Abstract
Presently there is limited research to suggest efficacious interventions for infants at-risk for autism. Pivotal response treatment (PRT) has empirical support for use with preschool children with autism, but there are no reports in the literature utilizing this approach with infants. In the current study, a developmental adaptation of PRT was piloted via a brief parent training model with three infants at-risk for autism. Utilizing a multiple baseline design, the data suggest that the introduction of PRT resulted in increases in the infants’ frequency of functional communication and parents’ fidelity of implementation of PRT procedures. Results provide preliminary support for the feasibility and utility of PRT for very young children at-risk for autism.
Keywords: Early intervention, Pivotal response treatment, Parent education, Infant siblings
Introduction
The genetic risk associated with autism is considered to be quite significant, with concordance rates in identical twin pairs of approximately 60 % for autism and 90 % for disorders on the autism spectrum (Veenstra-Vanderweele and Cook 2003; Dawson 2008), with siblings at a significantly higher risk than the general population (Bailey et al. 1996; Landa and Garrett-Mayer 2006; Zwaigenbaum et al. 2007). Siblings are also at an increased risk for developing features of the broader autism phenotype (BAP; Bailey et al. 1998; Constantino et al. 2006; Folstein et al. 1999; Losh et al. 2008) and other psychiatric and developmental problems (Landa et al. 1991, 1992; Pickles et al. 2000; Piven et al. 1997; Ruser et al. 2007; Smalley et al. 1995). Consequently, as infant siblings of children with Autism Spectrum Disorders (ASD) are followed prospectively at research centers across the country and internationally, emerging developmental vulnerabilities are identified months or years before these children would typically be referred for clinical evaluation (Zwaigenbaum et al. 2009). These concerns warrant referral for intervention, yet there is a critical need for research regarding evidence-based treatments for infants at risk for ASD as empirically supported interventions have not yet been clearly identified (Boyd et al. 2010; Chawarska et al. 2008; Dawson 2008).
It has been well-established through retrospective research methods that abnormalities in development associated with autism become apparent by as early as 12 months of age (Baranek 1999; De Giacomo and Fombonne 1998; Maestro et al. 2001; Osterling et al. 2002). Prospective studies of infant siblings of children with autism also provide documentation of impairments in the first 2 years of life (Cassel et al. 2007; Ozonoff et al. 2008; Zwaigenbaum et al. 2005). Furthermore, based on recent evidence of prospective studies of infant siblings of children with ASD (infants at-risk), the earliest clearly predictive symptoms appear to emerge by 12 months of age (e.g., Rogers 2009; Ozonoff et al. 2010; Paul et al. 2011). Though at 12 months of age infants at-risk who will go on to develop ASD cannot always be reliably differentiated from infants who will have either transient or persistent language or cognitive impairment but not ASD; these delays warrant intervention based on the infant’s high risk status. In particular, several studies have noted a developmental deceleration for infants at-risk between 12 and 24 months of age, including groups of children who do not go on to develop ASD, indicating an overall vulnerability for infants at-risk as a group (Brian et al. 2008; Landa and Garrett-Mayer 2006; Stone et al. 2007). This time period also corresponds to a typical developmental shift to more advanced forms of social-communication which may fail to develop for some infants in this cohort. These patterns of developmental slowing beginning at 12 months of age further suggest that this period may be a critical time for intervention to boost social and communication skills for infants at-risk prior to full syndrome expression of ASD.
Rationale for Intervention with Infants and Toddlers
Numerous studies document the efficacy of early intervention in preschool children with ASD, and there is emerging evidence that these techniques are also beneficial for toddlers with ASD (Dawson et al. 2010; Vismara and Rogers 2008). The literature suggests that some children may even be able to overcome many of their symptoms of autism with early intervention (Dawson and Osterling 1997; Harris and Handleman 2000; Harris and Weiss 1998; Koegel et al. 2006; McEachin et al. 1995; Sheinkopf and Siegel 1998; Volkmar et al. 2004). Analysis of developmental trajectories of young children participating in early intervention confirms the importance of initiating intervention before the gap between the child and typical peers is large (Koegel et al. 2006) due to greater opportunity to alter a child’s developmental trajectory toward a more typical path. Thus, intervening with children who are “at-risk” for developing ASD, but do not yet exhibit full syndrome expression, is likely to provide the greatest advantages from intervention (Dawson 2008).
Rationale for Pivotal Response Treatment Approach
Pivotal response treatment (PRT; Koegel et al. 1999) is an evidence-based manualized intervention for individuals with ASD and one of 10 comprehensive model programs for children with autism identified by the National Research Council (2001). PRT is an intervention approach deeply rooted in the principles of Applied Behavior Analysis (ABA) and also shares general instructional strategies with other empirically supported naturalistic behavioral programs, such as incidental teaching (Hart and Risley 1968), mand-model (Warren et al. 1984), Early Start Denver Model (Dawson et al. 2010), natural language paradigm (Koegel et al. 1987), and Prelinguistic Milieu Teaching (Yoder and Stone 2006). PRT places explicit emphasis on targeting “pivotal” areas which are likely to impact broad areas of functioning. For instance, the pivotal area of motivation is targeted by using specific behavioral procedures which are designed to increase a child’s motivation to interact with others. Thus, consistent with the social motivation hypothesis that impairments in ASD may be related to early failure to assign appropriate reward value to social stimuli (e.g., Dawson et al. 2005), PRT aims to address these core deficits in social motivation (Koegel et al. 1987) and alter the child’s developmental trajectory toward a more typical path by providing supplemental learning opportunities which enhance the reward properties of social communication interactions. Given the empirical research demonstrating the efficacy of the motivational PRT procedures in improving social and communication skills in older children with autism (Koegel and Koegel 2006; Koegel et al. 1987, 1999) and emerging evidence for the utility of naturalistic-behavioral procedures with infants and toddlers who demonstrate early signs of ASD (Dawson et al. 2010; Vismara and Rogers 2008), there is a need for further research aimed at systematically applying the PRT framework in a manner appropriate for infant siblings of children with autism.
