Abstract
Background:
Patients with obstructive sleep apnea/hypopnea syndrome (OSAHS), even those generally compliant with CPAP therapy, often intermittently discontinue CPAP.
Study Objective:
Examine the impact of CPAP withdrawal on sleep, sleep disordered breathing (SDB), and daytime function in subjects with varying severity of OSAHS.
Patients and Interventions:
Forty-two subjects (26M/16 F) with OSAHS (AHI4% = 45.2 ± 35.5/h pretreatment) on CPAP for 4 months were evaluated on the second night of CPAP withdrawal. Sleep architecture, SDB indices, and subjective/objective daytime function were assessed pretreatment, on CPAP therapy, and after CPAP withdrawal. Comparisons were made between pretreatment and CPAP withdrawal for the entire group, and for subgroups of mild/moderate (AHI4% < 30/h, n = 22) and severe (AHI4% > 30/h, n = 20) SDB.
Results:
Overall, and for mild/moderate subjects, SDB indices returned to pretreatment values on CPAP withdrawal but with fewer apneas and more hypopneas/RERAs. For severe SDB, the event frequency (AI, AHI4%, and RDI) was lower and O2 desaturation was improved on CPAP withdrawal. Across SDB severity, sleep architecture showed lower %REM (15.6% vs 12.9%, P = 0.009) on the CPAP withdrawal compared to pretreatment. Stanford Sleepiness Score, MSLT, and PVT measures were not significantly different between pretreatment and CPAP withdrawal.
Conclusions:
Over a wide range of SDB severity CPAP withdrawal results in recurrence of SDB, albeit with less severe O2 desaturation. Subjective/objective daytime function returned to pretreatment levels. Sleep architecture changes on CPAP withdrawal (acute SDB) may reflect reduced sleep pressure compared to pretreatment chronic SDB. Our data suggest detrimental effects of even brief withdrawal of CPAP in subjects with both mild and severe OSAHS.
Citation:
Young LR; Taxin ZH; Norman RG; Walsleben JA; Rapoport DM; Ayappa I. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. SLEEP 2013;36(3):405-412.
Keywords: CPAP withdrawal, daytime sleepiness, sleep disordered breathing
INTRODUCTION
Continuous positive airway pressure (CPAP) is the standard therapy for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS).1 When used, CPAP eliminates obstructive sleep disordered breathing (SDB) events by stabilizing the upper airway. Improvements in daytime sleepiness,2,3 neurocognitive function, and cardiovascular morbidity4 have been shown to correlate to consistent CPAP use, but despite the effectiveness of CPAP therapy, acceptance and adherence among patients is highly variable.5 Even normally compliant patients often intermittently spend several nights without CPAP, but data are limited on the effects of brief CPAP cessation.
CPAP withdrawal has been reported to cause immediate recurrence of SDB to levels of severity either similar to6–11 or slightly less12–17 than levels seen at pretreatment. Most published studies have reported that O2 desaturation on CPAP withdrawal is less severe than pretreatment.10–12,14–17 Potential mechanisms suggested for this effect include changes in upper airway anatomy, changes in ventilatory and upper airway control mechanisms, and effects of changes in sleep on the above. In two studies,13,14 subjective (Stanford sleepiness scale [SSS]) and objective measures (multiple sleep latency test [MSLT], psychomotor vigilance test [PVT]) of sleepiness were compared on CPAP withdrawal to pretreatment, but with conflicting results and limited statistical significance for some results. Limitations of all studies examining CPAP withdrawal effects include small sample size (8-30 subjects) and inclusion of subjects with predominantly severe SDB. The present study re-examines the effect of two nights of CPAP withdrawal compared to pretreatment for levels of SDB indices, O2 desaturation, sleep architecture, and daytime function in a larger group of subjects with a spectrum of SDB severity. Specifically, we contrasted the effect of CPAP withdrawal (compared to pre-treatment level) between subjects with mild/moderate SDB and subjects with severe SDB.
METHODS
Forty-two subjects (AHI4% = 45.2 ± 35.5/h) were recruited from 106 participants in a larger study relating SDB to daytime function. To be included in the present study, patients in the target population (i.e., complaining of snoring and/or excessive daytime sleepiness) had to have either a respiratory disturbance index (RDI) > 10/h or apnea hypopnea index (AHI4%) > 5/h (see full SDB index definitions below) on full in-laboratory nocturnal polysomnography (NPSG) and be effectively treated with CPAP with adherence objectively monitored for 2-3 months prior to undergoing two nights of CPAP withdrawal. All subjects were over 18 years of age and had stable medical histories with no significant comorbidities, as well as no change in medications that could affect excessive daytime somnolence during the study. Subjects were excluded if they were unable to provide consent. Also excluded from the study were subjects with suspected diagnosis of a non-SDB condition which could explain their EDS symptoms (e.g., periodic leg movements, narcolepsy, central sleep apnea, sleep hypoventilation syndromes, insomnia).
Of the initial 106 patients who underwent full NPSG, 19 were excluded: 7 had a diagnosis of SDB plus other sleep disorder (PLMSs, shift work disorder) and 12 were diagnosed with other sleep disorders (complaint of sleepiness/snoring, but AHI4% < 5/h and no evidence of elevated upper airway resistance by inspiratory flow limitation found on diagnostic PSG study). Forty-two of these 87 subjects completed the CPAP initiation period with sufficient CPAP use to be included in the present analyses and the 2-night CPAP withdrawal.
