Abstract
Study Objectives:
Humans with narcolepsy and orexin/ataxin-3 transgenic (TG) mice exhibit extensive, but incomplete, degeneration of hypo-cretin (Hcrt) neurons. Partial Hcrt cell loss also occurs in Parkinson disease and other neurologic conditions. Whether Hcrt antagonists such as almorexant (ALM) can exert an effect on the Hcrt that remains after Hcrt neurodegeneration has not yet been determined. The current study was designed to evaluate the hypnotic and cataplexy-inducing efficacy of a Hcrt antagonist in an animal model with low Hcrt tone and compare the ALM efficacy profile in the disease model to that produced in wild-type (WT) control animals.
Design:
Counterbalanced crossover study.
Setting:
Home cage.
Patients or Participants:
Nine TG mice and 10 WT mice.
Interventions:
ALM (30, 100, 300 mg/kg), vehicle and positive control injections, dark/active phase onset.
Measurements and Results:
During the 12-h dark period after dosing, ALM exacerbated cataplexy in TG mice and increased nonrapid eye movement sleep with heightened sleep/wake fragmentation in both genotypes. ALM showed greater hypnotic potency in WT mice than in TG mice. The 100 mg/kg dose conferred maximal promotion of cataplexy in TG mice and maximal promotion of REM sleep in WT mice. In TG mice, ALM (30 mg/ kg) paradoxically induced a transient increase in active wakefulness. Core body temperature (Tb) decreased after acute Hcrt receptor blockade, but the reduction in Tb that normally accompanies the wake-to-sleep transition was blunted in TG mice.
Conclusions:
These complex dose- and genotype-dependent interactions underscore the importance of effector mechanisms downstream from Hcrt receptors that regulate arousal state. Cataplexy promotion by ALM warrants cautious use of Hcrt antagonists in patient populations with Hcrt neurodegeneration, but may also facilitate the discovery of anticataplectic medications.
Citation:
Black SW; Morairty SR; Fisher SP; Chen TM; Warrier DR; Kilduff TS. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. SLEEP 2013;36(3):325-336.
Keywords: Cataplexy, mouse, narcolepsy, orexin, hypocretin
INTRODUCTION
The neurologic disorder narcolepsy is characterized by excessive sleepiness, fragmented sleep, short latency to rapid eye movement (REM) sleep and cataplexy—a sudden, emotionally triggered loss of muscle tone—in both human patients and animal models of the disease.1,2 Disruption of the hypocretin (Hcrt, also known as orexin) neuropeptide signaling system results in a narcoleptic phenotype.3–6 Neurodegeneration of approximately 90% of the sole population of Hcrt-producing neurons (located in the posterior lateral hypothalamus) underlies human narcolepsy7 and is recapitulated in the orexin/ataxin-3 transgenic (TG) mouse model in which the Hcrt cells have been genetically engineered to degenerate postnatally.8 Exogenous Hcrt administered to TG mice increases wakefulness and suppresses sleep and cataplexy to rescue the narcoleptic phenotype.9 Thus, small-molecule Hcrt agonists may prove useful as Hcrt replacement therapy, because both Hcrt receptors remain intact in human patients with narcolepsy.10,11
The development of anticataplectic medications will be facilitated by the use of tool compounds that provoke or exacerbate cataplexy in animal models. In canines with narcolepsy, physostigmine and prazosin have been used extensively to provoke cataplectic attacks.12 Although cataplexy has been well documented in multiple transgenic models affecting the Hcrt system,3,8,11,13 cataplexy expression is usually modest in rodents, which has led multiple laboratories to search for behavioral and pharmacologic interventions that could provoke cataplexy.14–17 In this context, the dopamine agonist quinpirole (QNP) has been reported to exacerbate cataplexy in orexin-deficient mice.15 To develop Hcrt agonists for the treatment of narcolepsy, pharmacologists will need an experimental tool that not only induces cataplexy, but can also be displaced from Hcrt receptors by test compounds to determine dose-response efficacy.
