Abstract
Stromal fibroblasts are a new prospective drug target. Mesenchymal stromal cells (MSCs) and monocyte-derived stromal cells, also known as fibrocytes, are distinct fibroblastic populations derived from separate lineages. Mesenchymal and myeloid fibroblast progenitors are multipotent, serve as progenitor cells in animal models, and are implicated in several diseases. In addition, epithelial-mesenchymal transition (EMT) has been established as a mechanism for generation of stromal cells. Organ sources, relative contributions, and functions of these populations in normal development and pathology are not well understood. Innovative approaches are needed to identify markers that can distinguish these stromal populations.
Introduction
Stromal cells, typically identified as tissue-resident fibroblasts, form a supportive scaffold for both healthy and pathological tissues. Stromal cells implicated in disease can be divided into three broad types: mesenchymal stromal cells (MSCs), monocyte-derived stromal cells and stromal cells arising through epithelial-mesenchymal transition (EMT). Our unpublished data (Fig. 1) show the appearance of cells derived from these three alternative lineages in ex vivo culture. These cell populations are important players in development and tissue remodeling, regeneration of damaged organs, and fibrosis because they secrete growth / immunomodulatory factors and extracellular matrix (ECM) components. There are three key questions about stromal cells. First, due to the lack of specific markers, we do not know the relative contributions of MSCs, fibrocytes and EMT-derived cells to stroma in healthy and pathological organs. Second, much remains to be understood about whether these fibroblastic populations execute synergistic or antagonistic functions in disease. Third, it is unclear to what extant systemic mobilization and recruitment of progenitors from the bone marrow as opposed to their migration from extramedullary organs or resident tissues contributes to the formation of stroma.
Figure 1.
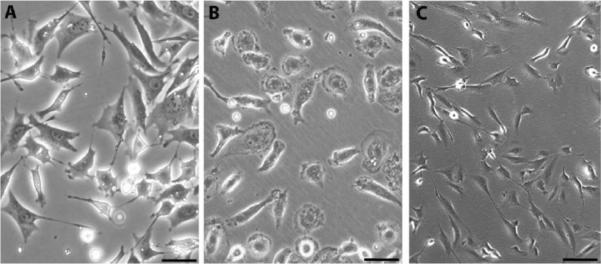
Morphology of human stromal populations in cell culture. (A) Primary MSC (passage 0) isolated as CFU-F from peripheral blood of a prostate cancer patient as described (Bellows et al., 2011a). (B) Primary adherent monocytes (passage 0) isolated from peripheral blood of a control cancer-free donor as described (Bellows et al., 2011a). (C) Mammary epithelial cells immortalized by retroviral-mediated expression of SV40 Large and Small T antigens and the catalytic subunit of telomerase and induced to undergo EMT through ectopic expression of Snail using the pBabe retroviral vector as described (Mani et al., 2008). Cells in A–C were cultured on uncoated plastic in αMEM medium containing 10% fetal calf serum. Scale bar: 50 μM.
Mesenchymal Stromal Cells
Mesenchymal stromal cells (MSCs) exist in many adult organs (da Silva Meirelles et al., 2006) and have a typical fibroblast appearance in culture (Fig. 1A). MSCs can be distinguished from hematopoietic cells based on the lack of the pan-leukocyte marker CD45 and distinguished from endothelial cells based on the lack of the pan-endothelial marker CD31/PECAM-1 (Bianco et al., 2008; Rodeheffer et al., 2008). A number of cell surface molecules, including platelet-derived growth factor receptor (PDGFR), Stro-1, CD13, CD29, CD44, CD73, CD90, CD105, and CD146, have been used for positive selection of MSCs (Gimble et al., 2007; Bianco et al., 2008). MSCs were first isolated from bone marrow stroma and termed fibroblast colony-forming units (CFU-F) based on their morphology (Friedenstein, 1980). The ability of MSCs to differentiate into cells of mesodermal lineages, such as osteoblasts, chondrocytes, and adipocytes, has resulted in the term “mesenchymal stem cells” (Prockop, 1997; Caplan, 2007). In addition to their mesenchymal progenitor function, MSCs serve as pericytes (mural cells) maintaining vascular integrity in homeostatic conditions (Crisan et al., 2008; Tang et al., 2008; Traktuev et al., 2008).
Differentiation of mesenchymal progenitors into fibroblasts is proposed to be a major source of stromal cells in both normal development and pathology (Bianco et al., 2008). MSCs are the primary source of collagen I in the ECM, deposition of which is an integral component of wound healing as well as fibrosis (Wynn, 2008). Preclinical studies and clinical trials with allografted MSCs indicate the intrinsic therapeutic potential of these cells and suggest that they are activated in disease to engage in tissue repair and regeneration (Toma et al., 2009; Caplan and Correa, 2011). This support involves angiogenic activity and the immunoprotection provided by the MSCs. The trophic activity of MSCs results from a number of bioactive molecules that they secrete to suppress apoptosis and scarring and to promote cell proliferation and vascularization. In addition, MSCs have immuno-modulatory properties (Jones and McTaggart, 2008), and their capacity to mute T-cells benefits autoimmune disease patients and favors the outcome of bone marrow transplantation through the suppression of graft-versus-host-disease.
MSCs are virtually absent in the peripheral circulation of healthy individuals, however, hypoxia and inflammation signals have been reported to result in MSC mobilization and migration from their niches (Rochefort et al., 2006; Okumura et al., 2009). Interestingly, systemic circulation of MSCs is observed in obesity (Bellows et al., 2011b) and is further elevated in cancer (Bellows et al., 2011a). This finding is reinforced by reports on mobilization of mesenchymal perivascular progenitors in cancer (Mancuso et al., 2011) as well as in acute stroke patients (Jung et al., 2011). Future studies will be needed to address bloodstream, as opposed to migration through solid tissues, as alternative routes of MSC trafficking to pathological sites.