All patients underwent comprehensive sleep evaluation by a physician at the NYU Sleep Disorders Center, diagnostic NPSG, and subjective and objective tests of daytime function on the following morning. Figure 1 outlines the protocol: CPAP was titrated using standard clinical guidelines to eliminate SDB events and inspiratory flow limitation. The validity of the pressure chosen was confirmed by reviewing the airflow tracing and pressure signals that were recorded continuously for 2 weeks on a custom CPAP machine (Fisher & Paykel Healthcare, Auckland, NZ). Following titration, patients used CPAP at this therapeutic pressure, with adherence (defined as hours of use at pressure) recorded on the device. After 2-3 months, subjects underwent a repeat laboratory NPSG on CPAP and evaluation of daytime function. Patients continued CPAP therapy at home and were then instructed to discontinue CPAP for 2 consecutive nights; the second night was monitored in laboratory with a full NPSG, followed by daytime testing. This protocol was approved by the NYU IRB, and all patients signed informed consent documents. The clinical trials registration number is NCT00393913.
Figure 1.

Outline of study protocol. Daytime function assessments included the multiple sleep latency test (MSLT), psychomotor vigilance test (PVT), Stanford Sleepiness Scale score (SSS), Epworth Sleepiness Scale score (ESS), and functional outcomes of sleep questionnaire (FOSQ). ESS and FOSQ were not administered at CPAP withdrawal, as they do not accurately assess acute sleepiness.
The diagnostic, on-CPAP, and CPAP withdrawal studies consisted of full-night in-laboratory NPSG performed according to AASM guidelines. Recordings of frontal, central, and occipital electroencephalogram (EEG), electrooculogram (EOG), and submental electromyogram (EMG) were used to monitor sleep metrics. Leg movements were monitored with an anterior tibialis EMG. A unipolar electrocardiogram (ECG) was used for cardiac monitoring. Oxygen saturation was monitored with a pulse oximeter (Masimo). Chest wall and abdominal movement were monitored with piezoelectric strain gauges. Sleep position was monitored using a multiposition switch. Respiratory airflow was measured using a nasal cannula pressure transducer system (Protech PTAF2), along with an oral thermistor (diagnostic NPSG only) to detect mouth breathing. During the CPAP studies, flow was recorded directly from the CPAP machine.
The NPSGs were scored for sleep and SDB events using the 2007 guidelines of the American Academy of Sleep Medicine (AASM),18 and all studies were scored by a single scorer. From these measures, absolute and relative amounts of each sleep stage, latency to each sleep stage, total sleep time (TST), sleep efficiency, and wake after sleep onset (WASO) were all calculated. Arousals were scored based on AASM criteria: the arousal index was calculated as the total number of all types of arousals per hour of sleep. Patients with clinically relevant SDB as defined above underwent manual CPAP titration. Apneas were defined as > 90% decrease in airflow relative to baseline flow lasting > 10 seconds. Hypopneas were defined as > 30% decrease in airflow with a 4% desaturation (AASM “recommended” criteria). Respiratory effort-related arousals (RERAs) were defined as visible reductions in airflow with flow limitation that were also associated with an arousal. AHI4% was calculated as the sum of all apneas and hypopneas with 4% oxygen desaturation divided by the TST. A respiratory disturbance index (RDI) was calculated, corresponding to the AASM alternate definition of AHI (and includes RERAs).
Mean apnea duration for each subject was calculated in the supine and non-supine positions, but the duration of hypopneas was not calculated as it was difficult to reproducibly apply a consistent definition for the start of hypopneas. Oxygen saturation was summarized for each NPSG by tabulating the %time < 90%saturation, the mean O2 saturation during sleep, and the minimum value of oxygen saturation across the entire study. We also tabulated the minimum value of oxygen saturation in the 30 sec following each apnea. Using these data, we were able to reliably calculate each subject's mean lowest saturation per apnea on the diagnostic and off-CPAP NPSGs.
Subjective and Objective Assessment of Daytime Function
The effects of CPAP withdrawal on daytime hypersomnolence were examined based on sleep tendency, subjective sleepiness, and performance. These criteria were assessed following each of the three NPSGs: diagnostic, on CPAP, and off CPAP. Subjective daytime sleepiness was evaluated using the Stanford Sleepiness Scale (SSS). The Epworth Sleepiness Score (ESS) was obtained at pretreatment on the diagnostic NPSG, but not on CPAP withdrawal. The ESS is not designed to assess daytime sleepiness after acute changes in sleep conditions; the questionnaire reflects a longer period of time and is not relevant to a 2-night CPAP withdrawal period. Vigilance was assessed using the psychomotor vigilance task (PVT). Each PVT lasted 20 min and was given four times during the daytime testing period at 2-h intervals, beginning 1.5 h after awakening. Average mean reaction time and frequency of lapses (defined as a RT > 500 milliseconds) were calculated. Sleep latency was assessed using the MSLT. Patients were given the opportunity to fall asleep during a 20-min period 4 times a day at 2-h intervals, and the tests were administered 30 min after the PVT. Sleep latency on each 20 minute test was calculated as elapsed time from lights out to the first epoch scored as sleep and averaged over the 4 tests.
The effects of CPAP withdrawal on SDB indices, O2 desatu-ration, sleep architecture, and daytime outcomes of subjective (SSS) and objective (MSLT, PVT) sleepiness were examined and compared to pretreatment and CPAP therapy values. Twelve patients averaged < 4 h/night of CPAP use, 2 did not have data, and 28 averaged > 4 h/night. To assess the impact of this imperfect adherence, we reanalyzed our data excluding all subjects with CPAP adherence < 4 h/night.
Data analysis was conducted with SPSS 17. Paired t-tests were used to assess differences between patients' pretreatment and CPAP withdrawal data. Analysis was for the entire group and subsets created by grouping subjects; (1) using standard clinical cut-points for pretreatment SDB severity: mild/moderate (AHI4% < 30/h, n = 22) and severe (AHI4% > 30/h, n = 20), (2) using the lowest and highest tertiles of AHI4% (AHI4% < 19/h), n = 14) and AHI4% > 69/h, n = 14). For comparisons between pretreatment and CPAP withdrawal data, a P value of 0.01 was used to identify statistically significant differences due to the large number of comparisons made. The term “trend” is used for P values between 0.05 and 0.01. Significance was assessed at P value of 0.05 for all other comparisons.
