Abstract
In this article, we will describe the malignant synaptic growth hypothesis of Alzheimer’s disease. Originally presented in 1994, the hypothesis remains a viable model of the functional and biophysical mechanisms underlying the development and progression of Alzheimer’s disease. In this article, we will refresh the model with references to relevant empirical support that has been generated in the intervening two decades since it’s original presentation. We will include discussion of its relationship, in terms of points of alignment and points of contention, to other models of Alzheimer’s disease, including the cholinergic hypothesis and the tau and β-amyloid models of Alzheimer’s disease. Finally, we propose several falsifiable predictions made by the malignant synaptic growth hypothesis and describe the avenues of treatment that hold the greatest promise under this hypothesis.
Keywords: β amyloid, acetylcholine, Alzheimer’s, computational model, dementia, excitotoxicity, long-term potentiation, neurofibrillary tangles, plasticity, tau
Alzheimer’s disease causes a progressive deterioration of cognitive function due to characteristic neuropathological changes in cortical structures, including amyloid plaques and neurofibrillary tangles. Current treatments for Alzheimer’s disease offer only a small reduction in behavioral symptoms [1,2] and do not halt the temporal progression of the disease – emphasizing the need for a functional framework for understanding the progression of the disease. The primary existing hypotheses of the root cause of Alzheimer’s disease typically focus on specific molecular pathways and do not address the characteristic anatomical progression of tangle pathology [3]. In particular, they do not address why neurofibrillary tangles first appear and attain their highest density in the lateral entorhinal cortex [4,5]. Exciting recent studies have shown that tau pathology can spread between structures, but even these studies do not yet address why neurofibrillary tangles appear to spread along specific pathways, but not others [6,7].
In this article, we describe a hypothesis that arises from the observation that neural network models of memory have a tendency to fall victim to malignant synaptic growth and consequently express symptoms analogous to those observed in Alzheimer’s disease patients. Malignant synaptic growth refers to the exponential growth in the magnitude and number of excitatory synapses caused when synaptic transmission at previously strengthened synapses directly enhances further synaptic modification in a mechanism of positive feedback [8–11]. This hypothesis can account for the initial appearance of tangles in regions giving rise to highly modifiable synaptic projections in limbic cortices, and can account for the functional spread of tangles along back-projections between cortical regions. This provides a potential functional framework for understanding Alzheimer’s disease not as an effect of toxicity imposed upon cortical circuits, but as a breakdown in the functional properties of cortical circuits that can induce a progression of breakdown in associated cortical regions.
The functional model of the initiation and progression of Alzheimer’s disease provides a framework for understanding a number of the empirical features of the disease, including the following:
The initial appearance of tangle pathology in areas giving rise to highly modifiable divergent connections;
The spread of tangle pathology along back-projections between cortical structures and associated progression from episodic memory to semantic memory and general cognition;
The initial increase and subsequent decrease in cholinergic innervation of cortical structures in Alzheimer’s disease;
The increase and subsequent decrease in synaptogenesis;
The role of APPs in the development of collaterals and synapses;
The role of MAPT protein in axonal transport, and the late-stage exponential increase in hyperphosphorylated tau;
The increased susceptibility to seizures associated with advanced Alzheimer’s disease.
In addition to addressing existing data, this theoretical framework suggests treatment strategies focused on regulating the network balance of encoding and retrieval, rather than targeting specific molecular pathology. For example, the framework suggests that the therapeutic effect on Alzheimer’s disease of the NMDA antagonist memantine (Namenda) arises from the reduction in synaptic modification and supports testing of other NMDA antagonists. In addition, the framework suggests the use of muscarinic M4 agonists for presynaptic regulation of synaptic transmission. These and other implications of the theory will be explored further below.
The malignant synaptic growth hypothesis
In this section, we will introduce the hypothesis, describe the existing simulation work supporting this hypothesis, and integrate the concepts from these simulations into more recent modeling work.
Introduction to the hypothesis
The malignant synaptic growth hypothesis of Alzheimer’s disease suggests that the physiological and behavioral correlates of the disease’s progression arise from the unchecked growth of synapses [8,9]. This results from a loss of balance between the dynamic strengthening and weakening of synaptic connections within cortical structures [8,10,12]. This problem can occur in many different types of associative memory models [11], but usually does not receive detailed analysis because it can be prevented by the separation of the dynamics of encoding and retrieval [13,14]. Thus, neural network simulations highlight the importance of enforcing the separation between new learning and the retrieval of previous memories. In the absence of a mechanism to enforce this separation, learning of new patterns is performed in the midst of memory retrieval. The result is something akin to a ‘collision’ of information encoding. New information is combined in a broader, merged representation with previously stored memories.
At a physiological level, this process leads to an imbalance in the amount of strengthening that occurs at each synapse. Over time, these collisions compound and, in neural network simulations, lead to profound retrograde amnesia and eventually anterograde amnesia and increase the susceptibility for seizures. The merging of memory representations is characterized by the exponential growth of synaptic strengths that could lead to excitotoxic cell death. The malignant synaptic growth hypothesis suggests that the primary pathological symptoms of Alzheimer’s disease are the result of excessive synaptic strengthening and the complications of this strengthening. In this framework, pathogenesis arises from an initial breakdown in the ability of the brain to perform novel encoding in the absence of retrieval of existing memories – an imbalance in the separation of encoding dynamics from retrieval dynamics.
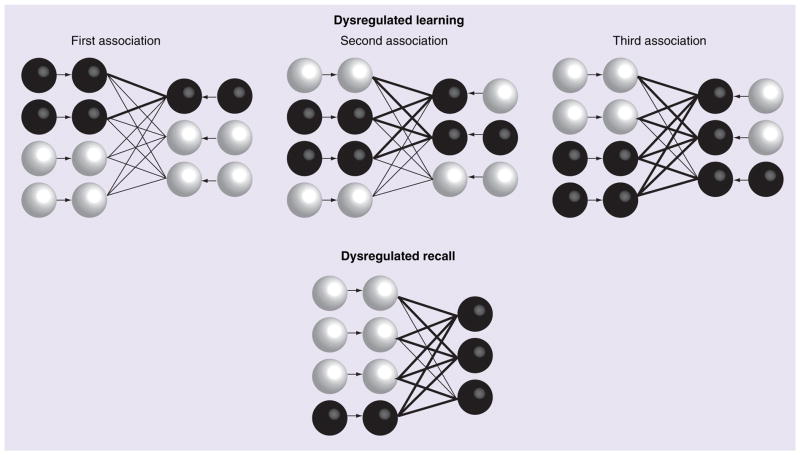
The malignant synaptic growth hypothesis was inspired by neural network simulations, and those interested in a detailed description of the hypothesis can refer to the mathematical structure of these simulations in other publications [8]. However, for those that are unfamiliar with neural network models, we provide a review of the properties of this model here. In the model, malignant synaptic growth results from poor separation of memory encoding and retrieval. When novel encoding is performed simultaneously with memory retrieval, the to-be-learned content is merged with the retrieved memory instead of forming its own representation. This process is shown schematically in Figure 1. The more often an existing memory is retrieved in the context of new encoding, the larger it grows, thereby increasing the probability that it will be retrieved again. Over time, this memory grows like a snowball rolling down a mountain or a malignant cancer spreading through the body. This growth occurs both within and across anatomical regions. Because the propensity for synaptic plasticity increases the risk for neural instability in the model, the breakdown in function affects portions of the network with the greatest propensity for synaptic modification. The spread of pathology is due to the exponential growth of synapses. As the excitatory synapses within and between neurons grow stronger, the amount of excitation received by each cell grows and could result in cell death due to too much excitation, a process known as excitotoxicity.