Hematopoietic-derived Stromal Cells
Not only mesenchymal, but also hematopoietic cells are recruited as components of stroma (Coussens and Werb, 2002). Leukocytes can display matrix adherence and plasticity in culture (Fig. 1B). When cultured for 2 weeks in the presence of heat-treated serum, or cultured for 4–5 days in the absence of serum, a small percentage of peripheral blood leukocytes differentiate into spindle-shaped cells called fibrocytes that express both hematopoietic markers and stromal markers such as collagen-I (Bucala et al., 1994; Chesney et al., 1997; Pilling et al., 2009). It is believed that fibrocytes originate from a subset of bone marrow-derived monocytes (Bucala et al., 1994; Abe et al., 2001). In this review, all hematopoietic origin fibroblast-like cells will be referred to as fibrocytes, although it is currently not clear whether they are all derived from the same leukocyte subtype through the same mechanism in vivo. Fibrocytes express the pan-hematopoietic cell marker CD45 along with CD11b and several other monocyte markers (Abe et al., 2001). On the other hand, in culture these cells can be induced to express markers typically used for MSC identification, such as CD13, and CD29 along with CD34, as well as collagen I and other ECM proteins (Chesney et al., 1998; Pilling et al., 2007).
Although there is no single marker that can be used to identify fibrocytes, staining cells or tissues for combinations of markers can be used to distinguish human fibrocytes from certain differentiated monocytic populations and from mesenchymal fibroblasts (Pilling et al., 2009). An intriguing observation is that collagen I-positive cells can be found in the circulation, although it is currently unknown whether these are cells that are about to enter a tissue and become a tissue fibrocyte, or whether they are cells that had been a tissue fibrocyte and have left the stroma and entered the circulation (Mehrad et al., 2007; Reilkoff et al., 2011). It has been proposed that fibrocytes can differentiate into myofibroblasts, an event corresponding to the phenotypic cell transition clinically observed in cancer and other fibrotic pathologies (Mattoli et al., 2009; Strieter et al., 2009). Fibrocytes are capable of substantially contributing to stroma in solid tissues (Hartlapp et al., 2001; Bellini and Mattoli, 2007; Herzog and Bucala, 2010). Indeed approximately 50% of fibroblastic cells in non-malignant fibrotic lesions are bone marrow-derived and express CD45 (Ishii et al., 2005), and in tumors stroma is also composed of both CD45+ and CD45− cells (Worthley et al., 2009).
Like MSCs, in addition to enforcing a fibrotic state in pathological tissues, fibrocytes can promote angiogeneisis (Hartlapp et al., 2001; Hong et al., 2005). While there are numerous similarities between these two types of stromal progenitors, there are also striking differences that are worth emphasizing. While MSCs do not express MHC type-II, are immunoprivileged, and suppress cytotoxic T-lymphocytes, fibrocytes express MHC-II and activate T-cells (Chesney et al., 1997; Abe et al., 2001). Thus, while possibly having similar ECM-producing and proangiogenic activities, MSCs and fibrocytes may have opposite effects on the immune response. Interestingly, fibrocytes, like MSCs, can differentiate into specialized mesenchymal cells, such as adipocytes (Hong et al., 2005; Ishii et al., 2005). Moreover, recent studies indicate that monocytic bone marrow-derived cells can provide a substantial contribution to the pool of adipocytes composing white adipose tissue (WAT) in normal development (Crossno et al., 2006; Majka et al., 2010), although these results have been disputed (Koh et al., 2007; Tomiyama et al., 2008).
EMT-derived Stromal Cells
The epithelial-to-mesenchymal transition is a series of cellular remodeling events that facilitates a number of normal biological processes, such as gastrulation and wound healing, as well as several pathological processes, such as fibrosis and cancer metastasis (Yang and Weinberg, 2008; Thiery et al., 2009). At the most fundamental level, EMT is defined as the process through which epithelial cells lose their epithelial traits and gain characteristics associated with mesenchymal cells (Hay, 1995). Epithelial cells, which form the lining of glandular organs, normally exist as sheets of cells tightly bound to one another and to the basement membrane. However, during EMT, epithelial cells dissociate these cell-to-cell and cell-to-basement membrane contacts in order to migrate independently and invade through the extracellular matrix. As illustrated in Figure 1C, through the loss of these adhesions and extensive cytoskeleton remodeling, the epithelial cells also lose their typical apical-basolateral polarity and take on a spindle-shaped morphology (Shook and Keller, 2003; Yang and Weinberg, 2008; Thiery et al., 2009). At the molecular level, EMT is associated with decreased expression of epithelial adhesion molecules, such as E-cadherin and epithelial cytokeratins. This loss of epithelial gene expression is accompanied by de novo expression of mesenchymal markers, such as vimentin, fibronectin, and N-cadherin (Shook and Keller, 2003; Thiery and Sleeman, 2006).
EMT events were originally described during embryonic development. During gastrulation, some of the cells located at the primitive streak undergo EMT prior to ingression, and these EMT-derived cells eventually form the embryonic endodermal and mesodermal tissues (Bellairs, 1986). In fact, EMT events are essential for embryonic development. If the ability of embryonic cells to undergo EMT is impaired, embryonic development ceases at the blastula stage (Thiery and Sleeman, 2006). Following gastrulation, EMT events occur during the development of neural crest cells from the neural tube, cardiac valve formation, palatal fusion, Müllerian duct regression, and peripheral nervous system formation (Hay, 1995; Shook and Keller, 2003; Yang et al., 2008). In addition, EMT may facilitate natural remodeling events in some epithelial organs, such as the branching morphogenesis of the mammary gland (Fata et al., 2004).
