Abstract
Pheromone-binding proteins (PBPs) in lepidopteran moths selectively transport the hydrophobic pheromone molecules across the sensillar lymph to trigger the neuronal response. Moth PBPs are known to bind ligand at physiological pH and release it at acidic pH while undergoing a conformational change. Two molecular switches are considered to play a role in this mechanism: (i) Protonation of His70 and His95 situated at one end of binding pocket, and (ii) Switch of the unstructured C-terminus at the other end of the binding pocket to a helix that enters the pocket. We have reported previously the role of the histidine-driven switch in ligand release for Antheraea polyphemus PBP1 (ApolPBP1). Here we show that the C-terminus plays a role in ligand release and binding mechanism of ApolPBP1. The C-terminus truncated mutants of ApolPBP1 (ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142) exist only in the bound conformation at all pH levels, and they fail to undergo pH- or ligand- dependent conformational switch. Although these proteins could bind ligands even at acidic pH unlike the wild-type ApolPBP1, they had ~4 fold reduced affinity towards the ligand at both acidic and physiological pH than that of ApolPBP1wt and ApolPBP1H70A/H95A. Thus, apart from helping in the ligand-release at acidic pH, the C-terminus in ApolPBP1 also plays an important role in ligand binding and/or locking the ligand in the binding pocket. Our results are in stark contrast to those reported for BmorPBP and AtraPBP, where C-terminus truncated proteins had similar or increased pheromone-binding affinity at any pH.
Keywords: PBP, NMR, delipidation, ApolPBP1, Open conformation, close conformation
Chemical communication in insects occurs via sensing of variety of small, volatile organic molecules called semiochemicals. Indeed, chemical sensing guides the most fundamental behaviors of insects including feeding, mating, protection of sites of oviposition, and escape predation. Pheromones, a class of semiochemicals that elicit behavioral response in the members of the same species, serve as chemical stimuli in intraspecies communication. In lepidopteran moths, sex pheromones secreted by females are detected with extreme selectivity and specificity by the males of the same species initiating the mating process. Pheromone-binding proteins (PBPs) present in the antennae of male moths, transport the hydrophobic pheromone molecules across the sensillar lymph to the membrane bound receptors (ion channels) (1, 2) while protecting them against the degrading enzymes to trigger the neuronal response. PBPs found in different moth species are highly similar exhibiting > 50% sequence identity (3). These acidic proteins are very soluble in water and have molecular masses between 14–16 kDa. The six cysteine residues conserved in all moth PBPs form three disulfide bonds that holds six helices together forming the binding pocket (3).
There are several reports suggesting the role of insect PBPs in either transportation and release of pheromone at the olfactory neuron site triggering neuronal response (4–10) or activation of the pheromone-sensitive neuron by the PBP-pheromone complex (11–14). However, it is widely accepted that moth PBPs present in the sensillar lymph of male insect antenna bind ligand at physiological pH and release it at acidic pH near the site of the olfactory neuron. This ligand release is facilitated by a well defined, dramatic conformational change in moth PBPs that is associated with pH change. Since pheromone fails to bind PBP below pH 6.0 (4, 6, 9, 10, 15, 16), the pH of the sensillar lymph has to be more than 6.0 to facilitate pheromone binding. Furthermore, release of pheromone driven by the pH-induced conformation change in PBP near the olfactory neuron is supported by the fact that the negatively charged dendritic membrane reduces the pH at the site of the pheromone-sensitive neuron (17). Thus, acidic pH at the site of the neuron facilitates the conformational change in PBP resulting in the release of pheromone (10).
Although majority work on moth PBPs suggests that pheromone is released by PBP near membrane while undergoing a pH-driven conformational change, it is still possible that PBP-pheromone complex may activate the receptors directly. However, it is important to note that ligand-bound moth PBP undergoes a well-defined conformational change in acidic pH (4). This conformational change should not be interpreted as acid denaturation of the protein, since ligand-free PBPs have more organized conformation at any pH containing 7 α-helices including the structured internalized C-terminus (4, 6, 15, 18, 19). In contrast, the bound conformation of PBP is composed of 6 α-helices with an unstructured exposed C-terminus at pH above 6.0 (4, 7, 8, 20). This pH-dependent conformational change might be important in PBP recycling if not for unloading the ligand at the site of receptor (19). Therefore, it is important to study the different elements responsible for this switch in order to understand the role of PBPs in moth olfaction.
Lepiodopteran moth PBPs exist in two conformations: “open” or “bound” exists in the presence of ligand at high pH and “closed” or “free” is observed in the absence of ligand and/or at low pH (4). Open conformation is characterized by the unstructured C-terminal tail that extends to the solvent leaving the binding cavity open. In the closed conformation, the C-terminus occupies the binding cavity in the form of the 7th α-helix thus closing the cavity. In our earlier work, we have shown that unliganded (delipidated) PBP from the giant silk moth, Antheraea polyphemus (ApolPBP1), is generally pH insensitive, existing in the closed conformation at acidic and physiological pHs, whereas the ligand-bound PBP undergoes a dramatic pH-dependent conformational change (4). At low pH, the protein remains in the closed or free conformation regardless whether the protein is undelipidated (ligand not removed by delipidation) or delipidated (ligand removed by delipidation) (4, 21).