Figure 1. Illustration of network dynamics during learning and recall under Alzheimer’s disease conditions.
Circles represent neurons: white circles represent inactive neurons, while black circles represent active neurons. The lines between circles represent synapses: thick black lines represent strong synapses and thin black lines represent weak synapses. Activity spreads through previously potentiated synapses as the second and third associations are learned causing the retrieval of the previously stored associations during the new encoding. The connections to these inappropriately active units grow further with each new learned association. During recall, all of the output units activate.
Adapted from [8] with permission from Elsevier.
Existing simulations supporting this hypothesis
Hasselmo provides a thorough description of the phenomena and quantitative explorations of the root causes of malignant synaptic growth induced by failures to maintain separation between encoding and retrieval [8]. For such details, we refer the reader to this paper. Here, we will provide a basic review of the simulation architecture, how the simulation performs under ‘healthy’ conditions, the changes that produce the network instability and the failures of the network following onset of the network instability.
The architecture of the simplest neural networks used in these simulations contains the basic elements of cortical circuits. The simulations contain a single population of neurons that receives input either from other neurons in the network (intrinsic connections) or from neurons outside of the network (extrinsic connections). Intrinsic connections allow neurons within the network to mutually excite one another allowing the network to fill in the missing portions of partial patterns (i.e., to perform retrieval). Extrinsic connections to the population provided input patterns controlled by the experimenter. These connections were used either to present novel stimuli to be encoded by the neural network or to present portions of previously encoded patterns to cue the retrieval of previously encoded memories.
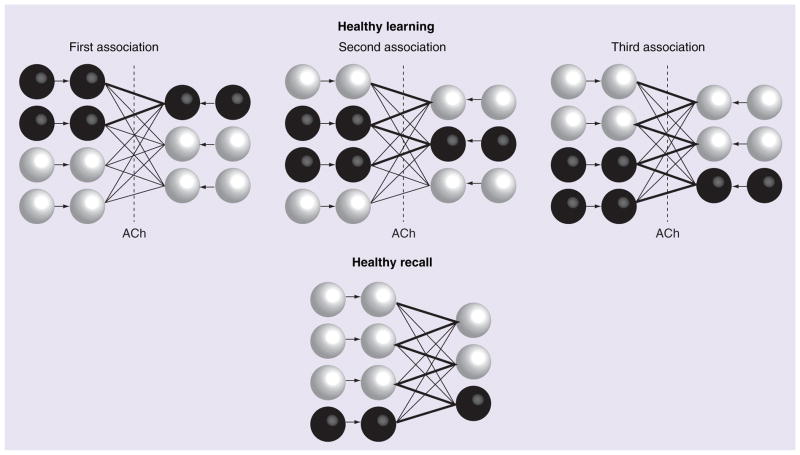
Healthy functioning of neural network models of associative memory require that the encoding of novel information occurs in the absence of retrieval of previously formed memories. Most models of associative memory implement a difference in network dynamics between encoding and retrieval [13,14], without describing a physiological mechanism [8]. When presented with a novel pattern, these networks allow the input activity to clamp the activity of neurons in the network, and the effect of intrinsic connections between neurons within the network is reduced to prevent retrieval. At the same time, the ability to modify the strength of these intrinsic synapses is simultaneously increased to boost encoding of the new pattern. Intrinsic synapses are modified using a Hebbian rule, often described informally as: ‘Neurons that fire together, wire together’. This allows selective strengthening of excitatory connections between neurons that are active in the pattern. This pattern is summarized in Figure 2.
Figure 2. Illustration of network dynamics during learning and recall under normal (non-Alzheimer’s disease) conditions.
ACh helps limit activity from spreading through previously potentiated synapses as the second and third associations are learned. This prevents extra units from activating and having their connections strengthened. During recall, only the correct output unit activates. See Figure 1 for a description of the schematic representation.
ACh: Acetylcholine.
Adapted from [8] with permission from Elsevier.
Conversely, when presented with a less novel input, the intrinsic projections are allowed to excite other cells in the network and the propensity of synapses for synaptic modification remains at a baseline level. Consequently, when a portion of a familiar input is presented to the network through the extrinsic projections, excitation spreads through the previously potentiated synapses of the intrinsic projections and activates neurons that may receive little direct excitation through the extrinsic connections. In this manner, the network is able to retrieve, or fill in, the missing portions of the cued representation.
The effects of acetylcholine provide a potential physiological mechanism for the change in dynamics during encoding and retrieval [15]. Empirical investigations into the modulatory influences of acetylcholine demonstrate that increased cholinergic tone both selectively suppresses the transmission at intrinsic synapses while lowering the threshold for the induction of long-term potentiation (LTP) [16,17] as shown in Figure 3. An alternate mechanism for preventing malignant synaptic growth involves balancing the strengthening of synapses via Hebbian long-term synaptic modification with the activity-dependent weakening of synapses [18,19].
Figure 3. Schematic summary of the effects of acetylcholine on synaptic transmission and plasticity.
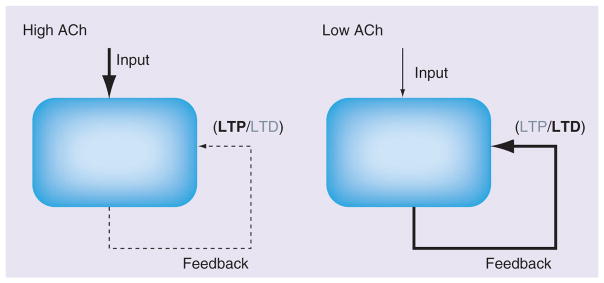
Increased levels of ACh suppress intrinsic connections, boost transmission rates of extrinsic projections and reduce the threshold for the induction of LTP. Reduced levels of ACh boost transmission rates of intrinsic projections and increase the threshold for the induction of LTP, increasing the probability that LTD is induced.
ACh: Acetylcholine; LTD: Long-term depression; LTP: Long-term potentiation.
Adapted from [8] with permission from Elsevier.
In these models, malignant synaptic growth occurs when the separation between encoding and retrieval breaks down, or when the balance of synaptic strengthening and synaptic weakening favors synaptic strengthening. Once induced, network instability accelerates due to positive feedback loops, as if synapses were boosting their own growth in small explosions, or spreading like a malignant growth (as shown in Figure 1). The malignant synaptic growth hypothesis does not specify a single mechanism for the breakdown between encoding and retrieval. This could involve a number of different factors that regulate the relative dynamics within cortical circuits. For example, this could involve a loss of cholinergic modulation, or a shift in the mechanisms that regulate the relative magnitude of synaptic strengthening and synaptic weakening.