Induction of EMT in immortalized human mammary epithelial cells not only imparts fibroblast-like qualities (e.g. migratory and invasive capabilities) to these cells, but also endows them with traits similar to mammary stem cells (Mani et al., 2008). Moreover, induction of EMT in immortalized human mammary epithelial cells by exposure to a range of EMT-inducing stimuli, including ectopic expression of the transcription factors Twist or Snail, or treatment with TGF-beta, leads to an increased ability to self-renew in mammosphere culture and acquisition of the CD44high/CD24low “cancer stem cell” profile (Al-Hajj et al., 2003; Mani et al., 2008; Morel et al., 2008). Mammary stem cells isolated from normal mouse or human mammary glands, or from human breast cancer tissues, express high levels of mesenchymal markers and EMT-inducing transcription factors (Mani et al., 2008; Lim et al., 2010), which is consistent with the notion that the EMT phenotype is linked with stem cell properties.
Interestingly, both MSCs and EMT-derived epithelial cells (Fig. 1C) exhibit mesenchymal traits (e.g. independent migration, invasiveness) and stem cell properties. EMT-derived mammary epithelial cells and bone marrow-derived MSCs share similar antigenic profiles and express relatively similar levels of the mRNAs encoding several EMT-inducing transcription factors (Battula et al., 2010). Similar to MSCs, EMT-induced epithelial cells can differentiate into multiple mesodermal lineages, including osteoblasts, adipocytes, and chondrocytes, and home to wounded tissues upon intravenous injection into mice (Battula et al., 2010).
In addition to epithelium, other tissues have also been shown to undergo mesenchymal transdifferentiation. Most notable are the reports on the conversion of vascular cells into multipotent mesenchymal progenitors (Medici et al., 2010). This phenomenon of endothelial-to-mesenchymal transition takes place in both malignant and non-malignant settings (Zeisberg et al., 2007). Interestingly, MSCs can induce EMT in adjacent malignant epithelial cells in tumors (Klopp et al., 2010). Together, these observations suggest parallel biological roles for MSCs and endogenous organ cells induced to undergo mesenchymal transition during wound healing, fibrosis, and other pathologies and calls for further analysis of the diversity of MSC-like cells in these processes.
Organ Origins of Stromal Cell Progenitors
Since the original work on macrophages pioneered by Metchnikov (Rossiianov, 2008), it has been considered that cells can be recruited to pathological sites both from the surrounding solid tissues and from remote organs through the circulatory system (Fig. 2). While extravasation of various circulating leukocytic populations is a well-characterized process, invasion of cells from local niches through the ECM is a phenomenon that is so far not well documented due to the lack of tools and cell tracking markers (Coussens and Werb, 2002). In addition, it is becoming apparent that the numbers and types of circulating cells is dynamic and can change in disease. This can result in a rapid increase in accessibility of cells that could become attracted by pathological tissues. Mobilization of stem cells and their descendent progenitor cells from the endogenous pools residing in the bone marrow as well as extramedullary organs may have clinical importance (Pelus, 2008; Kolonin and Simmons, 2009). The notion that endogenous stem cells may be activated to mend damaged tissues during injury repair and organ regeneration has prompted efforts to identify factors mobilizing stem cells.
Figure 2.
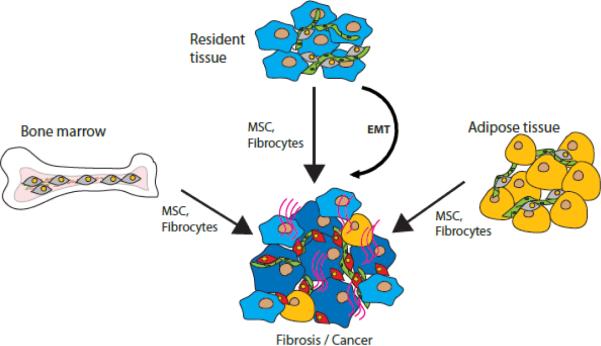
A model of pathological stroma. Mesenchymal stromal cells (MSCs) or fibrocytes from resident tissue, bone marrow, or adipose tissue can be recruited into the site of disease along with epithelial-mesenchymal or endothelial-mesenchymal transition (EMT) in the organ undergoing pathological remodeling. Parenchymal tissue transformation (light blue to dark blue) in cancer/ fibrosis is accompanied by functional changes in the pathological stromal cells (grey to red). Red lines: matrix accumulation in fibrosis / cancer desmoplasia. Green: vasculature. Yellow: adipocytes.
Experiments aimed to distinguish bone marrow from extramedullary pools of progenitors demonstrate that medullary cells expressing myofibroblast markers such as α-smooth muscle actin (αSMA) contribute to tumor stroma (Quante et al., 2011). Moreover, there is evidence that bone marrow MSCs can contribute to epithelial parenchyma in pathology (Okumura et al., 2009). These findings confirm other studies based on bone marrow transplantation in mouse models showing that medullary MSCs could give rise to tumor fibroblasts (Direkze et al., 2004; Udagawa et al., 2006; Worthley et al., 2009). Conflicting with these reports is experimental evidence that cells of mesenchymal stroma do not transplant along with the marrow hematopoietic progenitors (Simmons et al., 1987). This controversy could be explained by the induced expression of myofibroblast and mesenchymal markers in non-mesenchymal cells in the tumor microenvironment. Such conversion can reportedly take place not only through EMT, but also through endothelial-to-mesenchymal transition (Zeisberg et al., 2007). Identification of possibly existing organ-specific MSC promoters would be necessary for lineage tracing experiments that could resolve the ongoing debate.
