Abstract
Oncogenic signaling, such as HER2/neu signaling, has been shown to play major role for tumorigenesis in a subset of breast cancer patients. The use of anti-HER2/neu antibody has not only revealed the mechanisms for HER2/neu signaling but also shown a therapeutic advantage of its blockade. Indeed, the use of trastuzumab has greatly improved the treatment of HER2-positive breast cancer. Although this therapy has been used in the clinic for over twenty years, recent data is still uncovering new mechanisms by which this antibody exerts its anti-tumor activity. In addition to an improved understanding of the molecular mechanisms by which this therapy inhibits growth of tumor cells, the discovery that anti-HE2/neu therapy initiates and requires the adaptive immune system is one of these new mechanisms. The presence of anti-HER2/neu initiated adaptive immunity gives credence to efforts targeted at stimulating the immune system in treating HER2 positive breast cancer. This review focuses on the role of the inflammatory response in HER2 positive breast cancer with particular emphasis on trastuzumab therapy.
Keywords: Adaptive immunity, Innate immunity, HER2/neu, Oncogenic signaling, Breast cancer, Antibody therapies, Immunotherapy, Combination therapies, Antibody drug conjugates, Herceptin, Trastuzumab, Immune responses, Inflammation, Pathobiology
Introduction
The use of antibody therapies has changed the landscape for treating multiple cancer types. Over the past twelve years the FDA has approved multiple antibodies for use against human malignancies, establishing this therapy to be effective in treating cancer. In fact, antibody-based therapeutics are now essential components of many cancer treatment regimens (1). Moreover, the success these therapies are having in the clinic is driving further development of novel immunotherapies (2). Among the oncoproteins to which antibodies have been generated, the human epidermal growth receptor-2 (HER2/neu/ErbB2) is among the most targeted with two molecular targeting agents approved by the FDA (3).
The HER2 oncogene is the human homologue of the rat neu oncogene (HER2/neu). First identified in DNA from ethyl-nitroso-urea induced neuroglioblastomas in rats, HER2/neu is a 185 KDa protein with homology to epidermal growth factor receptor (EGFR) (4-6). Along with HER3 (ErbB3) and HER4 (ErbB4), these proteins constitute the type 1 growth receptor gene family (7). These transmembrane proteins are receptor tyrosine kinases that have intrinsic kinase activity and are activated when ligands bind and promote homo- or heterodimerization (7,8). However, no specific ligand for HER2/neu (ErbB2) has been identified (8,9). When normally expressed, heterodimerization of HER2/neu induces downstream signals through the AKT and MAP kinase pathways. Signaling through these receptors supports many physiological processes including embryogenesis, cell proliferation, differentiation, adhesion, motility and apoptosis (10,11).
Studies using monoclonal antibodies specific for the HER2/neu protein revealed that targeting this surface protein could block oncogenic signaling and inhibit tumor growth. In vitro treatment of neu-transformed fibroblasts or a human mammary gland adenocarcinoma cell line with an anti-neu antibody (clones 7.16.4 and 4D5 respectively) resulted in down-regulation of surface neu protein, and growth inhibition (12,13). When used to target xenographs of human tumors in nude mice, anti-neu antibody therapy inhibited tumor growth in vivo (14). Early studies also demonstrated overexpression of HER2/neu provided transformed cells with resistance to TNFα-mediated tumor inhibition in vitro (15). However, treatment of these cells with anti-HER2/neu antibody countered the resistance to TNFα and induced tumor cell death (13).