Relevance to recent neural network models
The simulations described above demonstrate the importance of incorporating mechanisms for preventing the malignant growth of synapses in neural network models. This topic has received substantial attention since the original simulation work carried out on this topic. A theme among several relatively recently proposed mechanisms for maintaining stability in neural networks is the use of oscillatory dynamics. In this section, we will describe two such mechanisms and how they relate to the malignant synaptic growth hypothesis. Models by Hasselmo et al. [20] and Norman et al. [12] present methods of maintaining separation between existing knowledge and novel encoding that utilize neural oscillations.
Electroencephalographic recordings from the hippocampus and entorhinal cortex show a high amplitude, clearly observable 6–12 Hz rhythmic signal known as theta [21] that has also been observed in humans [22]. Theta rhythms vary in frequency and amplitude in response to behavioral demands and have been the focus of considerable physiological investigation [23,24].
There is a strong correspondence between the known physiological properties of theta and the dynamics observed in the malignant synaptic growth model during healthy function. Current source density analyses of the inputs to area CA1 of the hippocampus reveal, for example, phasic alternations between strong input from the entorhinal cortex (extrinsic projections) and strong input from area CA3 (intrinsic projections) during each theta cycle [25,26]. Furthermore, LTP is induced most easily when the extrinsic projections are the strongest, while long-term depression is induces more readily when the intrinsic projections are the strongest [27]. Given this correspondence, Hasselmo et al. suggest that theta serves to separate encoding from retrieval and describe a series of simulations to demonstrate the effectiveness of theta to serve in this capacity [20].
Norman et al. describe a distinct, but compatible, model of theta mediated learning dynamics [10]. In this model, activation dynamics driven by theta, as described above, interact with a synaptic modification rule that strengthens strongly active neurons and weakens moderately active neurons [28]. Together, theta and this modification rule have the effect of limiting new strengthening to novel associations and proactively reducing the strength of excessively strong existing associations. The precise mechanisms of this model are beyond the scope of this article but are described in detail elsewhere [10,12,29].
In both models, theta is proposed to play an important role in maintaining a balance in the strength of synaptic connections and to limit strengthening of memories. As such, breakdowns in these mechanisms may contribute to the generation of Alzheimer’s disease as described by the malignant synaptic growth hypothesis.
Relationship to other hypotheses
In the following section, we discuss the relationship between the malignant synaptic growth hypothesis and the most prominent and relevant alternate hypotheses of Alzheimer’s disease. These will include the cholinergic hypothesis, the tauopathy model, the β-amyloid model and the pathological plasticity hypothesis. For each, we will introduce the defining properties and the points of alignment and contention between the other hypothesis and the malignant synaptic growth hypothesis.
It is worth noting at the outset that the malignant synaptic growth hypothesis describes the pathologies of Alzheimer’s disease at a functional level, whereas most other models described here focus on descriptions at the level of molecular and cellular processes. As such, the malignant synaptic growth hypothesis is not mutually exclusive with these alternate descriptions. Indeed, the pathologies characterized by each hypothesis are accurate descriptions of component processes relevant to Alzheimer’s disease. The hypotheses only differ in a fundamental way in defining what they view as the root cause of the disease. As spelled out below, the malignant synaptic growth hypothesis states that excessive strengthening of synapses is the first stage of Alzheimer’s disease and that other pathologies (e.g., cholinergic loss, β amyloid and phosphorylated tau build-up) result from the demands placed on the system to support or counteract this accelerating plasticity.
Cholinergic hypothesis
The cholinergic hypothesis suggests that the dominant factor in the cognitive decline of Alzheimer’s patients is the loss of cholinergic tone [30]. This hypothesis was first proposed by Bartus et al. based on clear demonstrations of significant enzyme reductions that are required for processing acetylcholine in postmortem examination of Alzheimer’s disease patients’ brains [31]. Investigations into the importance of acetylcholine for healthy cognitive function support the idea that the loss of cholinergic function may lead to the behavioral impairments observed in Alzheimer’s disease [17].
Both the cholinergic hypothesis and the malignant synaptic growth hypothesis ascribe important function to acetylcholine in healthy function and in the breakdown observed in Alzheimer’s disease. Both hypotheses suggest that without proper cholinergic tone, the cognitive state of an Alzheimer’s disease patient will deteriorate. For example, the malignant synaptic growth hypothesis depends upon acetylcholine for maintaining the separation between encoding a nd retrieval. Thus, without proper levels of acetylcholine, the rate of spread of the malignant synaptic growth will increase, thus accelerating the progression of the disease.
A fundamental difference between the cholinergic hypothesis and the malignant synaptic growth hypothesis resides in the way in which they view the role of acetylcholine in the breakdown observed in the progression of Alzheimer’s disease. Specifically, the cholinergic hypothesis suggests that the loss of cholinergic tone is, in and of itself, the reason for the cognitive decline. By contrast, exponential growth of synapses and the resulting excitotoxicity are the primary drivers of cognitive decline in the malignant synaptic growth hypothesis. While this process could be instigated by a reduction of cholinergic tone, it may equally be driven by other factors that upset the balance of synaptic strengthening and weakening. Instead, the malignant synaptic growth hypothesis suggests that the loss of cholinergic tone results from over taxation of the cholinergic system by way of attempting to reduce the interference between new encoding and existing memories. There is evidence to support the idea that cholinergic decline is a consequence of other factors and is not the root cause: cholinergic function is suppressed relatively late into the progression of the disease and has been found to be upregulated early in the progression of Alzheimer’s disease [32–34].
β-amyloid model
The β-amyloid model focuses on mutations in, and the aberrant processing of, the APP in proposing the origin and primary pathology underlying Alzheimer’s disease. This theory was initially described two decades ago [3,35–37]. The theory suggests changes in the processing of APP leading to the formation of β amyloid 1–42 (Aβ42), which induces the neurodegeneration that ultimately manifests in dementia. This theory was born out of the observation, among others, that Aβ deposition forms neuritic plaques that are found ubiquitously during histological examination of the brains of late-stage Alzheimer’s disease patients. Furthermore, when studied in cultured cells, application of Aβ42 oligomers leads to down regulation of surface NMDA-type glutamate receptor expression, LTP and synaptic spine density [38–40], each of which can be viewed as a form of neurodegeneration.
The β-amyloid model is easily integrated into the framework set out by the malignant synaptic growth hypothesis as a downstream process resulting from the excessive strengthening of synapses. The processing of APP is a naturally occurring component of synaptic plasticity [41]. Pathologies that lead to the production of Aβ42 when APP is processed will have a greater impact if synaptic plasticity occurs at increased rates. As such, the detrimental effects of Aβ42 oligomers will be substantially amplified as a consequence of malignant synaptic growth, which is itself detrimental.
The downstream influences of Aβ may, however, counteract the primary toxic effects of malignant synaptic growth. Specifically, the ability of Aβ oligomers to reduce NMDA receptor expression, slow LTP and remove synapses all limit the excitation of neurons and may thus serve to protect cells from excitotoxicity. In this light, the production of Aβ may serve as a natural form of chemotherapy that blocks the malignant growth of synapses. Like chemotherapy used to treat cancer, however, Aβ itself is harmful. Consequently, the relationship between Aβ concentration and a patient’s psychological condition may be complicated by the fact that the creation of Aβ may be a reactive mechanism to limit the progression of the disorder while additional accumulation introduces an additional form of toxicity. Indeed, there are cases in which patients with no cognitive impairment or only mild cognitive impairment, that nonetheless are found to have similar densities of senile plaques during postmortem examination as Alzheimer’s patients [42], potentially reflecting individuals for which this response compensated for the synaptic growth in a relatively balanced fashion until death.