The route through which fibroblast progenitors are recruited to pathological organs is unclear. While monocytes, which can become fibrocytes, are abundant in the peripheral blood, circulating MSC progenitors are virtually absent in the blood of healthy individuals (Kuznetsov et al., 2007). A body of evidence indicates that hypoxia and inflammation signals can cause MSC mobilization and migration from their niches (Rochefort et al., 2006). MSCs appear to “sense” wounds and malignant lesions, which explains their homing to the corresponding sites (Spaeth et al., 2009). Transplantation experiments have shown that the bone marrow serves a source of progenitor cells that can traffic to pathological sites (Peters et al., 2005; Papayannopoulou and Scadden, 2008). While bone marrow is generally accepted as a source of hematopoietic stem cells, marrow-derived pericytes and stromal cells have been also reported in a number of studies (Bababeygy et al., 2008). However, there also is evidence accumulating that pools of mesenchymal progenitors are autonomously maintained in extramedullary organs (Koh et al., 2007; Tomiyama et al., 2008). Thus, organs other than bone marrow may contribute to the pool of mesenchymal stromal progenitors mobilized in pathology and implicated in disease progression (Kolonin and Simmons, 2009).
Adipose Tissue as a Potential Source of Stromal Cell Progenitors
In addition to adipocytes and vascular endothelial cells (EC), WAT contains abundant perivascular MSCs termed adipose stromal cells (ASCs), as well as infiltrating leukocytes (Hausman et al., 2001; Daquinag et al., 2011b). ASCs comprise a large proportion of the stromal/vascular fraction of WAT, and display multipotency and proliferation capacities comparable to those of bone marrow MSCs, while also having unique features (Gimble et al., 2007; Noel et al., 2008). ASCs are a heterogeneous population of cells lacking hematopoietic and endothelial markers, at least some of which express CD34 (Traktuev et al., 2008; Zimmerlin et al., 2010). The relevance of CD34 expression on ASCs, which is rapidly suppressed in culture, is unclear but may have functional relevance (Suga et al., 2009). Both pericytes (Rodeheffer et al., 2008; Tang et al., 2008; Traktuev et al., 2008) and tunica adventitia cells (Corselli et al., 2011) have been identified as ASCs with the CD45-CD31-CD34+ immunophenotype.
ASCs promote endothelial cell proliferation and blood vessel formation at least in part via trophic effects of secreted vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and other angiogenic molecules (Traktuev et al., 2008). Systemically administered ASCs localize to sites of injury and contribute to revascularization of injured mouse organs, including the heart (Cai et al., 2009). On the other hand, monocytes/macrophages infiltrate WAT in obesity and significantly contribute to its cell content. The inherent capacity of monocytes/macrophages to migrate raises the possibility that WAT-resident monocytes may have overlooked pathological roles. Because monocytes are highly plastic and can efficiently acquire fibroblast functions in culture and in vivo (Abe et al., 2001; Hartlapp et al., 2001; Pilling et al., 2003; Pilling et al., 2007), both mesenchymal and hematopoietic cells from WAT are potential sources of pathological stromal progenitors. Interestingly, CD34+CD45+ fibrocytes have been observed in WAT (Traktuev et al., 2008) but have not been systematically characterized.
Obesity, hallmarked by overgrowth of WAT, is associated with various fibrotic conditions, and WAT may represent a potential source of progenitor cells mobilizable in disease (Kolonin and Simmons, 2009). In obese individuals, there is increased circulation of CD34-positive MSCs (Bellows et al., 2011b). Our studies suggest that MSCs and fibrocytes from the bone marrow as well as WAT might be mobilized in response to pathological signals (Fig. 2). These cells might serve as a reserve of pathological stroma progenitors in obese patients upon recruitment into the lesion. Excess WAT-derived progenitors released into the bloodstream in obesity could positively influence post-operative healing and organ regeneration, and could explain the “obesity paradox” of better post-surgical recovery observed for obese patients (Mullen et al., 2009). The flip side of excessive stromal cell mobilization may be that WAT-derived cells overabundant due to WAT expansion become recruited by tumors at an increased frequency in obese individuals. This phenomenon may at least partially account for the epidemiological association between obesity and disease progression reported for several types of cancer (Flegal et al., 2007; Kant and Hull, 2011). We have proposed that infiltration of WAT-derived cells into tumors results in higher concentrations of adipokines that affect cancer cells in a paracrine fashion, and could therefore be more potent than the endocrine signalling from remote WAT depots (Zhang et al., 2010). The possibility of ASCs, as well as of monocyte-derived cells from WAT, shaping the tumor microenvironment is consistent with recent reports on tumor stroma changes resulting from increased adiposity (Zyromski et al., 2009; Park et al., 2010; Dirat et al., 2011; Zimmerlin et al., 2011).
Stromal cells in Normal Physiology
Stromatogenesis is an integral component of development. In adulthood, every organ also relies on migration and proliferation of stromal cells that generate the ECM, proper composition of which is essential for normal tissue homeostasis and physiology. MSCs, hematopoietic progenitors, as well as EMT-derived cells, participate in this process. Postneonatally, stromal cells maintain organ architecture. While their turnover in healthy organs has not been systematically assessed, they certainly undergo self-renewal (Jackson et al., 2002). The current dogma is that MSCs are the primary pool of cells serving as progenitors for connective tissues, and transplantation experiments have demonstrated the organogenic potential of MSCs (Bianco et al., 2008). Stromal cell content in certain tissues is supported by the import of progenitors from other organs. For example, according to recent reports adipocytes can originate from bone marrow monocytes (Crossno et al., 2006; Sera et al., 2009; Majka et al., 2010), indicating mesenchymal transition as a contributor to homeostatic stroma maintenance.