Two molecular switches have been proposed to play a role in the pH-dependent ligand-release mechanism of moth PBPs: (i) protonation of His70 and His95 situated at one end of the binding pocket at pH below 6.0 that opens the histidine gate due to charge repulsion (9, 16, 20); and (ii) switch of the unstructured C-terminus to a helix at low pH that enters the binding pocket from the opposite end of the pocket as the ligand is released through the histidine gate (16, 19, 22). Thus, the exit of the odor molecule is accompanied by the entry of the C-terminal tail to the binding pocket of PBP. We have previously demonstrated the role of the two histidine residues (His70 and His95) in the ligand release mechanism of ApolPBP1 (4). At low pH, the repulsion between the two charged histidines opens the gate to unload the ligand (4). When these two histidines were mutated to alanines, the ApolPBP1H70A/H95A double mutant remained in bound conformation at all pH levels as the alanines permanently shut the gate prohibiting ligand release. Thus, the neutral forms of His70 and His95 shut the gate at pH above 6.0 to facilitate ligand binding while the repulsion between these two charged histidines opens the gate at low pH allowing ligand release (4). Clearly the exit of the ligand is controlled by the histidine gate located at one end of the binding pocket.
However, questions remain for the second molecular switch: the C-terminal tail of the protein. What is the role of this C-terminus switch? Is this C-terminal tail necessary for pheromone unloading at low pH? Can the ligand escape on its own when the histidine gate opens at low pH or is it pushed out as the C-terminal helix occupies the binding cavity? It is now clear that the ligand free ApolPBP1 is in the closed (or free) conformation at all pH levels, where the C-terminus is tucked inside the binding pocket as the 7th helix (4). The C-terminus truncated PBPs from the silk moth Bombyx mori (BmorPBP) and navel orangeworm moth Amyelois transitella (AtraPBP) were able to bind the ligand with similar affinities at both acidic and physiological pH as that of the wild type protein at physiological pH (22, 23). In contrast to them, the C-terminus truncated PBP of the gypsy moth Lymantria dispar (LdisPBP2) was found to have ~ 10× reduced affinity towards ligand (24). The moths Bombyx mori, Amyelois transitella and Lymantria dispar belong to the same insect order Lepidoptera. Although these PBPs have close to 50 % sequence identity and retain many conserved features including six strictly conserved cysteine residues, it is very interesting that their C-terminus truncated mutants behave differently with respect to ligand binding. Thus it is clear from these contradictory reports that the general mechanism of odor perception varies even across the same insect order. The giant silk moth, Antheraea polyphemus, also belongs to the order Lepidoptera. However, there are no reports on the role of the C-terminus of ApolPBP1 in either ligand binding or release although this is the first PBP on which most biochemical work has been conducted. Most importantly, no detailed investigation has been carried out on the effect of pH and ligand on the conformational transition of a C-terminus truncated PBP as well as a PBP where both molecular switches have been altered (C-terminus truncated and both critical histidines (His70 and His95) mutated to alanines).
To investigate the role of the C-terminus tail along with the two critical histidine residues in the ligand binding/releasing mechanism in ApolPBP1, we initiated a comprehensive structural study of this protein. The terminal 14 residues (Pro129-Val142) were deleted from the C-terminus of ApolPBP1wt as well as ApolPBP1H70A/H95A. The two distinct hypotheses tested here were: (i) the C-terminus is essential for ligand release only and its deletion would cause the protein to bind ligand freely at any pH since the binding pocket would be unoccupied at all pH levels, and (ii) the C-terminus is essential for ligand release as well as binding and its deletion would cause a reduction in ligand binding at any pH. To test these hypotheses, we studied the effects of pH and ligand on the conformation of both C-terminus truncated and C-terminus truncated H70A/H95A mutants of ApolPBP1 by high resolution solution NMR spectroscopy. The effects of C-terminus deletion alone and that of both C-terminus deletion along with His70 and His95 mutations on the binding of ligand to these proteins at different pH values were also investigated by fluorescence spectroscopy. Our results suggest that regardless of whether C-terminus truncated ApolPBP1 is bound to a ligand or not, the protein is always in open conformation without undergoing pH- or ligand-dependent conformational switch. This means unloading of the ligand for a C-terminus truncated ligand-bound ApolPBP1 at low pH is not possible even when the histidine gate is opened. Thus the ligand is not released through the opened histidine gate unless the C-terminal helix occupies the binding cavity. Therefore, the histidine gate at one end of the binding pocket and the C-terminal gate at the other end work hand-in-hand for the ligand release. Additionally, the ligand-free forms of the C-terminus truncated proteins exist only in open conformation at all pH levels as the unoccupied binding pocket remains open in the absence of the C-terminus. The important finding of our study is that the C-terminus truncated proteins have reduced affinity towards the ligand as seen in 2D-HSQC NMR and fluorescence-based AMA binding assay. Thus, our study implies that the C-terminus is not only necessary in ligand releasing mechanism but also plays an equally important role in ligand binding in the case of ApolPBP1. This is the first report detailing the effect of pH and ligand on the conformation of C-terminus truncated and when both switches (C-terminus gate and histidine gate) are eliminated in a moth PBP.
Materials and Methods
Cloning, Overexpression & Purification
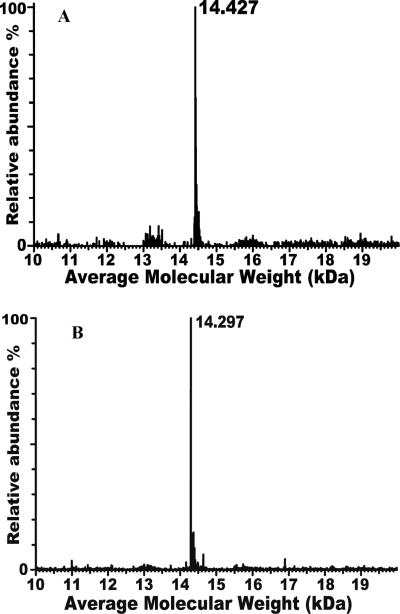
Truncation of 14 residues (Pro129 to Val142) from the C-terminus of ApolPBP1wt and ApolPBP1H70A/H95A was achieved using a PCR based approach. ApolPBP1wt and ApolPBP1H70A/H95A genes, cloned into pET-21a vector, were amplified with the following primers: forward, 5' GGAATTCCA|TATGTCGCCAGAGATCATGAAG 3' & reverse, 5' GCG|GATCCCTAAACCCAGTTCAGCTTATGGATCTC 3' (restriction sites are underlined) and sub-cloned between the restriction sites of NdeI and BamHI of pET-21a vector. The correct orientations of both constructs were determined by DNA sequencing. All plasmid constructs were transformed into E. coli origami cells and expressed as described previously (4). M9 minimal medium supplemented with 15NH4Cl (Cambridge Isotope Laboratories, MA) was used for the expression of isotope-labeled recombinant proteins. Unlabeled and 15N labeled proteins were expressed, purified and delipidated as described earlier (4). The purity of the proteins was assessed by LC/ESI-MS (Fig. 1, A and B). Protein concentrations were determined spectrophotometrically using the theoretical E280= 14230 M−1 cm−1.
