Abstract
Extracellular matrix (ECM) supplies both physical and chemical signals to cells and provides a substrate through which fibroblasts migrate during wound repair. To directly assess how ECM composition regulates this process, we used a nested 3D matrix model in which cell-populated collagen buttons were embedded in cell-free collagen or fibrin matrices. Time-lapse microscopy was used to record the dynamic pattern of cell migration into the outer matrices, and 3-D confocal imaging was used to assess cell connectivity and cytoskeletal organization. Corneal fibroblasts stimulated with PDGF migrated more rapidly into collagen as compared to fibrin. In addition, the pattern of fibroblast migration into fibrin and collagen ECMs was strikingly different. Corneal fibroblasts migrating into collagen matrices developed dendritic processes and moved independently, whereas cells migrating into fibrin matrices had a more fusiform morphology and formed an interconnected meshwork. A similar pattern was observed when using dermal fibroblasts, suggesting that this response in not unique to corneal cells. We next cultured corneal fibroblasts within and on top of standard collagen and fibrin matrices to assess the impact of ECM composition on the cell spreading response. Similar differences in cell morphology and connectivity were observed – cells remained separated on collagen but coalesced into clusters on fibrin. Cadherin was localized to junctions between interconnected cells, whereas fibronectin was present both between cells and at the tips of extending cell processes. Cells on fibrin matrices also developed more prominent stress fibers than those on collagen matrices. Importantly, these spreading and migration patterns were consistently observed on both rigid and compliant substrates, thus differences in ECM mechanical stiffness were not the underlying cause. Overall, these results demonstrate for the first time that ECM protein composition alone (collagen vs. fibrin) can induce a switch from individual to collective fibroblast spreading and migration in 3-D culture. Similar processes may also influence cell behavior during wound healing, development, tumor invasion and repopulation of engineered tissues.
Keywords: Extracellular Matrix, Fibrin, Collagen, 3-D Culture, Cell Motility
1. INTRODUCTION
The cornea is an optically clear tissue that forms the front surface of the eye, and accounts for nearly two-thirds of its refractive power. The corneal stroma, which makes up 90% of corneal thickness, is a highly ordered structure consisting of approximately 200 collagen lamellae (Pepose and Ubels, 1992). Corneal stromal cells (keratocytes) reside between the collagen lamellae, and are responsible for secreting ECM components required to maintain normal corneal structure and function (Hassell and Birk, 2010). From a mechanical standpoint, resting keratocytes are considered quiescent; they do not express stress fibers or generate substantial contractile forces (Jester et al., 1994; Lakshman et al., 2010). However, following injury, quiescent corneal keratocytes surrounding the area of injury generally become activated, and transform into a fibroblastic phenotype (Jester et al., 1999b; Stramer et al., 2003). The behavior of these corneal fibroblasts during wound healing has a profound impact on corneal transparency and refractive outcome (Moller-Pedersen et al., 2000; Netto et al., 2005).
Extracellular matrix, ECM, provides both physical and chemical signals that can play important roles in regulating cell mechanical behavior during wound healing (Schultz et al., 2011; Shaw and Martin, 2009) and other biological processes such as development, morphogenesis and cancer (Cox and Erler, 2011; Ghosh and Ingber, 2007; Nelson and Bissell, 2006). Following a full thickness injury in the cornea, a fibrin plug forms in the early stage of wound healing (Jester et al., 1995b; Phan et al., 1989; Zieske, 2001). This plug also contains fibronectin, an adhesive protein that can link cells to other ECM proteins, including fibrin and collagen (Singh et al., 2010). In addition to sealing the anterior chamber, the fibrin plug also provides a substrate through which fibroblasts can migrate. In vivo studies have shown that following full thickness corneal incisions in the rabbit, fibroblasts form an interconnected mesh as they migrate into the wound space, and these interconnections are hypothesized to mediate force transduction during wound contraction (Jester et al., 1995a; Petroll et al., 1993). In contrast, during migration through the collagenous corneal stroma during development or following transcorneal freeze injury, cells assume a spindle shaped morphology, and do not appear to cluster or form an interconnected mesh (Ichijima et al., 1993; Tomasek et al., 1982). Due to the complex nature of the in vivo environment, it is not known whether these two distinct patterns of migration are mediated by differences in the growth factor environment, or differences in the protein composition of the ECM with which the fibroblasts interact (fibrin vs. collagen).
In the current study we directly investigate whether collagen and fibrin differentially regulate the pattern of corneal fibroblast spreading and migration, using a previously described nested matrix model (Karamichos et al., 2009; Kim et al., 2012). We found striking differences in the pattern of corneal fibroblast migration into fibrin and collagen ECM. Fibroblasts migrating into collagen matrices developed dendritic processes and moved individually, whereas cells migrating into fibrin matrices had a fusiform morphology and formed an interconnected network. We also demonstrate that dermal fibroblasts exhibit a similar switch from individual to collective cell migration. Similar differences in cell connectivity were observed when corneal fibroblasts were cultured within or on top of un-nested collagen and fibrin matrices. Overall, these findings suggest that collagen and fibrin differentially modulate the mechanism of fibroblast spreading and migration in 3-D culture. Similar processes may also influence cell behavior during development, tumor invasion and repopulation of engineered tissues.
2. MATERIAL AND METHODS
2.1 Materials
Dulbecco’s modified Eagle medium (DMEM) and 0.25% trypsin/EDTA solution were purchased from invitrogen (Gaithersburg, MD). Platelet-derived growth factor BB isotype (PDGF) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Fetal bovine serum (FBS), fatty acid-free and fraction V bovine serum albumin (BSA), RPMI vitamin mix, Hepes, Sodium bicarbonate, Thrombin from human plasma, and monoclonal Anti-Pan Cadherin antibody were obtained from Sigma-Aldrich (St. Louis, MO). Penicillin, streptomycin, and amphotericin B were obtained from Lonza inc. (Walkersville, MD). Type I rat tail collagen and plasma fibronectin were purchased from BD Biosciences (Bedford, MA). Fibrinogen (purity > 95%; plasminogen, von Willebrand Factor and fibronectin depleted) was obtained from Enzyme Research Laboratories (South Bend, IN). Alexa Fluor 488 and Propidium Iodine (PI) were obtained from Molecular Probes, Inc. (Eugene, OR). RNase (DNase free) was purchased from Roche (Indianapolis, IN). Anti-fibronectin antibody was obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). FITC Goat-anti-rabbit conjugated and Goat-anti-mouse were obtained from Jackson ImmunoResearch (West Grove, PA).