RESULTS
Table 1 shows subject demographics, pretreatment levels of sleepiness, and CPAP adherence data for the entire dataset and also grouped by SDB severity. The dataset consisted of 26 males/16 females with the following race and ethnicity distribution; 31% White, 29% Black, 12% Asian, and 28% Other; 74% non-Hispanic. As expected, the severe SDB group had a higher BMI (P = 0.02) and lower Functional Outcomes of Sleep Questionnaire (FOSQ) score (P = 0.03) than the mild/ moderate SDB group, although they had similar ESS scores. Mean overall CPAP adherence at three months was 5.3 ± 1.8 h per night (n = 40) but was higher in subjects with severe SDB (P = 0.02). Objective CPAP adherence data were not available for two subjects for the entire 3-month period due to CPAP recording malfunction.
Table 1.
Pretreatment (baseline) data in all subjects, and grouped by SDB severity.
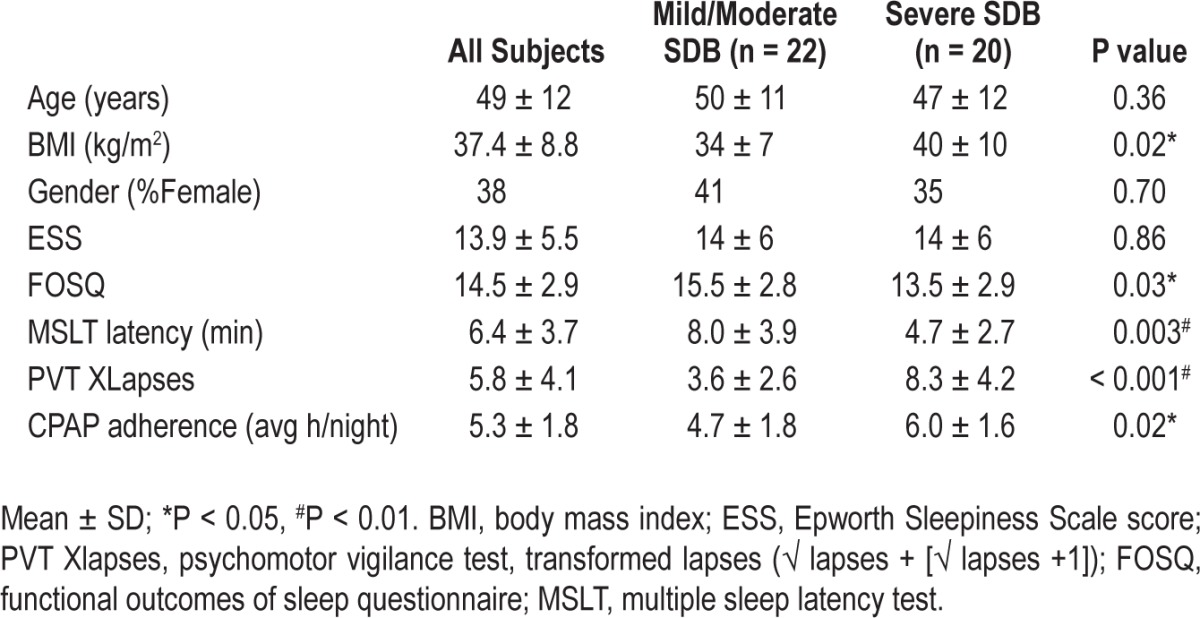
As expected, after approximately 3 months on therapeutic CPAP (mean ± SD = 91 ± 32 days) all subjects showed significant improvement in sleep architecture and SDB metrics on the CPAP NPSG compared to the pretreatment NPSG. These data are summarized in Tables S1A, S1B, S2A, and S2B in the supplemental material. There were also improvements in subjective (ESS, P = 0.002; SSS, P < 0.001; FOSQ, P = 0.04) and objective (MSLT latency, P = 0.02; PVT lapses, P = 0.001) measures of sleepiness on CPAP compared to pretreatment values (see Table S3A). Improvement in ESS was similar in both mild/moderate and severe SDB subject groups, but significant changes in the other outcome variables we seen only in the severe SDB group (see Table S3B).
The mean interval between the pretreatment NPSG and the CPAP withdrawal NPSG was 4 months (mean ± SD = 126 ± 37 days), and all subjects had been on CPAP ≥ 2 months prior to the CPAP withdrawal NPSG. There was no difference in BMI measured at these time points (37.4 ± 8.8 vs 37.2 ± 8.6 kg/m2; P = NS).
When comparing the metrics of SDB and O2 saturation between the pretreatment NPSG and CPAP withdrawal NPSG in all subjects (see Table S4 in supplemental material), there were no differences between the two off-CPAP studies in the time spent supine. Although the RDI was similar in both NPSGs, (pretreatment 50.6 ± 33.7/h vs CPAP withdrawal 45.8 ± 27.7/h, P = 0.10) for the entire group, the apnea index (AI) was lower (32.4 ± 31.3/h vs 24.7 ± 25.0/h P = 0.008), and the AHI4% showed a trend towards being less severe (45.2 ± 35.5/h vs 38.2 ± 29.9, P = 0.02) on the CPAP withdrawal NPSG. The lower ratio of apneas to all events on the CPAP withdrawal NPSG (0.53 ± 0.29 vs 0.42 ± 0.28, P = 0.002; fewer apneas and more hypopneas/RERAs) suggests a lesser degree of upper airway collapsibility.19 Figure 2 shows the AI, AHI4%, and RDI indices from the pretreatment NPSG plotted against the CPAP withdrawal NPSG in all subjects and suggests that effects were greatest in the patients with severe SDB. Table 2 addresses this directly separating patients into groups based on SDB severity. In the mild/moderate SDB group, there were no differences between any of the SDB indices or levels of O2 saturation on the CPAP-withdrawal and pretreatment NPSGs except for a trend towards a lower ratio of apneas to all events (P = 0.04). In the severe SDB group, however, all indices of SDB and O2 saturation showed improvement on the CPAP withdrawal night compared to the pretreatment NPSG. The results (data not shown) were similar when the analyses were repeated using groups based on AHI4% tertiles rather than the groups defined by AHI4% > or < 30/h.