Figure 1.
(A) Deconvoluted mass spectrum of the delipidated unlabeled ApolPBP1ΔP129-V142 showing a peak at 14.427 kDa; the theoretically calculated molecular weight is 14.433 kDa. (B) Deconvoluted mass spectrum of the delipidated unlabeled ApolPBP1H70A/H95AΔP129-V142 showing a peak at 14.297 kDa; the theoretically calculated molecular weight is 14.301 kDa. The difference between the masses of these two proteins corresponds to the substitution of two histidines by alanines.
NMR Measurements
All NMR data were collected at 35 °C on a Bruker Avance 600-MHz spectrometer at the Department of Chemistry and Biochemistry, Auburn University. pH titrations were carried out on 400 μl of uniformly 15N labeled 0.3 mM proteins in 50 mM sodium phosphate buffer, pH 6.5 or pH 4.5, 1 mM EDTA, 0.01% NaN3, and 5% D2O (used as a lock solvent) in a Shigemi tube. Two-dimensional {1H, 15N} heteronuclear single quantum coherence (HSQC) spectra were collected for 15N labeled undelipidated and delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142, samples at pH 4.5 and 6.5. Ligand titration studies were carried out for 15N labeled delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 4.5 and 6.5 with palmitic acid as a ligand. The proteins (310 μL of 220 μM in 50 mM phosphate buffer, pH 6.5 or 4.5, containing 5% D2O, 1 mM EDTA, and 0.01% (w/v) NaN3) were titrated with increasing concentrations of palmitic acid (0–2.2 mM) and the corresponding two-dimensional HSQC spectra were recorded at each titration point.
Fluorescence Spectroscopy
Fluorescence experiments were done on a 55B spectrofluorimeter (PerkinElmer Life Sciences) as described previously (4). All experiments were repeated at least twice to confirm reproducibility.
AMA Binding Studies
The binding of AMA to delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 6.5 and 4.5 was assessed by monitoring the increase in the AMA fluorescence at 480 nm as described previously (4). Phosphate buffer with the appropriate amount of AMA served as control for each data point. All PBP mutants showed maximum fluorescence intensity at 5 μM AMA concentration hence this data point was chosen to compare the relative binding affinity of all proteins. The fluorescence intensity of ApolPBP1wt at 1:5 protein: AMA mixture was considered as 100% binding affinity and relative binding affinities for other proteins were calculated accordingly.
Results
1. Role of C-terminus in Ligand Binding and Release
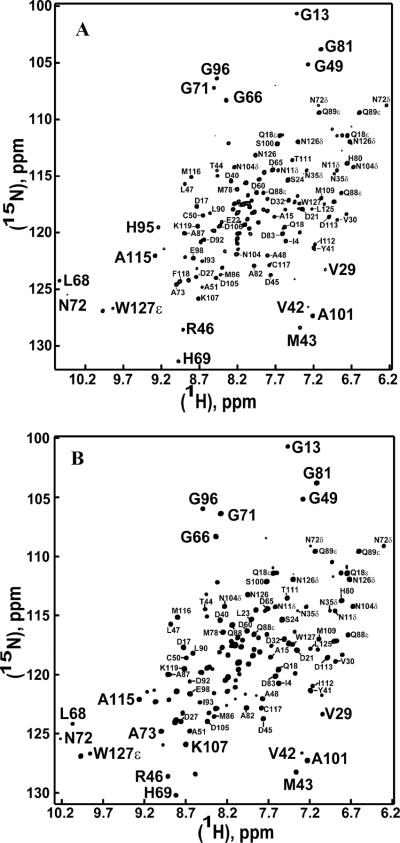
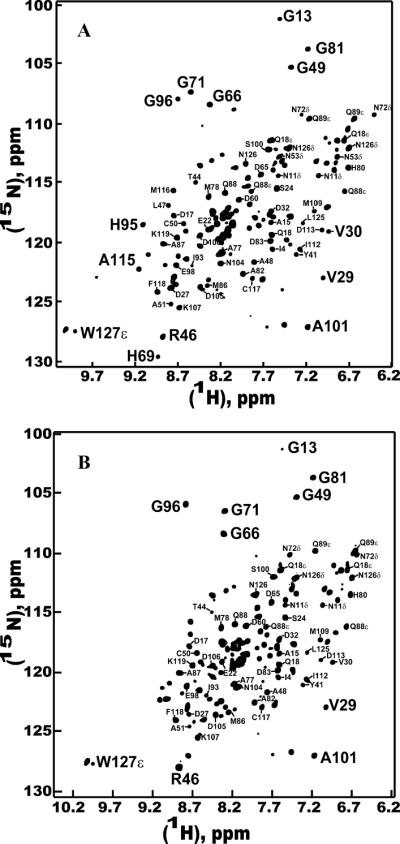
Lepidopteran moth PBPs are believed to release ligand at low pH near the membrane-bound receptors (ion channels) by undergoing a conformational change, which is facilitated by two molecular switches: the histidine switch and the C-terminus switch. In the present work, we have studied the role of C-terminus switch in the ligand binding and release of ApolPBP1. The two-dimensional {1H, 15N} HSQC spectrum is considered to be the fingerprint of a protein, as it is very sensitive to the environmental changes like pH, temperature, substrate binding, mutations etc. Local or global conformational changes occurring in the protein are reflected in the HSQC spectrum as the changes in the chemical shift positions of resonances of the amino acid residues involved. The HSQC spectra of delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 6.5 exhibited well-dispersed resonances indicating that both proteins were properly folded. These spectra largely resembled that of the open conformation of ApolPBP1wt, with marked disappearances of the resonances belonging to the residues in the C-terminal tetradecapeptide segment (Fig. 2, A and B). Using the assignment of undelipidated ApolPBP1wt at pH 6.5 (19), about 85% and 81% of the original peaks could be located in the HSQC spectra of delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142, respectively. Very interestingly, these delipidated proteins, where the endogenous ligand of the expression system has been removed through delipidation procedure, are still in open (bound) conformation although the hydrophobic cavity is empty. This data suggests that for the C-terminus truncated mutants, the binding pocket is not closed by the C-terminus anymore. Thus the conformation of the protein is open even when no ligand is bound to the pocket.