2.2 Cell Culture
Human corneal fibroblasts (HTK cells) (Jester et al., 2003) and dermal fibroblasts immortalized with human telomerase (Miron-Mendoza et al., 2010), were cultured in tissue culture flasks with DMEM containing 10% FBS and supplemented with 1% penicillin, 1% streptomycin, and 1% amphotericin B. Cell culture was carried out at 37°C in a 5% CO2 humidified incubator. Experiments were carried out using basal media supplemented with 5 mg/ml BSA and 50 ng/ml PDGF. Basal media was composed of DMEM containing pyruvate, supplemented with non-essential amino acids, 1% RPMI vitamin mix and ascorbic acid (Jester et al., 1994). Nested matrix experiments (below) were also carried out using a dermal fibroblasts cell line (a generous gift of Dr. Fred Grinnell) (Miron-Mendoza et al., 2010).
2.3 Nested Cell Migration Model
In order to study 3-D cell migration, cell-populated compressed ECM constructs were nested within acellular uncompressed matrices, using a variation of previously published models (Karamichos et al., 2009; Kim et al., 2010). In this model, a 2 ml neutralized collagen solution was prepared by mixing high concentration rat type I collagen with 0.1 N NaOH, 10X DMEM, and H2O to achieve a final concentration of 4 mg/ml. Collagen solution was then poured onto a rectangular metal mold (3 cm length, 2 cm width, 1 cm height) and placed in a humidified incubator (37°C, 5% CO2) for 30 minutes for polymerization. During polymerization time, cells were harvested from monolayer cultures with 0.25% trypsin/EDTA and washed with DMEM/10% FBS followed by DMEM. Cells (6×106) were mixed with a second 2ml collagen solution, which was added on top of the first collagen layer. After 30 minutes to allow collagen polymerization, the sandwich construct was compressed as previously described (Brown et al., 2005; Karamichos et al., 2009; Kim et al., 2010; Neel et al., 2006). This produced a 200 μm thick construct with an acellular ECM on the bottom and a cell-populated ECM on top. The first collagen layer serves as a spacer that prevents cells from contacting the glass substrate as they migrate out of the matrix, thus ensuring that they interact with the outer fibrin or collagen ECM.
To prepare the nested models, 6 mm diameter buttons were cut with a trephine blade and gently placed on glass bottom culture dishes. Buttons were then covered with a 100 μl solution of collagen or fibrin. Samples were then placed for 30 minutes in a humidified incubator (37°C, 5% CO2) to polymerize. After polymerization, migration media was added to each sample. Collagen was prepared by mixing high concentration rat type I collagen with 0.1 N NaOH, 10X DMEM, and H2O to achieve a final concentration of 2 mg/ml. For fibrin matrices, fibrinogen was warmed for 20 minutes and mixed with DMEM to achieve a final concentration of 1 mg/ml, and this solution was mixed with 0.5 U/ml thrombin to initiate polymerization. Concentrations for collagen and fibrin were selected because they produced similar fibril density and packing, based on confocal reflection imaging. Additional experiments were performed using collagen concentrations of 1mg/ml, 3mg/ml and 4mg/ml.
To prepare outer collagen and fibrin matrices containing fibronectin, collagen and fibrin solutions were mixed with soluble fibronectin in a ratio of 1:10. Subsequently 100 μl of this solution was immediately added to cover the compressed collagen buttons, and allowed to polymerize for 30 minutes prior to adding migration media.
2.4 Standard (Un-nested) Matrix Models
For experiments with cells within collagen and fibrin matrices, 100 μl neutralized solutions of collagen (2 mg/ml) and fibrin (1 mg/ml) containing 20×103 cells/matrix were allowed to polymerize for 30 min and then incubated with experimental media containing PDGF. For experiments with cells on the surface of matrices, 100 μl neutralized solutions of collagen (2 mg/ml) and fibrin (1 mg/ml) were allowed to polymerize for 30 min. After matrices polymerized, experimental media with PDGF containing 20×103 cells/ml were added to each matrix. For experiments with coated 2-D surfaces, dishes were completely covered with 50 μg/ml neutralized solutions of collagen and fibrin and placed for 1 hour in a humidified incubator (37°C, 5% CO2). Subsequently dishes were rinsed twice with DMEM serum free media and then migration media with PDGF containing 203cells/ml were added to each dish.
2.5 Time-Lapse Imaging
Live-cell imaging of cell migration was performed as previously described (Petroll et al., 2003). Briefly, a Nikon TE300 inverted microscope (TE300; Nikon, Tokyo, Japan) was used. The hardware was controlled using a PC running MetaMorph. To maintain cell viability during imaging, an environmental chamber at 37°C with 5% CO2 supply was mounted on microscope. A z-stack of images was taken at 10 minutes intervals using a 20X dry objective with Nomarski DIC, or a 10X dry phase contrast objective. Imaging was carried out for 6–24 hours depending on the experimental condition. To create movies of cell movements, a single z-plane image was selected at each time point, so that the same cells were in focus for the entire sequence. MetaMorph software version 7.7 (Molecular Devices Inc.) was used to generate movies of cell movements.
2.6 Immunostaining
Immunostaining was carried out by fixing cells with 3% paraformadehyde in phosphate buffer, PBS, for 10 min, and permeabilizing with 0.5% Triton X-100 in PBS for 15 min. Subsequently samples were washed for 30 minutes with PBS and then incubated with 1% BSA fraction V in PBS. For f-actin labeling, cells were incubated with Alexa Fluor 488 Phalloidin (1:150 ratio) for 60 minutes and then washed for 30 minutes to remove dye excess. For fibronectin (rabbit polyclonal) and cadherin (mouse monoclonal) staining, samples were incubated for 120 minutes with the corresponding antibody in a ratio of 1:100, washed for 60 minutes with PBS, and then incubated for 60 minutes with FITC goat anti-rabbit conjugated antibody (for fibronectin), or FITC goat anti-mouse (for cadherin). Samples were finally washed for 30 minutes to remove excess label. Nuclear staining was carried out after f-actin labeling, by incubating samples for 30 minutes with Propidium Iodide (1:100 ratio) in PBS containing 1:100 RNase-DNase free. All staining procedures were performed in original culture plates to avoid cell or matrix distortion.