The current study examined the application of a developmentally appropriate downward extension of PRT, delivered via a parent education model for infant siblings of children with ASD at 12 months of age. Specifically, we evaluated the program’s preliminary efficacy, in terms of (a) whether the application of PRT for infants could increase the frequency of functional communication attempts made by these children, and (b) whether a brief parent training intervention would be effective in teaching parents how to elicit more frequent functional communication from their children. Additionally, to address acceptability, we also examined parental satisfaction with the program.
Method
Participants
Participants were recruited from an ongoing prospective study of infant siblings of children with an ASD through a large University Medical Center. Given the challenges in accurately predicting with current methods which children at 12 months of age will go on to receive an ASD diagnosis later on, children were selected regardless of degree of apparent developmental concern. The first three consecutive infants at 12 months of age were offered the opportunity to take part in the pilot study and all three families consented to participate. All infants were administered the Mullen Scales of Early Learning (Mullen 1995) and the Autism Diagnostic Observation Schedule-Toddler module (ADOS-T; Luyster et al. 2009) at 12 months of age (see below, developmental testing). Participant characteristics and the results of these measures are outlined in Table 1. Based on developmental testing and clinical observation during routine evaluation, Child 1 and 2 demonstrated significant clinical concerns at 12 months of age, while subthreshold social and communication delays were noted for Child 3.
Table 1.
Results of developmental testing at 12 months of age
| Mullen scalesa
|
ADOS-T classification | |||||
|---|---|---|---|---|---|---|
| GM | VR | FM | RL | EL | ||
| Child 1 | 37 | 52 | 60 | 25 | 25 | 18 (moderate/severe concern) |
| Child 2 | 43 | 46 | 55 | 55 | 37 | 15 (moderate/severe concern) |
| Child 3 | 62 | 60 | 65 | 44 | 42 | 7 (little/no concern) |
GM gross motor, VR visual reception, FM fine motor, RL receptive language, and EL expressive language
T scores are provided for the Mullen Scales, mean = 50, standard deviation = 10
Child 1
Child 1 was the full biological sibling of a 4-year-old child diagnosed with Autistic Disorder. At 12 months, Child 1 presented with numerous symptoms of ASD. He demonstrated significant delays in both receptive and expressive language as evidenced by low scores on the Receptive and Expressive Language subscales on the Mullen, as well as poor responsivity to adults and infrequent initiation of communication during the ADOS (See Table 1). Child 1 rarely vocalized, and did not use gestures, pointing, giving, or eye contact for communicative purposes. He also evidenced some unusual sensory interests, including prolonged unusual visual regard for objects.
Child 2
Child 2 was the full biological sibling of a 5-year-old child diagnosed with Pervasive Developmental Disorder - Not Otherwise Specified (PDD-NOS). Child 2 demonstrated significant vulnerabilities in social and language domains at 12 months (See Table 1). In particular, Child 2 had delays in expressive language on both the Mullen and ADOS, as he vocalized infrequently, using primarily single-syllable sounds. He also rarely initiated interactions with others, and did not regularly use eye contact, reaching, or vocalizations to communicate. He did not readily respond to bids from others for interactions. In addition, Child 2 also evidenced hand-flapping and repetitive bouncing.
Child 3
Child 3 was the full biological sibling of an 8-year-old child diagnosed with PDD-NOS. Child 3 performed in the average range on developmental measures, and evidenced subthreshold areas of concern on the ADOS (See Table 1). Child 3 engaged in frequent babbling as well as social referencing and initiated joint attention. However, Child 3 did not demonstrate pointing or gesture use (other than a communicative reach), and did not clearly show or give objects to others. He also demonstrated some brief finger posturing.
Procedures
Design
The study utilized a multiple baseline across participants design, with 2, 3, and 4 weeks of baseline sessions collected for Child 1, 2, and 3, respectively. Subsequent to baseline data collection, intervention consisted of 10 1-h parent education sessions, occurring approximately weekly, over the course of 3 months (between approximately 12 and 15 months of age), with eight clinic and two home sessions. Data were also collected at post-intervention, approximately 1 week after the final intervention session, when the child was 15 months of age.
Data Collection
Videotaped probes were collected during baseline, clinic treatment sessions 2 through 8, and post-treatment phases for behavioral coding of the primary dependent variable. The first parent education session, as well as the two in-home sessions were not videotaped, as these were primarily psychoeducational in nature (e.g., introducing PRT) or focused on generalization (e.g., setting up the home for success).
To establish the frequency of child communicative attempts prior to intervention, during baseline sessions at the clinic parents were asked to play with their child in a room with age-appropriate toys and to attempt to elicit communication from their child for 10 min. To capture the frequency of child communication during clinic parent education sessions 2 through 8, the first 10 min during which the child was consistently provided with opportunities to respond (i.e., extended verbal discussion of techniques or review of materials between parent and clinician was not occurring) were analyzed. As both the parent and clinician were present and interacting with the child during parent education sessions, communicative bids to either the parent or clinician were analyzed. Subsequent to intervention, at the age of 15 months, a post-treatment probe was collected for each parent–child dyad to assess child communication and parent implementation of intervention strategies.
