Abstract
Study Objectives:
To characterize tongue and lateral upper airway movement and to image tongue deformation during mandibular advancement.
Design:
Dynamic imaging study of a wide range of apnea hypopnea index (AHI), body mass index (BMI) subjects.
Setting:
Not-for-profit research institute.
Participants:
30 subjects (aged 31-69 y, AHI 0-75 events/h, BMI 17-39 kg/m2).
Interventions:
Subjects were imaged using dynamic tagged magnetic resonance imaging during mandibular advancement. Tissue displacements were quantified with the harmonic phase technique.
Measurements and Results:
Mean mandibular advancement was 5.6 ± 1.8 mm (mean ± standard deviation). This produced movement through a connection from the ramus of the mandible to the pharyngeal lateral walls in all subjects. In the sagittal plane, 3 patterns of posterior tongue deformation were seen with mandibular advancement—(A) en bloc anterior movement, (B) anterior movement of the oropharyngeal region, and (C) minimal anterior movement. Subjects with lower AHI were more likely to have en bloc movement (P = 0.04) than minimal movement. Antero-posterior elongation of the tongue increased with AHI (R = 0.461, P = 0.01). Mean anterior displacements of the posterior nasopharyngeal and oropharyngeal regions of the tongue were 20% ± 13% and 31% ± 17% of mandibular advancement. The posterior tongue compressed 1.1 ± 2.2 mm supero-inferiorly.
Conclusions:
Mandibular advancement has two mechanisms of action which increase airway size. In subjects with low AHI, the entire tongue moves forward. Mandibular advancement also produces lateral airway expansion via a direct connection between the lateral walls and the ramus of the mandible.
Citation:
Brown EC; Cheng S; McKenzie DK; Butler JE; Gandevia SC; Bilston LE. Tongue and lateral upper airway movement with mandibular advancement. SLEEP 2013;36(3):397-404.
Keywords: Obstructive sleep apnea, mandibular advancement splint, pterygomandibular raphe, lateral walls
INTRODUCTION
While it is well known that chin lift increases airway paten-cy,1 the mechanism by which a mandibular advancement splint (MAS) improves obstructive sleep apnea (OSA) is poorly understood. An MAS fixes the mandible in an anterior position with the aim of decreasing airway collapsibility but is effective in only ∼50% of patients.2 Clinical variables which are favorable for MAS treatment include lower apnea hypopnea index (AHI), body mass index (BMI), female gender, primary oropharyngeal site of collapse, and some cephalometric and polysomnographic variations.2–5 Amount of mandibular advancement achieved also is related to treatment success.2
Mandibular advancement increases the lateral dimensions of the nasopharynx.6–10 Proposed mechanisms for this action include: mechanical effects via the genioglossus6 or palatine arches6,11; improvement in the nasopharyngeal airway due to anterior forward movement of the soft palate via its lateral connections6,8,12; decreased extraluminal tissue pressure in the anterior and lateral walls of the airway13; and increased airway muscle activity.14,15 The mechanism is thought to be largely mechanical because mandibular advancement improves airway patency during paralysis,6 although MAS increases neuromuscular activation.15 Mandibular advancement changes airway shape,6,8 but it is unclear why airway dimensions are improved in the lateral more than antero-posterior dimension in the nasopharynx.7,8,16 To our knowledge, the effect of mandibular advancement on the soft tissues surrounding the upper airway has not been dynamically imaged.
Spatial modulation of magnetization (SPAMM) is a tagged magnetic resonance imaging (tMRI) technique which superimposes a grid of “tag lines” on the tissue. The tissue magnetization is spatially modulated in the grid pattern prior to imaging, so these regions appear dark on subsequent images. Grid movement and deformation are measured from ultra-fast images taken after tagging. SPAMM has previously been used to measure the deformation of the tongue in young healthy individuals.17 Our aim was to quantify the movement of the tissues surrounding the airway during mandibular advancement to clarify the mechanism of action. Mechanical connections in the tongue and between upper airway structures, such as the mandible and the lateral walls of the nasopharynx were of particular interest. The hypothesis was that mandibular advancement would move the lateral walls of the nasopharyngeal airway and deform the tongue base.
METHODS
Thirty subjects (aged 31-69 years) with no contraindications for MRI were recruited through the Prince of Wales Hospital Sleep Clinics. Two subjects had technically inadequate axial images excluded from analysis. Subjects covered a wide spectrum from normal to severe OSA (apnea-hypopnea index [AHI] range 0-75 events/h sleep, 3 normal subjects) and BMI (range 17-39 kg/m2). Some subjects not usually considered for MAS were included to investigate the mechanics of the tongue with increasing BMI. No patient had received prior treatment for OSA. Subjects with severe chronic illness, medication that could affect upper airway muscle, or previous upper airway surgery were excluded, but minor orthodontic work was allowed. The study was approved by the University of New South Wales and Prince of Wales Hospital Human Research Ethics Committees. Written informed consent was obtained, and the study conformed to the Declaration of Helsinki.
Polysomnography
Normal subjects were either recruited through the sleep laboratory and underwent standard clinical polysomnography or were studied at home using an Embletta X50 (Medcare, Iceland) with one EEG lead, nasal airflow, pulse oximetry, position, and thoracic and abdominal bands. Home studies were scored by a sleep physician. In OSA subjects, in-laboratory polysomnography was performed using standard techniques, including electroencephalography (EEG), electrooculography, electrocardiogram, submental electromyogram (EMG), anterior tibialis EMG, nasal airflow measured with pressure transducer, pulse oximetry, thoracic and abdominal respiratory effort, and position. Sleep was scored in 30-s epochs by an experienced technician. All studies were scored according to AASM alternative criteria,18 with hypopnea defined as 50% reduction in airflow accompanied by desaturation of 3% or an arousal.
