Abstract
Study Objectives:
To compare the efficacy of telephone-delivered cognitive-behavioral therapy for insomnia to an information pamphlet control on sleep and daytime functioning at pretreatment, posttreatment, and 12-wk follow-up.
Design:
Randomized controlled parallel trial.
Setting:
N/A.
Participants:
Thirty individuals with chronic insomnia (27 women, age 39.1 ± 14.4 years, insomnia duration 8.7 ± 10.7 years).
Interventions:
Cognitive behavioral therapy for insomnia (CBTI) delivered in up to eight weekly telephone sessions (CBTI-Phone, n = 15) versus an information pamphlet control (IPC, n = 15).
Measurements and Results:
Sleep/wake diary, sleep-related questionnaires (Insomnia Severity Index, Pittsburgh Sleep Quality Index, 16-item Dysfunctional Beliefs and Attitudes about Sleep), and daytime symptom assessments (fatigue, depression, anxiety, and quality of life) were completed at pretreatment, posttreatment, and 12-wk follow-up. Linear mixed models indicated that sleep/wake diary sleep efficiency and total sleep time improved significantly at posttreatment in both groups and remained stable at 12-wk follow-up. More CBTI-Phone than IPC patients showed posttreatment improvements in unhelpful sleep-related cognitions (P < 0.001) and were classified as “in remission” from insomnia at follow-up (P < 0.05). Posttreatment effect sizes on most daytime symptoms were large (Cohen d = 0.8–2.5) for CBTI-Phone patients and small to moderate (Cohen d = -0.1–0.6) for IPC patients. All CBTI-Phone patients completed posttreatment and 12-wk follow-up assessments, but three IPC patients discontinued the study.
Conclusions:
The findings provide preliminary support for telephone-delivered CBTI in the treatment of chronic insomnia. Future larger-scale studies with more diverse samples are warranted. Some individuals with insomnia may also benefit from pamphlet-delivered CBTI with brief telephone support.
Citation:
Arnedt JT; Cuddihy L; Swanson LM; Pickett S; Aikens J; Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. SLEEP 2013;36(3):353-362.
Keywords: Cognitive behavioral therapy, insomnia, randomized controlled trial, sleep, telephone, treatment
INTRODUCTION
Chronic insomnia affects 10-20% of American adults1 and exacts a major personal and societal burden. Chronic insomnia has been linked to reduced quality of life, decrements in perceived health, increased risk for psychiatric and substance use disorders, and exacerbation of comorbid health conditions.1–6 In the workplace, insomnia is associated with presenteeism -- lost productivity as employees attend work but underperform--that equates to 11.3 work days annually.7 Moreover, the average 6-mo direct and indirect costs for adults with untreated insomnia, in comparison with those without insomnia, are estimated to be at least $1,200 greater.8
Despite the magnitude of the problem, the availability of effective treatment for individuals with insomnia remains suboptimal. First-line prescription and over-the-counter agents provide rapid symptomatic relief and are widely available, but they may be less appropriate for select patient groups, can lead to tolerance with repeated use, and for many patients are not as preferable as nonmedication approaches.9,10 Cognitive behavioral therapy for insomnia (CBTI) is a multicomponent nonmedication treatment targeting behavioral and cognitive factors that contribute to chronic sleep disturbances. Multiple controlled trials indicate that 70-80% of patients show benefit from CBTI,11–13 approximately 40% achieve remission from insomnia,14 and treatment benefits are sustained over time.15–18 Although highly efficacious, CBTI remains underutilized as a primary therapy for chronic insomnia.
Insomnia researchers and clinicians have highlighted the need for wider and more rapid dissemination of CBTI, proposing healthcare models such as “stepped care” to help propel CBTI to first-line treatment status.19 Whatever the optimal healthcare solution, a range of effective treatment delivery modalities will be required to address the scope of the problem. Most clinical trials of CBTI have used group or individual in-person modalities,11–13,20 which yield large effect sizes but are time-intensive, economically inefficient, and available to only select patient groups. At the other end of the spectrum, self-help treatments in the form of books or pamphlets21–24 and Internet interventions25–28 are accessible to more individuals with insomnia, but their outcomes have been more modest and treatment attrition more significant than for in-person trials. In addition, self-help insomnia treatments may be primarily appropriate for patients with less severe insomnia and those without comorbid disorders.29
Telephone-delivered interventions offer a compromise between traditional in-person CBTI and self-help interventions. They are more readily accessible and cost-effective than in-person services, and incorporate human touch, interaction, and therapeutic alliance that are missing from self-help interventions. Telephone-delivered interventions have been used successfully to have a positive influence on other health behaviors such as smoking, physical activity, and diet.30,31 In previous insomnia trials, the telephone has generally played a secondary role to support nonmedication interventions delivered in-person or as self-help.21,22,32,33 Only one controlled trial has directly compared CBTI delivered via three different modalities--telephone, individual, and group–in 45 individuals with chronic primary insomnia.34 Sleep/wake diary outcomes, self-rated insomnia severity, and daytime anxiety and depression symptoms all had similar degrees of improvement among the three treatment groups. These improvements, which were sustained at 3- and 6-mo follow-up, provide preliminary evidence that CBTI delivered by telephone may be as effective as CBTI delivered individually or in group format. However, this study design could not support efficacy of CBTI beyond that of any simpler intervention or placebo.
The primary aim of the current study, therefore, was to compare the efficacy of a telephone-delivered CBTI intervention to that of a simple information control consistent with nonmedication insomnia treatments provided in typical primary care settings. We hypothesized that individuals with chronic insomnia who received telephone-delivered CBTI rather than minimal treatment would show greater improvement in sleep quality (higher sleep efficiency and greater total sleep time) and larger reductions in ratings of insomnia severity immediately after treatment and at 12-wk follow-up. We also hypothesized that individuals in the telephone-delivered CBTI group would experience improvement on assessments of common sequelae of insomnia, including fatigue, depression and anxiety symptoms, unhelpful sleep beliefs, and quality of life.