In addition, to assess parent acquisition of PRT skills during the course of parent training and to provide supplemental information regarding parent independent implementation of the skills learned the prior week and associated child functional communication, weekly 10-min video generalization probes of parent independent implementation of PRT techniques were collected at baseline, weekly, and at post-treatment.
Intervention
PRT for Infants
As described in the introduction, PRT directly incorporates underlying principles of ABA and shares empirically validated instructional strategies with other established and extensively researched behavioral programs. The general approach for using PRT procedures to teach social communication by targeting the pivotal area of motivation is described by Koegel et al. (1987). Specifically, intervention incorporated the following individually validated motivational procedures: (a) following the child’s choice of stimulus materials used to elicit communication, (b) providing clear prompts for communication, (c) interspersal of maintenance and acquisition tasks, (d) immediate and contingent reinforcement, (e) the use of natural reinforcers that are directly and functionally related to the interaction, and (f) reinforcing goal-directed communicative attempts. A new manual was adapted with lessons, worksheets, and assignments addressing the six motivational procedures (above described) consistent with PRT, with specific examples of how these principles apply to teaching social and communicative behaviors to infants. In addition to vocalizations and word attempts which are typically the focus of initial PRT intervention, target behaviors also included pointing, giving, showing, and other developmentally appropriate gestures. Within the ten sessions, lessons were also provided in regards to other pivotal areas (i.e., initiation) and the teaching of other types of prelinguistic social behaviors appropriate for infants (i.e., responding to and initiating joint attention) using the motivational PRT principles (see Appendix for an outline of topics covered in each parent education session).
Format of Parent Education Sessions
Pivotal response treatment parent training was conducted consistent with procedures described in the literature (e.g., Koegel et al. 2003; Gilett and LeBlanc 2007). Specifically, during parent education sessions, the parent was introduced to one PRT strategy each week and guided in practicing the application of the PRT techniques with the child. Sessions followed a “practice with feedback” format, whereby the clinician modeled the procedures with the child, provided opportunities for the parent to practice the techniques with the child, and provided constructive feedback regarding procedure use. For instance, when introducing the topic of reinforcing attempts (week 4), the clinician demonstrated several examples of child attempts which could be reinforced while working with the child directly, then instructed the parent to try prompting for child communication and reinforcing effortful attempts, and subsequently provided verbal feedback regarding correct and incorrect implementation of the technique. Additional clinician modeling of procedures was provided throughout the 1-h session as needed for clarification of techniques.
Dependent Measures
Baseline, intervention, and post-intervention video probes were coded by two independent coders, blind to the purposes of the study.
Child Functional Communication
Child functional communication was the primary dependent measure. First, in order to obtain information about the overall percent of intervals in which the children were using any type of functional communication, coders used partial interval coding to identify the presence or absence of any functional communicative behavior during 10-s intervals for each 10-min probe. Specifically, they identified intervals in which the child clearly used eye contact, gesture, showing, giving, and/or vocalization to communicate to the examiner or parent. Functional communication was operationalized based on the literature (Openden 2005; Pierce and Schreibman 1997; Symon 2005) to include the use of the above described modes of communication, alone or in combination, with body/facial orientation to the adult or task, and for the purpose of communicating a clear message which was task-related. Responsive babbling and undirected vocalizations were excluded, as were gestures which occurred as part of a social routine but did not serve a clearly communicative purpose. Tantrumming, screaming, or whining were not included unless combined with functional communication as operationalized above (e.g., a clear gesture).
To obtain supplemental information regarding the type of child communication utilized, for each interval in which child functional communication occurred, the coder subsequently identified the presence or absence of eye contact, vocalization, gesture, or a response in which the child integrated two or more means of communication. The average percent of intervals containing each type of communication bid across sessions was calculated for each child.
Level of Prompting
For each interval containing child functional communication, coders also identified the level of prompting used by the adult in order to calculate the average percentage of intervals with child communication attempts made in response to physical, model, and open-ended prompts as well as child initiations across treatment probes. The average percent of intervals containing each type of prompt across sessions was calculated for each child. For any interval which contained more than one type of prompting, or a child initiation as well as a prompt, the most intrusive type of prompt was scored. Level of prompting from most to least intrusive was operationalized as follows. Physical prompts were defined as any time the child’s body was touched or physically guided to make a response (e.g., physically prompting the child to give an object). Model prompts were defined as any time the adult provided a model of the desired behavior for the child to imitate (e.g., demonstrating a point, modeling a sound). Open-ended prompts were defined as any indication from the adult that the child should respond which did not directly model the target behavior (e.g., adult holding up several items for the child to choose from or asking “What do you want?”). Child initiations were defined as any functional child communication which occurred spontaneously and was not preceded by any of the prompts described above.
Parent Independent Implementation of PRT
Parent fidelity of implementation of PRT was scored for generalization probes during and after intervention in which the parent had been asked to try to implement PRT independently without the clinician present. Standardized measures of fidelity of implementation of PRT (e.g., Minjarez et al. 2011; Bryson et al. 2007; Symon 2005) were adapted based on the developmental adaptations of the treatment protocol. For all parents, each 10-min probe was scored using ten 1-min intervals. For each interval, raters scored parents’ use of each PRT technique as either correct (parent used the technique correctly) or incorrect (parent did not use the technique, used the technique improperly, or used the technique both properly and improperly in the same interval). Fidelity of implementation was scored for the following variables: (a) follow child’s choice of stimulus materials (e.g., toys/objects) used to elicit communication, (b) provide clear opportunities to elicit communication, (c) intersperse maintenance and acquisition tasks, (d) use natural and contingent reinforcers, and (e) reinforce goal-directed communicative attempts. An overall fidelity score was calculated for each probe, by calculating the percent of correct intervals. Fidelity of implementation is considered to be 80 % correct or greater (Schreibman and Koegel 2005).