Due to the accessibility of HER2/neu at the cell membrane, and its low expression on normal tissues and over-expression on a high percentage of tumors, this protein was an ideal candidate for immunotherapeutic intervention (16). Trastuzumab is a humanized monoclonal antibody that was engineered by inserting the complementary regions of the murine antibody (clone 4D5) into a human IgG1 framework (17,18). Since its development, trastuzumab has been tested in several clinical trials and proved to be an effective adjuvant therapy for HER2/neu-positive metastatic breast cancers (19). In 1998, the FDA approved trastuzumab (Herceptin), for use in treating human breast cancer patients. It was the first monoclonal antibody to be approved for treating solid tumors. The therapeutic activity of trastuzumab has been evaluated in women with metastatic breast cancer as a single agent given before or after traditional chemotherapy, and in combination with a variety of chemotherapy agents (11,20-22). The first phase II trials demonstrated objective response rates from 12-15% (22,23). These studies established that trastuzumab therapy can be effective in patients (24). Based on the convincing preclinical studies, clinical trials were conducted and demonstrated the benefits of combining chemotherapy administration with trastuzumab (24-26), one such study enrolled women that had not received previous adjuvant therapy to examine the combination of trastuzumab with chemotherapy (20,24). The addition of trastuzumab to chemotherapy was associated with a longer time to disease progression, a higher objective response rate and longer survival (20). This study was instrumental, and on the basis of these results, the FDA approved trastuzumab, given either alone or in combination with chemotherapy, for treating patients with metastatic breast cancer overexpressing HER2/neu (1,9).
Immune Responses to Breast Cancer
Multiple studies have been published regarding the mechanisms by which anti-HER2/neu therapy inhibits tumor growth. Anti-HER2/neu therapy has both direct and indirect effects that ultimately lead to tumor cell death. The direct effects include diminishing cell signaling, induction of cell cycle arrest, inhibition of receptor shedding and, when combined with chemotherapy, inhibition of DNA repair. The indirect effects involve inhibition of angiogenesis and inflammatory cell engagement. These multiple effects are due to the ability of anti-HER2/neu to prevent HER2/neu dimerization while the effector Fc arm of the antibody engages Fc receptor (FcR)-positive inflammatory cells, such as natural killer cells and macrophages. In fact, FcR-positive cells were shown to be essential for mediating the therapeutic effects of the anti-HER2/neu antibody (27). A study by Clynes et. al. demonstrated that the therapeutic effect of the anti-HER2/neu antibody was significantly reduced in FcγR KO mice. In addition, when a mutation was made in the CH2 domain of the mouse IgG2 heavy chain to inhibit Fc receptor binding, anti-HER2/neu maintained an ability to inhibit tumor growth in vitro, but did not induce ADCC by NK effector cells. Though Fc-mediated mechanisms do not exclude other mechanisms of action by anti-HER2/neu, these data promoted ADCC as the major mechanism for the in vivo effects of anti-HER2/neu therapy. Over the past few years, the direct effect anti-Her2/neu therapy has on HER2 positive tumors has been reviewed extensively (18,28). Therefore, this review will focus on new evidence for how anti-HER2/neu therapy induces an adaptive immune response.
Understanding the role for adaptive immunity in HER2 positive breast cancer has been an important area of study for many years. For example, in the early 1990's many studies demonstrated that both humoral and adaptive immune responses against HER2/neu are present in HER2/neu positive beast cancer patients (29). However, how anti-HER2/neu therapy induces this response, and if adaptive immunity was even necessary was largely underappreciated. This was due in part to the overwhelming evidence that anti-HER2/neu therapy exerts multiple direct effects on the tumor, and these mechanisms were observed and supported by in vivo evidence (24). One caveat to many of these studies, however, was the use of xenographs of human tumors into nude mice. As a result, the role of adaptive immunity in anti-HER2/neu therapy was underexplored.