Experimental Protocol
Subjects were positioned within the MRI head-neck coil with the Frankfort plane vertical and padding to minimize head movement. Subjects were instructed to hold their breath at a comfortable end-expiratory level while images were acquired (2 sec). The mandible was manually advanced with an oral device (Figure 1B, Elastoplast Sport, # 30320, adult or youth size) fitted over the bottom teeth, by pulling a nylon wire anteriorly (Figure 1A). Several practice runs were performed until reproducible movement was obtained with the subject's jaw muscles relaxed. The mandible was advanced until resistance was felt. Images were taken in the midline sagittal plane and 2 axial planes (Figure 1D). A parasagittal plane (10 mm lateral to the left lateral wall of the nasopharynx) was added if time allowed (n = 14, Figure 1E). The upper axial plane was defined as the narrowest part of the nasopharynx on the sagittal scout image. A lower axial plane through the base of the genioglossus muscle was also imaged. Three to five repeats were performed in each plane.
Figure 1.
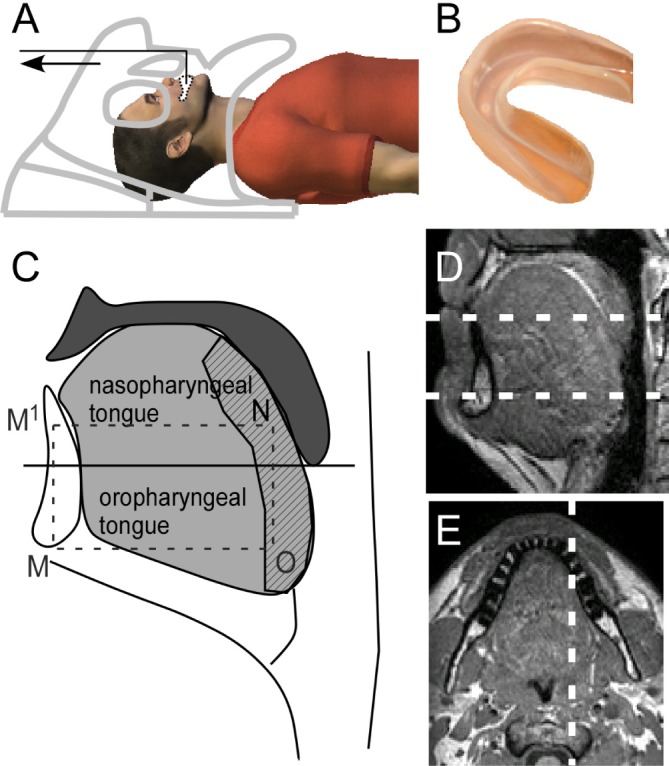
Experimental setup and imaging planes. (A) Subject in the MRI. The mandible is advanced when the nylon wire is pulled (arrow) with the head coil used as a fulcrum. (B) The mouth guard with nylon wire fits over the mandible. (C) Diagram of the tongue and upper airway which shows the anatomical definitions used. The tongue (light gray) is divided in two sections by a horizontal line through the tip of the uvula. The posterior tongue is shown hatched. The nasopharyngeal point used is designated N; the oropharyngeal point is O and correspond to the points used in the sagittal plane in Figure 2; and the tip of mandible M and M1 is the point superior to M at the level of N. The lines OM measured elongation of the oropharyngeal tongue, NM1 nasopharyngeal elongation, and NO posterior compression. (D) Midline sagittal anatomical image with white lines showing upper and lower axial planes. (E) Upper axial plane showing parasagittal imaging plane (white line) to the right of the midline through the lateral walls.
tMRI Technique
An available SPAMM sequence on a 3T MRI scanner was used (Philips, Achieva 3T, Best, The Netherlands). After grid production, 8 images were taken 250 ms apart during mandibular advancement. The imaging parameters were: flip angle 90 deg; spin echo repetition time 400 ms; echo time 16 ms; FOV = 178, 10, 200; 256 × 256 matrix and tag spacing 8.6 mm, slice thickness = 10 mm. Detailed anatomical images (T1TFE, 3D mode, FOV = 256 × 256× 130, 256 × 256 matrix, 130 slices, slice thickness = 1 mm) were taken at the end of the experiment. The hyoid mandibular plane distance (H-MP) and mandibular plane angle (MPA) were measured using bony landmarks in 3 dimensions.10
Analysis
Analysis of image sequences was performed using the harmonic phase (HARP) method19 implemented in Matlab (The MathWorks Inc, Natick, MA, USA). HARP allows tissue deformation to be computed at sub-pixel resolution, from the changes in spatial frequencies in the tMRI data.19,20 Image sequences with mouth opening > 1 mm, head movement > 1 mm, or mandibular advancement < 4 mm were excluded. When subjects had more than one suitable image sequence in that plane, all were analyzed and the results averaged.
Points of interest (Figure 2, anatomical images) were selected on the undeformed grid images and movement of the points tracked through 8 images. The points were defined as follows: (A) 1 cm anterior to the posterior border of the tongue at the level of the narrowest nasopharynx in the sagittal plane, (B) tip of the uvula, (C) midpoint of the oropharynx directly inferior to point A, (D) the mandible, (E and H) directly anterior to the left lateral wall (points F and I), 1 cm anterior to the anterior airway wall of the nasopharynx and oropharynx, respectively, (F and I) the left lateral wall of the nasopharynx and oropharynx, and (G and J) approximately two-thirds along a tissue plane from the left lateral wall to the mandible. Points surrounding these points were used to confirm that the tracks were representative of the movement. The axial planes were also examined for movement of the lateral walls using tracks at regular intervals overlaid on a matching anatomical scan (Figure 3).
Figure 2.
