 μ L proteinase K overnight at 55°C (see Note 2).
Dilute 2 μL lysate into 48 μ L ddH 2 O and transfer to a PCR tube (this is the template for PCR).
Add 28 μ L of Platinum PCR Supermix and 1 μL of each specific primer, diluted to 10 μ M.
Mix well and run PCR with 52°C annealing temperature (see Note 3).
Add 10× loading buffer and run on a 2% TAE agarose gel.
Banding patterns indicate genotype (see Table 1).
3.2. Purification of CD4 or CD8 T Cells
Isolate splenocytes/lymphocytes.
Resuspend in 1 mL Ack red blood cell (RBC) lysis solution (see Note 4).
Incubate at RT for 2 min.
Wash 1× with 10 mL PBS (see Note 5).
Count cells in an appropriate volume of PBS and spin down.
Aspirate the supernatant as well as possible.
Resuspend pellet in 3 μL magnetic sorting buffer and 0.75 μ L Antibody-Biotin Cocktail (CD4 isolation kit) per 106 cells.
Incubate at 4°C for 15 min (see Note 6).
Add 2 μL magnetic sorting buffer and 1.5 μ L anti-biotin microbeads (CD4 isolation kit) per 106 cells.
Incubate at 4°C for 30 min (see Note 6).
Add 10–20× the labeling volume magnetic sorting buffer and spin at 300× g for 5 min.
Equilibrate an LS MACS column with 3 mL magnetic sorting buffer, discarding the flow through.
Resuspend pellet in 1 mL of magnetic sorting buffer and add to the column, allowing it to enter the column by gravity flow, collecting the flow through (this is the “negatively selected fraction”).
Wash 3× with 3 mL of magnetic sorting buffer collecting all the flow through (“negatively selected fraction”).
The positively selected fractions (column bound) are CD4–; they can be discarded.
Count cells and resuspend in PBS or culture media, as necessary.
3.3. Vaccinia Infection and Adoptive Transfer
Isolate CD4+ T cells (Subheading 2.2) and resuspend in PBS at 10 × 106 cells/mL.
Resuspend at 20 × 106 cells/mL in PBS (see Note 7).
Thaw Vaccinia virus from frozen (−80°C) stock. Stocks are kept at 2 × 107 PFU/mL.
Place host mice (C57/B6 mice for OT-II transfer/Vaccinia-OVA infection) into a cage and heat them slowly using a radiant ceramic heat lamp (see Note 8).
After the mice have heated up, slide them into the immobilizer, tail sticking out, and inject 100 μL (2 × 106 CD4+ T cells) intravenously using the insulin syringe (see Note 9).
Bring up Vaccinia virus into a syringe. Inject 100 μL intraperitoneally (1–2 × 106 PFU).
Let the mice harbor the infection for 3–5 days. Sacrifice the mice and remove spleens (see Note 10).
3.4. Direct Ex Vivo Interrogation of T Cells
Splenocytes from host mice or directly from mutant mice should be in single-cell suspension.
Resuspend in RBC lysis buffer.
Incubate at RT for 2 min.
Wash 1× in 10 mL of PBS.
Resuspend at 20 × 106 /mL in culture medium.
Make up 2× stimulation medium (6 μg/mL anti-CD3 and 4 μg/mL anti-CD28, or 20 μ g/mL OVA peptide) (see Notes 11 and 12).
If performing intracellular cytokine staining, also supplement the stimulation medium with Golgi Plug (see Note 13).
Stimulate cells by adding equal volumes (1:1) of cells and 2× stimulation medium and move to an appropriate cell culture vessel.
Incubate overnight at 37°C.
3.5. Intracellular Cytokine Staining
If necessary, transfer the cells to a round-bottomed plate and pellet the cells (1,500 rpm for 5 min).
Stain the surface molecules diluted in 50 μL of surface staining buffer.
Incubate for 5–15 min at 4°C.
Wash with 150 μ L of unsupplemented surface staining buffer.
Resuspend the cells in 100 μ L BD Cytofix/Cytoperm solution.
Incubate at RT in the dark for 15 min.
Wash 2× with 100 μ L BD Permwash.
Resuspend in 50 μ L BD Permwash supplemented with: (a) FITC anti-IFN-g (1:500) (b) APC anti-IL-4 (1:100)
Incubate at RT in the dark for 30 min.
Wash with 150 μ L BD Permwash.
Resuspend in 200 μL PBS and run samples on a flow cytometer (see Fig. 1).
Fig. 1.