Wound healing is a specific setting in adulthood in which stromal progenitors play an important role. Bone marrow transplantation studies demonstrate that organ repair is assisted by hematopoietic progenitors, at least indirectly, through transient recruitment of blood cells sealing the wound and priming the response against infection. Fibrocytes recruited from the blood are incorporated into the scar tissue and become a component of the repaired organ at least for a period of time. Non-bone marrow-derived circulating progenitor cells also contribute to wound repair; however the organ sources remains unknown (Aicher et al., 2007). While tracking cell infiltration from the surrounding solid organs is more challenging technically, it is believed that not only hematopoietic descendents but also MSCs become a structural and functional component of the repaired tissue (Caplan and Correa, 2011). The long-term roles of fibrocytes, MSCs, and EMT-derived cells in the maintenance and resolution of scar tissue are unclear.
Stromal Cells in Fibrosis
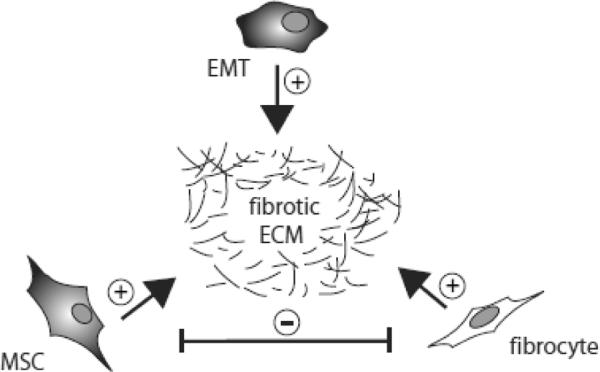
Progression of a number of pathologies resulting from genetic predisposition, injury / infection and chronically affecting respiratory, cardiovascular or digestive systems is associated with fibrotic tissue remodeling. Fibrosis is driven by activated stromal fibroblasts that deposit ECM proteins and become similar to myofibroblasts in that they start expressing αSMA (Neilson, 2006). Stromal progenitors are key players in chronic tissue scarring. Previous studies in fibrosis models demonstrated that fibrocytes, MSCs, and EMT-derived cells co-exist in non-malignant pathology; however their contributions and roles are not defined at present. The notion that EMT plays an important role in the formation of fibrotic scar tissue is widely accepted; however solid evidence is still lacking (Kriz et al., 2011). Because fibrocytes and MSCs also secrete collagen I and other ECM molecules, each one of them has the capacity to contribute to scar formation (Fig. 3). It could be expected that fibrocytes, which promote the immune response, support the chronic maintenance of the early tissue repair stage and scarring. On the contrary, it is believed that the anti-inflammatory properties of MSCs result in the eventual scar resorption (Caplan and Correa, 2011). According to this model, distinct stromal populations have the capacity to execute opposing functions that operate in succession in tissue repair. In chronic pathology, MSCs and fibrocytes might have opposing long-term roles with MSCs being “the good” and fibrocytes being “the bad”. However, this remains speculative until the tools to track and inactivate each population are developed and this hypothesis is tested experimentally. With cardiovascular disease being a major cause of death, understanding the role of pro-fibrotic progenitors in heart pathologies is very important, however the progress in this field is still minimal (Khan and Sheppard, 2006). Here, we will focus on renal and lung fibrosis as the examples of pathologies relying on stromatogenesis that are characterized comparatively well.
Figure 3.
Relationship between different populations of pathological stromal cells. A working model according to which fibroblasts derived from MSCs, fibrocytes, and through EMT, communicate in creating and maintaining the scar tissue in fibrosis. Indicated are positive (+) and negative (−) effects on ECM deposition and function of each other.
Chronic kidney disease is a debilitating condition occurring with an increasing incidence of over 13% in the US population (Coresh et al., 2007). Renal pathology usually initiates as a wound healing response aimed at the restoration of the normal architecture, and the recovery of function. Because tissue repair in the adult does not come to completion and is complicated by infections and inflammatory conditions such as obesity and diabetes, fibrosis does not become resolved and gradually progresses to a chronic state and results in tubulointerstitial and glomerular fibrosis. The excess accumulation of scar tissue composed of ECM molecules such as collagen leads to gradual loss of kidney function. Manifestations of chronic renal fibrosis (CRF), including glomerulosclerosis, vascular sclerosis, and tubulointerstitial fibrosis are the histological predictors of the end-stage disease (Liu, 2006). These transformations result in irreversible kidney failure over time and eventually require dialysis or transplantation for patient survival. Tissue and cell lineage origins of the stromal cells responsible for CRF are unclear. Interstitial fibroblasts, glomerular mesangial cells, and vascular smooth muscle cells are phenotypically similar, with the fibroblasts and mesangial cells acquiring features of smooth muscle when activated (Wynn, 2008). Renal EMT is proposed to be important at later stages of kidney disease progression (Rastaldi, 2006; Kriz et al., 2011). The relative contributions of EMT-derived cells, MSCs, and fibrocytes to CRF remain to be determined. The reported correlation between obesity and CRF (Thakur et al., 2006; Sachse and Wolf, 2008) could be explained by possible migration of MSCs from WAT.