Tauopathy model
The tauopathy model of Alzheimer’s disease centers its focus on the build-up of hyperphosphorylated tau and the neurofibrillary tangles that result from this build-up [43]. By this account, hyperphosphorylation reduces the binding of tau to microtubules, resulting in defective axonal transport (impeding delivery of supplies to distal portions of the cell’s axon) and aggregation of hyperphosphorylated tau in neurofibrillary tangles. The tangles themselves may also have detrimental effects by absorbing newly translated tau, blocking it from reaching the microtubule structure and by acting as a intracellular road block for cargo delivery (see [44] for a recent revision to this model). Empirical support for this model comes from repeated demonstrations that the progression of Alzheimer’s disease is well correlated with the build-up of neurofibrillary tangles [45]. However, it is also worth noting that neurofibrillary tangles have also been observed in the brains of cognitively healthy elderly patients and mild cognitive impairment patients [42,46].
The pathologies of particular interest to the tauopathy model of Alzheimer’s disease, like those described under the β-amyloid model, can be viewed as a downstream consequence of excessive synaptic modification. The uncontrolled growth of synapses would place enormous demands on the structural and axonal-transport mechanisms of afflicted cells.
Recent data on tauopathy have shown that the pathology can spread between regions. A mutated form of tau protein was expressed in the medial entorhinal cortex in mice, and caused the initial appearance of fibrillary tangles first in the axons of medial entorhinal neurons projecting to the dentate gyrus, and then in the cell bodies of medial entorhinal neurons [6,7]. The neurofibrillary tangle pathology then appeared subsequently in the hippocampal formation and in other associated cortical structures. The malignant synaptic growth hypothesis would attribute this spread of pathology to the fact that malignant synaptic growth in one region will cause enhanced overlap of network activity and induce malignant synaptic growth in other regions [9]. However, the authors of these recent articles attribute the effect to direct trans-synaptic transmission of the mutated tau protein. The trans-synaptic spread of mutated tau does not rule out a functional influence between regions, but the direct transmission of a mutated protein is not necessary for the hypothesis of malignant synaptic growth.
Pathological plasticity hypotheses
It is worth considering a class of hypotheses that are unified by their focus on plasticity itself as the root cause of Alzheimer’s disease. The first such account was presented by Ashford and Jarvik [47], but has been refreshed numerous times since [48–50]. Unlike the hypotheses described above that focus on individual molecular pathways in accounting for the development and progression of Alzheimer’s disease, pathological plasticity hypotheses take a more high-level, systemic view of the disease. These hypotheses serve to unify the mechanisms of the molecular-level hypotheses into a unified framework by highlighting the roles that each of the physiological pathways that the individual molecules are involved in play in neuroplasticity.
The malignant synaptic growth hypothesis shares a great deal in common with other pathological plasticity hypotheses. Examples of alignment include: a common coherent framework for linking the otherwise disparate pathologies observed in Alzheimer’s disease, a clear rationale for why anatomical regions that have a high capacity for plasticity are also the most vulnerable to the disease, a shared focus on the feedback loops that accelerate the progression of the disease following its onset and a natural account for the numerous nonmonotonic trends that are observed in the progression of different biomarkers.
However, other pathological plasticity hypotheses differ from the malignant synaptic growth hypothesis in a several important ways. First, they differ with respect to how they suggest the deregulation of synaptic plasticity begins. The malignant synaptic growth hypothesis focuses extensively on initial excess in the amount of strengthening that occurs at existing connections, caused by the functional process of encoding memories. By contrast, other pathological plasticity hypotheses suggest that the imbalance in plasticity results from a loss of connectivity, which triggers a compensatory response in the increased formation of new connections. Second, they differ with respect to what is referred to by neuroplasticity. The malignant synaptic growth hypothesis primarily focuses on plasticity at the synapse and only secondarily to morphological changes in the associated neurons. By contrast, pathological plasticity hypotheses focus largely on morphological changes and the balance between the rate of new growth and the breakdown of individual neurons when they describe plasticity. Third, the hypotheses differ with respect to the purported importance of acetylcholine. In the malignant synaptic growth hypothesis, acetylcholine is responsible for maintaining the separation between encoding and retrieval and thus plays a pivotal role in maintaining homeostasis. By contrast, acetylcholine plays little to no specific role in other pathological plasticity models.
Multifactorial hypotheses
A central tenant of the position described here is that symptoms observed in Alzheimer’s patients may result from multiple pathological factors. This viewpoint has received growing attention. For example, a recent review by Mufson et al. provided a clear and extensive review of the supporting evidence for the idea that Alzheimer’s disease results from a wide range of dysfunctions [51]. While their specific viewpoint differs from ours, in that they focus primarily on the degenerative factors that correlate with cognitive decline and we view these processes as reactive to the initial phase of synaptic growth, their and our positions share the view that Alzheimer’s disease is a systemic dysfunction. This is a position, we expect, that will appear with greater frequency in the future. It will be important, moving forward, that such theories go beyond stating that Alzheimer’s disease must be multifactorial and provide a coherent framework for understanding the constellation of factors involved. This will improve the ease with which new empirical data can be readily identified as fitting naturally into a given framework or challenging it and thereby drawing attention to the aspects of the framework that need greater investigation. We have made an explicit attempt to accomplish this here by linking the various well-established factors into the framework of malignant synaptic growth and the reactive downregulation of synaptic maintenance. Likewise, the next section highlights data points that support the malignant synaptic growth framework.
Empirical support for the hypothesis
The malignant synaptic growth hypothesis is consistent with much of the existing knowledge surrounding the physiological and behavioral pathology observed in Alzheimer’s disease. In this section, we will draw explicit links between characteristics of the simulations and empirical data. Examples and the correspondence in the model include the following.
Synapses with a strong propensity for synaptic strengthening will be the most susceptible to the induction of malignant synaptic growth. Thus, the model can account for the initial appearance of neurofibrillary tangles in the entorhinal cortex, which gives rise to the perforant path projection to synapses in the dentate gyrus [52], which show such prominent LTP that this is where LTP was first discovered in physiological studies [53]. Notably, the neurofibrillary tangles first appear in the lateral entorhinal cortex, which gives rise to broadly divergent inputs to the outer molecular layer of the dentate gyrus. Malignant synaptic growth at broadly divergent connections might put particularly strong demands on axonal transport and thereby enhance sensitivity to tangle formation. In contrast to the middle molecular, the outer molecular does not show the cholinergic presynaptic inhibition of synaptic transmission that has been shown to prevent malignant synaptic growth in simulations [54]. Notably, the deep layers of lateral entorhinal cortex show a more widely divergent pattern of projections to neocortex than any other area within the entorhinal cortex, which could contribute to the appearance of tangles in this region [55].
