Abstract
It has been shown that inhaled particulate matter such as air pollution and asbestos are linked to a number of immune diseases such as asthma, and Systemic Lupus Erythematosus (SLE), respectively. This research may contribute to understanding the mechanisms of how asbestos and air pollution particulate (PM10) produce oxidative stress on macrophages, as well as how the macrophages will respond to the oxidative stressors. Using Flow Cytometry, DCFDA Fluorescence, Glutamate Transport, and Cytokine Bead Array assays, we have shown that exposure to asbestos and PM10 up-regulates system xc- in macrophages, which reduces oxidative stress for the macrophage by providing substrates for antioxidants. The results demonstrate that asbestos, but not PM10, induces both expression and activity of system xc-. This led to differential cytokine production with a significantly increased expression of TNF alpha and MCP-1 for PM10 treatment but not for asbestos treatment. Inhibition of system xc- affected cytokine production only following asbestos exposure, further demonstrating a role for this antioxidant system in regulating immune outcomes for asbestos but not PM10. Understanding the mechanisms in which oxidative stress helps regulate macrophage responses may contribute to a better understanding of why certain diseases are brought on by asbestos and air pollution exposure.
INTRODUCTION
Systemic autoimmune diseases (SAID) are complex, chronic, hard to treat, and devastating to patients. Inhalation of particulates such as asbestos or silica was shown to increase the risk of SAID such as systemic lupus (Pfau et al. 2005; Noonan et al. 2006), but the mechanism is not well understood. Diseases such as autoimmunity develop when the immune system starts attacking self-tissues, which can be the result of overzealous signals such as cytokines. These damaging cytokines come from the macrophage, and while some research is being done on trying to understand the mechanisms behind asbestos related diseases, it is not clearly understood how air pollutants such as PM10 affects the macrophage's signal to the rest of the immune system. It is known that asbestos causes oxidative stress and is associated with autoimmunity (Blake et al. 2007;Pfau et al. 2005), and interestingly, PM10 causes oxidative stress as well but leads to a completely different disease outcome (MacNee & Donaldson 2003), and so it was hypothesized by the authors of this paper that the way in which macrophages handle the oxidative stress may play a role in the different disease outcomes.
The immune system is made up of specialized cells that work in specific ways, but as a whole, can produce a very effective response after being exposed to certain pathogens. Since macrophages are the primary immune cells found in the lung alveoli, they are often the first cell type present when it comes to producing an immune response to inhaled pathogens.
Macrophages can impact an immune response by secreting chemical messengers or cytokines to other cells in the body. Macrophage cytokine responses can be influenced by certain particles like asbestos and PM10 (particulate matter that are ≤ 10 microns in size) and by the amount of oxidative stress that they exert on the cell (MacNee & Donaldson 2003, Blake et al. 2007).
When a macrophage has been stressed via oxidation, the amount of reactive oxygen species (ROS) in the cytoplasm has been shown to be regulated by a specific amino acid transporter called xc- (Bannai et al. 1989). The transporter is a heterodimer consisting of the active subunit, xCT, and a stabilizing subunit called CD98. This transporter reduces ROS by exchanging intracellular glutamate for extracellular cystine. The cystine that is imported into the cell can then be converted to cysteine or glutathione, which are cellular antioxidants (Lo et al. 2008).
Both PM10 and asbestos particles generate ROS inside of the macrophage (Schneider et al. 2005, Blake et al 2007), which can lead to a specific cytokine response by the macrophage. These cytokines can influence the immune system to produce a TH1 or TH2 response. Amphibole asbestos has been linked with a combined TH1/TH2 response (Shah et al. 2010), while PM10 has been linked with a TH2 response (Hamilton et al. 2004). Understanding the mechanisms behind how asbestos and PM10 influence the body's immune system is important because disruption in the TH1/TH2 balance may result in disease. (Shah et al. 2010).