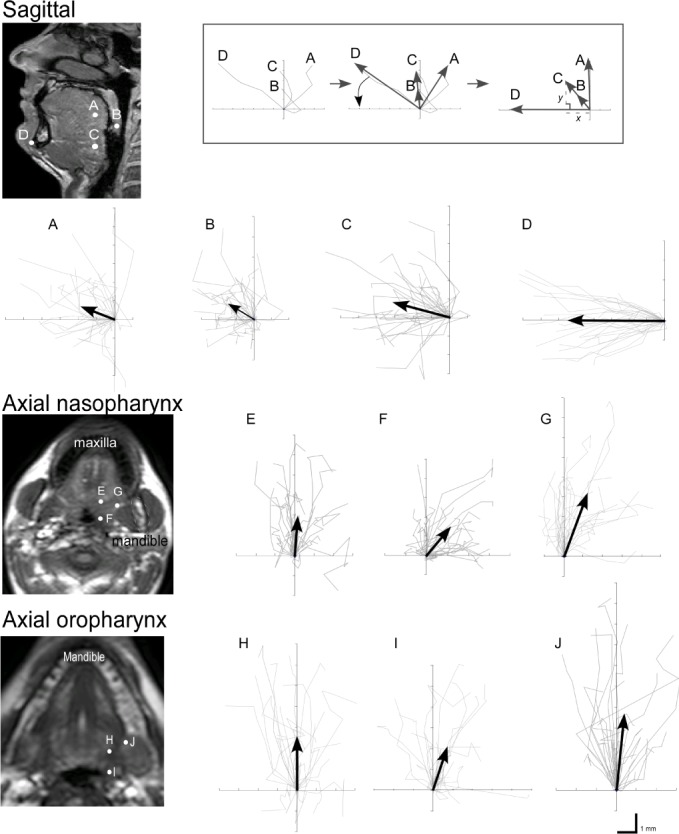
Mean tissue trajectories (gray) for all 30 subjects. The points tracked correspond to those shown on the anatomical diagrams. Black arrows show the direction and length of the average movement at each point. Inset top: the analysis method for the sagittal tracks of one subject with coordinate rotation to align the x-axis with the direction of mandibular advancement. Left: raw data. Middle: net trajectories (black arrows) and adjustment to mandibular x-y axis (curved arrow). Right: outcome after adjustment. In this example, the raw data show the nasopharyngeal (point A) moves posterior and the uvula (point B) and oropharyngeal point (point C) initially track posterior but then anterior with further advancement. Point A has moved perpendicular to the mandible, and point B and C demonstrate a superior (y) component relative to the mandibular advancement direction (point D).
Figure 3.
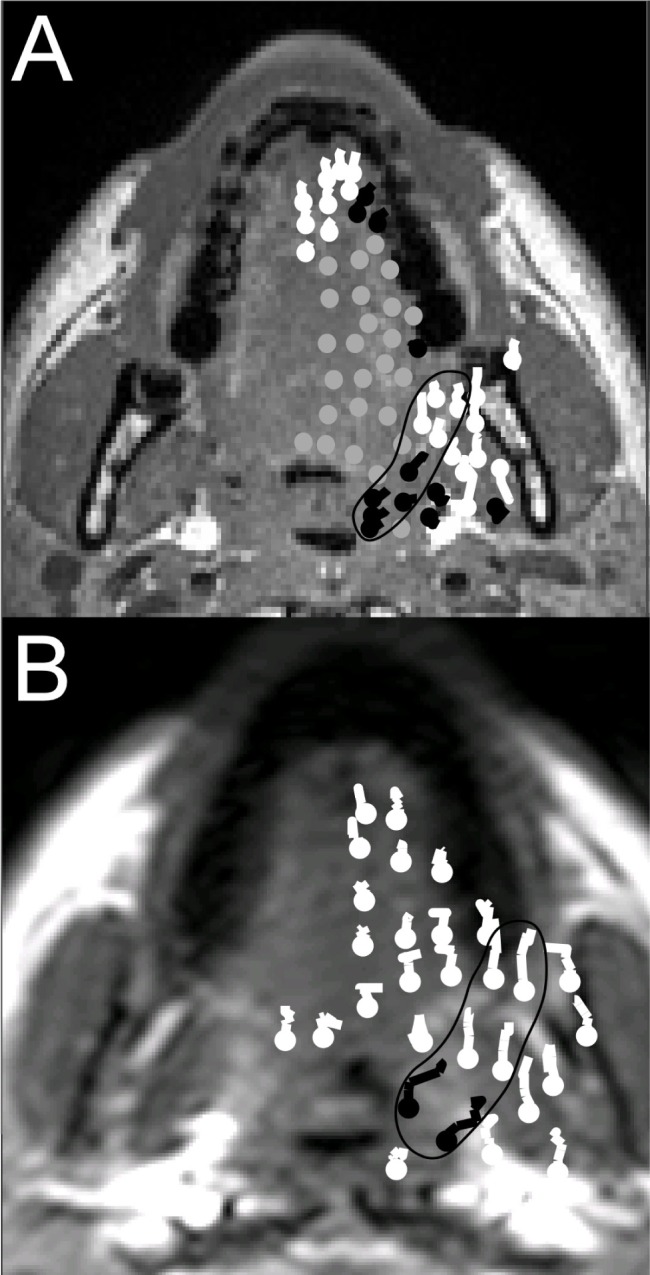
Movement tracks superimposed on axial anatomical images through the nasopharynx. Dots indicate the starting point and lines indicate the movement tracks. The tracks are colored according to direction of movement—black indicates predominantly lateral, white anterior, and gray minimal movement. The path of lateral movement from the lateral walls is circled in black. (A) Subject shows minimal movement anterior to the circled region. (B) The lateral connection shows increased movement compared to tissues anterior to the circled region.