Interobserver Agreement
One-third of the probes, randomly selected across children and phases, were scored for interobserver reliability for child and parent variables. Percent agreement was calculated using the following formula: number of agreements divided by the number of agreements plus disagreements, multiplied by 100. For child communication, an agreement was defined as both raters scoring either the occurrence or nonoccurrence of child communication for the same interval. For type of child communication, an agreement was defined as both raters identifying the same type of behavior for the same interval (e.g., both raters identifying a gesture occurred). For level of prompting, an agreement was defined as both raters scoring the same category of prompting for the same interval (e.g., model prompt). For parent fidelity, an agreement was defined as both raters scoring either correct or incorrect for a given PRT point for the same interval. Average percent agreement for child communication, type of communication, level of prompting, and parent fidelity were 92 (range 86–100), 87 (80–100), 91 (80–95) and, 84 % (74–100), respectively.
Parent Satisfaction Questionnaire
Following intervention, parents were asked to complete a brief anonymous questionnaire to assess satisfaction with the parent education provided. Specifically, parents rated satisfaction with their child’s progress, the skills taught, their likelihood to recommend the program to other families, as well as overall satisfaction with the program. Ratings were made on a 0–5 Likert scale, with 0 being not satisfied and 5 very satisfied. Parents also provided narrative responses regarding the most and least helpful aspects of the intervention.
Developmental Testing and Diagnostic Impressions at 12 and 36 months
All assessments were administered at 12 and 36 months of age by a doctoral-level clinician blind to the child’s sibling status and intervention participation.
Mullen Scales of Early Learning
The Mullen (1995) is an individually administered comprehensive measure of cognitive functioning for infants and preschool children, from birth through 68 months. The Mullen assesses the child’s abilities and provides standardized scores in five domains: Visual Reception, Receptive Language, Expressive Language, Fine Motor skills, and Gross Motor skills.
Autism Diagnostic Observation Schedule
The ADOS (Lord et al. 2000) is a comprehensive, investigator-based procedure that places the child in naturalistic social situations demanding specific social and communication reactions. Behaviors are coded in the areas of social communication, social relatedness, play and imagination, and restricted and/or repetitive behaviors. The ADOS provides a DSM-IV based algorithm for the diagnosis of autism, ASD, and non-PDD. The ADOS toddler module was administered at 12 months of age.
Diagnostic Outcomes at 36 Months
Given the variability in the development of infant siblings over time, assessments and diagnostic outcomes at the age of 36 months were examined to explore the relationship in this small sample of children between signs and symptoms at 12 months of age, this brief pilot intervention, and developmental outcomes. Diagnostic outcomes were determined by a doctoral-level clinician with expertise in the assessment and diagnosis of young children who was blind to the child’s sibling status and intervention participation. The diagnosis reflects the clinical impressions of the clinician following review of all available measures.
Results
Child Communication
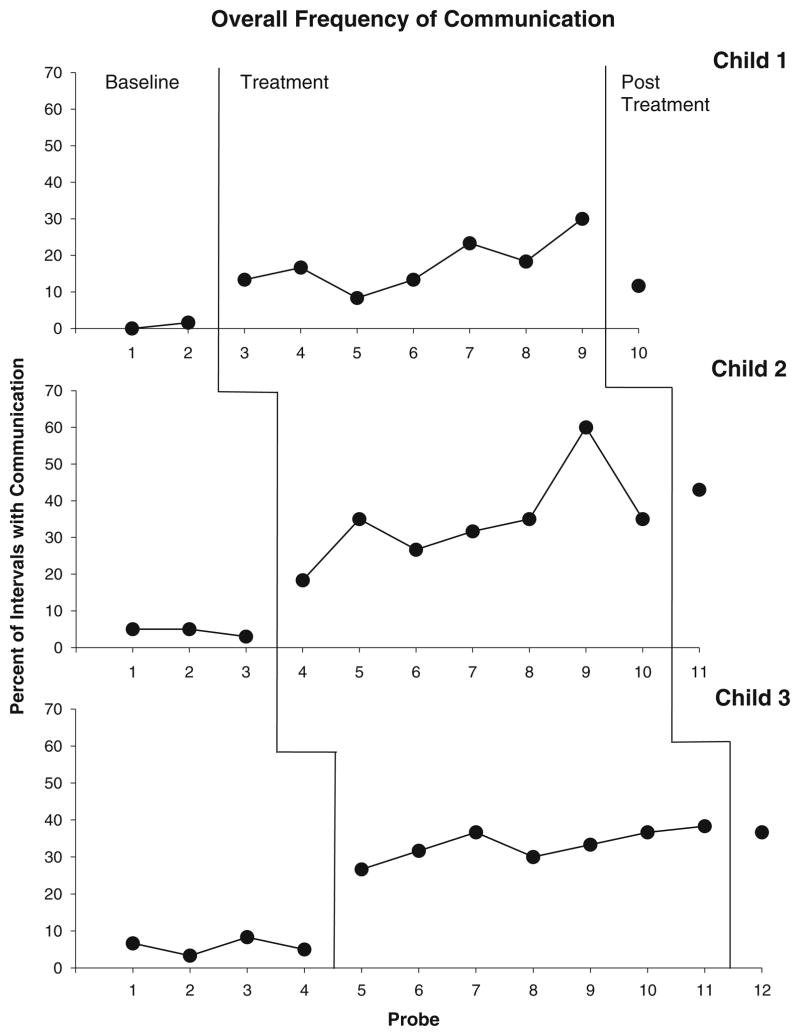
All three participants demonstrated low levels of communication during baseline probes (See Fig. 1), averaging 0.8, 4, and 6 % of intervals for Child 1, 2, and 3, respectively.