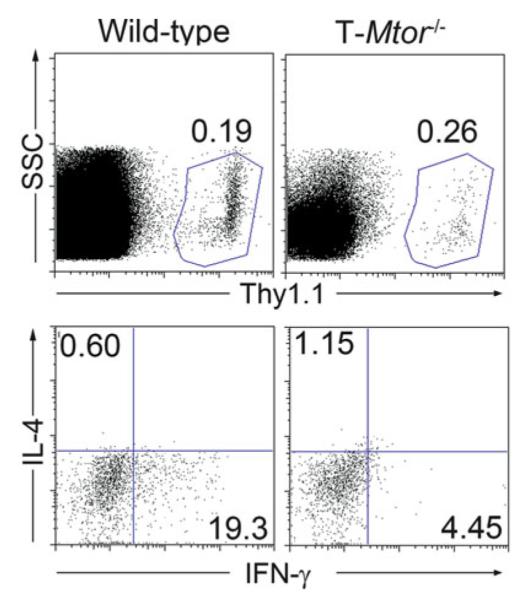
Wild-type and T-Mtor−/− mice were sacrifi ced. Spleens were harvested and subjected to CD4 purifi cation by magnetic sorting. 2 × 106 CD4 cells were injected intravenously into C57/BL6 host mice previously immunized with 2 × 106 PFU Vaccinia-OVA. Four days post transfer, host mice were sacrifi ced, and splenic cells were rechallenged with 50 μ g/mL OVA peptide overnight in the presence of a protein transport inhibitor. Splenocytes were then stained for Thy1.1 (upper panels) and intracellularly stained for IL-4 and IFN-γ to assess cytokine production (lower panels, gated on Thy1.1+ cells). Wild-type cells produce copious amounts of IFN-γ upon rechallenge, indicating that they have differentiated into Th1 cells. Cells deficient in mTOR fail to produce either cytokines when restimulated.
3.6. Multiparameter Phospho-FACS
Harvest stimulated cells by centrifugation (3 min at 1,500 rpm), transferring to a 96-well U-bottom plate if the stimulation was done in a separate vessel.
Wash 1× in 200 μ L PBS.
Resuspend in 50 μ L surface staining buffer supplemented with anti-CD4–biotin (1:500 dilution) (see Note 14).
Incubate at 4°C for 15 min.
Add 150 μ L of unsupplemented surface staining buffer and spin down.
Wash 1× in 200 μ L unsupplemented surface staining buffer and spin down.
Resuspend in 100 μ L fixation buffer.
Incubate at 37°C for 15 min.
Wash with 100 μ L PBS and spin down (see Note 15).
Wash 1× in 200 μ L PBS and thoroughly remove all supernatant.
Add 100 μ L ice-cold 90% methanol and gently pipet up and down twice (see Note 16).
Incubate at −20°C for 20 min.
Spin down.
Carefully remove the methanol.
Wash 2× in 200 μ L of PBS and spin down.
Resuspend cells in 100 μ L blocking buffer.
Incubate for 15 min at RT.
Wash with 100 μL 1% FCS in PBS.
- Resuspend cells in 50 μ L staining buffer supplemented with:
- Mouse anti-phospho-S6K (T389) (1:200)
- Rabbit anti-phospho-Akt (S473) (1:200)
Incubate at RT in the dark for 45 min.
Wash 1× with 1% FCS in PBS.
- Resuspend cells in 50 μ L intracellular staining solution supplemented with:
- Anti-mouse Oregon Green 488 (1:200)
- Anti-rabbit DyLight 649 (1:200)
- SA-conjugated PE (1:500)
Incubate at RT in the dark for 45 min.
Wash 2× with 200 μL unsupplemented intracellular staining buffer.
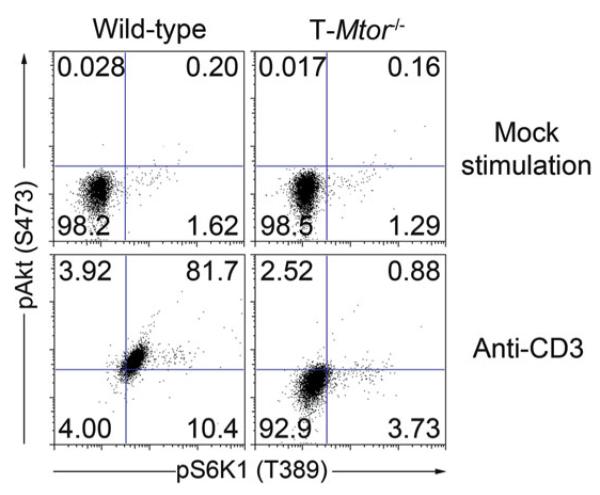
Resuspend in 200 μL PBS and run on cytometer (See Note 16, Note 17, and Fig. 2).