Several cytokines are released from macrophages in response to oxidative stressors, including MCP-1 and TNF-alpha. MCP-1 (Monocyte Chemotactic Protein-1) is one of the cytokines produced by macrophages, which encourages the immune system to produce a TH2 response (Rose et al. 2003; Deshmane et al. 2009). As the name suggests, MCP-1 is a protein that attracts other monocytes to the area. Macrophages under conditions of oxidative stress as a result of exposure to PM10 and asbestos secrete MCP-1 in order to attract other macrophages to that particular area to help clear debris. MCP-1 is an important primary response cytokine in cells under oxidative stress and is implicated as an important mediator in several inflammatory pathologies, including allergies and asthma (Holla et al. 2008; Rose et al. 2003). Another macrophage cytokine, TNF-alpha, is released in response to asbestos and plays a role in asbestos-related diseases including cancers and fibrosis (Helmig et al. 2010). However, it is released in response to many stressors, including particulate matter (Brown et al. 2004). Based on the information above, it was hypothesized that system xc- (xCT) plays a role in regulating differential cytokine production by macrophages stimulated via oxidative stress with either PM10 or amphibole asbestos, and might therefore be a target for therapeutic approaches in diseases related to these exposures. This research may be crucial to help understand how oxidative stress can affect the human body.
MATERIALS AND METHODS
Cell Culture
RAW 264.7 macrophages (ATCC, Manassas, VA) were cultured in RPMI with 10% fetal bovine serum and penicillin/streptomycin (100 units/mL penicillin, 100 ug/mL Streptomycin) at 37°C in a humidified 5% CO2 incubator (ThermoScientific). Cells were cultured for 48 hours and then scraped up and brought up to confluence (one million cells/milliliter) and transferred into two 12-well plates (one plate with the inhibitor present and one plate without the inhibitor) at two milliliters of RPMI per well. The inhibitor used was (S)-4-CPG ((S)-4-carboxyphenylglycine at 500 μM concentration (Tocris Scientific), which is a non-substrate inhibitor that has been shown to block xCT via blocking cystine/glutamate exchange by 97% when used at 500 μM (Patel et al. 2004). The inhibitor was allowed to react with cells 20-30 minutes before cells were exposed to challenge materials. Cells were challenged with amphibole asbestos called 6-Mix (from Libby, MT, courtesy: US Geological Survey) and PM10 (Urban dust PM1648. National Institutes of Standards & Technology) at 35μg/cm2 after blocking of xCT took place.
Expression of xCT and CD98
Cells were exposed to challenge material for 24 hours at specified dose, and then stained with a primary anti-xCT antibody(ABcam) and anti-CD98 (Becton Dickinson Biosciences), and then stained with secondary FITC and phycoerythrin conjugate antibodies, respectively for flow cytometry. Flow Cytometry was performed using a Becton Dickinson Biosciences FACS Calibur with CellQuest software, gating on live cells and setting the cut-off for negative staining based on staining with secondary antibody only.
Measurement of Cytokine Production
To determine whether blocking xCT affects the TH1 and TH2 response initiated by asbestos & PM10 respectively, the cytokine response was measured using flow cytometry. This was done by using a Mouse Inflammation Cytokine Cytometric Bead Array Kit from Becton Dickinson Biosciences that measures MCP-1, TNF-alpha, IL-6, IL-10, IL-12 and interferon gamma. Supernatants from the cultured cells following asbestos and PM10 treatment were incubated with the capture beads as described in the kit protocol, then washed and analyzed using the FACS Calibur.
Measurement of Oxidative Stress with Discholorflurescein diacetate (DCFDA)
This assay measures the oxidative stress of cells throughout a time course. Macrophages were cultured in RPMI with 10% fetal bovine serum and penicillin/streptomycin prior to assay and brought up to confluence (1 million cells/mL). A 96 well clear bottom plate with opaque sides was used, utilizing wells A1 through H6. Macrophages were plated so that wells A2 through H6 contained 100μL of the culture media/macrophage mixture (100,000 cells/well). In column 1 there were no macrophages plated, so this column of wells only contained PBS. Once the cells were plated, they were allowed to adhere for overnight in the incubator. The next day, the media was removed. In column 1, the PBS was removed and replaced with 10μM DCFDA dye to determine background. In column 2, the media was replaced with PBS only and no DCFDA dye, for determining autofluoresence of the macrophages. In column 3, the media was replaced with 3% H2O2 as a positive control for oxidative stress. After this, 10μM DCFDA dye was added. In columns 4-6, the media was also replaced with the 10μM DCFDA dye. After this, the plate was placed in a 37°C, 5% CO2 incubator for 1 hour so that the dye could load into the cells. After the dye was properly loaded into the cells, it was then removed from each well that contained it, and washed gently two times with warm PBS. After the wash, challenge suspensions were added. In column 4, 100μL of PBS was added to each well. Column 4 served as a no treatment control. In column 5, 100 μL of a concentration of PM10 to give 35μg/cm2 was added to each well. In Column 6, 100 μL of a concentration of 6 mix asbestos to give 35μg/cm2 was added to each well. In columns 1-3, PBS only was added to each well. After each challenge material was added, the plate was then returned back the incubator for 1-2 hours. After this incubation period, the plate was read with a BioTek MicroTiter plate reader at 520nm.