In the sagittal plane, the tracks were mapped in each subject in the image x-y coordinate system, then rotated so that the x-axis lay along the direction of mandibular motion (Figure 2, inset). For analysis, the components of the movement were expressed as a percentage of the movement of the mandible. Angle of movement was measured with reference to the positive x axis. In the upper axial plane, where the mandible motion was not in the imaging plane, the displacements are expressed as a percentage of the mandibular displacement in matching sagittal scans. In the sagittal plane, the tongue was divided into regions to describe where deformation occurred (Figure 1C). The nasopharyngeal region was the portion of tongue superior to a horizontal line through the tip of the uvula and the oropharyngeal region was below this line. The posterior tongue was defined as the 10-15 mm of muscle adjacent to the airway.
A sagittal mesh (Figure 4) was constructed for each subject using the image sequence with the largest mandibular advancement. The pattern of sagittal motion was classified according to movement of the posterior tongue in the oropharyngeal and nasopharyngeal region. These 3 groups were: (A) en bloc anterior movement of the posterior tongue in the nasopharyngeal and oropharyngeal regions > 2 mm, (B) anterior movement > 2 mm of the posterior tongue in the oropharyngeal region only, and (C) minimal anterior movement of the posterior tongue with mandibular advancement (< 1 mm). Group assignment was confirmed by a blinded review of each video sequence by a second researcher. The area of the mesh pre and post deformation was measured. Antero-posterior elongation was defined as the difference in distance of each point above from the mandible pre- and post-advancement. Supero-inferior compression of the posterior tongue was defined as the distance between the nasopharyngeal and oropharyngeal points pre- and post-advancement (see Figure 1C).
Figure 4.
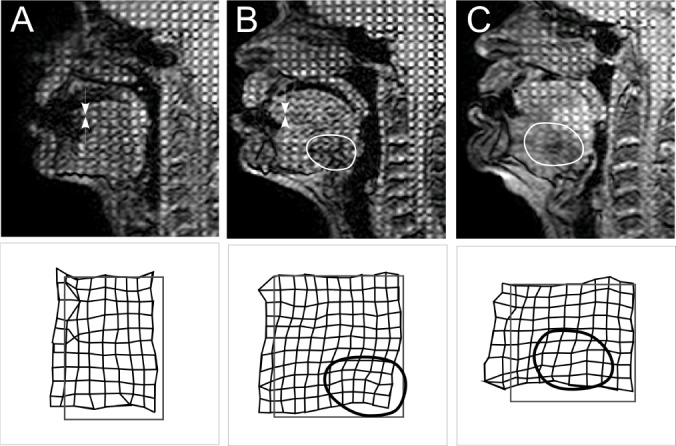
The three patterns of tongue displacement during mandibular advancement. MRI after 1.5 seconds of mandibular advancement for each pattern of movement is shown in the upper panels. The original grid spacing can be seen in adjacent brain tissue (top right), which has not moved. The lower panels are an enlarged view, showing the border of the original (undeformed) regular grid (gray rectangle) with the deformation after 1.5 seconds superimposed in black. The original tags were 8.6 mm apart. (A) The posterior tongue moves anteriorly en bloc. (B) The tongue moves anteriorly in the oropharyngeal region only (the oval shows deformation of the tags on the MRI image). Images A and B show supero-inferior compression of the tongue (white arrows on the MRI image). (C) Minimal anterior movement in either region. The oval indicates a region of antero-posterior elongation.
SPSS (Version 18, Chicago, IL) was used for all statistical analysis. Alpha was set at 0.05. Data are presented as mean ± standard deviation. All distributions were normal apart from the movement of the lateral upper airway, which became normal after logarithmic transformation. Paired Student's t-tests were performed to compare the movement of specific pairs of points. In the sagittal plane the nasopharyngeal point (Figure 2, point A) was paired with the oropharyngeal point (Figure 2, point B). In the upper axial plane movement at the lateral airway point (Figure 2, point F) was compared to the point anterior to the lateral point (Figure 2, point E). Pearson correlations were performed to assess the relationship between movement and AHI and other variables, and one-way ANOVA was used when there were > 2 groups. A priori power calculation indicated a sample size of 26 subjects was required to find a large effect (effect size 0.8) between the different points measured with a power of 0.8. This was based on assumed mean of 1 ± 0.5 mm movement of the nasopharyngeal point compared to 2.5 ± 0.5 mm movement of the oropharyngeal point.
RESULTS
Subject characteristics are shown in Table 1. Movies of mandibular advancement in 3 subjects, exhibiting the 3 characteristic motion patterns, are available in the supplemental material. Figure 2 shows the movement tracks for all subjects at all points studied, with mean displacements shown as arrows. Table 2 compares the amount of movement of the different points in the sagittal and axial planes, and Table 3 shows the correlations between movement and AHI, BMI, and MPA.
Table 1.
Subject characteristics, presented as mean ± standard deviation (range)
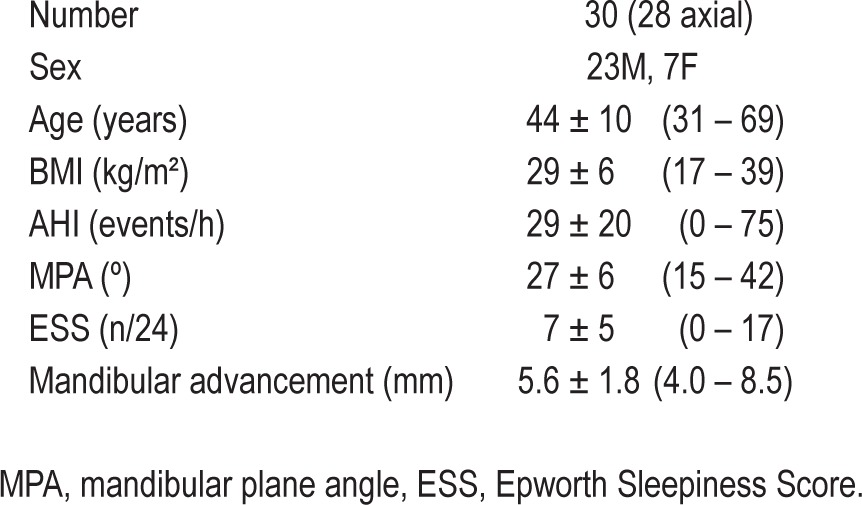
Table 2.