The excessive growth of excitatory synaptic connections will cause progressive increases in the neural activity within cortical regions. Consistent with this, mild cognitive impairment has been shown to be associated with increased activity within the hippocampal formation [56], and familial Alzheimer’s disease is associated with increased hippocampal activity in prodromal patients that carry the mutation [57]. In addition, Alzheimer’s disease, in advanced stages, is an identified risk factor for late-onset epilepsy [58]. The increase in excitatory drive could contribute to cell death in these regions via excitotoxicity.
Computational modeling of malignant synaptic growth shows that this effect can spread between interconnected associative memory models [9]. The feedback process spreads like fire between branches of a forest, progressively inducing similar positive-feedback mechanisms in previously inert circuits. This provides a framework for understanding the selective transmission of neurofibrillary tangle pathology from lateral entorhinal cortex into the connected regions at the border of region CA1 and the subiculum and subsequently along back-projections from the entorhinal cortex into other neocortical regions [5].
As noted above, cholinergic presynaptic inhibition of glutamatergic transmission can prevent malignant synaptic growth, or slow its progression by preventing retrieval mediated by synaptic transmission from enhancing subsequent synaptic modification. This suggests that feedback mechanisms might upregulate cholinergic modulation in response to excessive synaptic growth. This is supported by evidence showing that cholinergic innervation during Alzheimer’s disease initially increases in areas such as the molecular layer of the dentate gyrus and the neocortex [32,33]. Subsequently, this process might put excessively high demands on cholinergic modulation and result in the reduction of cholinergic innervation in cortical structures and a loss of basal forebrain neurons [59,60].
This framework also addresses features of the behavioral data on Alzheimer’s disease [9]. The greater susceptibility of highly modifiable synapses to malignant synaptic growth can explain the initial impairment of episodic memory function in Alzheimer’s disease, since this requires rapid associations of events with their spatial and temporal context. Subsequently, the spread of malignant synaptic growth will progress to affect consolidated episodic memories and more recent semantic representations [61,62]. The framework also suggests that the merging of mnemonic representations should result in greater proactive interference based on semantic relatedness [63,64], increased intrusions in some tasks [65], as well as a tendency toward hyperpriming effects that could result from excess connectivity at early stages of the disease [66]. This model provides a framework for linking the mechanisms of the disease at a circuit level to explicit cognitive manifestations of the breakdown in function.
The progressive spread of network dysfunction through the brain, as described in this framework, accounts for observed disruptions to cognitive functions such as language, motor skills and perception [67]. As the malignant synaptic growth begins to effect cortical areas of the brain, the information stored in these networks will begin to degrade, thereby reducing the efficacy of neural information processing in those areas. This occurs as neural representations merge and lose there ability to be distinctly activated without interference from other representations.
Empirical challenges for the hypothesis
There are also a number of empirical data points that the malignant synaptic growth hypothesis does not readily account for in its current form. Our goal in this section is to highlight these points and to provide potential avenues for reconciling these observations with the framework described here.
Early onset of synaptic loss
A long-standing characterization of Alzheimer’s disease is the pronounced reduction of synaptic densities observed throughout the brains of Alzheimer’s disease patients [68]. These reductions, observed through electron microscopy or through indirect measures such as concentrations of synaptic markers, are most pronounced in the hippocampus and frontal cortex but extend throughout the brain. Importantly, reductions in synaptic density correlate with premortem cognitive performance. Further, these reductions begin early and can be observed in patients with prodromal Alzheimer’s disease or mild cognitive impairment [69]. The observation of robust and widespread synaptic loss beginning early in the development of Alzheimer’s disease is a clear challenge for the malignant synaptic growth hypothesis.
Importantly, however, synaptic densities are also regularly found to change in a biphasic manner: first growing more dense in number before subsequently thinning. Supporting evidence comes from studies of synaptic markers synaptophysin, drebrin and PSD-95, as well as glutamatergic presynaptic bouton density in various brain regions in patients with mild Alzheimer’s disease or with mild cognitive impairment (for a related discussion see [68]). Similarly, functional MRI studies suggest that patients with prodromal Alzheimer’s disease exhibit increased levels of hippocampal activation relative to healthy controls and in contrast with the decreased level of hippocampal activation observed in patients with clinical Alzheimer’s disease [70]. Additional future modeling and empirical research will be required to better flesh out the alignment between the malignant synaptic growth hypothesis and observed changes in synaptic density.
Therapeutic effects of M1 agonists
Studies into the therapeutic effects of M1 cholinergic receptor agonists for Alzheimer’s disease patients have found promising results [71]. M1 agonists have been shown to generate improvements on a range of behavioral metrics including reductions in vocal outbursts, suspiciousness, delusions, agitation and hallucinations, as well as improvements in memory performance, selfcare, mood and instrumental activities of daily living [72]. Furthermore, a number of studies have found that M1 agonists reduce the levels of Aβ in cerebral spinal fluid in animal models. This is challenging for the malignant synaptic growth hypothesis because M1 receptor agonists have been shown to upregulate the induction of LTP. Thus, it is surprising that treatments that would otherwise be expected to upregulate synaptic growth would also be beneficial in the context of a model that suggests that Alzheimer’s disease results from excess synaptic strengthening.
Given the efficacy of the NMDA antagonist memantine in the treatment of Alzheimer’s disease (known to block the induction of LTP), it is unlikely that the therapeutic effects of M1 agonists are derived from their ability to upregulate LTP. Indeed, the signaling cascades activated by metabotropic M1 receptors, however, do more than upregulate LTP [73]. Most relevantly, the M1 receptors are also intimately tied into APP metabolism and tau phosphorylation. Thus, it is possible that the therapeutic effects of M1 agonists occur despite the propensity of these receptors to boost LTP induction. The malignant synaptic growth hypothesis would therefore suggest that the therapeutic effects of M1 agonists would be stronger if coadministered with NMDA receptor antagonists to offset the LTP boosting effects of M1 agonists.
Implications of the malignant synaptic growth hypothesis
Implications for treatment
The most straightforward implication of the malignant synaptic growth hypothesis is that treatments designed to slow the potentiation of synapses offer the most promise. There are many potential therapeutic targets that could serve this purpose including NMDA receptors. Indeed, the drug memantine, which acts as a partial antagonist for NMDA receptors, has been found to successfully slow the progression of the disease [74].
Second, the link between the malignant synaptic growth hypothesis and recent modeling work on the role of theta rhythms in regulating the dynamics of neural plasticity suggest that irregularities in neural rhythms may serve as effective biomarkers for Alzheimer’s disease. Irregular rhythms may be particularly effective because they would be detectable with relatively low-cost, noninvasive techniques and should be detectable from early-stage disease onset. However, additional research to validate this notion is required.
Many treatments for Alzheimer’s disease have focused on restoring basal cholinergic levels through blockade of central acetylcholinesterase [1,2], but these have been met with only moderate success. The malignant synaptic growth hypothesis provides a unique perspective on why cholinergic treatments may yield modest or inconsistent results. Because acetylcholine plays a crucial role in maintaining the separation of encoding and retrieval, and thus serves to limit positive feedback loops in synaptic strengthening, cholinergic tone is crucial to healthy function in this hypothesis. However, once the destabilization has occurred, and the synaptic modification has begun to grow uncontrollably, restoration or even augmentation of the cholinergic tone is likely to be insufficient to stop or reverse the consequences of this growth.