Fluorescence Images were collected with a laser confocal microscope (Leica SP2, Heidelberg, Germany) and reflected light was used to visualize fibrin and collagen fibers. HeNe laser (633nm) was used for reflected light, an Argon laser (488nm) was used for fibronectin and cadherin and a GreNe (543nm) laser was used for f-actin. Images were acquired sequentially to avoid cross talk between fluorescence channels. A stack of optical sections were acquired by changing the position of the focal plane in the z-direction with a step size of 2–5μm using a 20X air objective, or a step size of 1 μm using a 63X water immersion objective (1.2 NA, 220 μm free working distance).
2.7 Imaging Processing and 3D Reconstruction
Imaging processing, quantitative analysis of migrating cells and 3D reconstruction of z planes were carried out using MetaMorph software. Images taken in each z plane were combined into one single picture using the maximum intensity projection function. The amount of cell migration was determined by counting the number of cells that migrated into the outer matrix. Counts were averaged from four 20X microscopic fields in each construct, selected arbitrarily. Migrating cells were detected by nuclear staining with Propidium Iodide. Each field included the border of the button and the furthest moving cell. Duplicate samples were analyzed for each condition in each experiment. Final results for each condition are the mean and standard deviation of three separate experiments. For 3D reconstruction of cell migration, z-stacks of images were collected from the first migrating cell visualized on the top of the outer matrix to the last cell visualized in the bottom. The 3D reconstruction function was used in Metamorph to create movies of maximum intensity projections over a range of 3D angles.
2.8 Rheometry
The stiffness of collagen and fibrin matrices was measured by oscillation rheometry using an AR-G2 rheometer (TA instruments, New Castle, DE) with parallel plate geometry. 12 mm diameter collagen and fibrin samples were polymerized in special designed plates that were later mounted to the base plate of the rheometer. Subsequently a 12 mm diameter plate was lowered onto the sample until filling the gap between both plates. Measurements were carried out in a controlled 21°C room temperature. For initial characterization of matrix response, the shear modulus was measured using an oscillatory stress sweep at a frequency of 0.5 Hz until sample failure. Once the linear response of matrices was found, subsequent measurements were performed with an oscillating fixed 3 μNm torque at a frequency of 0.5 Hz for 20 min. Storage (G’) modulus was recorded for each sample. Data presented for each condition are based on measurement of three samples in two separate experiments.
3. RESULTS
3.1 Cells Can Migrate Individually in Collagen, but Remain Interconnected in Fibrin
In the nested migration model, cell-populated buttons are embedded in cell-free collagen or fibrin matrices. Cells migrate out of the inner button and into the outer matrix. We compared corneal fibroblast migration into collagen and fibrin outer matrices using media containing PDGF. PDGF is endogenously expressed in corneal tear fluid, and has been shown to increase Rac activity and induce cell spreading and migration in both dermal and corneal fibroblasts (Grinnell, 2000; Petroll et al., 2008). Consistent with previous studies (Karamichos et al., 2009; Kim et al., 2010), corneal fibroblasts stimulated with PDGF assumed an elongated morphology with dendritic processes at the leading edge when migrating into collagen ECM (Figure 1A). Fibroblasts generally migrated into collagen ECM as “individuals” that became separated from neighboring cells; this is best appreciated from time-lapse movies (Supplemental Video 1). As time progressed and the number of cells in the outer matrix increased, interconnections between cells were observed, but these were generally limited to the tips of dendritic processes and were usually transient in nature. 3D reconstructions show that cells also migrated at different Z angles, and did not coalesce into a single plane during migration (Supplemental Video 2).
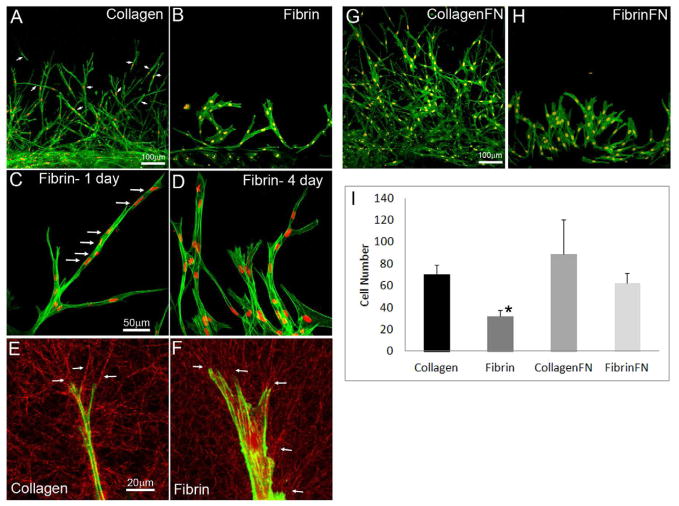
Figure 1.