Using an immunocompetent mouse model, we recently demonstrated that adaptive immunity and T cells are necessary for the tumor reduction by anti-HER2/neu therapy alone (30). Here, WT BALB/c mice were inoculated with a syngeneic HER2/neu positive breast cancer cell line and treated with a mouse anti-HER2/neu antibody. The critical role of adaptive immunity was supported by the observation that the efficacy of anti-HER2/neu therapy was greatly reduced in Rag1-KO mice that lack adaptive immunity. Anti-HER2/neu therapy had some effect in Rag1-KO mice suggesting that the direct effects of anti-HER2/neu therapy were intact, but the overall anti-tumor effect was significantly reduced in these mice. To explore the cell types involved, WT BALB/c mice depleted of CD8+ T cells were unable to completely respond to anti-neu therapy, and this CD8-dependency was recapitulated in tolerized neu-transgenic mice. Moreover, WT BALB/c mice with undetectable tumors for greater than 30 days after anti-HER2/neu therapy were subsequently resistant to a high dose tumor re-challenge, suggesting the presence of immune memory. Together these data suggest that, in addition to the direct effects anti-HER2/neu therapy has on inhibiting tumor growth, this treatment also induces and requires an adaptive immune response.
We also investigated the inflammatory events leading to the induction of adaptive immunity after anti-HER2/neu therapy. Stimulation of toll-like receptors (TLRs) via the MyD88 pathway is an important means by which antigen presenting cells are activated and respond to foreign pathogens (31). Accordingly, the anti-tumor effect of anti-HER2/neu therapy was abolished in MyD88-deficient mice. Typically, TLRs are activated via pathogen associated molecular patterns (PAMPs) from bacteria and viruses. However, some self-ligands associated with tissue damage, such as HMBG-1, are also capable of activating TLRs to increases cross-priming and activation of dendritic cells in both mice and humans (32,33). Neutralization of HMBG-1 alone greatly reduced the efficacy of anti-HER2/neu therapy. These data indicate that HMGB-1, an endogenous danger signal, is essential for antibody-mediated tumor regression, and it is conceivable that anti-HER2/neu antibody induces HMGB-1 release in the tumor microenvironment enhancing innate responses via the MyD88 pathway.
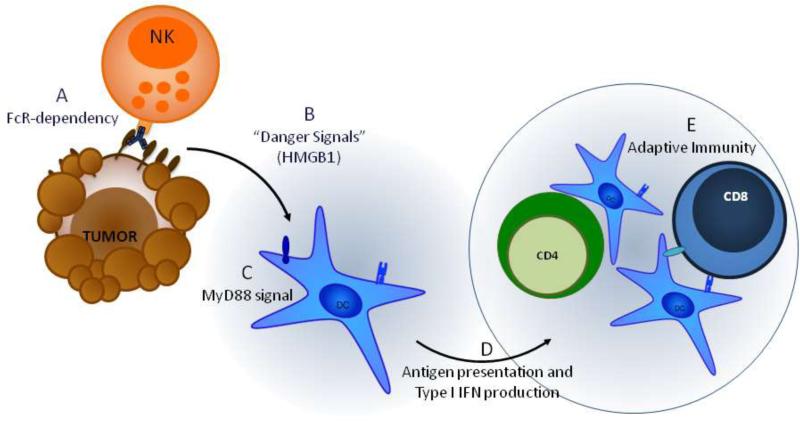
These observations were extended and confirmed by work from Mark Smyth's group who- through the use of another HER2/neu dependent tumor model and adoptive cell transfer experiments with CD8 and IFNγ KO mice- demonstrated that not only were CD8+ cells essential, but IFNγ from CD8+ cells was required for the anti-tumor effect of anti-neu therapy (34). Although this study also demonstrated that anti-HER2/neu therapy was dependent on TLR signaling through MyD88, they established the necessity of NK cells and the additional role for Type-I IFNs. Taken together these two studies suggest that NK cells induce ADCC after engaging the anti-HER2/neu antibody to promote the release of HMGB-1, stimulate MyD88 dependent TLRs, and thereby causing the release of Type-1 IFNs to prime the adaptive immune system (Figure 1).
Figure 1. Anti-HER2/neu therapy induces an adaptive immune response.