Figure 2.
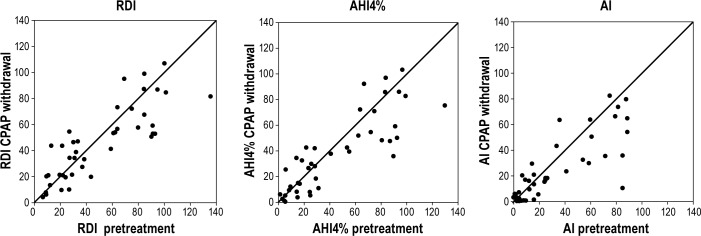
Figure plots RDI, AHI4%, and AI obtained on the pretreatment NPSG (x-axis) versus CPAP withdrawal NPSG (y-axis) along with the line of identity. Each point represents one subject and shows that there is little difference in RDI between pretreatment and CPAP withdrawal NPSG at all SDB levels. In subjects with severe SDB, the AHI4% and AI are lower on the CPAP withdrawal NPSG as seen by the predominance of data below the line of identity.
Table 2.
SDB metrics by patient groups based on SDB severity (mean ± SD). Comparisons are between pretreatment and CPAP withdrawal NSPG in each group.
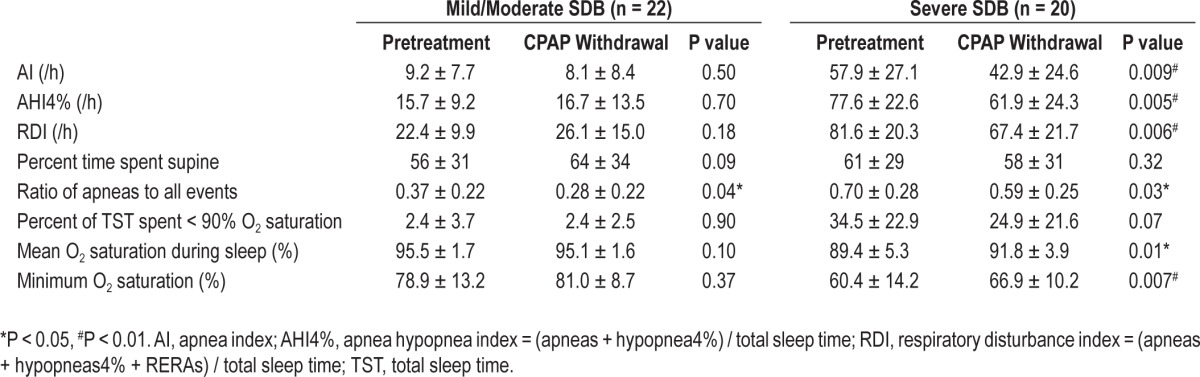
In the subjects with severe SDB, the minimum O2 saturation was higher (less severe O2 desaturation) on the CPAP withdrawal NPSG than on the pretreatment NPSG. In order to assess a possible cause for the improved oxygen levels on the CPAP withdrawal night compared to the pretreatment night, we compared the duration of apneas between the 2 NPSGs in the 20 subjects with severe SDB. There were, on average, 284 apneas per subject (range 4-554) during the CPAP withdrawal NPSG and 394 apneas per subject (47-721) during the pretreatment NPSG, with no differences in apnea duration between the nights, whether analyzed for all sleep (26.0 ± 7.3 vs 24.2 ± 7.0 sec, P = 0.06) or specifically for supine (25.1 ± 8.6 vs 24.3 ± 7.4 sec, P = 0.46) or non-supine (23.9 ± 6.5 vs 24.1 ± 8.1 sec, P = 0.46) sleep. The value of the minimum O2 saturation associated with apnea was, on average, higher (85.9% ± 5.5% vs. 82.4% ± 7.7%, P = 0.003) on the CPAP withdrawal NPSG. A sample plot of data of all apneas in one subject is shown in the supplemental material to illustrate this (Figure S1).
Table 3 shows data comparing metrics of sleep architecture between the CPAP withdrawal and the pretreatment NPSGs in groups based on SDB severity. Table S5 shows the same data in the entire group. Overall, TST was similar between pretreatment and CPAP withdrawal NPSG. Despite no demonstrable change in mean continuity of sleep, other sleep variables (WASO and sleep efficiency) were improved on CPAP withdrawal compared to pretreatment. These findings had stronger statistical significance in the severe than in the mild/moderate SDB group. Figure 3 plots the difference in %REM between pretreatment and CPAP withdrawal against pretreatment SDB severity. Overall there was consistently less %REM seen on the CPAP withdrawal NPSG than pretreatment, but there was no clear relationship to SDB severity.
Table 3.