METHODS
Participants
Potential participants age 18-65 yr were recruited from local primary care outpatient clinics and from the community via advertisement. To be eligible, participants had to meet Research Diagnostic Criteria criteria for chronic insomnia,35 with documented symptoms on three or more nights per week (> 60 min of total wake time during the night and sleep efficiency < 85%) based on two weeks of baseline daily sleep/wake diaries. Exclusion criteria included a diagnosis or high clinical suspicion of a sleep disorder other than insomnia; poorly controlled Axis I psychiatric disorder; uncontrolled medical disorder or pain syndrome that interfered with sleep, caused daytime sleepiness, or was likely to be causally related to the insomnia; current nonpharmacologic insomnia treatment or previous failed trial of CBTI; and routine overnight shift work. Participants taking sleep medications were not excluded if they met study criteria for insomnia, medications were stable for at least eight weeks, and they agreed to maintain their current medication regimen throughout the study. Of the 30 enrolled participants, seven reported using over-the-counter sleep aids either nightly (n = 1) or as needed (n = 6). No one reported taking prescription medication for insomnia.
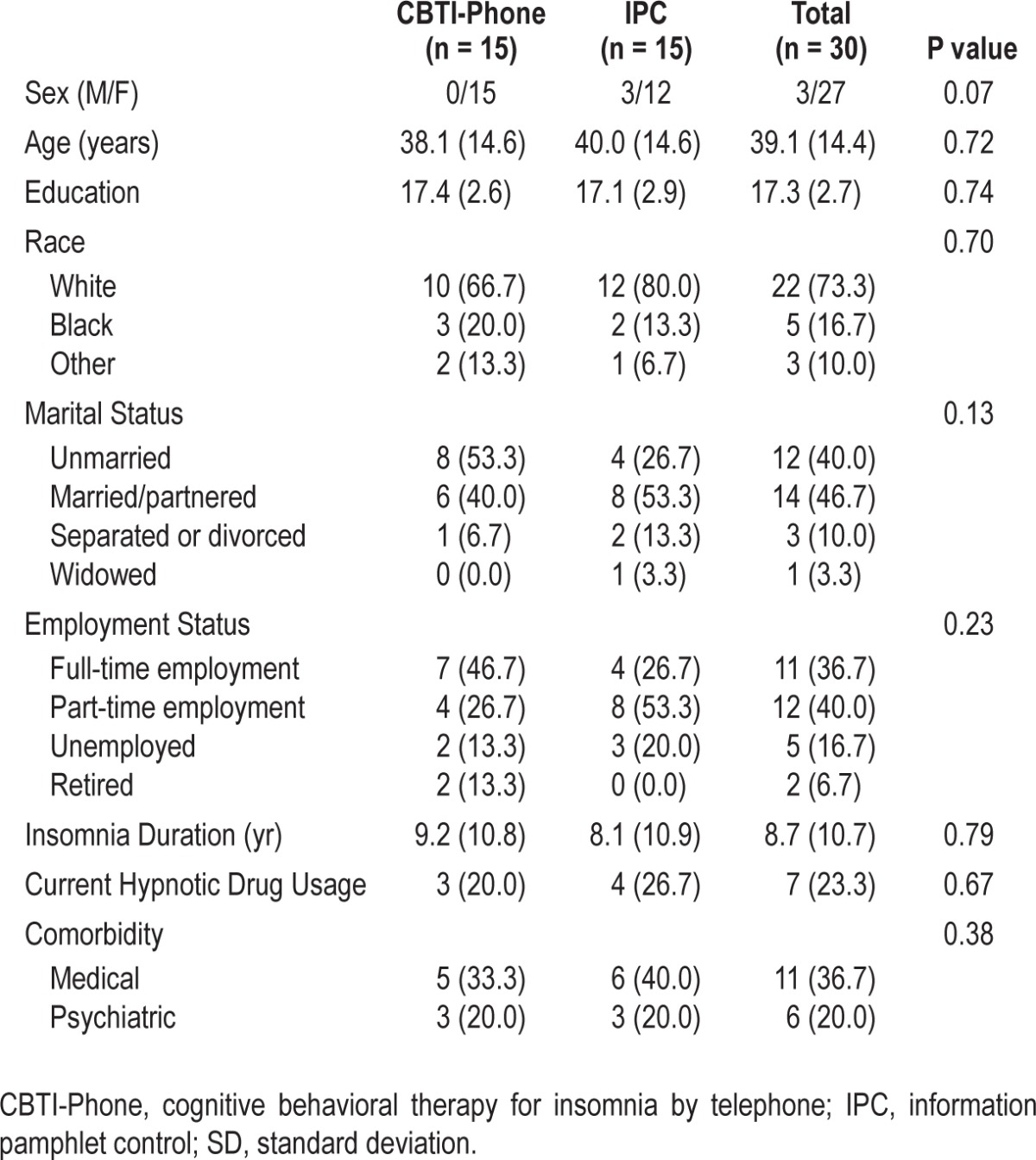
Potential participants were evaluated for initial eligibility using a mailed packet of screening questionnaires followed by telephone assessment to clarify responses and conduct further assessment as necessary. Those who were still eligible provided a two-week baseline sleep/wake diary to confirm that they met insomnia study criteria. Study procedures were approved by the University of Michigan Medical School Institutional Review Board and individuals earned up to $200 for study participation. Table 1 outlines study participant characteristics.
Table 1.
Demographic characteristics for randomized participants (mean [SD] or n [%]) by treatment group
Study Design
The study was a randomized, controlled parallel trial to compare the efficacy of a telephone-delivered cognitive-behavioral therapy for insomnia (CBTI-Phone) to an information pamphlet control (IPC). Thirty participants with chronic insomnia were randomized equally to the CBTI or IPC condition using a random number table. CBTI-Phone participants received four treatment modules with telephone intervention by experienced therapists in four to eight weekly 15- to 60-min sessions, contingent on treatment response. Participants randomized to the IPC condition were instructed to read and follow the recommendations in a CBTI pamphlet. Self-reported outcomes assessed insomnia severity and daytime functioning at pretreatment, posttreatment, and 12-week follow-up.