Fig. 1.
Percent of intervals with child communication across baseline, treatment, and post-treatment probes
Immediately after the introduction of the PRT procedures, all children evidenced a clear increase in the frequency of communication during intervention sessions with the parent and clinician. Specifically, Child 1’s communication increased to 13 % of intervals during the first intervention session, with similar increases for Child 2 (18 %) and Child 3 (26 %) in the first session. Child communication during intervention sessions continued to increase for all participants as treatment progressed, and Child 2 and Child 3 demonstrated particularly dramatic responses to intervention procedures, with Child 2 communicating in as many as 60 % of intervals during Probe 9, and Child 3 in 38 % of intervals by Probe 11. Gains in child frequency of functional communication were maintained during post-treatment probes, although Child 1, who presented with the most significant autistic symptomatology, demonstrated more modest levels of communication gains at post-treatment (12 %).
Table 2 presents supplemental information regarding the type of communicative bids ranging from eye contact to integrated communication. The numbers represent percentage of communicative bids that fall within a given category. Child 1 made the fewest average overall communicative bids, with a fairly even distribution across communicative types. Both Child 2 and Child 3 predominantly integrated two or more communicative strategies to communicate (24 and 23 %, respectively).
Table 2.
Treatment phase: frequency and variety of communicative attempts
| Total | Eye contact | Vocalization | Gesture | Integrated | |
|---|---|---|---|---|---|
| Child 1 | 17.6 | 4.5 | 3.5 | 6.4 | 7.4 |
| Child 2 | 34.5 | 2.4 | 1.7 | 13.1 | 23.8 |
| Child 3 | 33.3 | 9.5 | 0.4 | 2.9 | 23.1 |
Average percent of intervals with any communicative attempt (total), and average percent of intervals with a specific type of communicative attempt across treatment probes
Average Level of Prompting
On average during intervention for all three children, physical prompting and model prompting was used relatively infrequently (see Table 3). Although these strategies were often used to initially teach a new type of communicative behavior (e.g., giving, reaching, nodding), children readily learned to use these skills independently. The most common type of prompting was the open-ended prompt which indicated to the child that a response was expected but did not specifically model the expected behavior. These prompts were used on average between 65 % (Child 3) and 82 % (Child 1) of the time across treatment sessions. In addition, environmental arrangements, communicative temptations, and contingent responding by parents appeared to promote independent responding. Initiations ranged from an average of 11 % (Child 1) to 29 % (Child 3) of child functional communication. Table 3 provides a summary of the average percentage of child communication attempts made in response to physical, model, and open-ended prompts as well as child initiations across treatment probes.
Table 3.
Treatment phase: level of prompting
| Physical | Model | Open | Initiate | |
|---|---|---|---|---|
| Child 1 | 4.1 | 2.7 | 82.4 | 10.8 |
| Child 2 | 8.9 | 5.5 | 71.2 | 14.4 |
| Child 3 | 0.7 | 5.7 | 64.5 | 29.1 |
Average percentage of total intervals with child communication attempts made in response to physical, model, and open-ended prompts as well as child initiations across treatment probes
Parent Independent Implementation of PRT During Treatment
Analysis of parent fidelity of PRT implementation indicated that as parents were taught to implement PRT during weekly sessions with the clinician, they also demonstrated independent implementation of PRT during generalization probes taken without the clinician present (See Table 4). Prior to parent training, as expected, all parents demonstrated relatively low levels of implementation of PRT procedures (14–18 % average fidelity), although many parents did typically follow their child’s lead as shown in Table 4, with correspondingly low levels of child communication as well (0.8–6 % average intervals with child communication). During intervention, parents demonstrated more use of PRT procedures when working with their children independently (49–84 % average fidelity), although their fidelity of implementation of PRT procedures varied as they were learning the procedures. Despite the fact that while learning PRT parents did not consistently demonstrate high levels of fidelity of implementation, significant increases in child communication during parent independent implementation were noted (16–32 % average intervals with child communication).
Table 4.
Parent independent fidelity of implementation of PRT and associated child communication
| Percent parent fidelity | Percent intervals with child communication | |
|---|---|---|
| Average (range) | Average (range) | |
| Child 1 | ||
| Baseline | 17.5 (15–20) | 0.8 (0–1.66) |
| Treatment | 84.3 (65–98) | 21.9 (15–30) |
| Post | 68 | 11.6 |
| Child 2 | ||
| Baseline | 17.6 (13–25) | 4.4 (3.3–5) |
| Treatment | 48.5 (23–79) | 16.1 (3.3–35) |
| Post | 85 | 43.3 |
| Child 3 | ||
| Baseline | 14 (10–18) | 5.6 (3–8.3) |
| Treatment | 60.3 (38–74) | 31.9 (10–40) |
| Post | 78 | 36.6 |
Percent parent fidelity of PRT implementation during generalization probes of parent independent PRT use without clinician present and percent of intervals with child communication
Parent Satisfaction
Results of the parent satisfaction questionnaires indicated that all three parents reported high levels of satisfaction with the program. Specifically, parents gave average ratings of greater than 4 out of 5 regarding their satisfaction with child progress, the skills taught, likelihood of recommending the program, and overall satisfaction. For example, one parent reported “I love the techniques. The child doesn’t get upset or frustrated and he accomplishes the goals. I loved it.” When asked what was most helpful about the treatment, parents reported “parent training” and “How to get my child to do the things I wanted him to do, but on his terms in a fun, playful way.”