Differences in sleep architecture between Pretreatment and CPAP withdrawal NPSG, separated into patient groups based on by SDB severity group (mean ± SD)
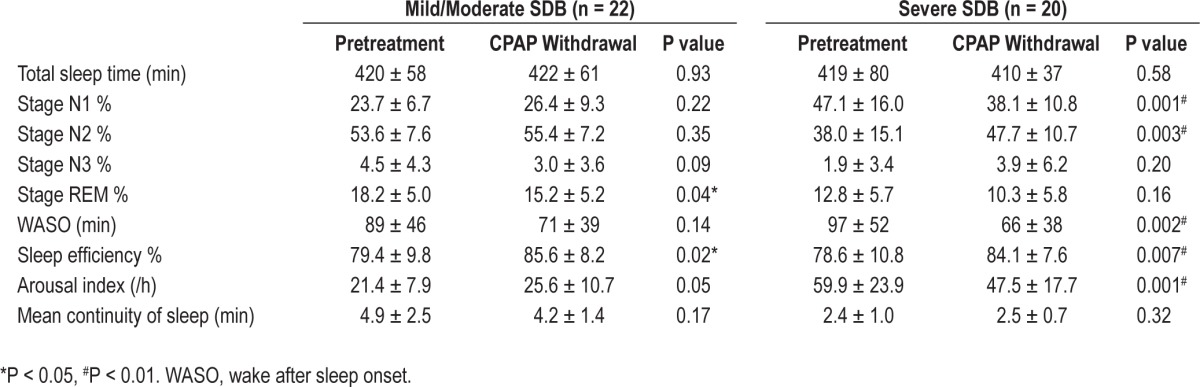
Figure 3.
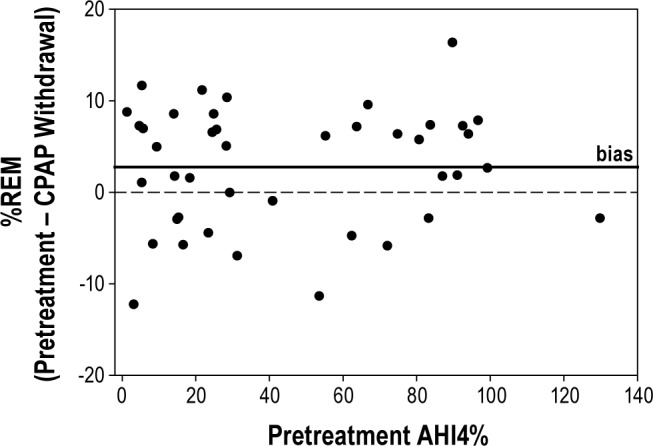
Pretreatment SDB severity assessed by AHI4% is plotted against the difference in %REM (pretreatment minus CPAP withdrawal NPSG). Each point represents one subject. Overall, subjects had higher %REM (2.8% ± 6.5%) on the pretreatment NSPG, and there was no clear effect of SDB severity.
When comparing the measures of daytime function on CPAP therapy to those obtained after CPAP withdrawal, there was a statistically significant worsening of subjective and objective outcomes compared to the values while on CPAP (see Table S3A in supplemental material). These differences in daytime function on and off CPAP were also seen when the severe and mild/ moderate SDB groups were examined separately, although the significance of the findings in the mild/moderate group is lower; this may be due to the much smaller demonstrable benefit of CPAP on this group (see Table S3B in supplemental material).
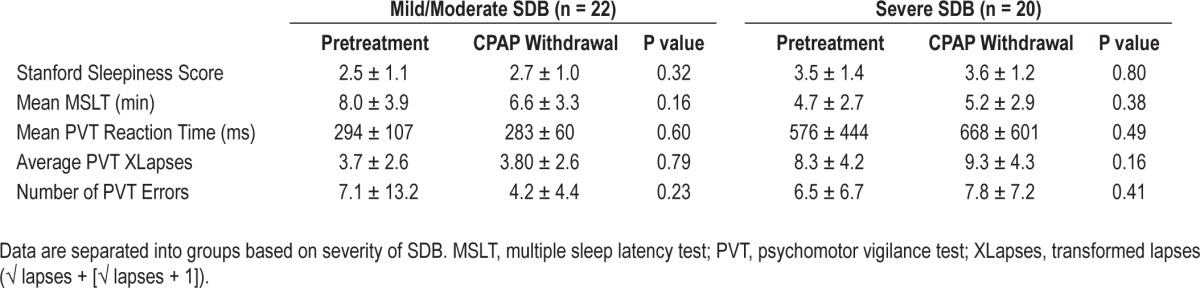
Table 4 shows the data comparing subjective (SSS) and objective measures of sleepiness (MSLT and PVT) obtained after the pretreatment and CPAP withdrawal nights, separating patients by pretreatment SDB severity. There were no differences between the measurements made after the pretreatment and the CPAP withdrawal NSPG in either group. Results (data not shown) were similar when the analyses were repeated using groups based on AHI4% tertiles rather than the groups defined by AHI4% > or < 30/h.
Table 4.
Comparison of measures of daytime function after Pretreatment and after CPAP withdrawal (mean ± SD)
When the subset of patients with poor CPAP adherence were excluded (n = 12), despite a smaller sample size (n = 28) we were able to reproduce essentially all the findings of the full study. With CPAP withdrawal, subjective (SSS) and objective daytime (MSLT, PVT variables) function returned to pretreatment values. The statistically significant changes in SDB variables seen in the entire data set were also seen in this subset with good CPAP adherence: AI (pretreatment: 35.1 ± 30/h, CPAP withdrawal: 25.9 ± 25/h, P = 0.02) and AHI4% (51 ± 35/h vs 41.9 ± 30.6/h, P = 0.03) were lower, RDI was not statistically different (56.8 ± 32.9/h vs 50.1 ± 28/h, P = 0.1), and O2 saturation was improved (mean O2 sat: 92.4% ± 4.6% vs. 93.6% ± 3.2%, P = 0.04, min O2 sat 69.5% ± 14.3% vs. 74.4% ± 11.2%, P = 0.007). As in the full dataset, WASO (96 ± 44 min vs 74.7 ± 36.5 min, P = 0.002) and sleep efficiency (78.6% ± 9.2% v 83.9% ± 7.1%, P = 0.002) were significantly improved on CPAP withdrawal compared to pretreatment; %stage N2 was higher (45.4 ± 11.8 vs 51.2 ± 9.9, P = 0.009); however, difference in %REM did not reach statistical significance (14.2 ± 6 vs 12.0 ± 6.8, P = 0.1).