Measurement of Glutamate Transport
Glutamate transported out of the cells was measured using the Amplex Red™ assay (Invitrogen), and quantified using a standard curve. Glutamate standards were included in the Amplex Red™ assay, which allowed for Glutamate quantification. Briefly, macrophages were challenged with PM10 or 6-Mix for 24 hr and then the media was collected and assayed according to the manufacturer's instructions. The amount of glutamate in RMPI media (135.9 μM) was subtracted from the totals, so that the data presented is only the amount transported by the cells.
Statistical Analysis
All experiments were replicated at least twice, and representative data is shown. Statistical analysis and graphing were done using Excel or Graphpad Prism software. Comparisons of means against control values were performed using an unpaired 2-tailed t-test. A p value of less than 0.05 was considered significant, which is consistent with other studies published in cell biology, immunology and immunotoxicology journals.
RESULTS
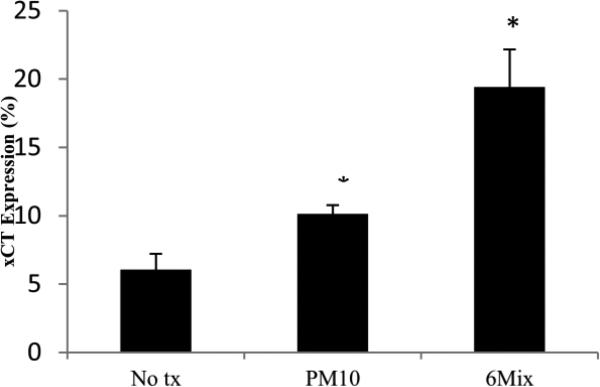
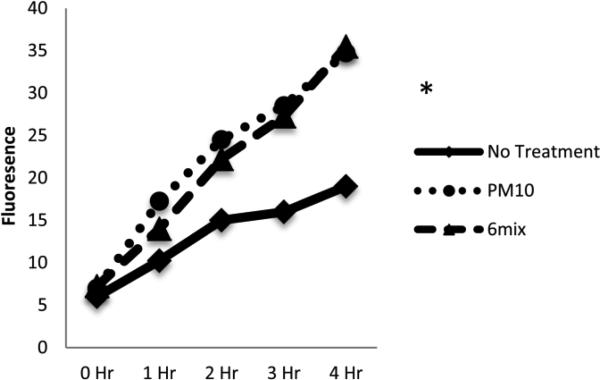
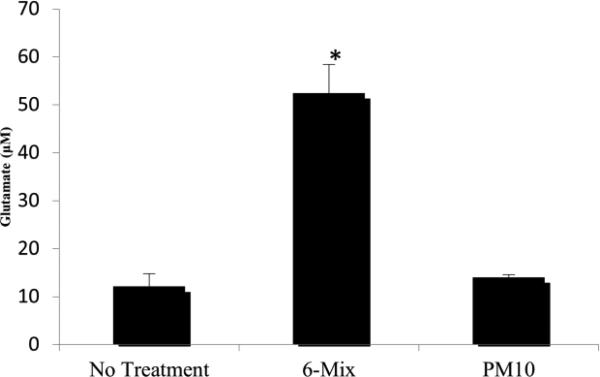
xCT expression on macrophages increased after challenge with both PM10 and asbestos. Both PM10 and asbestos elicited differences in the expression of xCT, as seen in Figure 1. All of the cells expressed high levels of CD98 even without treatment, suggesting that this molecule is constitutively present and available for dimerization with xCT (data not shown).Oxidative stress on a macrophage increases as it is challenged with both PM10 and asbestos. A significant difference was seen when the PM10-challenged group was compared to the control at the 2-hour time point. At the 3-hour time point a significant difference was also seen for asbestos when compared to the control (Figure 2). Despite the oxidative stress, the macrophages exposed to PM10 did not increase transport of L-glutamate. However, glutamate transport was significantly increased when the cells were treated with 6-Mix (Figure 3). These data suggests that the system xc- was activated by 6-Mix but not by PM10.