Treatment Groups
CBTI-Phone participants were mailed four patient modules developed by two of the authors (JTA, LMS) just before beginning treatment. Each session followed a standard structure,36 which began with a sleep/wake diary review and completion of the Insomnia Severity Index (ISI). The four treatment modules described in the following paragraphs—Module 1: Behavioral Strategies; Module 2: Sleep Hygiene; Module 3: Cognitive Therapy; and Module 4: Relapse Prevention—were delivered in the same order for all participants.
Module 1: Behavioral Strategies (Sleep Restriction and Stimulus Control)
Sleep restriction curtails the amount of time spent in bed each night to the patient's estimated average total sleep time to increase sleepiness at bedtime.37 The specific procedures followed for sleep restriction were to: (1) determine the participant's reported average total sleep time using pretreatment sleep diaries; (2) curtail the amount of time spent in bed to match this average or as close as is tolerable for the patient; and (3) gradually extend the amount of time in bed once sleep efficiency (time asleep/time in bed) exceeded 85%, until daytime functioning is optimized. Prescribed sleep schedules were never < 5 h per night. Stimulus control consists of a set of instructions designed to associate temporal (bedtime) and environmental (bed, bedroom) cues with rapid sleep onset and to establish a regular sleep-wake schedule.38 The general instructions are: (1) Go to bed only when sleepy or at the prescribed bedtime; (2) Do not use your bed for anything except sleep and sexual activity; (3) If you find yourself unable to fall asleep after 15-20 min, get up and go into another room. Stay up until you feel very sleepy and then return to the bedroom to sleep; (4) If you still cannot fall asleep within 15-20 min, repeat rule 3. Do this as often as necessary throughout the night; (5) Set your alarm and get up at the same time every morning regardless of how much sleep you got during the night; (6) Limit napping or avoid it entirely.
Module 2: Sleep Hygiene
Sleep hygiene education focuses on behaviors, substances, and environmental conditions that can help or hinder sleep. The following sleep-positive practices were promoted: regular meals, prebedtime routine, regular exercise, limited intake of caffeine, nicotine, and liquids in the evening, and a good-quality sleep environment (quiet, dark, and comfortable).
Module 3: Cognitive Therapy
Cognitive therapy alters dysfunctional beliefs about sleep that contribute to continued insomnia. We educated patients about the following cognitive themes from the 16-item Dys-functional Beliefs and Attitudes about Sleep scale (DBAS-16): perceived insomnia consequences, worry/helplessness about insomnia, sleep expectations, and medications. We also elicited patient-specific maladaptive cognitions, challenged their validity, and used cognitive restructuring strategies to modify them with more adaptive thoughts.
Module 4: Relapse Prevention
The final session with all participants reviewed treatment gains, emphasized the necessary behaviors and adaptive cognitions for maintaining these gains, and discussed relapse prevention. Relapse prevention identifies high-risk situations for insomnia, promotes realistic appraisals about future episodes of insomnia (e.g., distinguish between lapse, relapse, and collapse), and provides behavioral and cognitive strategies for dealing with these high-risk situations and the inevitable occasional poor night of sleep.36
Participants assigned to the IPC condition were mailed the American Academy of Sleep Medicine pamphlet Cognitive-Behavioral Therapy for Insomnia with instructions to review and implement the recommendations. To ensure that the material was reviewed, IPC participants received one 15- to 20-min telephone session with a study therapist, who summarized each page of the pamphlet without providing individualized recommendations.
Treatment Discontinuation
Treatment success for CBTI-Phone participants was defined as achieving ≥ 50% improvement in total wake time (sleep latency + wake after sleep onset) AND sleep efficiency ≥ 85% based on sleep/wake diaries AND ISI score ≤ 7 for two consecutive weeks. Participants who achieved this criterion before session 8 received the Relapse Prevention module and were classified as treatment completers. IPC participants completed posttreatment questionnaires 8 weeks after their phone session.
Study Therapists and Treatment Fidelity
Three clinical psychologists with expertise in CBTI served as study therapists for both conditions. They were supervised throughout the controlled trial by one of the study authors with behavioral sleep medicine credentialing. Phone treatment sessions were audiotaped with participant permission and therapists completed self-ratings of adherence to the treatment module and perceived competence at delivering each session. Ten percent of the audiotaped sessions were reviewed by the supervisor while completing adherence and competence scales, and protocol deviations were remediated immediately.
Assessments
Sleep and Insomnia
Daily sleep/wake diaries were maintained for two consecutive weeks at pretreatment, posttreatment, and 12-week follow-up. CBTI-Phone participants also maintained sleep/wake diaries during treatment. The primary dependent variables derived from these diaries were sleep efficiency ([total sleep time ÷ time in bed] × 100) and total sleep time; secondary variables included sleep latency, frequency of nighttime awakenings, min awake after sleep onset, and sleep quality ratings. Participants also completed the ISI,36 Pittsburgh Sleep Quality Index (PSQI),39 and DBAS-1640 at pretreatment, posttreatment, and 12-week follow-up.
Daytime Functioning
Participants completed validated measures of daytime functioning at pretreatment, posttreatment, and 12-week follow-up, including the Quick Inventory of Depressive Symptomatology– Self-Report (QIDS-SR) to measure depression symptom severity41; State-Trait Anxiety Inventory-Trait subscale (STAI-T) to assess trait anxiety level42; the Multidimensional Fatigue Inventory (MFI-20) to measure daytime fatigue43; and the 12-item Short Form Health Survey (SF-12)44 to measure health-related quality of life.
Statistical Analyses
Descriptive and inferential statistics were computed using SPSS 19.0 (IBM Corporation, Armonk, NY). Data are reported as mean ± standard deviation unless otherwise indicated, with significance level set at 0.05. To evaluate the magnitude of treatment effects, Cohen d effect sizes were calculated for the within-group difference (pretreatment to posttreatment and post-treatment to 12-week follow-up) separately for the CBTI-Phone and IPC conditions.