DISCUSSION
In 42 patients with a range of severity of SDB, our study showed that after two nights of CPAP withdrawal, the frequency of events (RDI) was similar to pretreatment; this was also true of subjective and objective sleepiness. Our results are in accord with several previously published studies, but there is considerable variability in the findings in other studies.7–8,10–11,13,14 Those of our SDB metrics that represent the most complete obstruction (AI, AHI4%) were lower on CPAP withdrawal than pretreatment. In addition, severity of the individual SDB events within each subject (AI, ratio of apneas to all events, and degree of O2 desaturation) was lower on the CPAP withdrawal NPSG than in the pretreatment NPSG, but this difference between CPAP withdrawal and pretreatment SDB levels was demonstrable only in the subjects with severe SDB. For the entire dataset indices of sleep disruption (WASO, sleep efficiency) were improved on CPAP withdrawal compared to pretreatment; this finding was also most evident in severe SDB subjects, with only a trend in the subjects with mild/moderate SDB (Table 3). In contrast to the apparent improvement of SDB on CPAP withdrawal, %REM was worse on CPAP withdrawal than pretreatment.
Data in our severe SDB subjects are consistent with some other studies,10–12,14–17 demonstrating a decrease in frequency of SDB events when comparing CPAP withdrawal to pretreatment values, but differ from other studies showing a return to pretreatment levels of SDB.6–11 Many of these studies were limited by small sample size and used insensitive tools to measure SDB, as they did not use the nasal cannula/pressure transducer to measure airflow. This reduced frequency of SDB events on CPAP withdrawal is consistent with the hypothesis that long-term CPAP results in physiologic changes that improve upper airway collapsibility (for example, reduce upper airway edema).20,21
Our data do not show a reduction in frequency of SDB in subjects with mild/moderate SDB, and this has previously not been specifically addressed. While the findings in milder SDB may be a consequence of lower numbers of events (resulting in a lower signal-to-noise ratio), we did not see even a trend to lower AI, AHI4%, or RDI on CPAP withdrawal compared to pretreatment. One possible explanation is that there may be less trauma to the upper airway in the pretreatment state in mild disease, and thus there is less long-term effect of CPAP. The difference in findings for severe and mild/moderate SDB in our data is consistent with findings of a previous study with a small number of subjects.7
The improvement in O2 saturation seen in our patients on CPAP withdrawal compared to pretreatment (see Table 2 and S4) can be partially explained by the reduction in number of apneas, since apneas generally produce the most severe desatu-ration. The minimum O2 saturation resulting from each apnea was higher on CPAP withdrawal. Lesser O2 desaturation from apnea on CPAP withdrawal than pretreatment has been reported in some studies10,15,17 and has been attributed to reductions in apnea duration.10 However, in our data, the average duration of the apneas themselves was not different between CPAP withdrawal and pretreatment (see text of Results section). Thus, earlier arousal from obstruction cannot explain the lesser de-saturation. In one study,17 a small but nonsignificant increase in O2 saturation preceding apnea was reported. Review of our tracings in the 20 subjects with severe SDB suggested that this inter-apnea maximum O2 saturation was indeed 1% to 2% higher on CPAP withdrawal than pretreatment. Because of the shape of the oxyhemoglobin curve, even a small increase in O2 saturation at the start of the apnea may explain a lesser desatu-ration following an apnea. The higher inter-apnea saturation suggests an increase in ventilatory drive during the unobstructed interval after apnea. If this is due to extended CPAP use, it could be explained by (1) higher level of CNS arousal in the inter-apnea period because of less sleepiness in the acute withdrawal study, or (2) changes in hypoxic/hypercapnic response at arousal compared to the chronic condition of OSAHS before treatment.22–24 Despite the suggestion of a greater “arousal” after SDB events implied by the higher inter-apnea O2 saturation, we were not able to confirm increased overall arousal using our PVT or MSLT data; but it should be noted that these are not direct assessments of the physiologic responses that occur immediately after arousal. Finally alteration of PCO2 by extended CPAP use (increased FRC while on CPAP) could have resulted in the higher inter-apnea O2 saturation. However, although not directly measured, there was little reason to believe that the subjects were hypercapnic or that this has changed on CPAP, and is supported by the lack of change in awake O2 saturation in these subjects (pretreatment: 97.3% ± 1.7% vs CPAP withdrawal: 97.6% ± 1.3%, P = NS).
Sleep architecture was also different during the CPAP withdrawal NPSG compared to the pretreatment NPSG. WASO was diminished and sleep efficiency improved, suggesting increased continuity of sleep on the CPAP withdrawal night compared to pretreatment. Possible explanations include (1) greater adaptation to the lab environment on the CPAP withdrawal NPSG, as subjects had by then experienced 3 prior nights (pretreatment, CPAP titration, CPAP therapy) in the laboratory, and (2) carryover of improved sleep because of long-term CPAP use. There may also have been a contribution from milder O2 desaturation in the subjects with severe SDB, but the changes in sleep were seen in both severe and mild SDB. Despite their statistical significance, the magnitude of the differences in WASO and sleep efficiency makes the clinical significance of these findings unclear.