Figure 1.
xCT expression differences between cells with no treatment, cells treated with PM10, and cells treated with Libby 6-mix. *: p < .05
Figure 2.
Oxidative stress differences, as measured by DCFDA fluorescence, between cells with no treatment, cells treated with PM10, and cells treated with Libby 6-mix. *: p < .05 when compared to No Treatment, n= 4 in each group.
Figure 3.
Glutamate transport out of the cells measured by Amplex Red Assay* : p< 0.05 compared to No Treatment, n=4 in each group.
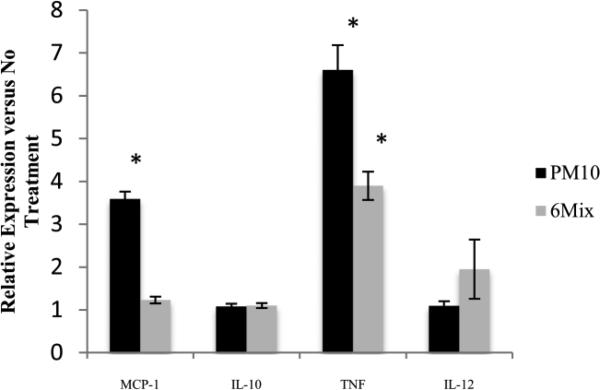
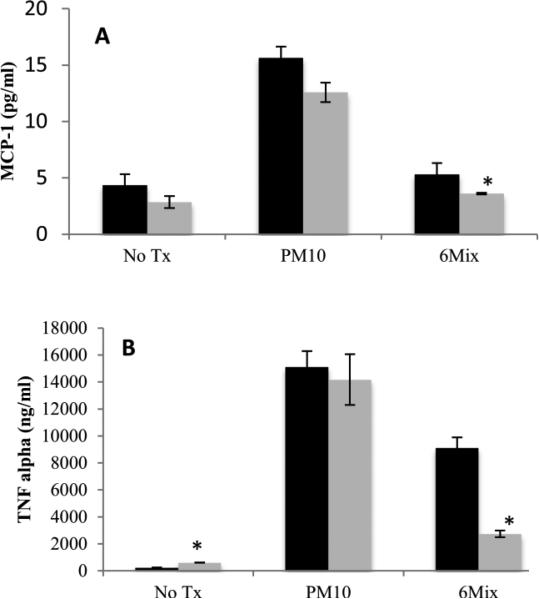
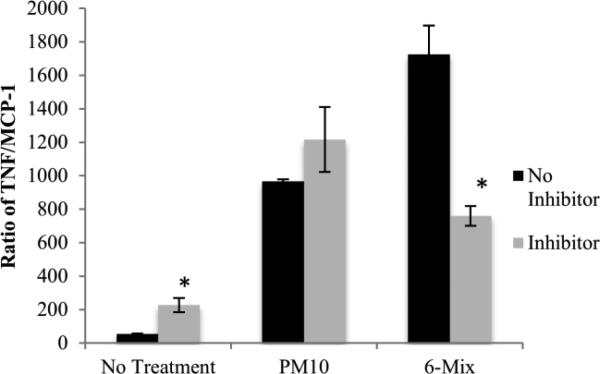
Macrophages produced a different pattern of cytokines in response to asbestos compared to PM10, with PM10 eliciting both TNF alpha and MCP-1, while 6-Mix only led to production of TNF alpha (Figure 4). There was no production of the other two cytokines in the kit, IL-6 and interferon gamma, in response to either challenge (Data not shown).To see if system xc- is important in determining macrophage cytokine production, the cells were treated with a system xc- inhibitor, (S)-4-CPG prior to challenge. Macrophages that were treated only with (S)-4-CPG had a non-significant decrease in MCP-1 concentration and a significant but low-level increase in TNF alpha, when compared to macrophages that had not been exposed to the inhibitor (Figure 5). Therefore, inhibitor alone had little effect on the production of these two cytokines. Consistent with the data showing that PM10 did not upregulate xCT, blocking the transporter with (S)-4-CPG had no effect on cytokine production elicited by PM10 (Figure 5 a & b). However, (S)-4-CPG significantly reduced both MCP-1 and TNF alpha from 6-Mix – treated cells. The inhibitor also reduced TNF alpha production in response to asbestos (but not PM10). This cytokine has been classified as a TH1 inducing cytokine (Helmig et al. 2010). This is shown in Figure 6, which illustrates the ratios between TNF and MCP-1 in each of the conditions. Treatment with (S)-4-CPG did not significantly alter the ratio for PM10, but the ratio for 6-mix was significantly reduced.