Figure 2.
Two-dimensional {1H,15N} HSQC spectra of delipidated 0.3 mM C-terminus deleted mutants of ApolPBP1 and ApolPBP1H70A/H95A in 50mM sodium phosphate buffer, pH 6.5 containing 5% D2O, 1 mM EDTA, and 0.01% sodium azide. Most of the peaks are labeled on the spectra except for a few from the crowded region. Some of the peaks from periphery are labeled in a larger font. (A) ApolPBP1ΔP129-V142 (B) ApolPBP1H70A/H95AΔP129-V142.
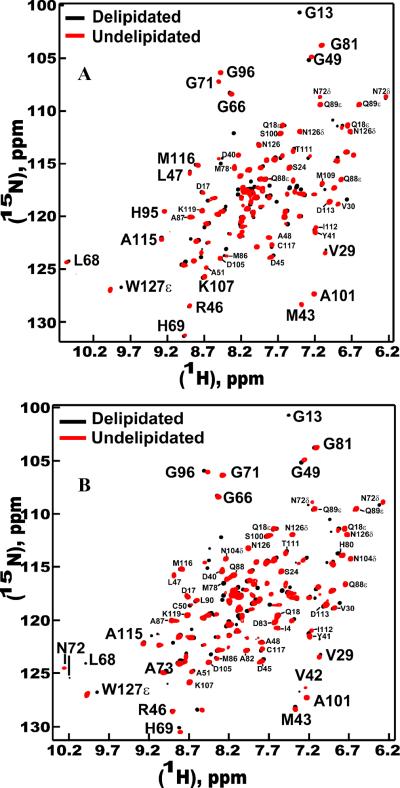
2D HSQC spectra of undelipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 6.5 matched very well with those of their delipidated counterparts (Fig. 3, A and B) and exhibited the characteristic open conformation pattern. Similar phenomenon was observed at pH 4.5 as well. Interestingly, several resonances such as those belonging to Gly13, Asn53δ, Trp127ε etc were found to disappear in the spectra of undelipidated proteins due to line broadening. Such resonances could be readily located in the spectra of delipidated counterparts of the same proteins, indicating that these residues must be in the intermediate exchange regime on NMR timescale in the ligand-bound protein.
Figure 3.
Effect of delipidation on ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 6.5. The figures represent an overlay of two-dimensional {1H,15N} HSQC spectra of 0.3 mM delipidated (colored black) and undelipidated (colored red) proteins in 50 mM sodium phosphate buffer, pH 6.5, containing 5% D2O, 1 mM EDTA, and 0.01% sodium azide. Note the disappearances of resonances such as those belonging to G13, W127ε and several others in the spectrum of undelipidated proteins. (A) ApolPBP1ΔP129-V142. (B) ApolPBP1H70A/H95AΔP129-V142.
2. Effect of pH and ligand on the conformation of ApolPBP1 C-terminus deleted mutants
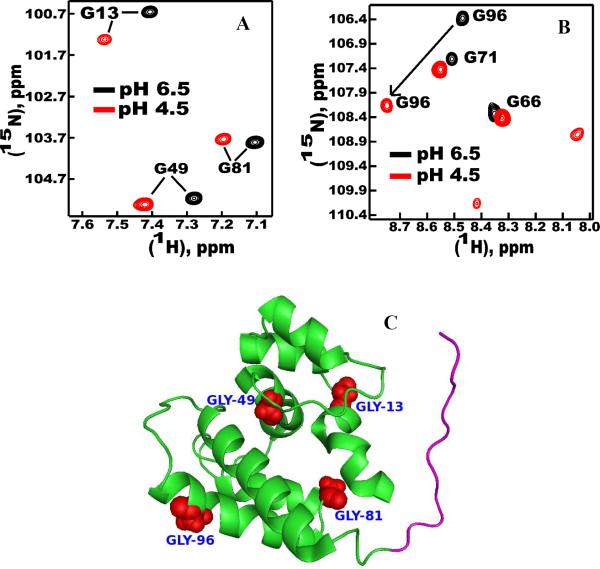
Delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 at pH 4.5 (Fig. 4, A and B) showed the same open conformation in HSQC spectra as observed at pH 6.5. The undelipidated proteins at pH 4.5 also exhibited the same characteristic open conformation as the delipidated proteins at pH 4.5. Thus, these proteins did not undergo the pH-dependent conformational change as that of the undelipidated ApolPBP1 wild-type protein reported earlier (4). These results are in agreement with those reported for the BmorPBP (1–128) variant (25). However, several resonances (for example, those belonging to Gly13, Gly49, Gly81, Gly96 etc) displayed a moderate-to-large change in their chemical shift positions (Fig. 5, A and B) as a result of pH change. This indicated that although the conformation remains open at both pH values, local environmental changes due to change in pH affect chemical shifts of these residues (Fig. 5, C).