Idiopathic pulmonary fibrosis (IPF) is a rapidly progressing fatal illness with incidence of 0.03 % in the U.S. population (Gross and Hunninghake, 2001). In IPF, the results of inflammation and fibrosis are evident radiographically and on biopsy as diffuse pulmonary infiltrates. Scarring resulting from ECM deposition is associated with alveolar epithelial cell injury, type II cell hyperplasia, progressive loss of normal lung architecture, dyspnea, and ultimately impaired respiratory function (Keane et al., 2005). The complexity of this pneumonia is revealed by heterogeneity of the fibrotic lesions / foci, and unpredictable progression to acute exacerbation. As in other fibrotic conditions, the origin of pathological stromal cells depositing ECM proteins is not understood. Conversion of resident fibroblasts and possibly alveolar epithelial cells through EMT takes place in advanced stages of fibrosis (Willis et al., 2006). However, it has become clear that differentiation of circulating progenitors derived from remote organs is important, in particular at early stages of lung disease. Interestingly, obesity is associates with prolonged survival in IPF patients (Alakhras et al., 2007), suggesting that WAT-derived factors may have beneficiary effects. These factors could in theory correspond to the migrating adipose MSCs and/or fibrocytes. Interestingly, survival of lung cancer patients positively correlates with obesity, while the opposite is the case for most other types of cancer (Flegal et al., 2007).
Stromal Cells in Cancer
Like wound healing, cancer progression relies on the recruitment of stem cells and partially differentiated progenitor cells (Dvorak, 1986). This occurs to a large extent through systemic cell mobilization and attraction from remote organs, as well as possibly via cell migration from surrounding tissues (Laird et al., 2008). Cancer progression relies on blood vessel formation (Folkman, 2006). Tumor neovascularization predominantly occurs through adjacent resident vasculature sprouting into the tumor (angiogenesis) in response to factors released by the hypoxic and inflammatory tumor microenvironment (Carmeliet and Jain, 2000). Recruitment of circulating progenitors into blood vessels (post-natal vasculogenesis) also plays an important role in formation of new vessels (Bertolini et al., 2006; Shaked et al., 2006). The numbers of circulating hematopoietic progenitor cells (HPC) endothelial progenitor cells (EPC), and mature EC are elevated in cancer (Bertolini et al., 2006; Folkman, 2006), and there is evidence for EPC recruitment by human tumors (Peters et al., 2005). The implication of distantly mobilized cells in cancer is illustrated by animal studies demonstrating that bone marrow-derived progenitors contribute to tumor microenvironment and promote cancer progression (Lyden et al., 2001; Gao et al., 2008).
Despite numerous therapeutic approaches aimed at neoplastic and vascular cells, resistance of cancer to treatment remains a challenge. This has led to realization that, in addition to vascular cells, stromal cells represent a key component of the tumor microenvironment contributing to therapy resistance (Fukumura et al., 1998; Bissell and Radisky, 2001; Wels et al., 2008). In addition to the EMT phenomenon, benign resident and recruited stromal cells are clearly implicated both at early stages of cancer and in metastasis. The tumor stroma cells are collectively termed cancer-associated fibroblasts (Karnoub et al., 2007). It has been proposed that mesenchymal progenitors, upon tumor infiltration, re-establish the trophic microenvironment that they normally maintain in the bone marrow (Wels et al., 2008). Cells of tumor stroma facilitate neovascularization by secreting pro-angiogenic and anti-apoptotic factors. In addition, the ability of mesenchymal tumor cells to suppress T cell-mediated cytotoxicity has been proposed to account for the resistance of cancer to the immune system (Fukumura et al., 1998; Bissell and Radisky, 2001). Activation and proliferation of cancer fibroblasts leads to their differentiation into myofibroblasts / reactive stroma (Coussens and Werb, 2002). The ensuing tumor fibrosis is manifested by desmoplastic ECM remodeling (Dvorak, 1986).
Gene expression profiling suggests that at least a proportion of tumor fibroblasts originate from MSCs (Galie et al., 2007). This notion is also supported by the presence of CD45-PDGFRβ+ cells in tumors (Fig. 4). Because MSCs has been shown to function as pericytes (Crisan et al., 2008; Tang et al., 2008; Traktuev et al., 2008), the stimulatory effects of MSCs on cancer progression (Hall et al., 2007; Lazennec and Jorgensen, 2008) are consistent with pericytes presenting a target complimentary to endothelial cells (Bergers et al., 2003; Lu et al., 2007; Sennino et al., 2007). In addition to their pro-angiogenic effects, MSCs secrete trophic and anti-apoptotic factors and have a protective anti-immune effect on tumors (Mishra et al., 2009). Combined, these effects may have a potent positive effect on tumor growth, which has been demonstrated in various models. In addition, MSCs secrete chemoattractants that may engage infiltration of fibrocytes and other types of leukocytes, some of which synergize in promoting cancer progression.
Figure 4.
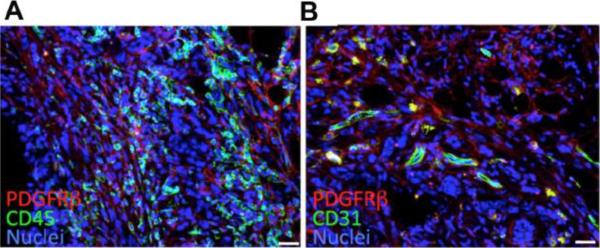
Tumor mesenchymal and hematopoietic stromal cells. Confocal immunofluorescence analysis of sections from Lewis lung carcinoma (LLC) tumors allografted into C57BL/6 mice. Shown are different tumor areas stained with antibodies against (A) mouse PDGFRβ (red) and mouse CD45 (green); or (B) mouse PDGFRβ (red) and mouse CD31 (green). Host mesenchymal stromal cells are PDGFRβ+. Host hematopoietic stromal cells are CD45+ (A). Host vascular endothelium is CD31+ (B). Malignant LLC cells are negative for CD31 and PDGFRβ. Nuclei are stained blue with TO-PRO-3. Scale bar: 50 μM.