Movement of upper airway tissues during simulated mandibular advancement, expressed as a percentage of the amount of mandibular advancement

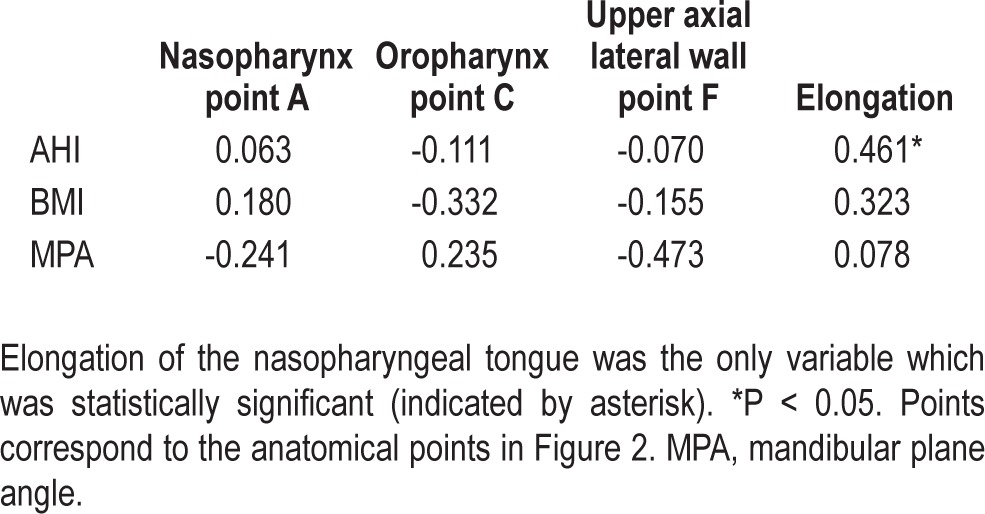
Table 3.
Pearson correlation coefficients (R) between movement and OSA severity, obesity, and mandibular plane angle.
Axial Imaging Planes
All 28 subjects had movement from the anterior of the ramus of the mandible to the lateral walls of the airway in the upper axial plane in the region of the pterygomandibular raphe. Movement did not always extend all the way from lateral mandible to the nasopharynx in the plane studied. This lateral connection moved in a different direction to tissues anterior to the connection during advancement (Figure 3). There was no difference in total movement anterior or lateral to the airway (Table 2, point E and point F), but the direction of movement of these 2 points was significantly different (P = 0.007). The parasagittal imaging plane showed < 1 mm supero-inferior outof-plane movement in the upper axial plane at the lateral walls of the airway (Figure 2, point F).
Sagittal Imaging Plane
Movement for a typical subject in the sagittal plane and the coordinate system rotation used to align mandibular advancement with the x axis are shown in the inset of Figure 2. With mandibular advancement this subject had posterior movement of the nasopharyngeal (point A) and oropharyngeal points (C) in the antero-posterior plane. The nasopharyngeal point (A) moved almost perpendicularly to the direction of mandibular advancement (D). Overall, there was greater displacement in the oropharyngeal region (31% ± 17% of mandibular advancement) compared to the nasopharyngeal region (20% ± 13%, Student t-test, P = 0.003). The superior component of movement of the nasopharyngeal point of 1.3 ± 2.0 mm (32% ± 27% of mandibular advancement superior component) and oropharyngeal point of and 1.8 ± 1.7 mm (29% ± 19%) were not significantly different (Student t-test, P = 0.468).
Tongue Deformation in the Sagittal Plane
On average the oropharyngeal tongue elongated anteroposteriorly by 2.5 ± 2.2 mm and the nasopharyngeal tongue 3.7 ± 1.7 mm. There was compression supero-inferiorly of 1.1 ± 2.2 mm. The posterior tongue compressed supero-inferiorly in 21/30 (70%) subjects. There were no cases where the oropharyngeal points and nasopharyngeal points moved in opposite directions during mandibular advancement. The mean mesh area pre-deformation (1,974 ± 736 mm2) and post-deformation (1,940 ± 734 mm2) were not significantly different (P = 0.081). Table 3 shows the correlations between antero-posterior elongation and the variables measured. AHI continued to show significant correlation with antero-posterior elongation in the nasopharyngeal region (R = 0.461, P = 0.010) after adjustment for BMI (R = 0.389, P = 0.037). Elongation was not related to BMI, despite a trend (R = 0.323, P = 0.082). Figure 4 shows the patterns of deformation and displacement of the tongue in the sagittal plane. In 10/30 (33%) subjects, the tongue elongated with mandibular advancement, but both the oropharyngeal and nasopharyngeal posterior tongue showed some anterior displacement (Figure 4, pattern A). In 8/30 (27%) the oropharyngeal tongue only showed anterior displacement (pattern B) and in 12/30 (40%) the tongue elongated antero-posteriorly with minimal displacement of the posterior oropharyngeal or nasopharyngeal tongue (pattern C). Pattern A was associated with a lower mean AHI (16 ± 16 events/h) compared to Pattern C (mean AHI = 39 ± 19 events/h, P = 0.04; Figure 5), but BMI and MPA were not significantly different among the 3 groups (P > 0.05).