Cell migration in nested collagen and fibrin matrices. (A, B) Overlays of f-actin and nuclear labeling showing cell migration from inner button into outside matrix after 48 hours of incubation. Outer matrix was either collagen (A) or fibrin (B). (C, D) Overlays of f-actin and nuclear labeling showing cell migration into fibrin after 1 day (C) or 4 days (D) of incubation. (E, F) Fluorescence and reflection microscopy of single migrating cells in collagen (E) and fibrin (F) matrix. Fiber alignment at the leading edge (arrows) shows that cells pull and reorganize matrix during migration. (G, H) Overlays of f-actin and nuclear labeling showing cell migration from inner button into outside matrix after 48 hours of incubation, with the addition of fibronectin to the outer collagen (G) and fibrin (H) matrices. (I) Graph showing cell counts in the outer matrix after 48 hours of incubation. Results are mean +/− SD based on three separate experiments with duplicate matrices at each matrix condition. (* P < 0.05 as compared to collagen and collagen-FN, ANOVA)
When fibrin was used as the outer matrix, the rate of cell migration was significantly reduced (Figure 1I). Importantly, there was also a striking difference in the pattern of cell movement observed in our nested model (Figure 1B). Unlike the individual cell migration observed in collagen ECM, invasion into fibrin was carried out by interconnected groups of cells extending from the inner button (Supplemental Video 3), and isolated cells were not observed. 3D reconstructions show that cells tended to coalesce into a single plane and form an interconnected meshwork as migration progressed (Supplemental Video 4). A recent study using dermal fibroblasts also demonstrated a reduction in the migration rate in fibrin as compared to collagen (Hakkinen et al., 2011); however differences in cell connectivity were not assessed, since the focus was on isolated cells plated at very low density. When we used dermal fibroblasts in our nested matrix model, similar differences in cell connectivity were observed between collagen and fibrin matrices (Figure 2, Supplemental Videos 5 and 6).
Figure 2.
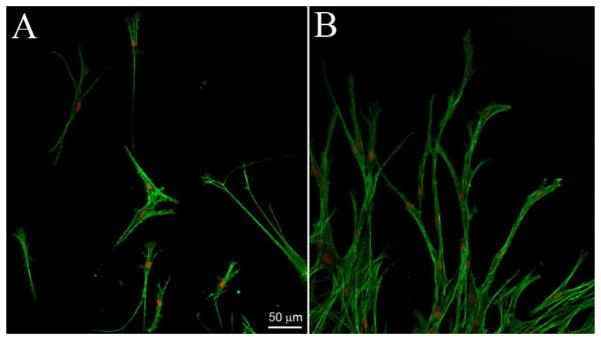
Overlays of f-actin and nuclear labeling of dermal fibroblasts migrating into collagen (A) or fibrin (B) matrix after 3 days of incubation. The leading edge of migratory fronts are shown. Note that cells orientate at different angles and can migrate individually in collagen matrices; whereas invasion into fibrin was carried out by long interconnected groups of cells extending from the inner buttons.
Cells initially migrated into fibrin matrices by axon-like extension of their leading edge, while maintaining connection to cells in the inner matrix at the rear. Other cells followed behind the leading cells along the same paths, producing long lines of interconnected cells (Figure 1C). When cells divided, they temporarily detached from other cells, then moved back along the same path to reattach (Supplemental Video 7). Prior to this reattachment, visible tracks were often observed in the fibrin ECM between cells. These observations suggest that tracks or conduits are created by the initial front of migrating cells, and cells behind them prefer to stay within these tracks, even if they become temporarily separated. As migration proceeded, cells having lateral protrusions often became connected to neighboring cells, producing an interconnected mesh. Migrating cells in fibrin were also broader and expressed more stress fibers than cells in collagen matrices (Figure 1B and 1D). To visualize cell-induced matrix reorganization, confocal reflection imaging was used (Lakshman et al., 2010). Cells reorganized matrix fibers in both conditions, as indicated by alignment of ECM fibrils surrounding cells, particularly at the leading edge of the cell processes (Fig. 1E and F, arrows).
3.2 Fibronectin Increases Cell Migration into both Collagen and Fibrin
It has been reported that fibronectin is necessary for cells to migrate (Greiling and Clark, 1997b), or that it plays a role increasing cell migration (Andresen et al., 2000b). In order to evaluate if fibronectin could increase cell migration in our model, fibronectin was added to the outer collagen and fibrin matrices. Addition of fibronectin increased the amount cell migration rate into both collagen and fibronectin matrices (Fig. 1I). However, addition of fibronectin did not change the patterns of migration (Fig. 1G and 1H). Cells still migrated primarily as individuals in collagen-fibronectin matrices, but were interconnected in fibrin-fibronectin matrices (Supplemental Video 8). Clusters of interconnected cells expressing stress fibers often formed in fibrin-fibronectin as migration proceeded (Supplemental Figure 2).
3.3. Cells Within and On Top of Standard Fibrin Matrices also Cluster and Interconnect
In order to determine whether the differences in migratory pattern were unique to our nested matrix model, we also cultured cells inside standard collagen and fibrin matrices and evaluated their interactions during spreading and migration. Figure 3 shows DIC images from time-lapse videos of cells within fibrin and collagen matrices. Cells were initially rounded and dispersed in both matrix types. After 9 hours of incubation, cells in collagen matrices were elongated, and repeated extension and retraction of cell processes was observed (Supplemental Video 9). Although cells interacted with each other via dendritic processes, these interactions were generally transient, and cells often migrated away from each other after making contact (compare positions of cells 1–4 at beginning and end of sequence). Clusters of cells did not form even after 18 hours of culture. On the other hand, cells in fibrin matrices did not elongate or spread as rapidly, and most cells remained somewhat rounded even after 9 hours of culture (Figure 3). As cells did spread and make contact with each other, they immediately formed broad and stable interconnections (Supplemental Video 10). This led to the formation of cell clusters by 18 hours (compare positions of cells 1, 3 and 4 at beginning and end of sequence). These data show that corneal fibroblasts inside standard matrices also become interconnected in fibrin, and behave more independently in collagen.
Figure 3.
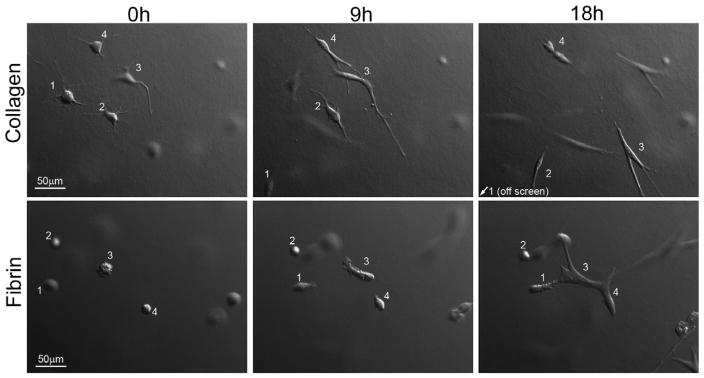
Motile activity of corneal fibroblasts within collagen and fibrin matrices. DIC images from time-lapse videos of fibroblasts incubated for 18 h within collagen or fibrin matrices. Cells within collagen matrices moved individually, while cells within fibrin matrices grouped together to form clusters.