A) Tumor cell death induced by oncogenic signal blockade and ADCC upon binding of anti-Her2/neu to the Her2 receptor induces release of danger signals, like HMGB-1, from apoptotic or stressed tumor cells. B) HMGB1 is recognized alone or in complex with other molecules, such as ssDNA, to activate dendritic cells and promote cytokine production via activation of the MyD88 pathway. C) MyD88 signaling enhances cross-presentation and activates dendritic cells and also induces the release of Type-1 IFNs. D) These dendritic cells can either remain in the tumor or travel to the lymph node and prime CD4+ and CD8+ T cells of the adaptive immune system. Primed T cells leave the lymph node and travel back to the tumor
Pertuzumab is a humanized monoclonal antibody that binds the extracellular domain II of the HER2 receptor, and has been effective in treating Trastuzumab resistant tumors when given in combination with Trastuzumab (35). The binding site of Pertuzumab differs from Trastuzumab, which binds the extracellular domain IV, but it nonetheless shares many of the same mechanisms of action (35). Because Pertuzumab can induce ADCC, it follows that induction of an adaptive immune response would be possible. It remains to be determined, however, if adaptive immunity is necessary for Pertuzumab therapy.
Although there is still much to learn about how anti-HER2/neu therapy inhibits tumorigenesis and how to combine it with standard care- be it radiation, chemotherapy or surgery- these studies identified a new mechanism of action for anti-HER2/neu therapy and support continued efforts aimed at harnessing this response for enhanced tumor reduction.
Immunotherapy to Breast Cancer
Prior to our study, other researchers have been attempting vaccination, regulatory T cell depletion and adoptive cell transfer; but these immunotherapies have had limited and various effects. Despite a measurable immune response, increased numbers of tumor-specific CD8+ T cells does not always lead to tumor reduction. Furthermore, recognition of the tumor antigen alone is not sufficient for the host to eradicate established solid tumors (36-38). Thus, natural immune responses fail to clear tumor cells and immune tolerance may occur throughout tumor progression. Several factors contribute to this dampened immune response. First, poor direct or indirect antigen presentation in lymphoid tissues reduces early T cell priming. This may be due to inadequate numbers of tumor cells migrating to the lymph node, low expression of MHC-I on tumor cells, or low expression of co-stimulatory molecules on APCs. Second, physical (e.g. hydrostatic pressure) and biological (poor adhesion molecule activation) barriers around tumor tissues can cause an inadequate number of immune cells to migrate into and though the tumor (39,40). Third, suppressive cell types residing within the tumor microenvironment can induce anergy or deletion of infiltrating T cells to promote tumor growth (41). Fourth, release of soluble factors such as IL-10, TGFβ, IL-17 or VEGF by the tumor or the surrounding stroma can inhibit T cell function or promote angiogenesis (38,42). Finally, immune tolerance and/or suppression can be induced by the tumor itself though the expression of the inhibitory molecule PD-L1.
Combination Therapies
Due to the multitude of factors limiting the immune response, the need for therapies that re-activate the immune system is imperative. Because targeted therapies such as anti-HER2/neu can hasten tumor reduction and reduce tumor immune suppression, they may also reset the microenvironment and open a window of opportunity for immunotherapies (43). Indeed, immunotherapy can lead to the reduction or even eradication of some established human tumors, but these responses are only achieved in a minority of patients (37,44,45). However, monotherapies are unlikely to overcome the multitude of suppressive mechanisms that inhibit anti-tumor immunity in all patients (43). Thus, new strategies and protocols are critically needed for treating the majority of cancer patients.
Among the recent strategies has been the combination of anti-HER2/neu with immunomodulatory therapies. Typically this involves combining monoclonal antibody therapy with tyrosine kinase inhibitors. However, some studies are focused on altering the adaptive immune system. One such strategy has been the combination of anti-HER2/neu therapy with an agonistic anti-CD137 antibody or with blocking PD-1. CD137 mediates lymphocyte survival, and can enhance the function of CD8+ T cells, natural killer cells and dendritic cells whereas PD-1 is an inhibitory receptor expressed activated and anergic T cells. Thus, combining either of these therapies with anti-HER2/neu may have synergistic effects involving both adaptive and innate immunity. Indeed, Stagg et. al. demonstrated that addition of an agonistic anti-CD137 or anti-PD-1 blocking antibody significantly enhanced the efficacy of anti-HER2/neu therapy when administered to both tumor-bearing WT and neu transgenic mice (34). In a similar fashion, Kohrt et. al. recently reported that CD137 is increased on human natural killer cells after anti-HER2/neu therapy, and selectively activating this protein with an agonistic anti-CD137 antibody enhanced the efficacy of anti-HER2/neu therapy in a xenograph model (46). Transplanting human tumors into aythmic mice allowed these researchers to identify how additional anti-CD137 treatment enhances antibody dependent cellular cytotoxicity; however, how activating signals through anti-CD137 therapy enhances adaptive immune responses still requires further elucidation.