Figure 4.
Two-dimensional {1H,15N} HSQC spectra of delipidated 0.3 mM C-terminus deleted mutants of ApolPBP1 and ApolPBP1H70A/H95A in 50mM sodium phosphate buffer, pH 4.5, containing 5% D2O, 1 mM EDTA, and 0.01% sodium azide. (A) ApolPBP1ΔP129-V142 (B) ApolPBP1H70A/H95ΔAP129-V142.
Figure 5.
(A) and (B) Expanded regions of the two-dimensional {1H, 15N} HSQC spectra of delipidated ApolPBP1ΔP129-V142 (0.3 mM) in 50 mM sodium phosphate buffer, containing 5% D2O, 1 mM EDTA, and 0.01% sodium azide, showing movements of certain peaks as a result of pH. The pH of the buffer is indicated on the spectra. (C) Residues that undergo moderate-to-large change in their chemical shift positions as a result of change in pH are shown as red spheres in the three-dimensional structure of ApolPBP1 (PDB id 1QWV). The C-terminus tail (residues 131–142) is colored magenta. The three-dimensional structure representation was prepared using PyMOL molecular graphics system.
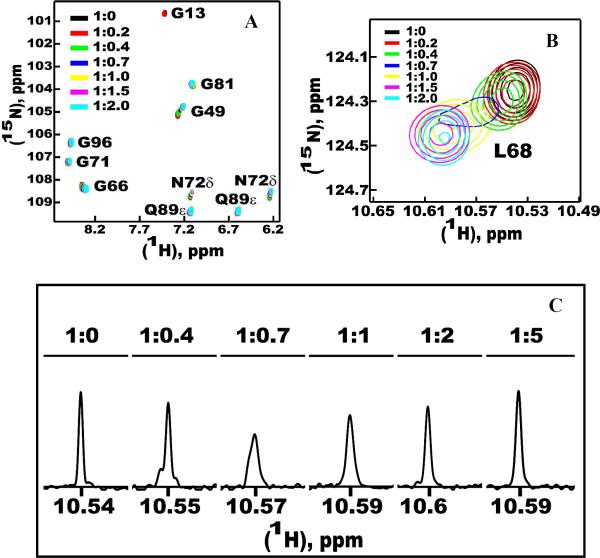
To investigate the effect of ligand on the conformations of delipidated forms of ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142, ligand titration studies were carried out at pH 6.5 and 4.5 using palmitic acid, (a fatty acid that binds to ApolPBP1). Protein: palmitic acid ratios were varied from 1:0 to 1:10. Ligand titration studies revealed that the C-terminus truncated proteins did not undergo conformational change with the addition of ligand. These delipidated proteins remained in the open conformation before and after the addition of excess ligand. However, some of the resonances (for example, Gly13, Leu68 etc) exhibited line-broadening and/or disappearance, indicating that they were in the intermediate exchange regime on the NMR time scale (Fig. 6, A–C). Several other resonances were seen to be in fast exchange regime on the NMR timescale, characterized by gradual changes in their chemical shift positions. These observations were in stark contrast to those observed earlier in the ligand titration assay of delipidated ApolPBP1wt and ApolPBP1H70A/H95A, in which almost all resonances were seen to be in slow exchange regime characterized by two sets of peaks for ligand-free (closed) and ligand-bound (open) conformations (4). Thus while delipidated ApolPBP1wt and ApolPBP1H70A/H95A exhibited very strong (nanomolar) affinities for ligands, their C-terminus-deleted counterparts had much reduced affinities in micromolar-to-millimolar range.
Figure 6.
(A) Expanded region of the two-dimensional {1H,15N} HSQC spectra of delipidated ApolPBP1ΔP129-V142 (220 μM) in 50mM phosphate buffer, pH 6.5, upon titration with palmitic acid. Protein: ligand ratios are indicated in the figure. Note the disappearance of the resonance belonging to Gly13 due to line broadening. Some other resonances are in intermediate-to-fast exchange regime. (B) Resonance corresponding to Leu68 showing the phenomenon of intermediate exchange, from the same spectrum as in (A). (C) One-dimensional slices from the 1H axis taken in the midpoint of the resonance corresponding to Leu68 (same as in (B)) showing the intermediate exchange phenomenon. Protein: ligand ratios are indicated on top of each slice. All slices are scaled relative to the same y axis.