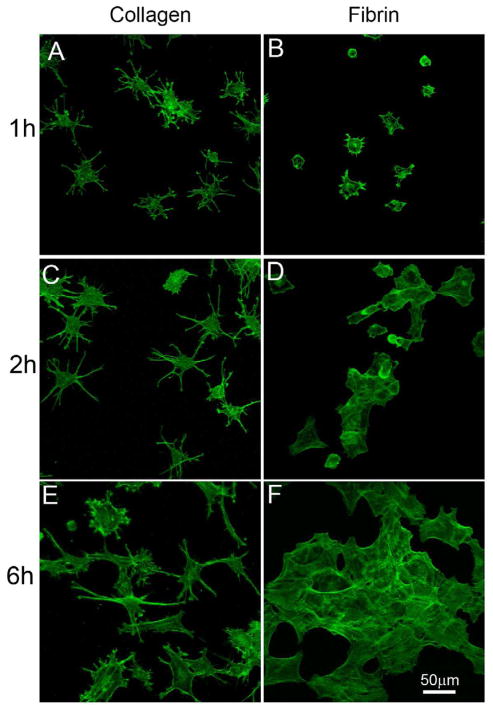
In order to evaluate the effects of the 3D environment on these differences in cell behavior, we plated cells on top of collagen and fibrin matrices. Cells in this 2D environment spread faster than observed in 3D, therefore only the first 6 hours of spreading and migration were analyzed. Corneal fiboblasts spread more rapidly on collagen matrices than on fibrin matrices (Fig. 4A,B). Furthermore, cells on collagen matrices developed a thin dendritic morphology (Fig. 4C), as reported previously by other labs (Jiang et al., 2008; Miron-Mendoza et al., 2010), while cells on fibrin assumed a spread, flattened morphology (Fig. 4D). Time lapse imaging demonstrated that cells on collagen interacted with each other transiently via their dendritic processes, but rarely formed stable interconnections or clusters (Supplemental Video 11). In the case of fibrin matrices, as cells spread and made contact with each other, they immediately coalesced and formed broad and stable interconnections (Supplemental Video 12). This led to the formation of cell clusters by 6 hours (Fig. 4E, F). Together our data show that in both 2-D and 3-D environments, cells interacting with collagen move individually, while cells interacting with fibrin form clusters.
Figure 4.
Spreading pattern of corneal fibroblasts on top of collagen and fibrin matrices. F-actin labeling after incubation for 6 hours on top of collagen or fibrin matrices. Corneal fibroblasts on collagen matrices spread rapidly and developed a dendritic morphology, while cells on top of fibrin matrices grouped together to form clusters.
3.4 Cadherin is Present between Interconnected Cells
Cadherins are transmembrane proteins found in cell-cell adhesions during collective migration (Ilina and Friedl, 2009). To evaluate if cadherin proteins were present in corneal fibroblast clusters, cells were cultured on fibrin matrices overnight, then fixed and stained for f-actin and cadherin. As shown in Figure 5A, cadherin was present between interconnected cells (arrows), suggesting it is involved in mediating cell-cell adhesion.
Figure 5.
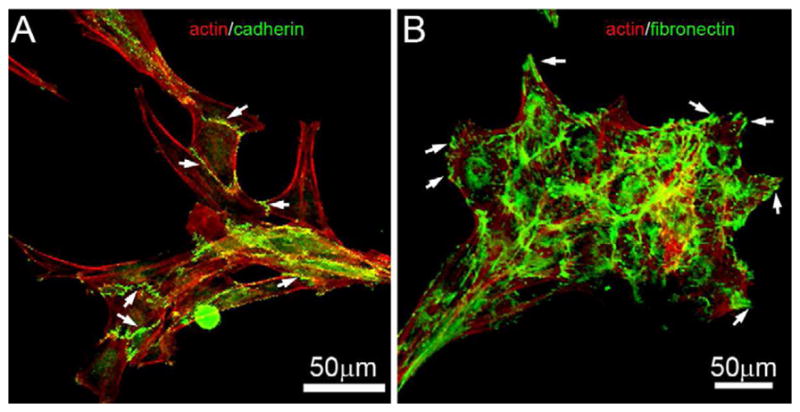
Cadherin and fibronectin organization for cells on fibrin matrices. Corneal fibroblasts were incubated overnight (18 h) on top of 1 mg/ml fibrin matrices. At the end of the incubation, cells were labeled for f-actin and cadherin (A), or f-actin and fibronectin (B). Cadherin was localized between interconnected cells (A, arrows). Fibronectin was located between cells in the clusters that formed on fibrin. In addition, fibronectin was often concentrated at the tips of cell processes extending at the edge of the cluster (B, arrows).
3.5 Fibronectin is Secreted by Cells on Fibrin ECM
Fibronectin is an adhesive protein that binds cells to other matrix components such as collagen and fibrin (Singh et al., 2010). Since fibroblasts do not bind well to fibrin, fibronectin may mediate attachment of cells to fibrin and thereby promote cell migration (Andresen et al., 2000b). In order to evaluate the distribution of fibronectin, cells were cultured overnight on fibrin matrices, and labeled for f-actin and fibronectin. Fibronectin was located between cells in the clusters that formed on fibrin, and was often concentrated at the tips of cell processes extending at the edge of the cluster (Figure 5B). Thus fibronectin may play a role in mediating cell attachment to the fibrin, and/or stabilizing or forming the cell-cell attachments within the cluster.