No differences were found on baseline demographic, sleep, and daytime functioning variables. The primary analyses were based on a 2 (treatment groups) × 3 (pretreatment, posttreatment, 12-week follow-up) split-plot randomized design, with all analyses based on the intent-to-treat model. We used linear mixed models to test main effects of group and time, and the group × time interaction effects for the sleep and daytime functioning variables. For each analysis, the pattern of covariances was modeled using Akaike Information Criteria to determine goodness of fit.45 Significant interactions or main effects involving time were followed up with post hoc independent samples t-tests on difference scores (for interactions) or paired samples t-tests (for main effects) comparing pretreatment to posttreatment and post-treatment to 12-week follow-up.
RESULTS
Participant Recruitment and Retention
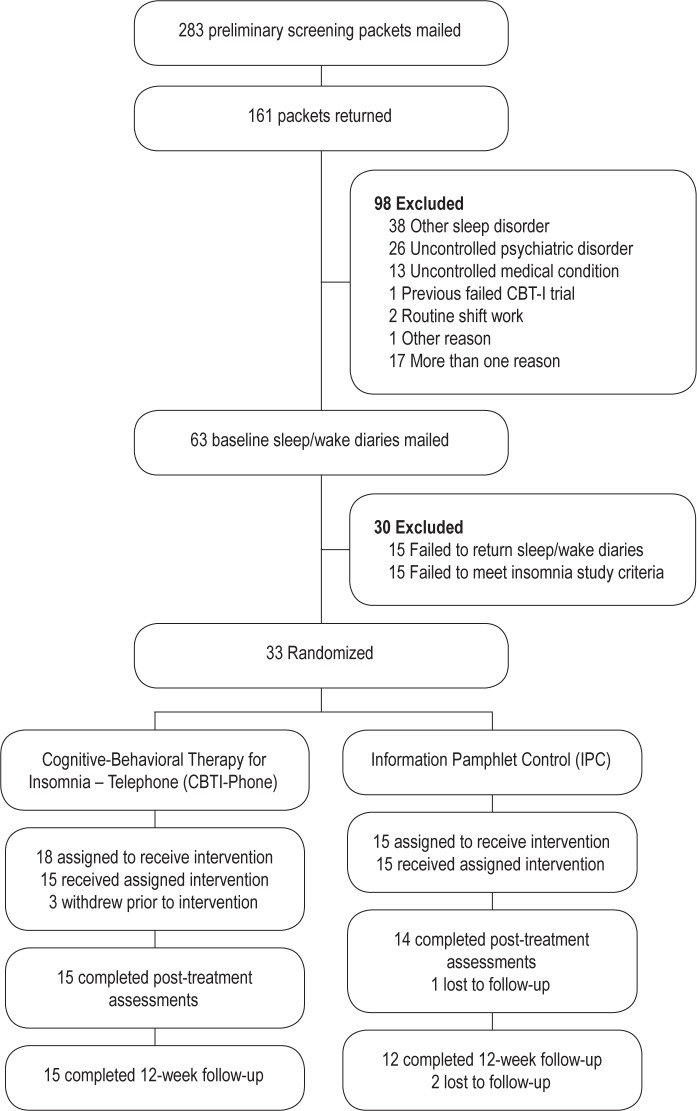
As shown in Figure 1, approximately 20% of study volunteers (33 of 161) who returned completed screening packets and sleep/wake diaries were randomized to one of the treatment conditions. The most common individual reasons for initial exclusion were suspicion of another sleep disorder and poorly controlled medical or psychiatric conditions. Almost one in five respondents had more than one reason for exclusion. Among those individuals who returned baseline sleep/ wake diaries, 23% (15 of 63) were subsequently excluded for failure to meet study criteria for insomnia.
Figure 1.
Participant recruitment and retention in randomized controlled trial of telephone-delivered cognitive-behavioral therapy for insomnia (CBTI-Phone) versus information pamphlet control (IPC).
Six of the 33 randomized participants (18%) discontinued treatment: three CBTI-Phone participants dropped out before treatment initiation, 1 IPC participant was lost to follow-up after the phone session, and 2 IPC participants were lost to follow-up at 12 weeks. All participants who initiated the CBTI-Phone treatment completed treatment and follow-up assessments.
Sleep/Wake Diary and Sleep Questionnaire Outcomes
Means and standard deviations for the sleep/wake diary variables and sleep-related questionnaires are shown in Table 2. Linear mixed-model analyses showed a significant group- by-time interaction (F[2,38.1] = 3.5, P < 0.05) and a time main effect (F[2,38.1] = 46.7, P < 0.001) for sleep efficiency. Post hoc analyses indicated that posttreatment sleep efficiency improved by 18.7 ± 8.2% in the CBTI-Phone group and by 14.5 ± 10.2% for the IPC group (P = not significant). At 12-week follow-up, sleep efficiency dropped slightly in the CBTI-Phone group relative to posttreatment and improved slightly in the IPC group (t[25] = 2.6, P < 0.05), although both groups continued to show clinically relevant improvements relative to pretreatment. Analyses of total sleep time indicated only a significant main effect of time (F[2,40.3] = 19.9, P < 0.001). Post hoc analyses indicated that total sleep time improved from pretreatment to posttreatment by 49.4 ± 54.5 min (t[28] = 4.9, P < 0.001) and by an additional 17.3 ± 54.9 min between posttreatment and 12-week follow-up (P = not significant).
Table 2.