Although the search for Hcrt agonists has been largely unsuccessful to date, considerable progress has been made in the development of Hcrt antagonists for the promotion of sleep in insomnia. Dual Hcrt receptor antagonism by almorexant (ALM) dose-dependently increases REM and non-REM (NREM) sleep and decreases wakefulness apparently without inducing either cataplexy18 or deficits in next-day performance.19 Similar results have been reported in studies of suvorexant in multiple species.20 To our knowledge, neither ALM nor other Hcrt antagonists have been evaluated for contraindications in patients with narcolepsy or other patient populations with various degrees of Hcrt neurodegeneration.21–24 Parkinson disease, Huntington disease, and traumatic brain injury have all been associated with Hcrt neurodegeneration, excessive sleepiness, and inappropriately timed wakefulness.21–23 Because some patients with these diseases that involve partial Hcrt cell loss are likely currently being treated for insomnia, it is important to understand the efficacy and risk profile (i.e., cataplexy) of the evolving class of hypnotic agents based on Hcrt antagonism in these populations. Whether Hcrt antagonists can exert an effect on any residual Hcrt that remains after neurodegeneration has not yet been determined.
The purposes of the current study were (1) to demonstrate hypnotic and cataplexy-inducing efficacy of a Hcrt antagonist in an animal model with very low (but not absent) Hcrt tone and (2) to compare this efficacy profile in the disease model with the effects produced in wild-type (WT) control animals. Orexin/ataxin-3 transgenic mice and WT mice were administered three doses of ALM at the beginning of the dark/active period and arousal state variables were assessed for the subsequent 12 h, when the propensity for cataplexy is greatest and hypnotic effects are most evident. Core body temperature (Tb) and locomotor activity (LMA) were also assessed to help evaluate the quality of the sleep/wake states that were measured and to determine the relationship between sleep and these physiologic variables under these experimental conditions. The effects of ALM were compared with both a negative control (vehicle, VEH) and a positive control—the dopamine D2/D3 receptor agonist QNP used at a dose that has previously been shown to induce cataplexy in orexin ligand knockout mice.15 We found that acute Hcrt receptor blockade exacerbated cataplexy, promoted sleep in a dose × genotype dependent manner, and decreased body temperature. By contrast, the chronic reduction in Hcrt signalling in TG mice was associated with attenuation of the decrease in Tb that normally accompanies the wake-to-sleep transition.
MATERIAL AND METHODS
Animals
Male, hemizygous transgenic (TG, n = 9) C57BL/6-Tg(orexin/ataxin-3)/Sakurai mice and WT (n = 10) littermates bred in our colony at SRI International were used. All experimental procedures were approved by the Institutional Animal Care and Use Committee at SRI and were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
Surgery
Mice (32 ± 0.9 g, age 15 ± 0.5 wk) were prepared for sterile surgical implantation of biotelemetry transmitters (F20-EET, Data Sciences Inc., St Paul, MN) for chronic recording of electroencephalograph (EEG), electromyograph (EMG), Tb, and LMA. Under isoflurane anesthesia (5% induction, 2-3% maintenance), midline cranial and abdominal incisions were made. After irrigation of the peritoneum with sterile 0.9% physiologic saline, transmitters (sterilized with a cold disinfectant/sterilant) were placed intraperitoneally along the midline and anchored to the abdominal wall during incision closure with a polyviolene 5-0 suture. Biopotential leads were routed subcutaneously to the head and neck via a trochar tube inserted through the cutaneous abdominal and cranial incisions. After the cutaneous abdominal incision was closed with polyviolene 5-0 sutures and wound clips, the skull was dehydrated and cleaned with 3% hydrogen peroxide. Cranial holes were drilled 1 mm anterior to bregma and 1 mm lateral to midline and, contralaterally, 2 mm posterior to bregma and 2 mm lateral to midline. EEG leads were wedged subcranially over the dura and were attached to the skull with cyanoacrylate and dental acrylic. EMG leads were positioned bilaterally through the nuchal muscles and were anchored with polyamide 4-0 suture. The cranial incision was closed with polyviolene 5-0 sutures. Mice were administered postoperative analgesic agents and subcutaneous hydration (buprenorphine, 0.05-0.1 mg/kg and ketoprofen, 2-5 mg/ kg, 0.7 mL lactated Ringer's solution), thermal support and soft chow for at least 2 days.