3.6 The Role of ECM Stiffness on Fibroblast Spreading and Migration Patterns
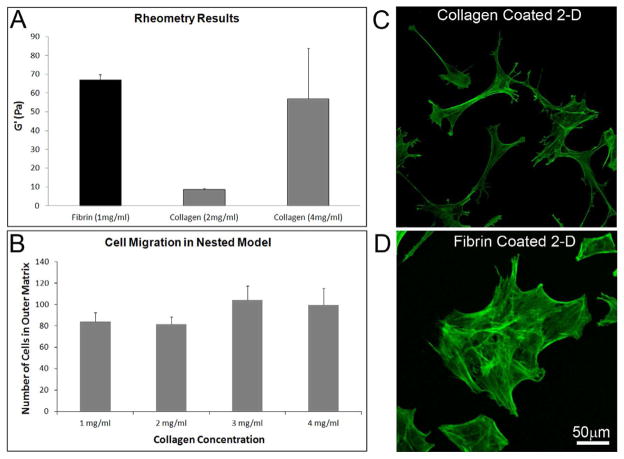
Concentrations for collagen (2mg/ml) and fibrin (1 mg/ml) were selected because they produced similar fibril density and packing, based on confocal reflection imaging. In order to determine whether the stiffness of these matrices was different, rheometry was performed. Rheometry demonstrated that the elastic (storage) modulus of 1 mg/ml fibrin matrices was higher than that of 2 mg/ml collagen matrices (67 Pascals vs. 8.5 Pascals). We also tested 4 mg/ml collagen matrices, and found the stiffness to be similar to fibrin (Figure 6A). To determine whether the differences in cell morphology and connectivity between fibrin and collagen were due to differences in matrix stiffness, we studied cell migration using a range of outer matrix collagen concentrations. The cell migration number was similar for all collagen concentrations tested (Figure 6B), as was the cell morphology and patterning. We also studied cell spreading on collagen- and fibrin-coated glass surfaces, which should have much higher stiffness. Cells on collagen-coated rigid surfaces continued to develop dendritic processes and remained separated in most areas. In some areas with higher cell density, interconnections between dendritic processes were observed, but cell clustering never occurred (Figure 6C). In contrast cells on fibrin-coated surfaces developed broad interconnections which consistently led to cluster formation (Figure 6D). Thus overall, matrix composition appears to be the primary factor inducing differences in cell clusteri – not matrix stiffness.
Figure 6.
Role of ECM stiffness on cell connectivity. (A) Matrix Stiffness was measured using an AR-G2 rheometer with parallel plate geometry. The elastic (storage) modulus of 1 mg/ml fibrin matrix was higher than that of 2 mg/ml collagen matrix, but was similar to that of 4 mg/ml collagen matrix. Pa = Pascals. (B) Collagen matrix concentration did not play a significant role in cell migration. Cell migration number and pattern was similar for all concentrations tested (P=0.153, ANOVA). Results are mean +/− SD based on three separate experiments with duplicate matrices at each matrix condition. (C and D) Collagen and fibrin coated surfaces (which should both have the same high stiffness), also showed the same differences in cell behavior as hydrated matrices. On collagen-coated surfaces, dendritic processes sometimes interconnected in areas of high cell density, but cell clustering was not observed. In contrast cells on fibrin-coated surfaces consistently grouped together and formed clusters.
4. DISCUSSION
In this study, we used a nested matrix construct to directly compare the pattern of keratocyte migration into collagen and fibrin matrices under controlled 3D culture conditions. Corneal fibroblasts generally migrated into collagen ECM as “individuals” that broke free of cells in the inner matrix and also remained separated from neighboring cells, as previously reported for dermal fibroblasts using a similar model (Miron-Mendoza et al., 2008). As time progressed and the number of cells in the outer matrix increased, interconnections between cells were observed, but these were generally transient in nature. In contrast, cells migrating into fibrin matrices extended their leading edge into the fibrin while maintaining connection to cells in the inner matrix at the rear. Other cells followed behind these cells along the same paths, producing long lines of interconnected cells. This pattern of collective cell migration has been termed “multicellular streaming” (Friedl and Wolf, 2009), which is thought to play a role in directional guidance of neural crest cells during embryonic development (Davis and Trinkaus, 1981; Kasemeier-Kulesa et al., 2005; Teddy and Kulesa, 2004). As migration into fibrin proceeded, adjacent cells having lateral protrusions become interconnected, resulting in the formation of a mesh-like structure. This migration pattern differs from the forward movement of an interconnected cell sheet characteristic of epithelial cells (Farooqui and Fenteany, 2005). It is also somewhat different than the classical “collective” cell migration pattern used by cancer cells, in which lateral connections between invading cell clusters are not typically observed (Ilina and Friedl, 2009). Interestingly, in vivo studies have shown that following full thickness corneal incisions, fibroblasts form an interconnected mesh as they migrate into the wound space, and these interconnections are hypothesized to mediate force transduction during wound contraction (Jester et al., 1995a; Petroll et al., 1993). Importantly, we observed a similar pattern of interconnected migration into fibrin when using dermal fibroblasts, suggesting that his behavior is not unique to corneal cells.
Following cell division in collagen ECM, cells always migrated in opposite directions and did not re-attach to one another. However, when cells in fibrin divided, they detached from each other only temporarily, then quickly moved back along the same paths to reattach. Similar differences were observed following division when cells were plated on top of collagen or fibrin matrices. Interestingly, following cell division inside fibrin ECM, visible tracks were sometimes observed in the fibrin ECM between cells by DIC imaging, and cells remained within these tracks when reattaching. These observations suggest that tracks or conduits are created by the initial front of migrating cells, and cells behind them prefer to stay within these conduits, even if they become temporarily separated. Microtracks of aligned collagen been identified previously during cell migration in 3D matrices, and they seem to be necessary for the transition from individual to collective cell migration in cancer cell invasion (Wolf et al., 2007).