Follow-Up Developmental Testing and Diagnostic Outcomes
Table 5 provides results of developmental testing conducted for each of the three children at 36 months of age as part of the ongoing prospective study of infant siblings of children with ASD. In addition, diagnostic outcomes determined by clinicians at that time are reported. Child 1 demonstrated developmental delays on the Mullen, a calibrated severity score in the autism range on the ADOS Module-1, and was given a diagnosis of Autistic Disorder at the age of 36 months. Child 2 and Child 3 both performed within the average range across domains on the Mullen, did not demonstrate significant autism symptomatology on the ADOS, and were given no clinical diagnosis at 36 months of age.
Table 5.
Developmental testing and clinical diagnosis at 36 months of age
| Mullen scalesa
|
ADOS severity score | Clinical diagnosis | ||||
|---|---|---|---|---|---|---|
| VR | FM | RL | EL | |||
| Child 1 | 40 | 26 | 33 | 38 | 7 | Autism |
| Child 2 | 51 | 48 | 58 | 58 | 4 | None |
| Child 3 | 53 | 59 | 56 | 56 | 1 | None |
GM gross motor, VR visual reception, FM fine motor, RL receptive language, and EL expressive language
T scores are provided for the Mullen Scales
Discussion
The purpose of this pilot study was to examine (a) whether the application of PRT adapted for infants could increase the frequency of functional communication attempts made by infants at-risk for autism, and (b) whether a brief parent training intervention would teach parents how to elicit more frequent functional communication from their children. Results of this study demonstrate preliminary efficacy for a developmental adaptation of PRT to infants at-risk for an ASD. The PRT intervention resulted in gains in child functional communication and parent implementation of motivational procedures to elicit more frequent communication attempts from their children. This study makes a significant contribution to the search for evidence-based interventions appropriate for infants at-risk for ASD by providing preliminary evidence of the efficacy and acceptability of PRT in this population. Given the preliminary nature of this study, further research will be necessary to confirm the findings with larger numbers of children and stronger experimental controls. For instance, the small number of baseline probes across the three children limits the strength of experimental control for maturational changes and is an important limitation which will need to be addressed in future research. In spite of these limitations, this study is one of the first reports of the use of PRT procedures in children under 24 months of age (Schreibman et al. 2009).
Though PRT has traditionally been applied with pre-school and school-age children, the results of this study demonstrate that the procedures can be adapted for use with very young children when the motivational principles are applied to elicit prelinguistic communication attempts so critical at this age (Osterling et al. 2002). In this at-risk population, low frequency of functional communication, both verbal and nonverbal, is a significant concern (Boyd et al. 2010; Rogers 2009). Following the start of intervention, all three children demonstrated immediate increases in the use of functional communication both during intervention sessions and during probes taken of their parents independently using the procedures. The speed with which clinicians and parents were able to evoke these changes suggests that even a brief intervention applied early in development can rapidly increase a child’s opportunities to practice functional communication.
As a critical means of prelinguistic communication, gestures were a primary target of intervention and preliminary data regarding the types of communication used by children across probes indicated that all three children learned to use gestures regularly to communicate during treatment. Child 1 used gestures predominantly, while Child 2 and 3 often integrated multiple means of communication into a single bid. This integration represents a more advanced communicative skill and, given that integration of communication acts was not specifically targeted in intervention, this result suggests the possibility of an encouraging collateral gain which can be explored in future research.
Given the short duration of this intervention, it is quite promising that parents of infants at-risk for ASD were able to learn the principles of PRT procedures and reach or approach the 80 % level considered adequate for fidelity of implementation of PRT (Schreibman and Koegel 2005). Prior research has suggested that parents can reach fidelity of implementation criteria following 25 h of parent training (Koegel et al. 2002), therefore, implementation close to 80 % is considered good progress after only 10 h of training. These results provide evidence that the developmentally adapted procedures are easy for parents to learn and that parents can make substantial progress in learning skills for motivating their children to communicate even in a short period of time. Evidence of enhanced frequency of child communication when parents began implementing at least some PRT procedures appears to suggest that even partial implementation of PRT strategies may enhance child communication to some degree during parent–child interactions. For parents to consistently meet fidelity of implementation criteria, additional hours of parent training are likely to be needed. Additionally, parent reports of satisfaction with the parent education aspects of the intervention further support the use of a parent training approach and suggest that for parents who may be extremely worried about their at-risk infant, short-term parent training in strategies for eliciting functional communication may provide a constructive way to support their child’s social-communication development.
Examination of child functional communication during post-treatment probes indicated that Child 2 and Child 3 maintained their level of communication during interactions with parents once treatment had ended. However, particularly concerning are the data from Child 1 whose communication during the post-treatment probe, though higher than initial baseline levels, was relatively infrequent, occurring during only 12 % of intervals during the probe. For this child who demonstrated emerging symptoms of autism at 12 months, the brief nature of this intervention was effective in enhancing communication but not sufficient to address the severity of his social communication impairments. In this case, as is considered best-practice in the field, intensive early intervention programming appropriate for a child with autism is warranted as soon as a diagnosis of autism is suspected. Child 1 received referrals to local early intervention at 12 months of age, but due to a delay in the initiation of services by local providers, ultimately did not began services until after his participation in the study. Diagnostic outcomes at 36 months of age indicated that Child 1 continued to present with significant symptoms of autism at that age whereas Child 2 and Child 3, in spite of transient evidence of social communication impairment during the second year of life, did not meet criteria for a clinical diagnosis of autism at 36 months of age.