3. AMA binding studies by fluorescence
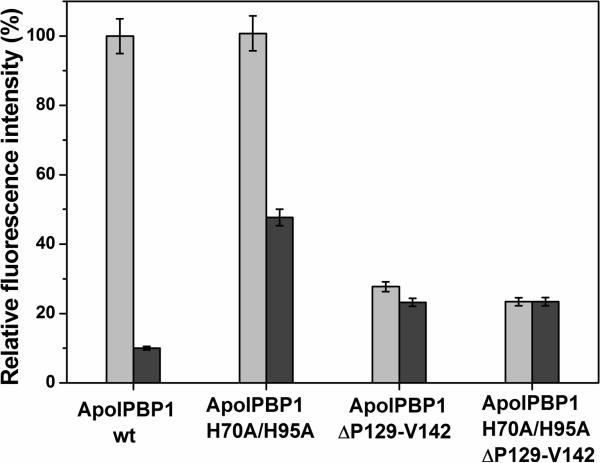
The hydrophobic fluorescent dye 1-aminanthracene (AMA) fluoresces weakly in the aqueous environment after excitation at 256 or 298 nm with a λmax of 563 nm. In hydrophobic environments such as the binding cavity of ApolPBP1, the fluorescence of AMA enhances considerably with a λmax of ~ 480 nm. We have already used AMA titration studies to compare the binding affinities of various proteins in different conditions (4). In the present work, we carried out the titrations of delipidated ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 with AMA at pH 6.5 and 4.5. Our studies revealed that the affinity of the C-terminus truncated proteins towards AMA at pH 6.5 was greatly reduced in comparison to that of the ApolPBP1wt and ApolPBP1H70A/H95A. Similarly C-terminus truncated proteins could bind AMA at low pH unlike the ApolPBP1wt although the affinities were greatly reduced as compared to ApolPBP1H70A/H95A mutant (Fig.7). The maximum fluorescence intensity observed for all C-terminus deleted proteins was about 23–28 % of what was observed for the wild type protein, under the identical experimental conditions (Fig. 7). These results are consistent with the ligand titration studies monitored by NMR.
Figure 7.
Relative fluorescence intensities of delipidated ApolPBP1 mutants upon addition of 5 μM AMA to 1 μM protein samples. Light grey: pH 6.5; Dark grey: pH 4.5. Error bars indicate standard deviation of the data. ApolPBP1wt and ApolPBP1H70A/H95A show similar binding at pH 6.5. ApolPBP1wt has negligible binding at pH 4.5 while ApolPBP1H70A/H95A shows considerable binding at same pH. Both ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 show reduced binding (23–28 % of the wild type protein at pH 6.5) at both pH 6.5 and 4.5.
Discussion
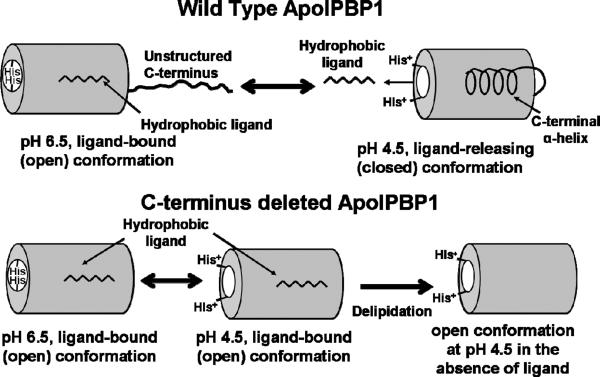
In the present work, we are reporting for the first time the importance of C-terminus in the ligand binding and releasing mechanisms of ApolPBP1. Truncation of C-terminus in ApolPBP1wt and ApolPBP1H70A/H95A resulted in proteins which exhibited the open (bound) conformation regardless of pH and presence/absence of ligand (Fig. 1, A, B; Fig. 2, A, B; Fig. 3, A, B). Although change of pH did not cause any drastic conformational change in these proteins from open to closed conformation as was observed for undelipidated (ligand-bound) ApolPBP1wt (4), it did affect chemical shifts of several resonances indicating that local changes occurred in the protein due to the effect of pH (Fig. 5, A–C). Delipidation also did not bring any major conformational change in these proteins (Fig. 3, A and B) in a striking contrast to what was seen earlier for the ApolPBP1wt and ApolPBP1H70A/H95A at pH 6.5 (4). Thus, the C-terminus truncated ApolPBP1 stays in the open form at all pH regardless of presence or absence of ligand as the unoccupied binding pocket remains open in the absence of the C-terminus (Fig. 8).
Figure 8.
Schematic representation of the role of C-terminus in ligand binding and release mechanism in ApolPBP1. The ligand-bound wild type protein (in open conformation) at physiological pH undergoes a pH dependent conformational change to release ligand at acidic pH (closed conformation). The C-terminus truncated protein, however, remains in the open conformation at both physiological and acidic pH, in the presence/absence of ligand.
Several resonances that had disappeared in the spectra of undelipidated proteins could be easily located in the spectra of delipidated proteins. These resonances again underwent line broadening and/or disappearance during ligand titration (Fig. 6, A). Most of the resonances in all C-terminus truncated proteins exhibited intermediate-to-fast exchange phenomena on the NMR time scale during ligand titration (Fig. 6, A–C), implying micromolar-to-millimolar affinities towards the ligand. Thus, while ApolPBP1wt and ApolPBP1H70A/H95A had nanomolar affinities towards ligands (characterized by the slow exchange seen for almost all resonances in HSQC during ligand titration), their C-terminus truncated counterparts had much less affinities. In fluorescence spectroscopic studies, ApolPBP1ΔP129-V142 and ApolPBP1H70A/H95AΔP129-V142 showed the ability to bind AMA even at low pH; unlike the wild type protein (Fig. 7). However, their binding affinities at both pH 6.5 and 4.5 were greatly reduced (by ~4 fold) as compared to ApolPBP1wt and ApolPBP1H70A/H95A (Fig. 7). Our results are in contrast to those reported for BmorPBP and AtraPBP using cold binding assay (22, 23). For BmorPBP, the C-terminus deleted mutant retained its binding ability at pH 7 nearly same as that of the wild type and displayed significant binding at pH 5 (22). In the case of AtraPBP, the C-terminus truncated mutant showed a 1.5 fold increase in the binding at pH 7 and retained the binding at pH 5 albeit lesser than at pH 7 (23). However, C-terminus truncated LdispPBP2 showed higher Kd (and hence, lesser affinity) than that of wild type protein in a GC assay (24), which is consistent with our observations.