A reduction in %REM on CPAP withdrawal seems initially at odds with the lesser frequency of SDB and with lesser O2 desaturation. However, this sleep finding is consistent with a lower sleep pressure carrying over from a period of CPAP use compared to the sleep pressure built up during chronic untreated SDB. In acute CPAP withdrawal, the lesser sleep pressure results in more difficulty initiating and maintaining REM in the face of recurrent SDB. Previous studies comparing sleep architecture on CPAP withdrawal to pretreatment were performed in small numbers of subjects and show conflicting trends. While some studies show no significant differences7,12,13,16 in any sleep variable, small reductions in TST and SE were shown by Sforza et al.14 Similar to our findings in 42 subject with a range of severity of SDB, %REM was less on CPAP withdrawal in two studies of subjects with exclusively severe SDB (total n = 33).10,15 It is interesting to speculate that acute CPAP withdrawal could thus have effects on neurologic processes like memory even before sleep pressure accumulates and that these could be acutely greater than the pretreatment effects.
In our dataset, two nights of CPAP withdrawal was associated with a return to pretreatment levels of subjective (SSS) and objective sleepiness (MSLT and PVT). This was true in both the severe and mild/moderate SDB groups and is consistent with the data of Kribbs et al.,13 although they studied predominantly severe SDB subjects and PVT findings did not show statistical significance. In contrast, Sforza et al.14 found that subjective sleepiness did not return to pretreatment levels and that the sleep latency from the MSLT was slightly improved at CPAP withdrawal compared to pretreatment. Although it is possible that differences in the duration of CPAP use between the studies could account for these contrasting results, in the Sforza study there was also the confounding effect of weight loss in the subjects.
It is also possible that the return to pretreatment levels of sleepiness on CPAP withdrawal that we saw in our data could have been solely a psychological effect of awareness by the subject of lack of their usual CPAP therapy. We did not use a sham intervention, but this has been done in other studies, and subjective sleepiness has consistently been shown to recur on CPAP withdrawal in studies with25 and without sham intervention.13
In our study, subjects with mild SDB showed an ESS of 14 (similar to those with severe SDB). This is similar to findings of a poor relationship of ESS to SDB severity in prior studies (e.g., Chervin et al.26). Because our study excluded any subject with clinical suspicion of reasons for sleepiness other than SDB (including clear self-restricted sleep time), we do not believe that other causes of sleepiness explained the high ESS in the mild/moderate SDB group. In addition, these subjects showed the same improvement as the subjects in the severe SDB group for their ESS when put on CPAP, supporting that the SDB was the cause of their sleepiness (Table S3B in supplemental material).
The effect of CPAP withdrawal in our data may have been influenced by the duration of time on CPAP. With the design of the present study this duration may not have been sufficient to produce all the long-term improvements associated with CPAP treatment.27,28 It remains possible that the return to baseline sleepiness on CPAP withdrawal was a consequence of only 4 months of therapy and would not have been seen after more prolonged CPAP use prior to the withdrawal. While a residual susceptibility to recurrent SDB cannot be excluded in our subjects without a longer trial than we performed, subjects did use CPAP long enough for us to observe significant improvement in daytime function compared to pretreatment.
Finally, our interpretation that CPAP withdrawal from the fully treated state results in recurrent manifestations of SDB could be challenged, as not all of our patients showed optimal CPAP adherence while on therapy. However when we restricted the analysis to only the subset of patients with good CPAP adherence the findings were essentially unchanged (see Results).
In conclusion, our data in 42 subjects with a wide range of SBB severity confirm that withdrawal of CPAP results in recurrence of SDB with similar frequency to pretreatment, but less severity by several measures (degree of airflow obstruction and O2 desaturation). These findings were more evident in subjects with severe SDB. Both subjective and objective measures of sleepiness return to baseline levels with two nights of CPAP withdrawal after being significantly improved by CPAP therapy. Although there are changes in sleep architecture suggesting subjects have overall improved sleep during CPAP withdrawal compared to pretreatment values, there are also suggestions that %REM is lower at this time, perhaps due to lower sleep pressure. In contrast to the SDB itself, the pattern of the effect of CPAP withdrawal on daytime function and sleep did not appear to differ in patients with severe or mild/moderate SDB. The return to pretreatment levels of subjective and objective sleepiness after only two nights without CPAP add to other evidence25 against intermittent CPAP use in patients with OSAHS regardless of SDB severity.
ACKNOWLEDGMENTS
The authors acknowledge Rakhil Kanevskaya and Ming Chen for technical assistance and Nikia Scott and Joseph Keating for management of the study. Work for this study was performed at the Sleep Disorders Center of Pulmonary, Critical Care and Sleep Medicine Department, New York University School of Medicine, New York, NY. Supported by grants from: NIH R01HL81310, NIH 1 UL1RR029893, Foundation for Research in Sleep Disorders.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Norman holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring. Dr. Rapoport has received research support for grants and clinical trials from Fisher& Paykel Healthcare, Ventus Medical; speaking and/or consulting engagements for Fisher & Paykel Healthcare, Ventus Medical, and BioMarin. In addition, he holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare, Advanced Brain Monitoring and Tyco (Health C'Aire). Dr. Ayappa has received research support for grants and clinical trials from Fisher& Paykel Health-care and Ventus Medical. She also holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring. The other authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Effect of CPAP therapy on SDB metrics for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on SDB metrics. Patients grouped by SDB severity (mean ± SD)
Plot of apnea duration (x-axis) and minimum O2 saturation (y-axis) associated with each apnea for all apneas in one subject from the pretreatment NPSG (closed circles) and CPAP withdrawal NPSG (open circles). The mean duration of apneas was similar on the two NPSGs (28.1 ± 8.2 vs 31.8 ± 9.3 sec), but the minimum value of O2 saturation associated with each apnea was higher on the CPAP withdrawal NPSG (87.9% ± 5.2%) compared to pretreatment (79% ± 8.1%).