It is expected that infant siblings of children with ASD will have diverse outcomes and that some will develop ASD and some will not (Landa and Garrett-Mayer 2006). The current study indicates that children with a variety of symptom presentations responded positively to the intervention. Future research will be needed to address the question of which children are likely to benefit most from this type of very early intervention and at what point in the development of a child at-risk for autism should intervention services be initiated. Based on the data from the current study, it is not possible to make conclusions about the effect of the brief intervention on subsequent observations of autism symptoms. However, it is possible that more intensive intervention provided over a longer period of time could significantly alter the developmental trajectories of these children at risk (Rogers 2009) and further research will be needed to explore the extent to which intervention can alter the development of autism symptoms or the severity of impairment. Additionally, starting intervention even earlier, that is, within the first year of life, may provide even greater benefit in altering the developmental trajectory toward a more typical path. Given continued advances in early detection of autism symptoms (Ozonoff et al. 2010) and increased monitoring of infant siblings of children with autism who are at risk for atypical social communication development (e.g., High Risk Baby Siblings Research Consortium), there is an urgent bioethical imperative to develop evidence-based interventions appropriate for this very young population.
Overall, this study provides promising preliminary data in support of the developmental application of PRT to target early functional communication skills in infant siblings at risk for ASD. This approach was well-accepted by families of these infants and provided parents with useful strategies for eliciting more frequent social communication and providing opportunities for the infant to practice meaningful reciprocal communication throughout daily routines at home. The initial efficacy of this line of research is supported and the results of this study constitute a first step toward investigating whether PRT can impact the trajectory of autism symptom development in infants at-risk. Additional research is now warranted to establish a more intensive program of intervention for these infants, and to track the lasting effects of a more intensive intervention on the developmental trajectories and condition of autism over time.
Acknowledgments
The project was supported by Award Number R01 MH087554 from the National Institute of Mental Health (PI: K. Chawarska) and P01 HD03008, Project 1 (PI: K. Chawarska) from the National Institute of Child Health and Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, National Institute of Child Health and Development, or the National Institutes of Health.
Appendix
See Table 6.
Table 6.
Outline of topics covered in parent education sessions
| Week | Title | Topics |
|---|---|---|
| 1 | Let’s get started | Introduce program, PRT, and “ABC’s” of behavior |
| 2 | Follow the leader | Discuss child choice, creating opportunities, use of routines |
| 3 | Good things come to those who play | Natural and contingent reinforcement |
| 4 | Try, try again | Reinforcing attempts, differential reinforcement |
| 5 | Keep it clear | Clear opportunities, use of prompting, maintenance and acquisition |
| 6 | Home sweet home | Arranging the home to promote communication |
| 7 | Communication checklist | Fidelity, taking data |
| 8 | Make yourself at home | Generalization to home |
| 9 | Wow–look at that! | Joint attention |
| 10 | Wrap-up | Progress and goal setting |
Contributor Information
Amanda Mossman Steiner, Email: amanda.mossman.steiner@gmail.com, Yale Child Study Center, 40 Temple Street, New Haven, CT 06510, USA.
Grace W. Gengoux, Stanford University School of Medicine, Stanford, CA, USA
Ami Klin, Emory University, Atlanta, GA, USA.
Katarzyna Chawarska, Yale Child Study Center, 40 Temple Street, New Haven, CT 06510, USA.
References
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28:369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37(1):89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Odom SL, Humphreys BP, Sam AM. Infants and toddlers with autism spectrum disorder: Early identification and early intervention. Journal of Early Intervention. 2010;32(2):75–98. [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12:433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Koegel LK, Koegel RL, Openden D, Smith IM, Nefdt N. Large scale dissemination and community implementation of pivotal response treatment: Program description and preliminary data. Research & Practice for Persons with Severe Disabilities. 2007;32(2):142–153. [Google Scholar]
- Cassel T, Messinger D, Ibanez L, Haltigan J, Acosta S, Buchman A. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Autism in infants and toddlers: Diagnosis, assessment, and treatment. New York: Guilford Press; 2008. [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorder. American Journal of Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J. Early intervention in autism: Effectiveness and common elements of current approaches. In: Guralnick, editor. The effectiveness of early intervention: Second generation research. Baltimore: Brookes; 1997. pp. 307–326. [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Wijsman E, Schellenberg G, Estes A, Munson J, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European Child and Adolescent Psychiatry. 1998;7:131–136. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Folstein S, Santangelo S, Gilman S, Piven J, Landa R, Lainhart J, et al. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Gillett JN, LeBlanc LA. Parent implemented natural language paradigm to increase language and play in children with autism. Research in Autism Spectrum Disorders. 2007;3:247–255. [Google Scholar]
- Harris S, Handleman J. Age and IQ at intake as predictors of placement for young children with autism: A four- to six- year follow-up. Journal of Autism and Developmental Disorders. 2000;30(2):137–142. doi: 10.1023/a:1005459606120. [DOI] [PubMed] [Google Scholar]
- Harris S, Weiss M. Right from the start: Behavioral intervention for young children with autism. Bethesda, MD USA: Woodbine House; 1998. [Google Scholar]
- Hart BM, Risley TR. Establishing the use of descriptive adjectives in the spontaneous speech of disadvantaged children. Journal of Applied Behavior Analysis. 1968;1:109–120. doi: 10.1901/jaba.1968.1-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Bruinsma Y, Koegel LK. Developmental trajectories in early intervention. In: Koegel RL, Koegel LK, editors. Pivotal response treatments for autism. Baltimore: Brookes; 2006. pp. 131–140. [Google Scholar]