In our lab, we have focused on the immunostimulatory properties of LIGHT. Expression of LIGHT, a TNF family member, within the tumor microenvironment can attract various immune cells, including substantial numbers of FcR+ cells, dendritic cells, and T cells (47). In the context of HER2/neu-positive breast cancer, targeting tumors with an adenovirus expressing LIGHT enhanced the efficacy of anti-HER2/neu therapy (30). Moreover, tumor-free mice after combination therapy were resistant to a lethal re-challenge of HER2-positive tumor cells but not HER2-negative cells. These data suggest that expression of LIGHT works by enhancing the adaptive immune response and promoting antigen specific memory. Together these pre-clinical studies support further effort aimed at enhancing the adaptive immune response initiated by anti-HER2/neu therapy.
Antibody Drug Conjugates
Given the harsh side effects of chemotherapy, approaches that selectively target cytotoxic agents to cancer cells is an attractive strategy. This approach is very promising in the treatment of HER2/neu-positive cancer. In particular, Trastuzumab-emtansine (T-DM1), an antibody drug conjugate (ADC) which has a chemotherapeutic agent linked to Trastuzumab, has proven to be effective in treating tumors that had progressed on previous therapies, including Trastuzumab (48). We have demonstrated that timing of chemotherapeutic administration can have different effects on the immune response initiated by anti-HER2/neu therapy (30). It will be important, therefore, to determine whether and to what extent Trastuzumab-emtansine induces an immune response.
Conclusion
The discovery of the association between HER2/neu overexpression and breast cancer prognosis has promoted a succession of therapies aimed at exploiting this tumor oncoantigen, and the introduction of anti-HER2/neu antibody therapy revolutionized the treatment of HER2 positive breast cancer. Since the approval Trastuzumab by the FDA, many studies have sought to understand the mechanisms by which this therapy reduces tumor burden. The recent identification that the adaptive immune response is necessary for mediating the effects of this antibody treatment has furthered the understanding of anti-HER2/neu therapy and given credence to studies testing immunotherapies to fight HER2/neu-positive breast cancer. Further understanding the mechanisms behind current therapies, like anti-HER2/neu antibody, will benefit the development of these strategies.