Arousal State Recording
Mice were permitted at least 3 wk postsurgical recovery and at least 1 wk adaptation to running wheels, handling, and dosing procedures prior to data collection. Throughout the study, mice were housed individually in home cages with access to food, water, and running wheels ad libitum. Room temperature (24 ± 2°C), humidity (50 ± 20% relative humidity), and lighting conditions (light-dark 12:12) were monitored continuously via computer. Animals were inspected daily in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and SRI guidelines. Physiologic data and video-recorded behavioral data were simultaneously acquired with DataQuest Art 4.2 software (Data Sciences Inc., St. Paul, MN). EEG and EMG were sampled at 250 Hz. Digital videos were recorded at 10 frames/sec, 4CIF deinterlacing resolution.
Drugs
ALM [(2R)-2-[(1S)-6,7-dimethoxy-1-[2-(4-trifluoromethylphenyl)-ethyl]-3,4-dihydro-1H-isoquinolin-2-yl]-N-methyl-2-phenyl-acetamide]18 was synthesized by chemists at SRI International (> 99% purity as determined by nuclear magnetic resonance, mass spectrometry, and high-performance liquid chromatography) according to procedures described in the patent literature.25 (-)-Quinpirole hydrochloride (QNP, (4aR-trans)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline hydrochloride, Tocris Bioscience, Ellisville, MO) and ALM were dissolved in 1.25% hydroxypropyl methyl cellulose/0.1% dioctyl sodium sulfosuccinate/0.25% methylcellulose in water. Aliquots of QNP (0.5 mg/mL) were prepared prior to dosing and stored at -20°C. ALM was weighed individually for each animal, sonicated for 60 min, and vortexed immediately prior to dosing. ALM concentrations of 3, 10, and 30 mg/mL were used. All doses were delivered at 10 mL/kg final volume. Doses were chosen based on previous studies.15,26,27
Histology
Mice (age 28.4 ± 0.2 wk) were transcardially perfused with 30 mL phosphate buffered saline (PBS, 1×) followed by 50 mL chilled paraformaldehyde (4% in 1× PBS). Brains were harvested, postfixed overnight at 4°C in paraformaldehyde, and subsequently cryopreserved in 30% sucrose with sodium azide. Coronal sections, 40-μm thick, were sliced on a freezing microtome in a one-in-six series. Sections containing the posterior hypothalamus were sampled to visualize Hcrt2-containing cell bodies and varicosities. Sections that contained the posterior hypothalamus or the locus coeruleus were incubated (1:2,000) with goat anti-Hcrt2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by donkey anti-goat secondary antibody (Jackson Immunoresearch, West Grove, PA) in blocking solution (1% triton X-100 and 5% donkey serum in 1× PBS). Posterior hypothalamic sections were also incubated (1:10,000) with rabbit anti-melanin-concentrating hormone (MCH, Phoenix Pharmaceuticals, Burlingame, CA) followed by donkey anti-rabbit secondary antibody in blocking solution. All sections were developed with the avidin-biotin-peroxidase system using diaminobenzidine (Vector Laboratories, Burlingame, CA). The posterior hypothalamus was delineated and Hcrt2-immunoreactive somata were identified at 40× magnification. Hcrt2-positive somata were counted unilaterally in three adjacent sections in a series -1.58 to -1.82 mm from bregma. Only somata with complete and intact cell membranes were included in the counts.
Experimental Design
In a two-way mixed (between-within) design, TG and WT mice were dosed intraperitoneally with ALM (30, 100, and 300 mg/kg),26,27 QNP (0.5 mg/kg) as a positive control for cataplexy,15 or VEH. Treatments were administered in a counterbalanced crossover design once every 3 days. Dosing occurred at the beginning of the dark/active phase at Zeitgeber Time (ZT) 12. Physiologic data and video-recorded behavioral data were collected over the following 12 h when the propensity for cataplexy is greatest and hypnotic effects are most evident in nocturnal species.