Figure 5.
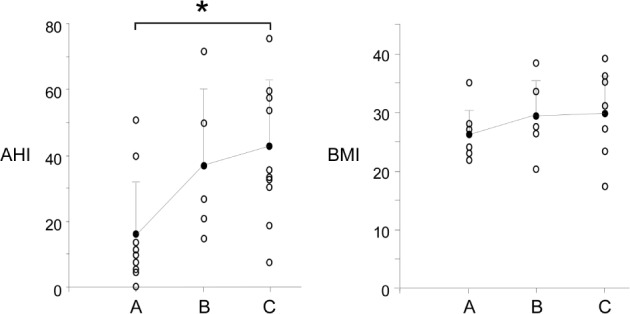
AHI and BMI of each subject (open circles) plotted against the movement pattern group and the mean for each group (closed circles). Error bars indicate the standard deviation. Group A is en bloc movement, group B is posterior oropharyngeal movement, and group C is minimal posterior movement. Difference in mean AHI was statistically significant between groups A and C (asterisk, ANOVA, Tukey post hoc P = 0.04).
DISCUSSION
This study is the first to define the movement and deformation of the soft tissues surrounding the upper airway during mandibular advancement. The study was conducted during wakefulness. There are three main findings. First, lateral wall tissues moved laterally with mandibular advancement via a connection from the lateral walls of the nasopharynx to the lateral mandible. Anatomically, the connection appeared to correspond to the region of the pterygomandibular raphe (see Figure 6). Second, the majority of subjects showed antero-posterior elongation of the tongue and rostro-caudal compression in the posterior tongue during mandibular advancement. Finally, the tongue elongated more with mandibular advancement in subjects with higher AHI. Low AHI was associated with en bloc forward movement of the posterior tongue, but high AHI was associated with minimal movement of the posterior tongue. This difference was independent of BMI. This study suggests that there are two mechanisms by which mandibular advancement improves airway collapsibility in some OSA patients, firstly by en bloc antero-posterior motion of the tongue in subjects with lower AHI and secondly by increasing lateral airway dimensions via a direct connection from the lateral ramus of the mandible.
Figure 6.
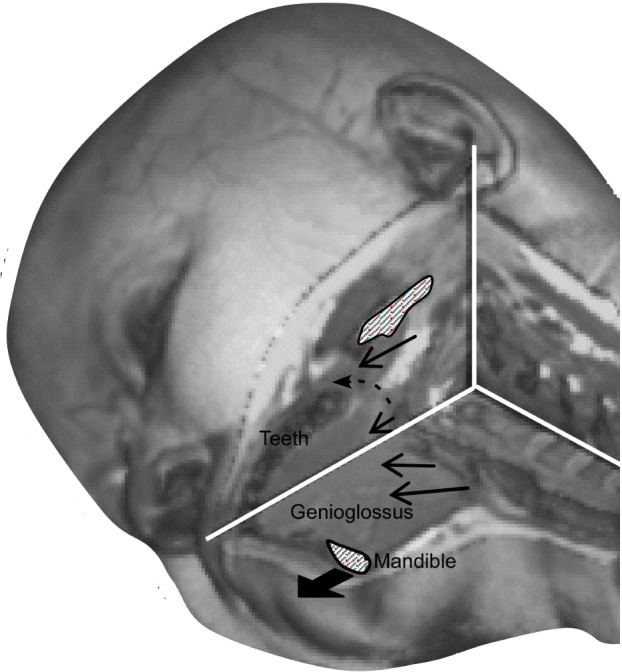
Detailed T1 anatomical image of the axial and sagittal planes intersecting at the narrowest point of the airway, which shows the proposed model of movement with mandibular advancement. The black dashed arrow is slightly posterior to the pterygomandibular raphe. The other arrows surrounding the airway indicate average movement at each point and are roughly to scale.
Early studies suggested that the MAS increased the space between bony structures and provided increased room for soft tissues,2 but recent research has shown that the action is more complex with substantial inter-subject variation. Lateral cephalometry demonstrated that mandibular advancement increases the antero-posterior dimensions of the oropharynx6,21,22 but has variable effects on the nasopharynx. Some studies have shown enlarged antero-posterior dimensions of the nasopharyngeal airway,6,21,23,24 others have shown no significant increase.22,25 The hyoid moves closer to the mandibular plane,10,21,23,25–27 but with a wide range of direction and amount of movement.28 Mandibular advancement changes airway shape,6,16 which can increase airway stability in some subjects.29 Oliven and colleagues11 suggested that mandibular advancement may optimize the mechanics of the tongue so that the fibers are closer to their most efficient mechanical length. The elongation of the tongue demonstrated here in the antero-posterior dimension could produce this effect.30
Movement of tissues with mandibular advancement is influenced by anatomical and physiological differences between subjects and possibly altered local mechanics related to fat deposition and mouth opening.27,31 The inverse correlation between elongation of the nasopharyngeal tongue and AHI adjusted for BMI, although significant, accounted for less than half of observed variation (R2 = 0.16), which suggests other factors also influence how the tongue responds to mandibular advancement. Previous imaging studies have not found clinically useful variables that consistently predict response to MAS, but suggest that MPA, neck circumference,3 mandibular retrognathism,32 distance of the hyoid from the mandibular plane,16 nasopharyngeal airway space and oropharyngeal site of collapse5 correlate with response. Here, the pattern of tongue deformation during mandibular advancement was significantly associated with AHI but not BMI. The en bloc movement associated with low AHI suggests the tongue deforms less and mandibular advancement increases airway size along its length in these subjects. The amount of movement at the nasopharyngeal point was significantly negatively correlated with BMI, possibly due to increased load from the combined effects of increased extraluminal positive pressure and negative pressure in the airway. OSA patients have an elevated critical pressure of the upper airway, which is related to BMI.33 Fat deposition in the tongue may also have a structural effect to decrease motion.34
Internal deformation of the tongue with mandibular advancement has not been characterized previously. Although computer modeling suggests that mandibular advancement may rotate the tongue in the sagittal plane,35 we found no evidence of tongue rotation. This study showed evidence of the tongue acting as a muscular hydrostat.17,36 The area of the tongue grid in the sagittal plane was not different pre and post mandibular advancement. In a muscular hydrostat, the shape changes but the volume remains constant.36 Thus, deformation of the tongue base influences the whole tongue. In this study, elongation of the posterior tongue base produced supero-inferior compression, in keeping with the tongue acting as a muscular hydrostat. Superior movement of the tissues could be explained by increased tension in the ligaments supporting the tongue from the skull base, but our data suggest this is less likely because the ligaments are lateral to the movement observed (see also below).