Acknowledgments
This research was supported in part by the National Institutes of Health grants CA141975 and CA97296 to Y.X.F.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005 Sep.23(9):1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Griggs J, Zinkewich-Peotti K. The state of the art: immune-mediated mechanisms of monoclonal antibodies in cancer therapy. Br J Cancer. 2009 Dec.101(11):1807–12. doi: 10.1038/sj.bjc.6605349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Experimental Cell Research. 2010 Apr.316(7):1083–100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar.290(5803):261–4. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- 5.Padhy LC, Shih C, Cowing D, Finkelstein R, Weinberg RA. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982 Apr.28(4):865–71. doi: 10.1016/0092-8674(82)90065-4. [DOI] [PubMed] [Google Scholar]
- 6.Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur. J. Cancer. 1998 May;34(6):791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar T, Gullick WJ. The type I growth factor receptors in human breast cancer. Breast Cancer Res Treat. 1994 Jan.29(1):3–9. doi: 10.1007/BF00666177. [DOI] [PubMed] [Google Scholar]
- 8.Shepard HM, Brdlik CM, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Invest. 2008 Nov.118(11):3574–81. doi: 10.1172/JCI36049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. Nature Publishing Group. 2012 Jul.12(8):553–63. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 10.Quaglino E, Mastini C, Forni G, Cavallo F. Coligan John E, et al., editors. ErbB2 transgenic mice: a tool for investigation of the immune prevention and treatment of mammary carcinomas. Current protocols in immunology. 2008 Aug. doi: 10.1002/0471142735.im2009s82. Chapter 20:Unit 20.9.1–20.9–10. [DOI] [PubMed] [Google Scholar]
- 11.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003 Sep.22(42):6570–8. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 12.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul.41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 13.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989 Mar.9(3):1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci USA. 1986 Dec.83(23):9129–33. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudziak RM, Lewis GD, Shalaby MR, Eessalu TE, Aggarwal BB, Ullrich A, et al. Amplified expression of the HER2/ERBB2 oncogene induces resistance to tumor necrosis factor alpha in NIH 3T3 cells. 1988. [DOI] [PMC free article] [PubMed]
- 16.Eccles SA. Monoclonal antibodies targeting cancer: “magic bullets” or just the trigger? Breast Cancer Res. 2001;3(2):86–90. doi: 10.1186/bcr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992 May 15;89(10):4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006 Feb.232(2):123–38. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Abramson V, Arteaga CL. New strategies in HER2-overexpressing breast cancer: many combinations of targeted drugs available. Clin Cancer Res [Internet] 2011 Mar.17(5):952–8. doi: 10.1158/1078-0432.CCR-09-1947. Available from: http://clincancerres.aacrjournals.org/cgi/doi/10.1158/1078-0432.CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. Mass Medical Soc. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Vogel C, Cobleigh M, Tripathy D, Gutheil J. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of HER2-Overexpressing Metastatic Breast Cancer. Journal of Clinical Oncology. 2002 doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 22.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999 Sep.17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996 Mar.14(3):737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 24.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007 Jul.357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 25.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005 Oct.353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 26.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. New England Journal of Medicine. 2005 Oct.353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 27.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000 Apr.6(4):443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 28.Raja S, Luan H, Naramura M, Bailey T, Clubb R, Band V, et al. Mechanisms of Trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J Carcinog. 2011;10(1):28. doi: 10.4103/1477-3163.90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladjemi MZ, Jacot W, Chardès T, Pèlegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010 Sep.59(9):1295–312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010 Aug.18(2):160–70. doi: 10.1016/j.ccr.2010.06.014. [This study is the first to identify the necessity of adaptive immunity in anti-HER2/neu therapy and the implications on current therapies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006 Apr.440(7085):808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 32.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 33.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007 Dec.220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 34••.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proceedings of the National Academy of Sciences. 2011 Apr.108(17):7142–7. doi: 10.1073/pnas.1016569108. [This study highlights the critical role for Type I and II IFNs and how anti-HER2/neu therapy may be enhanced by combination therapies such as anti-PD-1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capelan M, Pugliano L, de Azambuja E, Bozovic I, Saini KS, Sotiriou C, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2012 Aug. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 36.Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006 May;6(5):383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg SA. The emergence of modern cancer immunotherapy. Ann Surg Oncol. 2005 May;12(5):344–6. doi: 10.1245/ASO.2005.01.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29(3):233–40. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 39.Ryschich E, Schmidt J, Hämmerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002 Feb.97(6):719–25. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 40.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 41.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005 Mar.201(5):779–91. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature Publishing Group. 2012 Apr.:1–15. doi: 10.1038/nrc3237. [This review thoroughly covers immunotherapy in breast cancer as well as other cancer types.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996 Mar.183(3):725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul WE, editor. Fundamental Immunology. 4th ed. Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 46.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012 Mar.122(3):1066–75. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007 Aug.179(3):1960–8. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy CG, Morris PG. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anticancer Drugs. 2012 Sep.23(8):765–76. doi: 10.1097/CAD.0b013e328352d292. [DOI] [PubMed] [Google Scholar]