The affinity of 1-AMA towards ApolPBP1wt and various mutants was compared under identical experimental conditions. While ApolPBP1H70A/H95A retained 100% of the affinity towards AMA at pH 6.5 and nearly 50 % affinity at pH 4.5 (Fig. 7), the C-terminus truncated ApolPBP1 and C-terminus truncated ApolPBP1H70A/H95A lost 75% of their affinity towards AMA at both pH values: 6.5 and 4.5 (Fig. 7). Thus, based upon the AMA binding assay data, we can conclude that the C-terminus truncated proteins have much less affinity towards ligands at any pH values in comparison with that of ApolPBP1wt and ApolPBP1H70A/H95A under identical experimental conditions.
The reduced binding affinities of the delipidated C-terminus-truncated mutants of ApolPBP1 can be attributed to inefficient delipidation due to the absence of the C-terminus that helps in ejecting the ligand out at low pH. It is possible that the delipidation is not as effective in C-terminus-truncated proteins as in ApolPBP1wt and ApolPBP1H70A/H95A. As a result, the endogenous ligand could compete with the added ligand resulting in relatively less binding affinity as compared to that of the ApolPBP1wt and ApolPBP1H70A/H95A. However, based on the following observations we conclude that this is not the case: (i) even undelipidated proteins showed much reduced ligand binding affinity at any pH compared to their counterparts with intact C-terminus, (ii) we see disappearance of certain resonances in the undelipidated samples in the HSQC spectrum, and (iii) addition of palmitic acid to the delipidated proteins caused the same resonances to broaden and disappear resulting in the spectra which matched to that of undelipidated proteins. This indicates that the delipidation of these proteins was effective and complete.
Thus, it is important to note that even when the C-terminus is missing, the ligand removal is possible at low pH by delipidation procedure. This is quite surprising especially for ApolPBP1H70A/H95AΔP129-V142 where both molecular switches forming the ligand release mechanism (histidines as well as C-terminus) are removed. Therefore even though these two switches are very important for ligand release; there might be some other residues playing a minor role in this mechanism. It is also possible that the delipidation is effective because the protein loses its overall binding affinity at acidic pH, or that the affinity of Lipidex resin towards the hydrophobic ligand is more than that of the protein at low pH. This phenomenon was observed even in the case of ApolPBP1H70A/H95A, where the affinity of the protein at pH 4.5 was lower than that at pH 6.5 (4).
The histidines are important only in the ligand releasing mechanism. As reported in our earlier work, their substitution to alanines confers the protein the ability to bind ligand even at low pH (4). This substitution does not affect the ligand binding affinity of the protein at high pH either since the delipidated forms of both ApolPBP1wt and ApolPBP1H70A/H95A have similar binding affinities towards AMA and acetate pheromone at pH 6.5 (4). Unlike ApolPBP1wt and ApolPBP1H70A/H95A, the C-terminus truncated mutants behave very differently. Although C-terminus truncated proteins can bind the ligand at low pH, their affinities at low pH are much less than that of the ApolPBP1H70A/H95A. Additionally, the affinities of the C-terminus truncated proteins towards a ligand at high pH are also drastically reduced as compared to the wild type and double mutant ApolPBP1. Indeed, one would expect a considerably tight protein-ligand association in the case of ApolPBP1H70A/H95AΔP129-V142 even at low pH, where both molecular switches have been removed, as compared to ApolPBP1wt or ApolPBP1H70A/H95A. Our results, on the contrary, indicate reduced ligand binding affinities for both C-terminus deleted proteins in all of the conditions tested. Thus, it is evident that C-terminus plays a major role in the ligand-release mechanism at low pH as well as in the ligand-binding in the case of ApolPBP1. It is also possible that in addition to helping with the binding of the ligand in the binding pocket, the C-terminus also acts as a gate to “lock” the ligand in the binding pocket (24), until released by the protonation of histidines at low pH. Hence removal of C-terminus might be hampering effective association resulting in faster dissociation of the ligand from the protein, reducing the overall affinity.
Conclusion
We are reporting here for the first time the effects of ligand and pH on the C-terminus truncated and C-terminus truncated with histidine gate mutated PBPs. Both NMR and fluorescence data suggest that unlike highly homologous BmorPBP C-terminus, the C-terminus of ApolPBP1 is involved in both ligand binding and release. It is clear from this work that even though PBPs from moths belonging to the same insect order have very high sequence identity, the C-terminus deleted proteins behave differently under identical conditions. Thus, the mechanistic details of the role of the ligand-binding/release of moth PBPs can not be generalized across an entire insect order; rather there are subtle species-specific difference/s.
Acknowledgments
Funding statement: This research was financially supported by United States Department of Agriculture PECASE award 2003-35302-12930, and grant 2011-65503-20030, National Science Foundation grant IOS-0628064, and National Institutes of Health grant DK082397 to Smita Mohanty.
List of Abbreviations
- PBP
pheromone-binding protein
- ApolPBP1
A. polyphemus PBP1
- BmorPBP
B. mori PBP
- LdisPBP2
Lymantria dispar PBP2
- HSQC
heteronuclear single quantum correlation
- AMA
1-aminoanthracene
- undelipidated ApolPBP1
ApolPBP1 bound to the hydrophobic endogenous ligand from the host expression system
- delipidated ApolPBP1
ApolPBP1 after the removal of hydrophobic ligand by delipidation.
Footnotes
AUTHOR CONTRIBUTION S. M. conceived, designed the strategies and techniques employed and supervised the research and analyzed data, U.K. designed the primers, Suman M. cloned the genes, U.K. and Suman M. performed protein expression, purification, NMR sample preparation and delipidation, Suman M. collected 2D HSQC data, U.K performed NMR data processing and analysis, U.K. and Suman. M. performed fluorescence experiments and analysis, S. M. and U. K. wrote the paper, U.K. prepared all figures.