While accumulating evidence indicates that MSCs give rise to tumor stroma, hematopoietic cells contribute to a comparable extent (Du et al., 2008; Dawson et al., 2011). Bone marrow transplantation studies in mice show that 25% of cancer myofibroblasts, especially at the periphery of the tumor, are derived from the bone marrow (Direkze et al., 2004) and express CD45 (Udagawa et al., 2006) as illustrated by our unpublished data (Fig. 4). While lymphocytes do infiltrate tumors and affect cancer progression (DeNardo et al., 2010), the majority of marrow-derived stromal cells are of myeloid lineage (Shojaei et al., 2008). All independently reported monocytic populations including tumor-associated dendritic cells (TADC), recruited bone marrow-derived circulating cells (RBCC), TIE2-expressing monocytes (TEM), and myeloid-derived suppressor cells (MDSC) appear to promote cancer angiogenesis in a paracrine manner (Bertolini et al., 2006; Folkman, 2006). There is also evidence that bone marrow-derived myeloid progenitors may give rise to a subpopulation of tumor pericytes (Kim et al., 2009). Whether these populations and fibrocytes are truly distinct, or if they all represent the same plastic myeloid lineage pool collectively playing a role in the tumor stroma, remains to be determined.
Whether MSCs, monocytic cells, and EMT-derived stroma affect tumor progression positively or negatively may be context-dependent, which offers an interesting subject of investigation. There is evidence for epigenetically altered fibroblasts driving not only cancer progression, but also initiation (Bhowmick et al., 2004), which makes the origin of stromal progenitor cells in cancer particularly important. We recently analyzed migration of WAT-derived cells in response to cancer signals (Zhang et al., 2009). Our experiments showed that adipose MSCs become mobilized from WAT implants and migrate to tumors, which is associated with accelerated tumor growth. We also showed that WAT-derived MSCs migrate to tumors through the systemic circulation upon subcutaneous administration, and engraft the perivascular niche, which is sufficient to promote cancer progression. We propose this phenomenon to at least partially explain the epidemiological association between obesity and cancer (Zhang et al., 2010). Interestingly, recruitment of ASCs, but not of marrow-derived or lung-derived MSCs, promoted tumor growth in mice in our study (Zhang et al., 2009), indicating that MSCs from WAT have a unique trophic function. The cancer-promoting properties of adipose stroma have been recently reported by other groups (Dirat et al., 2011; Zimmerlin et al., 2011). Combined, the results of these studies suggest that different organs serve as reservoirs of stromal precursors that may have distinct properties and tumor-promoting capacities. While clinical trials using stromal cells in regenerative medicine are underway, the accumulating evidence that stromal cells can promote cancer progression indicates that caution must be used.
Conclusion
A number of questions regarding the etiology of stromal fibroblasts remain unanswered. The relative contribution of bone marrow, as opposed to other organs and resident tissue, to the pools of pathological stromal cells is to be defined in different pathological settings (Fig. 2). The relative content of mesenchymal progenitors, monocytes, and EMT-derived cells in pathological stroma is also yet to be characterized in disease-specific contexts. An important question is whether EMT-, MSC- and monocyte-derived stromal cells execute complementary or opposing functions during fibrotic pathogenesis (Fig. 3). It is unclear to which extent trans-differentiation of plastic fibroblast progenitors recruited into the lesion complicates the issue. Progress has been limited by the lack of reliable markers that could distinguish the distinct populations of stromal cells. In the past, various approaches have been undertaken to characterize molecular differences between stromal populations, however success in this endeavor has been limited. Recent identification of a new marker of adipose MSCs (Daquinag et al., 2011a) sets the stage for efforts toward systematic characterization of stromal progenitor markers. In the future, identification of differentially expressed cell surface molecules may enable targeting of stromal cell populations. Understanding the properties and roles of EMT-derived cells, MSCs, and monocytic stroma from bone marrow and other organs may outline strategies to differentially control the functions of the respective cell populations in order to suppress disease progression. We envision therapies aimed at specific stromal cell pools becoming a complementary treatment of fibrosis and cancer. New stromal cell markers will make it possible not only to quantify distinct populations of pathological stromal cells in disease, but also to ablate a desired subpopulation. Conversely, the approach of using MSCs as vehicles (Hall et al., 2007) might be expanded to fibrocytes and EMT-derived cells for directing treatment to desired sites for therapeutic purposes.
Highlights.
Alternative origins of stromal fibroblasts in pathology
Mesenchymal stromal cells (MSC), monocyte-derived stromal cells and mesenchymal transition-derived cells are distinct fibroblastic populations that are implicated in development and disease progression.
Acknowledgements
We thank Paul J. Simmons for the critical reading of the manuscript and Alexes Daquinag for providing images.
This work was supported in part by the following awards to M.K: CNE119003 from the American Cancer Society, KG080782 from Komen for the Cure, and 0835434N from American Heart Association and P50 CA140388 SPORE from NIH-NCI. Work in the S.M. laboratory is supported by R01 CA155243 from NIH-NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: M.K. conceived the scope of the review and wrote the manuscript draft. K.E., S.E., and R.G. have expanded the contents and edited the manuscript.