Fig. 2.
Spleen and lymph node cells were harvested from wild-type and T-Mtor−/− mice. After RBC lysis and extensive washing, splenocytes were stimulated with 3 μ g/mL anti-CD3 and 2 μ g/mL anti-CD28 overnight. Cells were surface stained with anti-CD4–biotin, fixed in 4% formalin, and permeablized with 100% methanol. After a 10-min block in 10% FCS, cells were stained intracellularly for phosphorylation of S6K1 and Akt. Plots are gated on CD4 cells. Voltage was adjusted based on secondary-only controls and gates were set with an unstimulated control. Wild-type cells activate both mTORC1 (pS6K1) and mTORC2 (pAkt) signaling when stimulated, but mTOR-defi cient T cells lack activation of both mTORCs.
Acknowledgments
The authors would like to thank members of the Powell laboratory for technical assistance in optimizing these models. In addition, we would like to thank Dr. Sara C. Kozma (U. Cincinnati) for generating the original floxed mouse lines. This work was supported by R01AI077610-01A2.
4. Notes
A congenic marker (like Thy (CD90), CD45, or Ly isoforms) facilitates the ability to track adoptively transferred T cells. Likewise, a TCR transgene recognizing a cognate antigen expressed by Vaccinia virus (like OT-II and OVA-expressing Vaccinia) can be used to both stimulate and identify such cells in vivo. Backcrossing several generations to the host strain (B6, in this case) is highly recommended if the floxed line was made on a different background.
Our laboratory uses 96-well PCR plates to help streamline the genotyping by being able to use multichannel pipettors. After an overnight incubation, tails should be almost completely dissolved.
Optimizing for individual thermocyclers and primer pairs is highly recommended.
Red cell lysis uses a hypotonic solution (Ack lysing buffer, NH 4 Cl) to lyse RBCs. It is critical that the incubation during hemolysis is kept as short as possible to prevent damage to lymphocytes.
Unless otherwise stated, the term “wash” refers to resuspension of cell pellets in the solution mentioned followed immediately by centrifugation at 300 × g for 5 min.
Incubations at 4°C during magnetic sorting can be extended for up to 60 min with no effects on purity, yield, or viability.
Syringes typically have a void volume of 100 μ L; it is critical to account for this when preparing your sample so that you have enough cells to inject all the needed host mice.
It is critical that your donor mice have a distinguishing cytometric marker (our laboratory uses Thy1.1 and Thy1.2, but CD45.1 and CD45.2 or Ly markers can work).
Heating the mice allows easy visualization of the tail veins; they are on either side of the midline of the tail. When properly performed, there should be very little pressure on the plunger of the syringe when depressed. Start the furthest from the base of the tail. If pressure is felt, try injecting the vein again, slightly closer to the base of the tail. Precision generally comes with practice. Retro-orbital injections are easier to perform, but the delivery is not always consistent.
Spleens from Vaccinia -infected hosts should be generally larger than mice that have not been immunized.
Anti-CD3 and anti-CD28 cross-link all TCRs and deliver costimulation. This is done via the Fc receptor on APCs. This stimulation should be used for non-TCR transgenic T cells or when all cells should be activated. OVA peptide stimulates only the OT-II cells. For the Vaccinia experiment listed in Subheading 2.3, OVA peptide should be used as the stimulation of choice.
Always include a no-stimulation control, containing only medium and Golgi Plug. This is a critical experimental control for setting negative gates on the flow cytometer.
Golgi Plug (brefeldin A) is ideal for overnight stimulations and the majority of cytokines. Some cytokine procedures work better with Golgi Stop (monensin), which is ideal for short-term stimulations. This should be optimized based on your particular needs.
Some antigens, especially cell surface antigens, become degraded with the harsh methanol permeabilization step in this protocol. To overcome this hurdle, our laboratory stains cell surface molecules with a biotinylated antibody prior to fixation. The marker can then be detected with fluorochrome-conjugated streptavidin during the secondary incubation step.
Cells can be stored at 4°C at this point. It is critical for consistency that all the intracellular phospho-staining is done at the same time to compare across conditions.
90% ice-cold methanol should be added drop by drop, very gently.
If the samples are not to be run immediately, a second fixation step in fixation buffer is recommended.
References
- 1.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 2.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 7.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4 + CD25 + FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Xiao Z, Chen B, Han J, Gao Y, Zhang J, Zhao W, Wang X, Dai J. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One. 2008;3:e1856. doi: 10.1371/journal.pone.0001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/ PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]