Fibronectin is an adhesive protein secreted by cells that facilitates binding to other ECM proteins (Singh et al., 2010). In skin tissues, both fibrin and fibronectin are found in the fibrin clot, the provisional matrix following injury (Clark, 1996). In vivo corneal wounds also have high levels of fibronectin (Jester et al., 1995b). Fibronectin synthesis has also been shown to contribute to endothelial cell network formation and tubulogenesis in vitro (Reinhart-King, 2011; Zhou et al., 2008). In our model, fibronectin was secreted within the cell clusters that formed on fibrin ECM. Fibronectin was most concentrated at the tips of cell processes extending from the edge of these clusters, suggesting fibronectin may play a role in mediating cell attachment to the fibrin. Since most cells require fibronectin in order to bind to fibrinogen and fibrin (Greiling and Clark, 1997a; Grinnell et al., 1980), leading edge cells might secrete fibronectin to facilitate binding to the fibrin ECM during migration, and cells behind them likely prefer to stay within these fibronectin conduits, resulting in interconnected lines of cells. When fibronectin was added to the outer collagen and fibrin matrices in the current study, the cell migration rate increased in both cases, consistent with previous results (Andresen et al., 2000a). However, the pattern of migration did not change, suggesting that exogenous fibronectin does not replace the function of localized, cell-secreted fibronectin.
A hallmark of collective cell migration is the presence of cell-cell junctions (Ilina and Friedl, 2009). In collective cell migration, the rear of the front cell remains coupled to the cell behind it during migration (Ilina and Friedl, 2009). Cell-cell adhesion is mainly mediated by cadherins. Consistent with this result, we observed the presence of cadherin in regions of cell-cell interaction on fibrin ECM. Fibronectin may also play a role in mediating cell-cell adhesion, by serving as a bridge for integrin-integrin interactions between cells (Ilina and Friedl, 2009). For example, α5β1 integrin binds to fibronectin along interfaces between ovarian carcinoma cells (Casey et al., 2001) or fibroblasts (Salmenpera et al., 2008), and blocking of β1-integrin function in migrating multicellular melanoma clusters leads to loss of cell-cell cohesion, cell detachment and transition to single-cell migration (Hegerfeldt et al., 2002). We observed high levels of secreted fibronectin around and between cells that had formed clusters on fibrin matrices, suggesting a possible role in cell adhesion. Previous studies have shown that fibronectin binding is required for keratocyte transformation to a myofibroblast phenotype (Jester et al., 1999a). Consistent with these observations, cells interacting with fibrin matrices had a broader morphology and expressed more stress fibers than cells in collagen matrices. Experiments blocking α5 binding or fibronectin secretion are needed to bring more insight into the role of fibronectin in mediating both cell-cell and cell-matrix interactions in fibrin matrices.
3D ECMs are composed of a network of hydrated fibers that have the capacity to undergo reorganization (Chandran and Barocas, 2006). Cell migration and cell-matrix interactions are affected by both the elasticity of the ECM, and the geometrical organization of the fiber network, i.e. the density of the fibers and the space between fibers (Alexander et al., 2008; Miron-Mendoza et al., 2010; Zaman et al., 2006). For this study, we selected concentrations of collagen (2mg/ml) and fibrin (1 mg/ml) that produced similar densities of fibers, as visualized by confocal reflection imaging. However, rheometry measurements indicated that the fibrin matrices were much stiffer. To investigate whether differences in stiffness impacted fibroblast morphology and connectivity during migration, we performed additional experiments using 4mg/ml collagen, which had a similar stiffness to the 1mg/ml fibrin matrices. We also studied cell spreading on collagen- and fibrin-coated glass surfaces, which have very high stiffness as compared to hydrated 3-D matrices. In all cases, cells interacting with collagen assumed a dendritic morphology and continued to spread and migrate individually, whereas cells interacting with fibrin interconnected and formed clusters. Previous studies have shown that vascular endothelial cells tend to aggregate on compliant substrates, and remain separated on stiffer substrates (Reinhart-King, 2011). Furthermore, dermal fibroblasts on collagen matrices can form aggregates when they are in a pro-contractile growth factor environment (serum, LPA), but the amount of clustering is inversely related to matrix stiffness (Rhee et al., 2010). Thus based on matrix elasticity alone, one would expect less cell aggregation in fibrin ECM. Overall, the data suggest that differences in ECM stiffness are not the underlying cause for the difference in cell connectivity observed between collagen and fibrin matrices.
In conclusion, our results demonstrate for the first time that ECM composition (collagen vs. fibrin) can play an important role in modulating the pattern of fibroblast spreading and migration in 3-D culture. This may explain, in part, the different migratory patterns observed during corneal wound repair in vivo. Similar processes may also influence cell behavior during development, tumor invasion and repopulation of bioengineered tissues.
Supplementary Material
Overlay of f-actin and nuclear labeling of corneal fibroblasts migrating into fibrin-fibronectin matrix after 4 days of incubation.
Corneal fibroblast migration in nested model with 2 mg/ml outside collagen matrix. Migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
3D Reconstruction of migrating corneal fibroblast in nested model with 2 mg/ml outside collagen matrix. After 48h of cell migration, cells were fixed and labeled for f-actin. Confocal images taken at different z planes were reconstructed into a 3D dynamic movie, using maximum intensity projections from −50 to +50 degrees.
Corneal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix. Cell migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
3D Reconstruction of migrating corneal fibroblast in nested model with 1 mg/ml outside fibrin matrix. After 48h of cell migration, cells were fixed and labeled for f-actin. Confocal images taken at different z planes were reconstructed into a 3D dynamic movie, using maximum intensity projections from −50 to +50 degrees.
Dermal fibroblast migration in nested model with 2 mg/ml outside collagen matrix. Migration in nested model was recorded beginning 24 hours after plating using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Dermal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix. Cell migration in nested model was recorded beginning 24 hours after plating using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Corneal fibroblasts detach and re-attach during migration in nested model with a 1 mg/ml fibrin matrix. Cell migration into a fibrin matrix was recorded for 32 h using DIC imaging. Frames were collected every 20 minutes and display rate is 10 frames/sec.
Corneal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix containing 100 μg/ml fibronectin. Cell migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts inside 2 mg/ml collagen matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 18 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts inside 1 mg/ml fibrin matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 18 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts on top of 2 mg/ml collagen matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 6 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts on top of 1 mg/ml fibrin matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 6 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Highlights.
Corneal fibroblasts migrating into collagen move independently.
Corneal fibroblasts migrating into fibrin form an interconnected meshwork.
Dermal fibroblasts migrating into collagen and fibrin exhibit similar differences in cell behavior.
Fibroblasts cluster within or on top of standard fibrin matrices, but not on collagen matrices.