Effect of CPAP therapy on sleep architecture for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on sleep architecture. Patients grouped by SDB severity (mean ± SD)
Effect of CPAP therapy on daytime outcomes for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on daytime outcomes. Patients grouped by SDB severity (mean ± SD)
Comparison of SDB metrics between pretreatment and CPAP withdrawal NPSGs for the entire group (n = 42, mean ± SD)
Comparison of sleep architecture between pretreatment and CPAP withdrawal NPSG in total group (n = 42, mean ± SD)
Comparison of measures of daytime function after pretreatment and after CPAP withdrawal NPSG for the entire dataset
REFERENCES
- 1.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 2.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEvoy RD, Thornton AT. Treatment of obstructive sleep apnea syndrome with nasal continuous positive airway pressure. Sleep. 1984;7:313–25. doi: 10.1093/sleep/7.4.313. [DOI] [PubMed] [Google Scholar]
- 7.Collop NA, Block AJ, Hellard D. The effect of nightly nasal CPAP treatment on underlying obstructive sleep apnea and pharyngeal size. Chest. 1991;99:855–60. doi: 10.1378/chest.99.4.855. [DOI] [PubMed] [Google Scholar]
- 8.Fiz JA, Abad J, Ruiz J, Riera M, Izquierdo J, Morera J. nCPAP treatment interruption in OSA patients. Respir Med. 1998;92:28–31. doi: 10.1016/s0954-6111(98)90028-2. [DOI] [PubMed] [Google Scholar]
- 9.Rauscher H, Popp W, Wanke T, Zwick H. Breathing during sleep in patients treated for obstructive sleep apnea. Nasal CPAP for only part of the night. Chest. 1991;100:156–9. doi: 10.1378/chest.100.1.156. [DOI] [PubMed] [Google Scholar]
- 10.Boudewyns A, Sforza E, Zamagni M, Krieger J. Respiratory effort during sleep apneas after interruption of long-term CPAP treatment in patients with obstructive sleep apnea. Chest. 1996;110:120–7. doi: 10.1378/chest.110.1.120. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Phillips CL, Melehan KL, Rogers NL, Seale JP, Grunstein RR. Effects of short-term CPAP withdrawal on neurobehavioral performance in patients with obstructive sleep apnea. Sleep. 2006;29:545–52. doi: 10.1093/sleep/29.4.545. [DOI] [PubMed] [Google Scholar]
- 12.Leech JA, Onal E, Lopata M. Nasal CPAP continues to improve sleep-disordered breathing and daytime oxygenation over long-term follow-up of occlusive sleep apnea syndrome. Chest. 1992;102:1651–5. doi: 10.1378/chest.102.6.1651. [DOI] [PubMed] [Google Scholar]
- 13.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 14.Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep. 1995;18:195–201. [PubMed] [Google Scholar]
- 15.Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19:774–82. doi: 10.1093/sleep/19.10.774. [DOI] [PubMed] [Google Scholar]
- 16.Bonsignore MR, Parati G, Insalaco G, et al. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:279–86. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- 17.Marrone O, Salvaggio A, Bonsignore MR, Insalaco G, Bonsignore G. Blood pressure responsiveness to obstructive events during sleep after chronic CPAP. Eur Respir J. 2003;21:509–14. doi: 10.1183/09031936.03.00039803a. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules terminology and technical specifications. [Google Scholar]
- 19.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 21.Mortimore IL, Kochhar P, Douglas NJ. Effect of chronic continuous positive airway pressure (CPAP) therapy on upper airway size in patients with sleep apnoea/hypopnoea syndrome. Thorax. 1996;51:190–2. doi: 10.1136/thx.51.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–6. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 23.Berthon-Jones M, Sullivan CE. Time course of change in ventilatory response to CO2 with long-term CPAP therapy for obstructive sleep apnea. Am Rev Respir Dis. 1987;135:144–7. doi: 10.1164/arrd.1987.135.1.144. [DOI] [PubMed] [Google Scholar]
- 24.Kimoff RJ, Brooks D, Horner RL, et al. Ventilatory and arousal responses to hypoxia and hypercapnia in a canine model of obstructive sleep apnea. Am J Respir Crit Care Med. 1997;156:886–94. doi: 10.1164/ajrccm.156.3.9610060. [DOI] [PubMed] [Google Scholar]
- 25.Kohler M, Stoewhas AC, Ayers L, et al. The effects of CPAP therapy withdrawal in patients with obstructive sleep apnea: a randomised controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 26.Chervin RD, Aldrich MS. Characteristics of apneas and hypopneas during sleep and relation to excessive daytime sleepiness. Sleep. 1998;21:799–806. [PubMed] [Google Scholar]
- 27.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 28.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of CPAP therapy on SDB metrics for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on SDB metrics. Patients grouped by SDB severity (mean ± SD)
Plot of apnea duration (x-axis) and minimum O2 saturation (y-axis) associated with each apnea for all apneas in one subject from the pretreatment NPSG (closed circles) and CPAP withdrawal NPSG (open circles). The mean duration of apneas was similar on the two NPSGs (28.1 ± 8.2 vs 31.8 ± 9.3 sec), but the minimum value of O2 saturation associated with each apnea was higher on the CPAP withdrawal NPSG (87.9% ± 5.2%) compared to pretreatment (79% ± 8.1%).
Effect of CPAP therapy on sleep architecture for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on sleep architecture. Patients grouped by SDB severity (mean ± SD)
Effect of CPAP therapy on daytime outcomes for the entire group (n = 42, mean ± SD)
Effect of CPAP therapy on daytime outcomes. Patients grouped by SDB severity (mean ± SD)
Comparison of SDB metrics between pretreatment and CPAP withdrawal NPSGs for the entire group (n = 42, mean ± SD)
Comparison of sleep architecture between pretreatment and CPAP withdrawal NPSG in total group (n = 42, mean ± SD)
Comparison of measures of daytime function after pretreatment and after CPAP withdrawal NPSG for the entire dataset