Sleep/wake diary and sleep questionnaire outcomes at baseline, posttreatment, and 12-wk follow-up
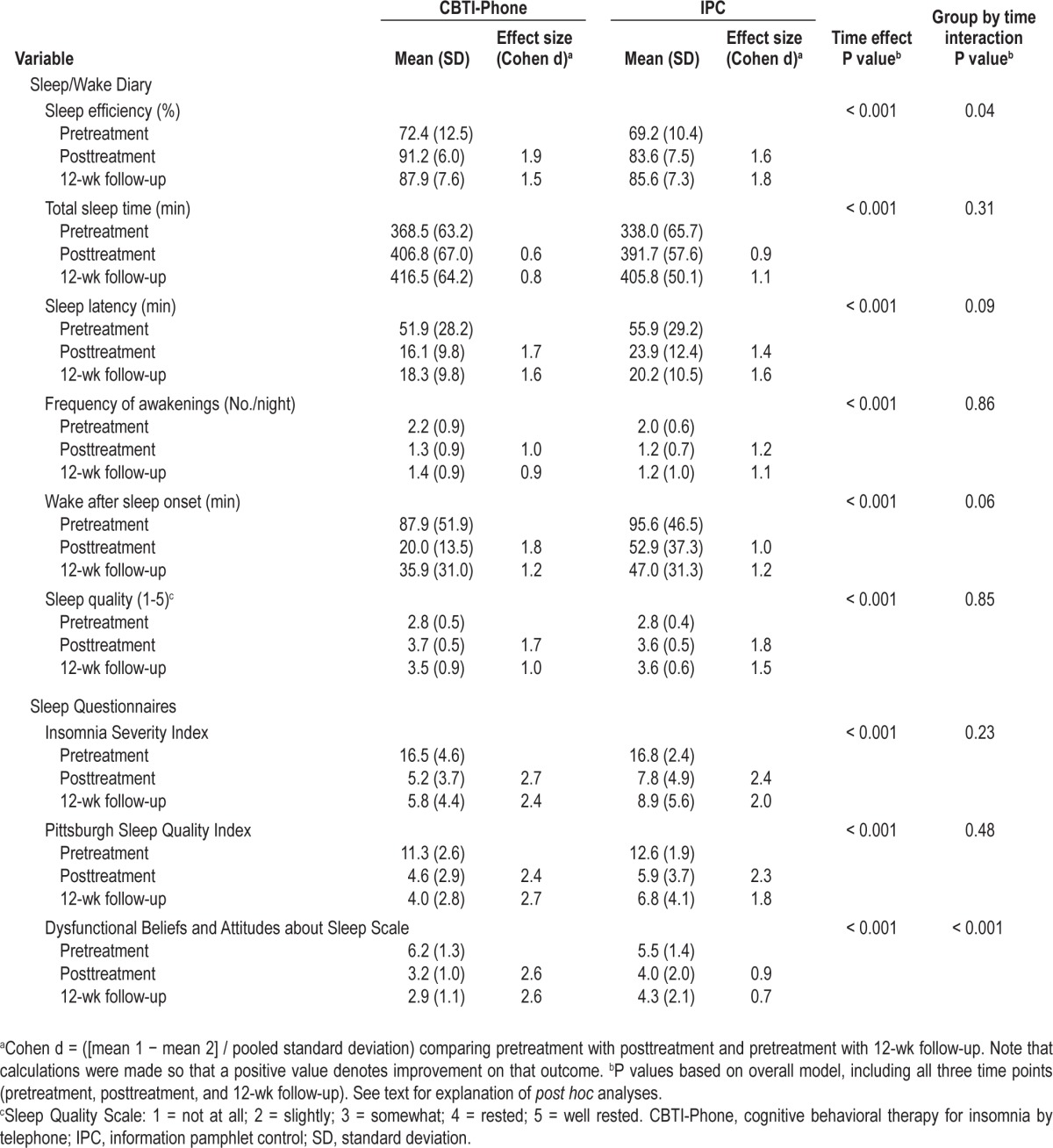
Significant main effects of time were found for the secondary sleep/wake diary outcomes, with no significant group-by-time interactions. Post hoc analyses indicated significant improvements from pretreatment to posttreatment for sleep latency (t[28] = 7.2, P < 0.001), frequency of nighttime awakenings (t[28] = 5.7, P < 0.001), wake after sleep onset (t[28] = 7.0, P < 0.001), and ratings of sleep quality (t[28] = 9.0, P < 0.001). Posttreatment improvements on these parameters were sustained at 12-week follow-up.
Consistent with most of the sleep/wake diary analyses, there were significant time main effects for the ISI (F[2,30.4] = 112.0, P < 0.001) and PSQI (F[2,30.8] = 120.1, P < 0.001), but no significant time-by-group interactions. Post hoc analyses indicated large pretreatment to posttreatment improvements in both groups for the ISI (t[27] = 12.8, P < 0.001) and PSQI (t[27] = 14.3, P < 0.001) with sustained improvements at 12-week follow-up. The group-by-time interaction was significant, however, for the DBAS-16 (F[2,46.5] = 11.2, P < 0.001), which indicated that scores improved more in the CBTI-Phone than the IPC group from pretreatment to posttreatment and from posttreatment to 12-week follow-up.
Treatment Responder and Remitter Analyses
We evaluated the clinical significance of the sleep treatment changes in both treatment conditions using published criteria based on the ISI.46 A marked treatment response was defined as a posttreatment ISI score that was > 8 points less than the pretreatment score; treatment remission was characterized as a posttreatment ISI score ≤ 7. Using these criteria, 13 of 15 CBTI-Phone participants had a marked treatment response at posttreatment compared with 7 of 15 IPC participants (X2 (1) = 5.40, P < 0.02). Additionally, 11 of 15 CBTI-Phone participants achieved remission to insomnia at posttreatment compared with 6 of 15 of the IPC participants (X2 (1) = 3.39, P < 0.06). At follow-up, 9 of 15 CBTI-Phone participants continued to experience a marked treatment response compared with 6 of 12 IPC participants (X2 (1) = 1.20, P = not significant). More participants in the CBTI-Phone group (12 of 15) than the IPC group (5 of 12) were classified as in remission from insomnia at 12-week follow-up (X2 (1) = 6.65, P < 0.01).