By way of analogy, the relationship between acetylcholine and Alzheimer’s disease may be thought of like a rock wedged below a snowball positioned at the top of a steep hill. The rock serves to prevent a stationary snowball from rolling down the hill, but is of limited utility for stopping the snowball from rolling and growing once it has begun its descent. Even if the snowball is brought to rest again, the rock may be of limited use in preventing it from beginning to roll again once it has grown beyond a certain point. In the context of developing therapies, this suggests that therapies targeted at restoring cholinergic tone may serve to slow the rate at which the disease progresses but may not address the root cause of the destabilization.
Beyond having limited utility in restoring function, it is possible that cholinergic-based therapies may also have counter-productive effects on the treatment of Alzheimer’s disease. While the dampening of transmission strength in intrinsic projections by increased cholinergic tone may limit the spread of activation, the simultaneously reduced threshold for the induction of LTP may lead to further strengthening of hyperpotentiated synapses. Pharmacological agents targeted at specific muscarinic receptors subtypes [75] may provide more selective benefits of acetylcholine by activating the M4 subtype of muscarinic receptors that could reduce retrieval by causing selective presynaptic inhibition of glutamatergic transmission [76,77] without activating postsynaptic M1 receptors [78] that enhance postsynaptic depolarization and enhance long-term synaptic modification [79–80].
Lines of therapy that target the production and clearance of Aβ for slowing the progression of Alzheimer’s disease are not well motivated by the malignant synaptic growth hypothesis. To understand why, it is important to highlight the potential neuroprotective properties that Aβ may have in the context of this hypothesis. Synthetic Aβ application to tissue slices or cultures have shown that it reduces surface expression of NMDA-type glutamate receptors, inhibits LTP and reduces dendritic spine density [38–40,81]. Each of these factors limit the excitation of, and plasticity in, affected cells. Thus, the influences of Aβ may provide relief for neurons that are overtaxed by malignant synaptic growth. On the other hand, Aβ is also a potent neurotoxin [82]. An analogy for this costly form of neuroprotection can be found in cancer, where chemotherapy itself is toxic and harmful to the body, but serves to combat the growth of a malignant tumor. However, it is important to highlight that we are not suggesting that there is no benefit from blocking the production, and thus toxic effects, of aβ. Instead, we are suggesting that while blocking its production may prevent some neurotoxicity, doing so may also prevent the slowing of the malignant growth of synapses, and therefore may not reduce the incidence of excitotoxicity. Importantly, the fact that mutations are a causative factor for the familial form of Alzheimer’s disease might not reflect the neurotoxic effects of aβ, but might instead reflect a weaker neuroprotective effect of Aβ42 in contrast with Aβ40, so that mutations that cause greater levels of Aβ42 may provide less neuroprotective control of synaptic modification.
Implications for future empirical research
The malignant synaptic growth hypothesis predicts that an increased incidence of LTP should precede the appearance of other pathological markers of Alzheimer’s disease. Empirical testing of this hypothesis is challenged by the lack of an adequate animal model for Alzheimer’s disease. At present, mouse models exist for both the tauopathy model and the β-amyloid model of Alzheimer’s disease, however, neither satisfactorily serves as a complete model. Despite this, the tauopathy mouse model supports the malignant synaptic growth hypothesis, in that, prior to pathogenesis, these animals express increased LTP and rapid learning [83]. In mouse models used to study the β-amyloid hypothesis, however, LTP has been repeatedly shown to be downregulated [48,84,85]. Another approach to address this question, however, may be to test if manipulations shown to boost LTP induction also increase the risk for Alzheimer’s disease-like pathologies. For example, Tang et al. derived a transgenic mouse that overexpressed NMDA receptors and was found to exhibit facilitated LTP and increased memory performance [86]. It would be expected that this mouse would exhibit Alzheimer’s disease like pathologies with a greater likelihood than wild-type mice, or that the rate of cognitive decline should be accelerated if crossed with another mouse model of Alzheimer’s disease.
The theoretical links between Alzheimer’s disease onset and changes to neural rhythms, such as theta, motivate additional investigation into their relationship in patient populations. Initial work on this domain is promising, showing that theta amplitude is significantly increased in early stages of Alzheimer’s disease onset [87]. However, additional work is required to understand the progression of these effects over the course of the disease and to understand the mechanisms underlying their generation.
Conclusion
Decades of research have elucidated individual molecular pathways involved in the progression of Alzheimer’s disease, but we remain without an effective treatment for the disease. The focus on specific molecular pathways may be too restricted. The individual pathways that have taken center stage in mainstream Alzheimer’s disease research (regulation of acetylcholine, phosphorylation of tau and processing of APP) are all components of the neural machinery that supports plasticity in synapses. Accordingly, we suggest that Alzheimer’s disease results from dysregulation of this machinery. Initially proposed almost two decades ago, this hypothesis remains a viable model of the functional and biophysical mechanisms underlying the development and progression of Alzheimer’s disease. Much of the empirical work done in the intervening decades bolsters this viewpoint and serves to motivate a broadening of the view of the disease.
Future perspective
The next decade of research on Alzheimer’s disease will undoubtedly yield countless invaluable insights at many levels but, we believe, will also see a paradigm shift in the mainstream focus of Alzheimer’s disease researchers. While many of the insights to come will remain focused on individual pathways, we also expect to see growing interest in perspectives that take a more systems-level approach to understanding Alzheimer’s disease. At present, there is great excitement within the field to see the birth of treatments that target the aberrant processing of APP. If expectations are not met come the maturation of these treatments, the field will be forced to take a step back to re-evaluate the facts on the table. At that point, we believe, the links between the individual molecular pathways that have held the focus of the field will begin to emerge and attention to neural plasticity mechanisms will grow rapidly.
Executive summary.
The malignant synaptic growth hypothesis
Previous hypotheses of Alzheimer’s disease suggest that cellular and molecular mechanisms (i.e., loss of synaptic proteins, β-amyloid plaques and neurofibrillary tangles) underlie cognitive decline.
The malignant synaptic growth hypothesis suggests a functional cause in Alzheimer’s disease described by an imbalance of synaptic strengthening. The increased demands placed on cortical circuits then triggers plaque and tangle pathologies.
Malignant synaptic growth is the result of overlap between encoding and retrieval processes. When a memory is retrieved in the context of new encoding, faulty associations are made between the two. The more this process occurs, the number, strength and spread of synapses become greater, serving to accelerate the progression of the pathologies.
In healthy individuals, acetylcholine prevents malignant synaptic growth by providing presynaptic inhibition to glutamatergic input of intrinsic synapses, while increasing long-term potentiation, thereby separating encoding and retrieval processes.
Relevance to recent neural network models
Recent computational simulations demonstrate that rhythmic oscillations may play an important role in separating new encoding from retrieval of previously stored memories and for maintaining homeostasis in synaptic plasticity mechanisms.
Relationship to other hypotheses
Other hypotheses regarding the pathogenesis of Alzheimer’s disease focus on individual cellular and molecular pathways. While accurate, these hypotheses are likely to be overly focused.