These differences are consistently observed on both rigid and compliant ECM environments.
Acknowledgments
The authors wish to thank Dr. Fred Grinnell for his helpful comments and suggestions, the dermal fibroblast cells, and for the use of the rheometer.
This study was supported in part by NIH R01 EY 013322, NIH P30 EY020799, and an unrestricted grant and Senior Scientific Investigator Award (WMP) from Research to Prevent Blindness, Inc., NY, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Hager H, Josephsen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp Eye Res. 2000a;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Hager H, Josephsen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp Eye Res. 2000b;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 2005;15:1762–1770. [Google Scholar]
- Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegama TR, Ruff LE, Skubitz AP. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159:2071–2080. doi: 10.1016/s0002-9440(10)63058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran PL, Barocas VH. Affine versus non-afine fibril kinematics in collagen networks: theoretical studies of network behavior. J Biomech Eng. 2006;128:259–270. doi: 10.1115/1.2165699. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Wound repair,: overview and general considerations. In: Clark RAF, editor. Molecular and cellular biology of wound repair. Plenum Press; 1996. pp. 3–50. [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EM, Trinkaus JP. Significance of cell-to-cell contacts or the directional movement of neural crest cells within a hydrate collagen lattice. J Embryol Exp Morph. 1981;63:29–51. [PubMed] [Google Scholar]
- Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2009;199:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1306–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997a;100:861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997b;110 ( Pt 7):861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibronectin and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin) Cell. 1980;19:517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparision of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue engineering: Part A. 2011;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, b1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- Ichijima H, Petroll WM, Jester JV, Barry PA, Andrews PM, Dai M, HDC In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea. Cornea. 1993;12:369–378. doi: 10.1097/00003226-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999a;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J Anat. 1995a;186:301–311. [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J Anat. 1995b;186 ( Pt 2):301–311. [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999b;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rhee S, Ho CH, Grinnell F. Distinguishing fibroblst promigratory and procontractile growth factor environments in 3-D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Lakshman N, Petroll WM. An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell Motil Cytoskeleton. 2009;66:1–9. doi: 10.1002/cm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–245. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zhou C, Lakshman N, Petroll WM. Corneal Stromal Cells use both High- and Low-Contractility Migration Mechanisms in 3-D Collagen Matrices. Exp Cell Res. 2012 doi: 10.1016/j.yexcr.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010;90:350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Mol Biol Cell. 2008;19:2051–2058. doi: 10.1091/mbc.E07-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–6435. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Neel EAA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Hutcheon AEK, Zieske JD, Wilson SE. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Pepose JS, Ubels JL. The Cornea. In: Hart WM, editor. Adler’s Physiology of the Eye. Mosby Year book; St. Louis: 1992. pp. 29–70. [Google Scholar]
- Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993;104 ( Pt 2):353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Ma L, Jester JV. Direct Correlation of Collagen Matrix Deformation with Focal Adhesion Dynamics in Living Corneal Fibroblasts. J Cell Sci. 2003;116:1481–1491. doi: 10.1242/jcs.00357. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008;217:162–171. doi: 10.1002/jcp.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TM, Foster CS, Wasson PJ, Fujikawa LS, Zagachin LM, Colvin RB. Role of fibronectin and fibrinogen in healing of corneal epithelial scrape wounds. Invest Ophthalmol Vis Sci. 1989;30:377–385. [PubMed] [Google Scholar]
- Reinhart-King CA. How matrix properties control the self-assembly and maintenance of tissues. Ann Biomed Eng. 2011;39:1849–1856. doi: 10.1007/s10439-011-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Ho CH, Grinnell F. Promigratory and procontractile growth factor environments differentially regulate cell morphogenesis. Exp Cell Res. 2010;316:232–244. doi: 10.1016/j.yexcr.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmenpera P, Kankuri E, Bizik J, Siren V, Virtanen I, Takahashi S, Leiss M, Fassier R, Vaheri A. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res. 2008;314:3444–3452. doi: 10.1016/j.yexcr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short-and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multistep pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Siemeski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Rowe RG, Hiraoke N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes & development. 2008;22:1231–1243. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overlay of f-actin and nuclear labeling of corneal fibroblasts migrating into fibrin-fibronectin matrix after 4 days of incubation.
Corneal fibroblast migration in nested model with 2 mg/ml outside collagen matrix. Migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
3D Reconstruction of migrating corneal fibroblast in nested model with 2 mg/ml outside collagen matrix. After 48h of cell migration, cells were fixed and labeled for f-actin. Confocal images taken at different z planes were reconstructed into a 3D dynamic movie, using maximum intensity projections from −50 to +50 degrees.
Corneal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix. Cell migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
3D Reconstruction of migrating corneal fibroblast in nested model with 1 mg/ml outside fibrin matrix. After 48h of cell migration, cells were fixed and labeled for f-actin. Confocal images taken at different z planes were reconstructed into a 3D dynamic movie, using maximum intensity projections from −50 to +50 degrees.
Dermal fibroblast migration in nested model with 2 mg/ml outside collagen matrix. Migration in nested model was recorded beginning 24 hours after plating using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Dermal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix. Cell migration in nested model was recorded beginning 24 hours after plating using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Corneal fibroblasts detach and re-attach during migration in nested model with a 1 mg/ml fibrin matrix. Cell migration into a fibrin matrix was recorded for 32 h using DIC imaging. Frames were collected every 20 minutes and display rate is 10 frames/sec.
Corneal fibroblast migration in nested model with 1 mg/ml outside fibrin matrix containing 100 μg/ml fibronectin. Cell migration in nested model was recorded for 27 h using phase contrast imaging. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts inside 2 mg/ml collagen matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 18 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts inside 1 mg/ml fibrin matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 18 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts on top of 2 mg/ml collagen matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 6 h. Frames were collected every 20 min, and display rate is 10 frames/sec.
Motile activity of corneal fibroblasts on top of 1 mg/ml fibrin matrix. Cell spreading and migration was recorded using DIC imaging. Time of observation was 6 h. Frames were collected every 20 min, and display rate is 10 frames/sec.