Figure 4.
Cytokine Bead Array measuring cytokines produced by treated cells, divided by values from No Treatment group. * : p< 0.05 compared to No Treatment for that cytokine.
Figure 5.
TNF alpha and MCP-1 measured from macrophages that were treated with (S)-4-CPG prior to challenge. Black bars represent cytokines produced in the absence of (S)-4-CPG, and gray bars are in the presence of (S)-4-CPG. *: p<0.05 compared to the same treatment without (S)-4-CPG, n=4 in each group.
Figure 6.
Ratios of TNF alpha to MCP-1 were calculated for each condition, to represent a relative TH1/TH2 ratio of these two cytokines. *: p< 0.05 comparing With CPG against No CPG in each pair of bars. n=4 in each group.
DISCUSSION
We have shown that RAW macrophages express a low level of xCT and high levels of CD98-the heterodimeric subunit of system xc- - constitutively. Upon stimulation, only asbestos led to a significant up-regulation of the expression of xCT. In addition, only asbestos led to an increase in the release of L-glutamate into the medium, suggesting that system xc- amino acid transport was not activated by PM10. System xc- is known to be the primary glutamate transporter on macrophages (Watanabe & Bannai. 1987) Therefore, it is unlikely that xCT plays a role in regulating cytokine release from macrophages stimulated with PM10. However, since both xCT expression and glutamate transport were increased following exposure to asbestos, it is likely that system xc- does play a role in regulating macrophage responses to asbestos. In order to further test that hypothesis, cytokine release was analyzed with and without an xCT inhibitor.
Under the conditions used in these experiments, the primary cytokines produced by the RAW macrophages were MCP-1 and TNF-alpha, but only the PM10 exposure induced expression of MCP-1. This is consistent with a predominantly TH2 response that is associated with PM10 exposure (Schneider et al. 2005). Because both 6-Mix and PM10 induced oxidative stress as measured by DCFDA, but only 6-Mix used system xc-to counteract that stress, it might be assumed that PM10-treated macrophages are in a more oxidized state over the 24 to 48 hour treatment period, possibly by other antioxidant systems being active. It has been shown that macrophages in an oxidized state tend to support a more TH2 response (Murata 2002), and our data appear to support that model. Therefore, we hypothesized that if system xc- were blocked, we would tend to see more MCP-1 production, even in the 6-Mix treated cells. In actuality, this was not the case: In fact, treatment with (S)-4-CPG decreased the production of MCP-1 from 6-Mix treated macrophages. In order to more clearly visualize what might be happening, we used these two cytokines to roughly represent a TH1-inducing cytokine (TNF alpha) and a TH2-inducing cytokine (MCP-1) and calculated their ratios (Figure 6).
While acknowledging that there are many other factors that could influence TH responses, our data show that 6-Mix induced a much more TH1 ratio than PM10, consistent with a more reduced intracellular environment that would occur due to cystine import. In addition, (S)-4-CPG pre-treatment did not affect PM10-induced cytokines significantly; consistent with the fact that PM10 did not induce upregulation of xCT.
It was hypothesized that when xCT was not available to help reduce ROS inside of the macrophage (i.e. xCT was blocked with an inhibitor), other antioxidant mechanisms would upregulate to aid in decreasing oxidative stress. From this knowledge, it can be deduced that macrophages treated with PM10 may be protecting themselves from cell death via oxidative stress through other anti-oxidant mechanisms other than system xc-. The most direct way that it would overcome this oxidative stress, would be by upregulating thrioredoxin production (Dai et al. 2008). Dai et al. has shown that homocysteine can create ROS in treated cells, which in turn, leads to MCP-1 production. As this happens, thioredoxin is upregulated to counteract the effects generated from homocysteine. This data can be used in future studies, to determine whether PM10 or 6-Mix induce expression of thioredoxin, especially in the absence of functional system xc-.
We have shown that while 6-Mix activates system xc- in macrophages, PM10 does not. This difference appears to cause differential cytokine responses. These data can help to explain how inhaled dusts drive certain pathologies brought on by amplifying immune responses to produce pathologies such as asthma or autoimmunity. As it was mentioned earlier, blocking system xc- does not work in terms of preventing asthma or diseases caused by an overzealous TH2 response. However, for diseases caused by an overactive TH1 response such as asbestos induced diseases, there is a clear implication that system xc- is active. This suggests that system xc- does play a role in TH1 diseases, and this knowledge could lead to future breakthroughs for treating TH1 diseases through the manipulation of system xc-.