Daytime Functioning Outcomes
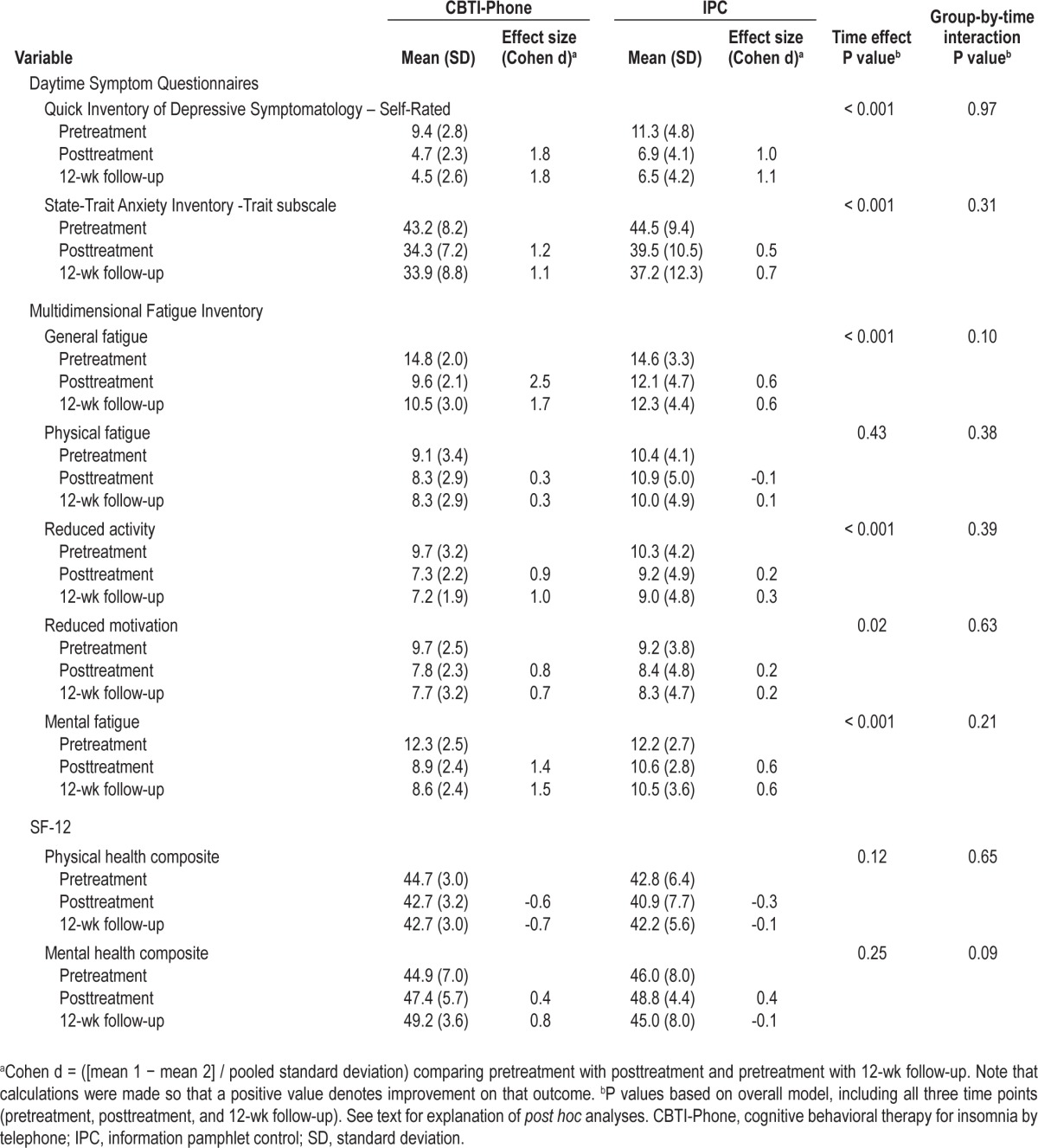
Means and standard deviations for the daytime functioning variables are shown in Table 3. Posttreatment reductions in depressive symptom severity as measured by the QIDS-SR (t[26] = 7.0, P < 0.001) and the trait subscale of the STAI (t[27] = 6.1, P < 0.001) were found for both groups, with no group-by-time interaction. Significant main effects of time were found for all MFI-20 subscales except Physical Fatigue, with no significant group-by-time interactions. Post hoc analyses indicated improvements across both groups on the General Fatigue (t[28] = 5.9, P < 0.001), Reduced Activity (t[27] = 3.7, P < 0.001), Reduced Motivation (t[28] = 2.6, P < 0.01), and Mental Fatigue (t[28] = 5.2, P < 0.001) subscales. No significant main effects or interactions were found for either the Physical Health or Mental Health Composite scores of the SF-12. Within-group improvements in the CBTI-Phone group were large for nearly all of the daytime functioning outcomes; in contrast, within-group effect sizes for IPC participants ranged from small to moderate for most outcomes. Daytime functioning variables that showed posttreatment improvements continued to remain at posttreatment levels at 12-week follow-up.
Table 3.
Daytime functioning outcomes at baseline, posttreatment, and 12-wk follow-up
Treatment Fidelity
All participants assigned to IPC received the 15- to 20-min telephone review session. CBTI-Phone participants completed an average of 5.1 ± 1.7 sessions (range, four to eight sessions). Session durations ranged from 16.7 ± 2.9 min for Session 7 to 59.4 ± 12.8 min for Session 1. Total session duration was not related to treatment outcome, using change in ISI score from pretreatment to posttreatment as an index of treatment response (r = 0.19, P = 0.52).
All of the treatment sessions were audiotaped with participant permission and 10% of sessions in both treatment conditions were randomly selected for independent review. Each taped session was evaluated against a checklist of all elements to be covered from the manualized treatment or from the information brochure. These sessions were rated as being 100% pure and meeting all of the requirements for each session.