Mandibular advancement increases the cross sectional area of the nasopharynx.6–10 The lateral walls of the airway are more compliant than the tissues anterior to the airway13,37 and are increasingly recognized as important in the pathogenesis of OSA. CT,38 MRI,8,10,16 and nasoendoscopic examinations6,7,9 demonstrate that mandibular advancement increases the nasopharyngeal lateral dimension airway more than the antero-posterior dimension. The apparent connection between the mandible and the lateral airway walls in this study might explain this. The axial videos through the nasopharynx presented here suggest a direct connection between the lateral walls and the ramus of the mandible. This has not been demonstrated before, although Isono et al. proposed that tongue advancement influenced the soft palate via the pharyngeal arches.6 A number of structures may be involved, but we speculate that the pterygomandibular raphe, an aponeurotic connection between the buccinator muscle and the superior pharyngeal constrictor, is a likely candidate after comparing the tagged images with detailed anatomical images and anatomical references.39 It attaches superiorly to the hamulus of the medial pterygoid plate and inferiorly to the mylohyoid line of the mandible but is absent in 36% of subjects.39 When absent, the superior pharyngeal constrictor is continuous with buccinator.39 It is unknown if the presence of a fascial component to this structure affects compliance in the lateral walls, but if it does, then its absence in some patients could influence the clinical response to MAS. We tried to identify a tendinous structure in the presumed region of pterygomandibular raphe as described in anatomical studies,39 but the MRI appearance of the pterygomandibular raphe is not well described, so we were unable to make firm conclusions. There are also a number of ligaments which run infero-anteriorly from the skull base to the ramus of the mandible in the lateral region, including the sphenomandibular ligament. The parasagittal plane was included in our experimental protocol to investigate the role of supero-inferior connections from the skull base in the region of lateral movement. They appeared less likely to explain lateral movement because there was minimal movement in the direction of these ligaments.
Our technique has limitations in additional to being conducted during wakefulness. First, our results are two-dimensional images of three-dimensional structures. In each plane there is the possibility of out-of-plane movement. While a number of planes were imaged, there was insufficient time to image the whole airway in three dimensions. Furthermore, despite instructions to remain passive, some voluntary and reflex muscle activation14,15 in response to the dynamic movement is possible, and this may be different to the effect of a static advancement during sleep, where tissues are likely to relax over time. Also, MAS holds the tissues in a static position during sleep, so our results may not reflect the position of the tissues with MAS in situ. We were unable to recruit a significant subset of subjects who subsequently had MAS treatment, so could not draw conclusions about MAS response. Hence further studies are needed to look at whether different movement patterns and/or the presence of the pterygomandibular raphe can influence clinical response to MAS. Also, the study was only powered to find large effects that were more likely to be clinically significant.
Dynamic imaging has revealed the movement of the tissues surrounding the airway with mandibular advancement and allowed us to quantify movement of the tongue in response to mandibular advancement during wakefulness. It revealed a likely connection between the lateral walls of the airway and the mandible at the nasopharyngeal level. The nature of this connection and the pterygomandibular raphe are new targets for investigation of the etiology of OSA and response to MAS. The internal deformation of the tongue with mandibular advancement was characterized here for the first time. The pattern of deformation of the tongue related to AHI and increased AHI was associated with increased elongation of the tongue independent of BMI, suggesting increasing OSA severity is associated with differences in upper airway mechanics, which may lead to unfavorable treatment outcome with MAS.
ACKNOWLEDGMENTS
This research was supported by an NHMRC project grant. LEB, SCG, and JEB are supported by NHMRC fellowships. ECB was supported by the ResMed Foundation Scholarship. The authors wish to thank A/Prof Peter Cistulli for commenting on the final version of the manuscript.