Each of the mechanisms of focus in these other hypotheses also play roles in mechanisms of neural plasticity. As a result these mechanisms would be expected to suffer as a result of being overtaxed in the course of malignant synaptic growth.
-
These pathways include:
Acetylcholine;
Tau phosphorylation;
Amyloid precursor protein processing.
The malignant synaptic growth hypothesis is intimately related to a class of theoretical models of Alzheimer’s disease that focus on neural plasticity mechanisms. Although similar in many respects, the malignant synaptic growth hypothesis differs in its focus on acetylcholine for maintaining healthy function and in its assumption that pathogenesis begins with unchecked strengthening of synapses.
Empirical support for the hypothesis
Many empirical observations about the neural and behavioral indicators of the progression of Alzheimer’s disease are consistent with this model.
-
Points of correspondence include:
Anatomical specificity of the initiation and progression of tangle formation and neural degeneration;
The progression of cognitive decline from deficits in episodic memory to semantic representations;
Nonmonotonic changes in cholinergic activity.
Empirical challenges for the hypothesis
Additional computational and empirical research is required to reconcile several empirical data points with the malignant synaptic growth hypothesis.
-
These empirical points and the avenue by which they may be reconciled include:
Substantial synaptic loss in early Alzheimer’s disease challenge our malignant growth hypothesis; however, a phase of increased synaptic densities has also been observed, suggesting a biphasic progression;
M1 agonists have been shown to have therapeutic effects; however, these may occur despite the tendency of M1 agonists to boost long-term potentiation induction.
Implications of the malignant synaptic growth hypothesis
The malignant synaptic growth hypothesis suggests that treatment of Alzheimer’s disease should focus on restoring the separation between encoding and retrieval and slowing mechanisms of neural plasticity.
This could be achieved by targeting the M4 subtype of muscarinic receptors to reduce retrieval by presynaptic inhibition of glutamatergic transmission.
Treatments focused on β amyloid are likely to yield inconsistent results as β amyloid may serve as a neural protective factor.
Additional research into the relationship between synaptic plasticity, as understood from a functional stand-point, theta rhythms and Alzheimer’s disease are merited given their predicted intimate relationship under this hypothesis.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by National Institute of Mental Health F32 MH090671, R01 MH60013, R01 MH61492 and the Office of Naval Research MURI award N00014-10-1-0936. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Bottini G, Berlingeri M, Basilico S, et al. GOOD or BAD responder? Behavioural and neuroanatomical markers of clinical response to donepezil in dementia. Behav Neurol. 2012;25(2):61–72. doi: 10.3233/BEN-2011-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 3.Goate A, Hardy J. Twenty years of Alzheimer’s disease-causing mutations. J Neurochem. 2012;120(Suppl 1):S3–S8. doi: 10.1111/j.1471-4159.2011.07575.x. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2006;9(Suppl 3):S35–S44. doi: 10.3233/jad-2006-9s305. [DOI] [PubMed] [Google Scholar]
- 6▪▪.de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–697. doi: 10.1016/j.neuron.2011.11.033. Shows spreading of human tau from where it was expressed in the mouse medial entorhinal cortex to other cortical regions. This progression is well aligned to that characterized by the Braak staging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. This article also demonstrates the spread of tau pathology between regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasselmo ME. Runaway synaptic modification in models of cortex: implications for Alzheimer’s disease. Neural Networks. 1994;7:13–40. [Google Scholar]
- 9.Hasselmo ME. A computational model of the progression of Alzheimer’s disease. MD Comput. 1997;14(3):181–191. [PubMed] [Google Scholar]
- 10.Norman KA, Newman EL, Detre G. A neural network model of retrieval-induced forgetting. Psychol Rev. 2007;114(4):887–953. doi: 10.1037/0033-295X.114.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein-Messica A, Ruppin E. Synaptic runaway in associative networks and the pathogenesis of schizophrenia. Neural Comput. 1998;10(2):451–465. doi: 10.1162/089976698300017836. [DOI] [PubMed] [Google Scholar]
- 12.Norman KA, Newman E, Detre G, Polyn S. How inhibitory oscillations can train neural networks and punish competitors. Neural Comput. 2006;18(7):1577–1610. doi: 10.1162/neco.2006.18.7.1577. [DOI] [PubMed] [Google Scholar]
- 13.Kohonen T. Self-Organization and Associative Memory. Springer-Verlag; Berlin, Germany: 1984. [Google Scholar]
- 14.Hopfield JJ. Neurons with graded response have collective computational properties like those of two-state neurons. Proc Natl Acad Sci USA. 1984;81(10):3088–3092. doi: 10.1073/pnas.81.10.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16(6):218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- 16.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman EL, Gupta K, Climer JR, Monaghan C, Tsuno Y, Hasselmo ME. Modeling the cholinergic modulation of cortical networks. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanton PK, Sejnowski TJ. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- 19.Grossberg S. Adaptive pattern classification and universal recoding: II. Feedback, expectation, olfaction, illusions. Biol Cybern. 1976;23(4):187–202. doi: 10.1007/BF00340335. [DOI] [PubMed] [Google Scholar]
- 20.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14(4):793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 21.Green JD, Arduini AA. Hippocampal electrical activity and arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 22.Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399(6738):781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- 23.Vinogradova OS. Expression, control, and probable functional significance of the neuronal theta-rhythm. Prog Neurobiol. 1995;45(6):523–583. doi: 10.1016/0301-0082(94)00051-i. [DOI] [PubMed] [Google Scholar]
- 24.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 25.Brankack J, Stewart M, Fox SE. Current source density analysis of the hippocampal theta rhythm: associated sustained potentials and candidate synaptic generators. Brain Res. 1993;615(2):310–327. doi: 10.1016/0006-8993(93)90043-m. [DOI] [PubMed] [Google Scholar]
- 26.Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8(3):244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23(37):11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman EL, Norman KA. Moderate excitation leads to weakening of perceptual representations. Cereb Cortex. 2010;20(11):2760–2770. doi: 10.1093/cercor/bhq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the complementary learning systems model: oscillating inhibition and autonomous memory rehearsal. Neural Netw. 2005;18(9):1212–1228. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Contestabile A. The history of the cholinergic hypothesis. Behav Brain Res. 2011;221(2):334–340. doi: 10.1016/j.bbr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 31.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 32.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51(2):145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 33.Geddes JW, Monaghan DT, Cotman CW, Loh IT, Kim RC, Chui HC. Plasticity of hippocampal circuitry in Alzheimer’s disease. Science. 1985;230:1170–1181. doi: 10.1126/science.4071042. [DOI] [PubMed] [Google Scholar]
- 34.Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8(11):1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selkoe DJ. Amyloid protein and Alzheimer’s disease. Sci Am. 1991;265(5):40–47. doi: 10.1038/scientificamerican1191-68. [DOI] [PubMed] [Google Scholar]