ACKNOWLEDGEMENTS
This work was supported by an ISU Undergraduate Research Fellowship (JO), and NIH/NIEHS grant number R15 ES018986, and P20 RR016454 (INBRE supported Flow Cytometry Core Facility).
REFERENCES
- 1.Bannai S, Sato H, Ishii T, Sugita Y. Induction of cystine transport activity in human fibroblasts by oxygen. The Journal of Biological Chemistry. 1989;264(31):18480–18484. [PubMed] [Google Scholar]
- 2.Blake DJ, Bolin C, Cox D, Cardozo-Pelaez F, Pfau JC. Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxilogical Sciences. 2007;99(1):277–288. doi: 10.1093/toxsci/kfm166. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Donaldson K, Stone V. Effects of PM10 in human peripheral blood monocytes and J774 macrophages. Journal of Respitory Research. 2004;5:29. doi: 10.1186/1465-9921-5-29. doi: 10.1186/I465-9921-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Wang X, Feng J, Kong W, Xu Q, Shen X, Wang X. Regulatory role of thioredoxin in homocysteine-induced monocyte chemoattractant protein-1 in monocytes/macrophages. FEMS Letters. 2008;582:3893–3898. doi: 10.1016/j.febslet.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Deshmane SL, Kremlev S, Amini S, Sawaya B. Moncyte Chemoattractant Protein 1 (MCP-1): An Overview. Journal of Interferon & Cytokine Research. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. Doi:10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton RF, Holian A, Morandi MT. A comparison of asbestos and urban particulate matter in the vitro modification of human alveolar macrophage antigen-presenting cell function. Experimental Lung Research. 2004;30(2):147–62. doi: 10.1080/01902140490266439. [DOI] [PubMed] [Google Scholar]
- 7.Helmig S, Aliahmadi N, Schneider J. Tumor necrosis factor-alpha gene polymorphisms in asbestos-induced diseases. Biomarkers. 2010;15(5):400–9. doi: 10.3109/1354750X.2010.481365. [DOI] [PubMed] [Google Scholar]
- 8.Holla L, Mrazek F, Petrek M. MCP-1 and CCR2 gene polymorphisms in Czech patients with allergic disorders. International Journal of Immunogenetics. 2008;36(1):69–72. doi: 10.1111/j.1744-313X.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Lo M, Wang Y, Gout P. The Xc-cysteine/glutamate antiporter: a potential target for therapy of cancer and other diseases. Journal of Cellular Physiology. 2008;215:593–602. doi: 10.1002/jcp.21366. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Macnee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles special reference to COPD. European Respiratory Journal. 2003;21(Suppl. 40):47s–51s. doi: 10.1183/09031936.03.00403203. (2003) [DOI] [PubMed] [Google Scholar]
- 11.Murata Y, Shimamura T, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. International Journal of Immunology. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 12.Noonan C, Pfau J, Larson T, Spence M. Nested case-control study of autoimmune disease in an asbestos exposed population. Environmental Health Perspectives. 2006;114(8):1243–1247. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SA, Warren BA, Rhoderick JF, Bridges RJ. Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology. 2004;46:273–84. doi: 10.1016/j.neuropharm.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of Autoimmune Responses Associated with Asbestos Exposure in Libby MT. Environmental Health Perspectives. 2005;113(1):25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose CE, Sung-Sang, Sung J, Man Fu S. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–288. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- 16.Schneider J, Card G, Pfau JC, Holian A. Air pollution particulate SRM 1648 causes oxidative stress RAW 264.7 macrophages leading to production of prostaglandin E2, a potential Th2 mediator. Journal of Inhalation Toxicology. 2005;17:871–877. doi: 10.1080/08958370500244498. [DOI] [PubMed] [Google Scholar]
- 17.Shah D, Kiran R, Wanchu A, Bhatnagar A. Oxidative stress in systemic lupus erythematosus: Relationship to Th1 cytokine and disease activity. Immunology Letters. 2010 doi: 10.1016/j.imlet.2010.01.005. (2010), doi:10.1016/j.imlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages. Journal of Experimental Medicine. 1987;165(3):628–40. doi: 10.1084/jem.165.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]