DISCLOSURE STATEMENT
This research was funded by the National Health and Medical Research Council (of Australia). This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Guildner CW. Resuscitation - Opening the airway: A comparative study of techniques for opening an airway obstructed by the tongue. JACEP. 1976;5:588–90. doi: 10.1016/s0361-1124(76)80217-1. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 3.Mehta A, Qian J, Petocz P, Darendeliler M, Cistuli P. A randomized controlled study of a mandibular advancement splint for obstructive sleep apnoea. Am J Respir Crit Care Med. 2001;163:1475–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 4.Marklund M, Hans Stenlund H, Franklin KA. Mandibular Advancement devices in 630 men and women with obstructive sleep apnea and snoring. tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 5.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29:666–71. [PubMed] [Google Scholar]
- 6.Isono S, Tanaka A, Sho Y, Konno A, Nishino T. Advancement of the mandible improves velopharyngeal airway patency. J Appl Physiol. 1995;79:2132–8. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Liu Y, Gao Y. Three-dimensional upper-airway changes associated with various amounts of mandibular advancement in awake apnea patients. Am J Orthod Dentofacial Orthop. 2008;133:661–8. doi: 10.1016/j.ajodo.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Chan ASL, Lee RWW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35:836–42. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 10.Chan ASL, Sutherland K, Schwab RJ, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65:726–32. doi: 10.1136/thx.2009.131094. [DOI] [PubMed] [Google Scholar]
- 11.Oliven R, Tov N, Odeh M, et al. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol. 2009;106:1668–73. doi: 10.1152/japplphysiol.91501.2008. [DOI] [PubMed] [Google Scholar]
- 12.Tsuiki S, Lowe AA, Almeida FR, Kawahata N, Fleetham JA. Effects of mandibular advancement on airway curvature and obstructive sleep apnoea severity. Eur Respir J. 2004;23:263–8. doi: 10.1183/09031936.04.00094304. [DOI] [PubMed] [Google Scholar]
- 13.Kairaitis K, Parikh R, Stavrinou R, et al. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–6. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K. Effect of a prosthetic appliance for treatment of sleep apnea syndrome on masticatory and tongue muscle activity. J Prosthet Dent. 1998;79:537–44. doi: 10.1016/s0022-3913(98)70175-1. [DOI] [PubMed] [Google Scholar]
- 15.Johal A, Gill G, Ferman A, McLaughlin K. The effect of mandibular advancement appliances on awake upper airway and masticatory muscle activity in patients with obstructive sleep apnoea. Clin Physiol Funct Imaging. 2007;27:47–53. doi: 10.1111/j.1475-097X.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep. 2011;34:469–77. doi: 10.1093/sleep/34.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283–94. doi: 10.1113/jphysiol.2008.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 19.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048–60. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng S, Butler JE, Gandevia S, Bilston L. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283–94. doi: 10.1113/jphysiol.2008.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Otsuka R, Ono T, Honda E, Sasaki T, Kuroda T. Effect of titrated mandibular advancement and jaw opening on the upper airway in nonapneic men: A magnetic resonance imaging and cephalometric study. Am J Orthod Dentofacial Orthop. 2004;125:191–9. doi: 10.1016/s0889-5406(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 22.Johal A, Battagel JM. An investigation into the changes in airway dimension and the efficacy of mandibular advancement appliances in subjects with obstructive sleep apnoea. Brit J Orthod. 1999;26:205–10. doi: 10.1093/ortho/26.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Poon KH, Chay SH, Chiong KFW. Airway and craniofacial changes with mandibular advancement device in Chinese with obstructive sleep apnoea. Ann Acad Med Singapore. 2008;37:637–44. [PubMed] [Google Scholar]
- 24.Tsuiki S, Hiyama S, Ono T, et al. Effects of a titratable oral appliance on supine airway size in awake non-apneic individuals. Sleep. 2001;24:554–60. doi: 10.1093/sleep/24.5.554. [DOI] [PubMed] [Google Scholar]
- 25.Sam K, Lamb B, Ooi CG, Cook M, Ip MS. Effect of a non-adjustable oral appliance on upper airway morphology in obstructive sleep apnoea. Res Med. 2006;100:897–902. doi: 10.1016/j.rmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol. 2001;531:677–91. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isono S, Tanaka A, Tagaito Y, Sho Y, Nishino T. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87:1055–62. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Battagel JM, Johal A, L'Estrange PR, Croft CB, Kotecha B. Changes in airway and hyoid position in response to mandibular protrusion in subjects with obstructive sleep apnoea. Eur J Orthod. 1999;21:363–76. doi: 10.1093/ejo/21.4.363. [DOI] [PubMed] [Google Scholar]
- 29.Cistulli PA, Gotsopoulos H, Marklund M, Lowe AA. Treatment of snoring and obstructive sleep apnea with mandibular repositioning appliances. Sleep Med Rev. 2004;8:443–57. doi: 10.1016/j.smrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibers. J Physiol. 1966;184:170–92. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31:543–7. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoekema A, Doff MHJ, de Bont LGM, et al. Predictors of obstructive sleep apnea-hypopnea treatment outcome. J Dent Res. 2007;86:1181–6. doi: 10.1177/154405910708601208. [DOI] [PubMed] [Google Scholar]
- 33.Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–24. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenig JS, Thach BT. Effects of mass loading on the upper airway. J Appl Physiol. 1988;64:2294–9. doi: 10.1152/jappl.1988.64.6.2294. [DOI] [PubMed] [Google Scholar]
- 35.Van Holsbeke C, De Backer J, Vos W, et al. Anatomical and functional changes in the upper airways of sleep apnea patients due to mandibular repositioning: a large scale study. J Biomech. 2010 doi: 10.1016/j.jbiomech.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol. 2007;210:4069–82. doi: 10.1242/jeb.007096. [DOI] [PubMed] [Google Scholar]
- 37.Kairaitis K, Stavrinou R, Parikh R, Wheatley J, Amis T. Mandibular advancement decreases pressures in the tissues surrounding the upper airway in rabbit. J Appl Physiol. 2006;100:349–56. doi: 10.1152/japplphysiol.00560.2005. [DOI] [PubMed] [Google Scholar]
- 38.Kyung SH, Park YC, Pae EK. Obstructive sleep apnea patients with the oral appliance experience pharyngeal size and shape changes in three dimension. Angle Orthod. 2005;75:15–22. doi: 10.1043/0003-3219(2005)075<0015:OSAPWT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K, Gasser RF. Morphology of the pterygomandibular raphe in human fetuses and adults. Anat Rec. 1989;224:117–22. doi: 10.1002/ar.1092240115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