References
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 2001;166:7556–75562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ. Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:1448–1453. doi: 10.1378/chest.06-2784. [DOI] [PubMed] [Google Scholar]
- Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R. The primitive streak. Anat. Embryol. 1986;174:1–14. doi: 10.1007/BF00318331. [DOI] [PubMed] [Google Scholar]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of Progenitor Cells in Obese and Lean Colorectal Cancer Patients. Cancer Epidemiol. Biomarkers Prev. 2011a doi: 10.1158/1055-9965.EPI-11-0556. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG. Influence of BMI on Level of Circulating Progenitor Cells. Obesity. 2011b;19:1722–1726. doi: 10.1038/oby.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat. Rev. Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc. Natl. Acad. Sci. U S A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J. Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The Tunica Adventitia of Human Arteries and Veins as a Source of Mesenchymal Stem Cells. Stem. Cells Dev. 2011 doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Crossno JT, Jr., Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An Isoform of Decorin Is a Resistin Receptor on the Surface of Adipose Progenitor Cells. Cell Stem Cell. 2011a;9:74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends. Pharmacol. Sci. 2011b;32:300–307. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Chae SS, Jain RK, Duda DG. Direct evidence for lineage-dependent effects of bone marrow stromal cells on tumor progression. Am. J. Cancer Res. 2011;1:144–154. [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol. Blood. Transfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- Galie M, Konstantinidou G, Peroni D, Scambi I, Marchini C, Lisi V, Krampera M, Magnani P, Merigo F, Montani M, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2007;27:2542–2545. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes. Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta. Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Bucala R. Fibrocytes in health and disease. Exp. Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- Ishii G, Sangai T, Ito T, Hasebe T, Endoh Y, Sasaki H, Harigaya K, Ochiai A. In vivo and in vitro characterization of human fibroblasts recruited selectively into human cancer stroma. Int. J. Cancer. 2005;117:212–220. doi: 10.1002/ijc.21199. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wulf GG, Goodell MA. Stem cells: a minireview. J. Cell. Biochem. Suppl. 2002;38:1–6. doi: 10.1002/jcb.10045. [DOI] [PubMed] [Google Scholar]
- Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp. Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Bahn JJ, Jeon D, Kim JH, Kim S, Won CH, Kim M, Lee SK, et al. Multipotent PDGFRbeta-expressing cells in the circulation of stroke patients. Neurobiol. Dis. 2011;41:489–497. doi: 10.1016/j.nbd.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Kant P, Hull MA. Excess body weight and obesity--the link with gastrointestinal and hepatobiliary cancer. Nat. Rev. Gastroenterol. Hepatol. 2011;8:224–238. doi: 10.1038/nrgastro.2011.23. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Keane MP, Strieter RM, Belperio JA. Mechanisms and mediators of pulmonary fibrosis. Crit. Rev. Immunol. 2005;25:429–463. doi: 10.1615/critrevimmunol.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am. J. Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, Spaeth E, Xu W, Zhang X, Lewis MT, et al. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5:e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J. Clin. Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin MG, Simmons PJ. Combinatorial stem cell mobilization Nat. Biotechnol. 2009;27:252–253. doi: 10.1038/nbt0309-252. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Mankani MH, Leet AI, Ziran N, Gronthos S, Robey PG. Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells. 2007;25:1830–1839. doi: 10.1634/stemcells.2007-0140. [DOI] [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:1–14. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, Spannuth W, Arumugam T, Han LY, Jennings NB, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin. Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc. Natl. Acad. Sci. USA. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Martin-Padura I, Calleri A, Marighetti P, Quarna J, Rabascio C, Braidotti P, Bertolini F. Circulating perivascular progenitors: a target of PDGFR inhibition. Int. J. Cancer. 2011;129:1344–1350. doi: 10.1002/ijc.25816. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoli S, Bellini A, Schmidt M. The role of a human hematopoietic mesenchymal progenitor in wound healing and fibrotic diseases and implications for therapy. Curr. Stem Cell Res. Ther. 2009;4:266–280. doi: 10.2174/157488809789649232. [DOI] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem. Biophys. Res. Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann. Surg. 2009;250:166–172. doi: 10.1097/SLA.0b013e3181ad8935. [DOI] [PubMed] [Google Scholar]
- Neilson EG. Mechanisms of disease: Fibroblasts--a new look at an old problem. Nat. Clin. Pract. Nephrol. 2006;2:101–108. doi: 10.1038/ncpneph0093. [DOI] [PubMed] [Google Scholar]
- Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Okumura T, Wang SS, Takaishi S, Tu SP, Ng V, Ericksen RE, Rustgi AK, Wang TC. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab. Invest. 2009;89:1410–1422. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr. Opin. Hematol. 2008;15:285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J. Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J. Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastaldi MP. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J. Nephrol. 2006;19:407–412. [PubMed] [Google Scholar]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rossiianov K. Taming the primitive: Elie Metchnikov and his discovery of immune cells. Osiris. 2008;23:213–229. doi: 10.1086/591875. [DOI] [PubMed] [Google Scholar]
- Sachse A, Wolf G. New aspects of the relationship among hypertension, obesity, and the kidneys. Curr. Hypertens. Rep. 2008;10:138–142. doi: 10.1007/s11906-008-0026-x. [DOI] [PubMed] [Google Scholar]
- Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK, et al. Hematopoietic stem cell origin of adipocytes. Exp. Hematol. 2009;37:1108–1120. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans. Am. Clin. Climatol. Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, Morse S, Reisin E. Functional and structural renal changes in the early stages of obesity. Contrib. Nephrol. 2006;151:135–150. doi: 10.1159/000095325. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ. Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama K, Murase N, Stolz DB, Toyokawa H, O'Donnell DR, Smith DM, Dudas JR, Rubin JP, Marra KG. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells. 2008;26:330–338. doi: 10.1634/stemcells.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev D, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A Population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Udagawa T, Puder M, Wood M, Schaefer BC, D'Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley DL, Ruszkiewicz A, Davies R, Moore S, Nivison-Smith I, Bik To L, Browett P, Western R, Durrant S, So J, et al. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells. 2009;27:1463–1468. doi: 10.1002/stem.63. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem. Biophys. Res. Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-tomesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J. Stem Cells. 2010;2:103–113. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Daquinag A, Traktuev DO, Amaya F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng. Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, Swartz-Basile DA, Nakshatri H. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]