DISCUSSION
The findings from this randomized controlled trial provide preliminary support for telephone-delivered CBTI in the treatment of chronic insomnia. Participants who received CBTI by telephone showed large effects at posttreatment for diary-based sleep efficiency, more improvements in cognitions related to insomnia than participants receiving an information pamphlet, and moderate to large changes in the common daytime consequences of insomnia. More CBTI-Phone than IPC participants were classified as “marked treatment responders” at posttreatment and more CBTI-Phone participants were in remission from insomnia at 12-week follow-up. Posttreatment effect sizes for CBTI-Phone on both the sleep/wake diary and daytime symptom outcomes generally ranged from moderate to large, which are consistent with effect sizes reported from both in-person randomized controlled trials of CBTI11–13,47 and hypnotic agents48 in middle-aged adults with chronic insomnia. Finally, the acceptability and feasibility of telephone-delivered insomnia therapy was supported by excellent retention rates (100%) throughout treatment and at 12-week follow-up.
Current standards for insomnia research advocate for the inclusion of measures to assess waking correlates of insomnia.49 In the current study, we found that both CBTI-Phone and IPC led to improvements in daytime symptoms of fatigue, depression symptoms, and trait anxiety. Depression symptoms for participants in the CBTI-Phone condition improved from mildly symptomatic to within normal limits with treatment, whereas IPC participants reported moderately high depression symptoms at baseline and only mild symptoms at treatment completion.41 Similarly, baseline mean scores on the trait subscale of the STAI were slightly elevated in both groups, but decreased to within normal limits by the end of treatment.42 Baseline fatigue scores for both treatment groups were equivalent to other insomnia populations with high levels of fatigue (e.g., patients with nonmetastatic breast cancer) and the magnitude of posttreatment improvement was similar.50 Although there were no significant group-by-time interactions for any daytime symptom measure, CBTI-Phone participants consistently showed large improvements in these outcomes, compared with small to medium treatment effects for IPC participants. It is important to note that the study excluded potential participants with poorly controlled psychiatric and medical conditions, which likely contributed to restricted ranges on these outcomes at baseline. As a result, our findings cannot be extended to individuals with chronic insomnia and clinically significant levels of depression, anxiety, and fatigue. Nevertheless, the findings do suggest that the CBTI-Phone intervention may improve daytime sequelae typical of chronic insomnia.
Acute sleep and daytime functioning treatment gains were sustained at 12-week follow-up in CBTI-Phone participants. We found no significant posttreatment to follow-up differences on any sleep/wake diary or daytime symptom outcome. Moreover, we found that 80% of CBTI-Phone participants (12 of 15) continued to be classified as “in remission” from insomnia 12 weeks after treatment completion. These findings lend preliminary support to the sustainability of treatment gains using telephone-delivered CBTI.
The findings from our study are consistent with those from the only other existing controlled study to evaluate telephone-delivered CBTI.34 In that study, telephone-delivered CBTI produced improvements in sleep and daytime symptoms that were equivalent in magnitude to those observed after treatment delivered individually and in groups. Acute treatment gains were also sustained 3 and 6 mo posttreatment. Components of treatment were similar to the ones used in our study; however, screening and data collection were conducted in person, even for participants assigned to receive CBTI by telephone. Thus, our study demonstrated the efficacy of CBTI for chronic insomnia, while providing a potentially less costly and more convenient alternative to in-person CBTI. Our study is also the first to compare telephone-delivered CBTI to a much simpler delivery format (pamphlet), and to show that CBTI-Phone offers advantages over improvements that may occur due to the pamphlet intervention, time, or placebo effect.
Our study had components that differed from most insomnia treatment trials. First, screening of participants, collection of study assessments, and treatment delivery were all conducted by mail and telephone rather than in person. The primary benefit of this approach is that study participation was not restricted by residence location. Although we limited the scope of our recruitment to a geographically small area, future studies should include individuals living in rural or remote locations with limited resources, and those with limited mobility or with travel restrictions. Screening and study outcomes could also be administered by computer, further enhancing feasibility and reducing costs. Second, we intentionally built into our study design an a priori criterion of “treatment success” to determine treatment discontinuation rather than delivering a predetermined number of treatment sessions (e.g., six or eight sessions). We opted for this method because it more accurately reflects the approach taken in most clinical settings. As a result, we were able to deliver the CBTI-Phone intervention in a relatively time-efficient manner (mean sessions = 5.1 ± 1.7, with 60% requiring only four sessions). The validity of our “treatment success” criteria was supported by the maintenance of acute treatment gains at 12-wk follow-up, with 80% of CBTI-Phone participants (12 of 15) being classified as “in remission” from insomnia. We believe that this approach to determine appropriate treatment termination is more likely to be adopted in clinical practice and thus should be considered in subsequent insomnia treatment trials.
CBTI-Phone participants showed clear benefits in terms of sleep and daytime symptoms at posttreatment and follow-up, but it is important to note that IPC participants showed improvements of a similar magnitude on most of the sleep and daytime symptom assessments. This finding was unexpected, because other recent insomnia trials using information controls have not found similar posttreatment sleep improvements.51 Differences in methods could account for a greater treatment response among our control participants. For example, we used a different information pamphlet from the American Academy of Sleep Medicine (Cognitive-Behavioral Therapy for Insomnia vs. Insomnia, Sleep as We Grow Older, and Sleep Hygiene), which may have had more content overlap with our active telephone-delivered intervention. Second, both studies conducted telephone follow-up with control participants but we specifically reviewed the pamphlet during the phone session, whereas participants in the study by Buysse et al.51 were encouraged to continue study participation. Although the pamphlet review was conducted without providing individualized recommendations, the approach in our study may have been more therapeutic than we anticipated, or at least the implicit endorsement of this information through the review may have motivated participants to adhere more closely to the recommendations. The phone consultation may have also been the initial review of the material for some participants. Nevertheless, our findings suggest that an educational pamphlet plus a single phone follow-up may be a cost-effective, efficacious, and minimally burdensome treatment for certain patients with insomnia. Similar simple interventions have been used successfully in other areas of sleep medicine, such as to improve continuous positive airway pressure adherence in patients with obstructive sleep apnea.52 Future studies should seek to clarify which insomnia subtypes are most likely to respond to these simple insomnia interventions. These evaluations should also include daytime symptom measures, because our study found consistently greater posttreatment improvement on these variables among CBTI-Phone participants.