References
- 1.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 2.Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 3.Ring J, Prusti R, Mohanty S. Chemical Communication: A Visit with Insects. Curr. Chem. Biol. 2008;2:83–96. [Google Scholar]
- 4.Katre UV, Mazumder S, Prusti RK, Mohanty S. Ligand binding turns moth pheromone-binding protein into a pH sensor: effect on the Antheraea polyphemus PBP1 conformation. J. Biol. Chem. 2009;284:32167–32177. doi: 10.1074/jbc.M109.013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damberger F, Nikonova L, Horst R, Peng G, Leal WS, Wüthrich K. NMR characterization of a pH-dependent equilibrium between two folded solution conformations of the pheromone-binding protein from Bombyx mori. Protein Sci. 2000;9:1038–1041. doi: 10.1110/ps.9.5.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horst R, Damberger F, Luginbühl P, Güntert P, Peng G, Nikonova L, Leal WS, Wüthrich K. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14374–14379. doi: 10.1073/pnas.251532998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee D, Damberger FF, Peng G, Horst R, Güntert P, Nikonova L, Leal WS, Wüthrich K. NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH. FEBS Lett. 2002;531:314–318. doi: 10.1016/s0014-5793(02)03548-2. [DOI] [PubMed] [Google Scholar]
- 8.Mohanty S, Zubkov S, Gronenborn AM. The solution NMR structure of Antheraea polyphemus PBP provides new insight into pheromone recognition by pheromone-binding proteins. J. Mol. Biol. 2004;337:443–451. doi: 10.1016/j.jmb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Zubkov S, Gronenborn AM, Byeon I-JL, Mohanty S. Structural consequences of the pH-induced conformational switch in A.polyphemus pheromone-binding protein: mechanisms of ligand release. J. Mol. Biol. 2005;354:1081–1090. doi: 10.1016/j.jmb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Wojtasek H, Leal WS. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J. Biol. Chem. 1999;274:30950–30956. doi: 10.1074/jbc.274.43.30950. [DOI] [PubMed] [Google Scholar]
- 11.Kowcun A, Honson N, Plettner E. Olfaction in the gypsy moth, Lymantria dispar: effect of pH, ionic strength, and reductants on pheromone transport by pheromone-binding proteins. J. Biol. Chem. 2001;276:44770–44776. doi: 10.1074/jbc.M104688200. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaissling KE. Olfactory perireceptor and receptor events in moths: a kinetic model. Chem. Senses. 2001;26:125–150. doi: 10.1093/chemse/26.2.125. [DOI] [PubMed] [Google Scholar]
- 14.Kaissling K-E. Olfactory perireceptor and receptor events in moths: a kinetic model revised. J. Comp. Physiol., A. 2009;195:895–922. doi: 10.1007/s00359-009-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Xu W, Rayo J, Ishida Y, Leal WS, Ames JB. NMR structure of navel orangeworm moth pheromone-binding protein (AtraPBP1): implications for pH-sensitive pheromone detection. Biochemistry. 2010;49:1469–1476. doi: 10.1021/bi9020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W, Leal WS. Molecular switches for pheromone release from a moth pheromone-binding protein. Biochem. Biophys. Res. Comm. 2008;372:559–564. doi: 10.1016/j.bbrc.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 17.Keil TA. Surface coats of pore tubules and olfactory sensory dendrites of a silkmoth revealed by cationic markers. Tissue Cell. 1984;16:705–717. doi: 10.1016/0040-8166(84)90004-1. [DOI] [PubMed] [Google Scholar]
- 18.Damberger FF, Ishida Y, Leal WS, Wüthrich K. Structural basis of ligand binding and release in insect pheromone-binding proteins: NMR structure of Antheraea polyphemus PBP1 at pH 4.5. J. Mol. Biol. 2007;373:811–819. doi: 10.1016/j.jmb.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 19.Lautenschlager C, Leal WS, Clardy J. Coil-to-helix transition and ligand release of Bombyx mori pheromone-binding protein. Biochem. Biophys. Res. Comm. 2005;335:1044–50. doi: 10.1016/j.bbrc.2005.07.176. [DOI] [PubMed] [Google Scholar]
- 20.Sandler BH, Nikonova L, Leal WS, Clardy J. Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 2000;7:143–151. doi: 10.1016/s1074-5521(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 21.Mohanty S. Moth Olfaction: A Model of Exquisite Sensitivity and Specificity. Biochem. Physiol. 2012;1:e106. [Google Scholar]
- 22.Leal WS, Chen AM, Ishida Y, Chiang VP, Erickson ML, Morgan TI, Tsuruda JM. Kinetics and molecular properties of pheromone binding and release. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5386–5391. doi: 10.1073/pnas.0501447102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Xu X, Leal WS, Ames JB. Extrusion of the C-terminal helix in navel orangeworm moth pheromone-binding protein (AtraPBP1) controls pheromone binding. Biochem. Biophys. Res. Comm. 2011;404:335–338. doi: 10.1016/j.bbrc.2010.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Y, Pace TCS, Castillo C, Bohne C, O'Neill MA, Plettner E. Ligand-interaction kinetics of the pheromone- binding protein from the gypsy moth, L. dispar: insights into the mechanism of binding and release. Chem. Biol. 2009;16:162–72. doi: 10.1016/j.chembiol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Michel E, Damberger FF, Ishida Y, Fiorito F, Lee D, Leal WS, Wüthrich K. Dynamic conformational equilibria in the physiological function of the Bombyx mori pheromone-binding protein. J. Mol. Biol. 2011;408:922–931. doi: 10.1016/j.jmb.2011.03.008. [DOI] [PubMed] [Google Scholar]