- 36.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 37.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17(9):1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 38.Shrestha BR, Vitolo OV, Joshi P, Lordkipanidze T, Shelanski M, Dunaevsky A. Amyloid β peptide adversely affects spine number and motility in hippocampal neurons. Mol Cell Neurosci. 2006;33(3):274–282. doi: 10.1016/j.mcn.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 40.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70(1):1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 42.Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimer’s Dis. 2010;19(1):221–228. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 44.Spires-Jones TL, Kopeikina KJ, Koffie RM, de Calignon A, Hyman BT. Are tangles as toxic as they look? J Mol Neurosci. 2011;45(3):438–444. doi: 10.1007/s12031-011-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer PL, Xu H, Woltjer RL, et al. Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiol Aging. 2011;32(12):2113–2122. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashford JW, Jarvik L. Alzheimer’s disease: does neuron plasticity predispose to axonal neurofibrillary degeneration? N Engl J Med. 1985;313(6):388–389. [PubMed] [Google Scholar]
- 48.Teter B, Ashford JW. Neuroplasticity in Alzheimer’s disease. J Neurosci Res. 2002;70(3):402–437. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- 49▪▪.Mesulam MM. A plasticity-based theory of the pathogenesis of Alzheimer’s disease. Ann NY Acad Sci. 2000;924:42–52. doi: 10.1111/j.1749-6632.2000.tb05559.x. Reviews the effects of memantine on Alzheimer’s disease, which suggests that the blockade of synaptic modification slows the progression of Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 50.Neill D. Alzheimer’s disease: maladaptive synaptoplasticity hypothesis. Neurodegeneration. 1995;4(2):217–232. doi: 10.1006/neur.1995.0027. [DOI] [PubMed] [Google Scholar]
- 51.Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathologica. 2012;123(1):13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29(12):671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Lomo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):617–620. doi: 10.1098/rstb.2002.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahle JS, Cotman CW. Carbachol depresses synaptic responses in the medial but not the lateral perforant path. Brain Res. 1989;482(1):159–163. doi: 10.1016/0006-8993(89)90554-4. [DOI] [PubMed] [Google Scholar]
- 55.Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7(2):146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 56.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57▪▪.Quiroz YT, Budson AE, Celone K, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann Neurol. 2011;68(6):865–875. doi: 10.1002/ana.22105. Shows hyperactivation of the hippocampus in presymptomatic carriers of Alzheimer’s disease mutations, supporting the concept of an initial phase of excessive excitatory synaptic growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharfman HE. Alzheimer’s disease and epilepsy: insight from animal models. Future Neurol. 2012;7(2):177–192. doi: 10.2217/fnl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikonomovic MD, Abrahamson EE, Isanski BA, Wuu J, Mufson EJ, DeKosky ST. Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch Neurol. 2007;64(9):1312–1317. doi: 10.1001/archneur.64.9.1312. [DOI] [PubMed] [Google Scholar]
- 60.Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD. Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr Alzheimer Res. 2007;4(4):340–350. doi: 10.2174/156720507781788855. [DOI] [PubMed] [Google Scholar]
- 61.Huff FJ, Corkin S, Growdon JH. Semantic impairment and anomia in Alzheimer’s disease. Brain Lang. 1986;34:269–278. doi: 10.1016/0093-934x(86)90103-3. [DOI] [PubMed] [Google Scholar]
- 62.Di Giacomo D, De Federicis LS, Pistelli M, et al. The loss of conceptual associations in mild Alzheimer’s dementia. J Clin Exp Neuropsychol. 2012;34(6):643–653. doi: 10.1080/13803395.2012.667393. [DOI] [PubMed] [Google Scholar]
- 63.Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. J Int Neuropsychol Soc. 2004;10(1):91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- 64▪.Loewenstein DA, Acevedo A, Agron J, Duara R. Vulnerability to proactive semantic interference and progression to dementia among older adults with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;24(5):363–368. doi: 10.1159/000109151. Shows the hyperpriming effects that would be expected if there were merging of semantic representations due to runaway synaptic modification. [DOI] [PubMed] [Google Scholar]
- 65.Fuld PA, Katzman R, Davies P, Terry RD. Intrusions as a sign of Alzheimer dementia: chemical and pathological verification. Ann Neurol. 1982;11:155–159. doi: 10.1002/ana.410110208. [DOI] [PubMed] [Google Scholar]
- 66.Giffard B, Desgranges B, Eustache F. Semantic memory disorders in Alzheimer’s disease: clues from semantic priming effects. Curr Alzheimer Res. 2005;2(4):425–434. doi: 10.2174/156720505774330582. [DOI] [PubMed] [Google Scholar]
- 67.Hasselmo ME. Runaway synaptic modification in models of cortex: implications for Alzheimer’s disease. Neural Networks. 1994;7(1):13–40. [Google Scholar]
- 68.Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathologica. 2009;118(1):167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 69.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann NY Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- 71.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodick NC, Offen WW, Levey, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 73.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s disease – the pivotal role of brain M1 receptors. Neurodegener Dis. 2008;5(3–4):237–240. doi: 10.1159/000113712. [DOI] [PubMed] [Google Scholar]
- 74.Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer’s disease. Drugs. 2006;66(11):1515–1534. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- 75.Kennedy JP, Bridges TM, Gentry PR, et al. Synthesis and structure-activity relationships of allosteric potentiators of the m(4) muscarinic acetylcholine receptor. Chem Med Chem. 2009;4(10):1600–1607. doi: 10.1002/cmdc.200900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shirey JK, Xiang Z, Orton D, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol. 2008;4(1):42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- 77.Dasari S, Gulledge AT. M1 and M4 receptors modulate hippocampal pyramidal neurons. J Neurophysiol. 2011;105(2):779–792. doi: 10.1152/jn.00686.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doralp S, Leung LS. Cholinergic modulation of hippocampal CA1 basal-dendritic long-term potentiation. Neurobiol Learn Mem. 2008;90(2):382–388. doi: 10.1016/j.nlm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Ovsepian SV, Anwyl R, Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci. 2004;20(5):1267–1275. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- 80.Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25(48):11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Almeida CG, Tampellini D, Takahashi RH, et al. β-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Wang HY, Lee DH, D’Andrea MR, et al. β-amyloid(1–42) binds to α7 nicotinic acetylcholine receptor with high affinity Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 83▪.Boekhoorn K, Terwel D, Biemans B, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26(13):3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. Shows enhanced long-term potentiation in mice with tau mutation suggesting a link between tau mutations and a possible trigger of runaway synaptic modification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor CJ, Ireland DR, Ballagh I, et al. Endogenous secreted amyloid precursor protein-α regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008;31(2):250–260. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Poirier R, Veltman I, Pflimlin MC, Knoflach F, Metzger F. Enhanced dentate gyrus synaptic plasticity but reduced neurogenesis in a mouse model of amyloidosis. Neurobiol Dis. 2010;40(2):386–393. doi: 10.1016/j.nbd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Tang YP, Shimizu E, Dube GR, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 87▪.Fonseca LC, Tedrus GMAS, Fondello MA, Reis IN, Fontoura DS. EEG theta and alpha reactivity on opening the eyes in the diagnosis of Alzheimer’s disease. Clin EEG Neurosci. 2011;42(3):185–189. doi: 10.1177/155005941104200308. Shows enhanced theta rhythm magnitude in Alzheimer’s disease, supporting the hypothesis that irregularities in neural rhythms, such as theta, may be linked to pathogenesis or may be used as low-cost noninvasive early detectable biomarkers. [DOI] [PubMed] [Google Scholar]