The major strengths of the current study were the randomized controlled trial design, active minimal intervention comparison condition, and inclusion of methods to ensure treatment integrity. The findings need to be interpreted, however, in the context of several limitations. The study sample included predominantly women with chronic primary insomnia, a minority of whom had medical/psychiatric comorbidities and/or used hypnotic medication. The generalizability of our findings to males with insomnia and those with more clinically relevant psychiatric and medical symptoms remains unknown. Moreover, the observed maintenance of treatment gains at follow-up need to be viewed cautiously given that assessments were conducted only 12 weeks posttreatment. Our primary outcomes were based on subjective reports, which are the preferred primary outcome for insomnia trials, but still subject to response bias. Inclusion of objective measures of sleep, such as actigraphy or polysomnography, would have strengthened the study design by providing a more detailed assessment of sleep. Sixty percent of CBTI-Phone participants required only four sessions to achieve “treatment success,” but the duration of some sessions was longer than would be practical in many clinical contexts (e.g., 75th percentile of session duration was 50.1 min). Thus, future work is required to refine the treatment sessions further and maximize treatment efficacy while minimizing participant burden. Finally, participant screening was conducted entirely by questionnaire and follow-up phone assessments. We therefore had no objective verification that participants in our study did not have other sleep or medical disorders that were causally related to their insomnia. The magnitude of improvements in the current study, however, suggest that sleep improved for most participants in spite of any other conditions that may have been present.
In summary, our findings support the efficacy of telephone-delivered CBTI to improve sleep and waking function in individuals with chronic primary insomnia. The results from this study can be placed in the context of other previously explored CBTI modalities, including brief in-person51,53 group,54 biblio-therapy with and without consultations,21,22,32 and Internet.25–27 Future large-scale controlled treatment studies are warranted in more diverse samples of individuals with chronic insomnia using both trained and naïve therapists to deliver the intervention. These studies should also include cost analyses to compare different modalities for delivering CBTI. If future studies support the benefits of this approach, telephone-delivered CBTI could be offered as an effective treatment option in a stepped-care model to treat insomnia.
ACKNOWLEDGMENTS
This project was supported by Award Number UL1RR024986 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors thank Sehar Minhas for her help with recruiting study participants and with data entry and verification.
Footnotes
A commentary on this article appears in this issue on page 303.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chervin serves on the board of directors for the AASM, ABSM, ASMF, and International Pediatric Sleep Association is on the advisory board of Sweet Dreamzzz. He has received research support from Philips Respironics, Fisher Paykel and has consulted for the NIH, Proctor & Gamble, and Zansors. He serves as a section editor for UpToDate and receives remuneration. Dr. Arnedt serves on the advisory board of Health Media Inc. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition Criteria: Results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 3.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 4.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 6.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34:997–1011. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of U.S. workers: results from the America Insomnia Survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–73. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients' acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15:302–5. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 10.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24:411–7. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 12.Murtagh DRR, Greenwood KM. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 16.Backhaus J, Hohagen F, Voderholzer U, Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251:35–41. doi: 10.1007/s004060170066. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–96. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 18.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295:2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 19.Espie CA. “Stepped Care”: A health technology solution for delivering cognitive behavioral therapy as first line insomnia treatment. Sleep. 2009;32:1549–58. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Mimeault V, Morin CM. Self-help treatment for insomnia: bibliotherapy with and without professional guidance. J Consult Clin Psychol. 1999;67:511–9. doi: 10.1037//0022-006x.67.4.511. [DOI] [PubMed] [Google Scholar]
- 22.Morin CM, Beaulieu-Bonneau S, LeBlanc M, Savard J. Self-help treatment for insomnia: a randomized controlled trial. Sleep. 2005;28:1319–27. doi: 10.1093/sleep/28.10.1319. [DOI] [PubMed] [Google Scholar]
- 23.Jernelov S, Lekander M, Blom K, et al. Efficacy of a behavioral self-help treatment with or without therapist guidance for co-morbid and primary insomnia: a randomized controlled trial. BMC Psychiatry. 2012;12:5. doi: 10.1186/1471-244X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorvatn B, Fiske E, Pallesen S. A self-help book is better than sleep hygiene advice for insomnia: A randomized controlled comparative study. Scand J Psychol. 2011;52:580–5. doi: 10.1111/j.1467-9450.2011.00902.x. [DOI] [PubMed] [Google Scholar]
- 25.Ström L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol. 2004;72:113–20. doi: 10.1037/0022-006X.72.1.113. [DOI] [PubMed] [Google Scholar]
- 26.Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807–15. doi: 10.1093/sleep/32.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66:692–8. doi: 10.1001/archgenpsychiatry.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive-behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–81. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13:61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006;3:CD002850. doi: 10.1002/14651858.CD002850.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change. Am J Prev Med. 2007;32:419–34. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Currie SR, Clark S, Hodgins DC, el-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcohlics. Addiction. 2004;99:1121–32. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 33.Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49:227–33. doi: 10.1016/j.brat.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastien CH, Morin CM, Ouellet MC, Blais FC, Bouchard S. Cognitive-behavioral therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72:653–9. doi: 10.1037/0022-006X.72.4.653. [DOI] [PubMed] [Google Scholar]
- 35.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 36.Morin CM. Insomnia: psychological assessment and management. New York: The Guilford Press; 1993. [Google Scholar]
- 37.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 38.Bootzin RR, Nicassio PM. Behavioral treatments for insomnia. In: Hers-en M, Eissler R, Miller P, editors. Progress in behavior modification. New York, NY: Academic Press; 1978. pp. 1–45. [Google Scholar]
- 39.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 42.Spielberger CD, Gorsuch RL, Luchene R. The State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 43.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 44.Ware JE, Jr., Kosinski M, Keller SD. A 12-item short-form survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 46.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 48.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–7. [PubMed] [Google Scholar]
- 49.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 50.Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol. 2003;71:189–200. [PubMed] [Google Scholar]
- 51.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997;20:284–9. doi: 10.1093/sleep/20.4.284. [DOI] [PubMed] [Google Scholar]
- 53.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2002;26:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 54.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]