- Koegel RL, Koegel LK. Pivotal response treatments for autism. Baltimore, MD: Brookes; 2006. [Google Scholar]
- Koegel RL, Koegel LK, Brookman LI. Empirically supported pivotal response interventions for children with autism. In: Kazdin AE, Weisz JR, editors. Evidence-based psychotherapies for children and adolescents. New York: Guilford Press; 2003. pp. 341–357. [Google Scholar]
- Koegel LK, Koegel RL, Harrower JK, Carter CM. Pivotal response intervention I: Overview of approach. Journal of the Association for Persons with Severe Handicaps. 1999a;24:174–185. [Google Scholar]
- Koegel LK, Koegel RL, Shoshan Y, McNerney E. Pivotal response intervention II: Preliminary long-term outcomes data. Journal of the Association for Persons with Severe Handicaps. 1999b;24:186–198. [Google Scholar]
- Koegel RL, O’Dell M, Koegel LK. A natural language teaching paradigm for nonverbal autistic children. Journal of Autism and Developmental Disorders. 1987;17:187–200. doi: 10.1007/BF01495055. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Symon JB, Koegel LK. Parent education for families of children with autism living in geographically distant areas. Journal of Positive Behavior Interventions. 2002;4:88–103. [Google Scholar]
- Landa R, Folstein S, Isaacs C. Spontaneous narrative-discourse performance of parents of autistic individuals. Journal of Speech and Hearing Research. 1991;34:1339–1345. doi: 10.1044/jshr.3406.1339. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa R, Piven J, Wzorek M, Gayle J, Chase G, Folstein S. Social language use in parents of autistic individuals. Psychological Medicine. 1992;22:245–254. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: From phenome to genome. Journal of Neuropathology and Experimental Neurology. 2008;67(9):829–837. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Whitney G, Coffing M, Petrak R, Pierce K, et al. The Autism Diagnostic Observation Schedule-toddler module: A new model of standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Barbieri F, Casella C, Cattaneo V, Cavallaro M, et al. Early behavioral development in autistic children: The first 2 years of life through home movies. Psychopathology. 2001;34:147–152. doi: 10.1159/000049298. [DOI] [PubMed] [Google Scholar]
- McEachin JJ, Smith T, Lovass OI. Long-term outcomes for children with autism who received early intensive behavioral intervention. American Journal of Mental Retardation. 1995;97(4):359–372. [PubMed] [Google Scholar]
- Minjarez M, Williams S, Mercier E, Hardan A. Pivotal response group treatment program for parents of children with autism. Journal of Autism and Developmental Disorders. 2011;41(1):92–101. doi: 10.1007/s10803-010-1027-6. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc; 1995. AGS edn. [Google Scholar]
- National Research Council. Educating children with autism. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Openden D. Unpublished Doctoral Dissertation. University of California; Santa Barbara: 2005. Pivotal response treatment for multiple families of children with autism: Effects of a 4-day group parent education workshop. [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio F, Cook IC, Moore Hill M, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:258–268. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12:457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52(5):588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, et al. Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry. 2000;40(1):491–502. [PubMed] [Google Scholar]
- Pierce K, Schreibman L. Multiple peer use of pivotal response training to increase social behaviors of classmates with autism: Results from trained and untrained peers. Journal of Applied Behavior Analysis. 1997;30(1):157–160. doi: 10.1901/jaba.1997.30-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, et al. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry. 1997;154(2):185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Rogers S. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruser TF, Arin D, Dowd M, Putnam S, Winklosky B, Rosen-Sheidley B, et al. Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders. 2007;37:1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- Schreibman L, Koegel RL. Training for parents of children with autism: Pivotal responses, generalization, and individualization of interventions. In: Hibbs ED, Jensen PS, editors. Psychosocial treatments for child and adolescent disorders: Empirically based strategies for clinical practice. Washington, DC: American Psychological Association; 2005. pp. 605–631. [Google Scholar]
- Schreibman L, Stahmer AC, Cestone-Bartlet V, Dufek S. Brief report: Toward refinement of a predictive behavioral profile for treatment outcome in children with autism. Research in Autism Spectrum Disorders. 2009;3:163–172. doi: 10.1016/j.rasd.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Siegel B. Home-based behavioral treatment for children with autism. Journal of Autism and Developmental Disorders. 1998;28(1):15–22. doi: 10.1023/a:1026054701472. [DOI] [PubMed] [Google Scholar]
- Smalley S, McCracken J, Tanguay P. Autism, affective disorders, and social phobia. Neuropsychiatric Genetics. 1995;60:19–26. doi: 10.1002/ajmg.1320600105. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatric and Adolescent Medicine. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Symon JB. Expanding interventions for children with autism: Parents as trainers. Journal of Positive Behavior Interventions. 2005;7(3):159–173. [Google Scholar]
- Veenstra-Vanderweele J, Cook E. Genetics of childhood disorders: XlVI. Autism, part 5: Genetics of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:116–118. doi: 10.1097/00004583-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Vismara L, Rogers S. The Early Start Denver model: A case study of an innovative practice. Journal of Early Intervention. 2008;31:91–108. [Google Scholar]
- Volkmar F, Lord C, Bailey A, Schultz R, Klin A. Autism and pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Warren SF, McQuarter RJ, Rogers-Warren AP. The effects of mands and models on the speech of unresponsive language-delayed preschool children. Journal of Speech and Hearing Disorders. 1984;49:43–52. doi: 10.1044/jshd.4901.43. [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone W. Randomized comparison of two communication interventions for preschools with autism spectrum disorders. Journal of Consulting and Clinical Psychology. 2006;74:426–435. doi: 10.1037/0022-006X.74.3.426. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Roger S, Carter A, Carver L, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: Insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]