Data Analysis
Data were manually scored by experts (≥ 96% interrater reliability) using Neuroscore 2.1 (Data Sciences Inc., St. Paul, MN). Data in 10-sec epochs were classified as wakefulness (W; mixed-frequency, low-amplitude EEG and high-amplitude, variable EMG), REM sleep (theta-dominated EEG and EMG atonia), NREM sleep (low-frequency, high-amplitude EEG and low-amplitude, steady EMG), wheel-running behavior, or cataplexy. Criteria for cataplexy were ≥ 10 sec of EMG atonia, theta-dominated EEG, and behavioral immobility preceded by ≥ 40 s of W.28 Data were analyzed as time spent in each scored classification per h. Latency to NREM or REM onset was calculated from the time of injection to the first three continuous epochs of NREM or REM sleep, respectively. Cumulative time spent in W, NREM, and REM, as well as the REM:NREM ratio, were calculated over the 12-h recording period. Gross LMA (counts per min) and Tb were measured to assess the quality of sleep/wake states (e.g., hypnotic-induced hypothermia beyond the normal reduction in Tb associated with sleep). State-specific Tb values were determined as the mean Tb across all epochs of W, NREM, or REM per hourly bin or for the total 12-h period. The change in Tb from W to NREM or REM sleep over the 12-h dark period was calculated for individual mice as the mean Tb in NREM or REM minus the mean Tb in W.
To determine whether any of the pharmacologic treatments affected the consolidation of arousal states, the duration and number of bouts for each state were calculated in hourly bins. A bout of NREM sleep consisted of a minimum of two consecutive 10-sec NREM epochs and was terminated by the occurrence of a single epoch of a different state. A bout of REM sleep, cataplexy, or wheel-running was defined as the occurrence of a minimum of one epoch. The inter-REM sleep interval (IRSI), a measure of the REM sleep cycle, was calculated as the time interval between the end of a REM bout and the start of the next REM bout. EEG spectra during NREM sleep were computed using the fast Fourier transform algorithm in Neuroscore on all epochs without visually detectable artifact. EEG delta power (0.5-4 Hz) in NREM sleep (NRD) was then calculated in hourly bins. NRD for each condition was normalized against the mean NRD value obtained during the 12-h VEH recording for each individual.
Statistical Analysis
All statistical tests were performed using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA). Histologic data (Hcrt cell counts, mean ± standard error [SE]) were compared between genotypes by two-tailed t-test. Cataplexy data (total min, total number of bouts, mean bout duration) were analyzed from TG mice per 12-h time block, or as an hourly time series, and are shown as the mean ± SE. One-way repeated measures (RM)-analysis of variance (ANOVA) was used to evaluate cataplexy data in 12-h bins across drug conditions. Cataplexy time series data were assessed using two-way RM-ANOVA on factors “drug condition” and “time.” Sleep/wake state data were analyzed as the summed total per 12-h time block, or as the cumulative hourly time series, and are shown as the mean ± SE. Sleep/wake state consolidation variables (number of bouts and bout duration), IRSI, number of wheel-running bouts, LMA (counts per min), and state-specific Tb (°C) are expressed as the mean ± SE for the 12-h period. Total time spent in W, NREM and REM, NRD, latency to NREM and REM, REM:NREM ratio, IRSI, state consolidation variables, wheel-running, LMA, and state-specific Tb were evaluated using a two-way mixed model ANOVA on factors “genotype” (between subjects) and “drug condition” (within subjects). Time series data per genotype group were evaluated using two-way RM-ANOVA on factors “drug condition” and “time.” For all analyses, statistical significance was set at P < 0.05. When ANOVA indicated significance, post hoc Bonferroni t tests were used to detect differences between drug conditions. The relationship between the number of Hcrt cells and physiologic variables was determined by linear regression.
RESULTS
To determine the efficacy of Hcrt receptor antagonism in mice with attenuated endogenous Hcrt ligand levels, the effects of ALM on arousal state and cataplexy were assessed during the dark/active period in TG mice compared with WT control mice. As expected at age 28 wk, TG mice had far fewer posterior hypothalamic cells that stained positive for Hcrt2 (10.4 ± 2.5) compared with WT mice (383.8 ± 44.4), t11 = 13.3, P < 0.001 (Figure 1A, B). Hcrt terminals in the locus coeruleus, a major extrahypothalamic projection site of Hcrt cells, were virtually absent in TG mice compared with WT control mice (Figure 1E-H). Despite degeneration of the Hcrt cells in TG mice, posterior hypothalamic neurons that contained the co-extensive neuropeptide MCH were spared (Figure 1C, D), as described previously.8
Figure 1.
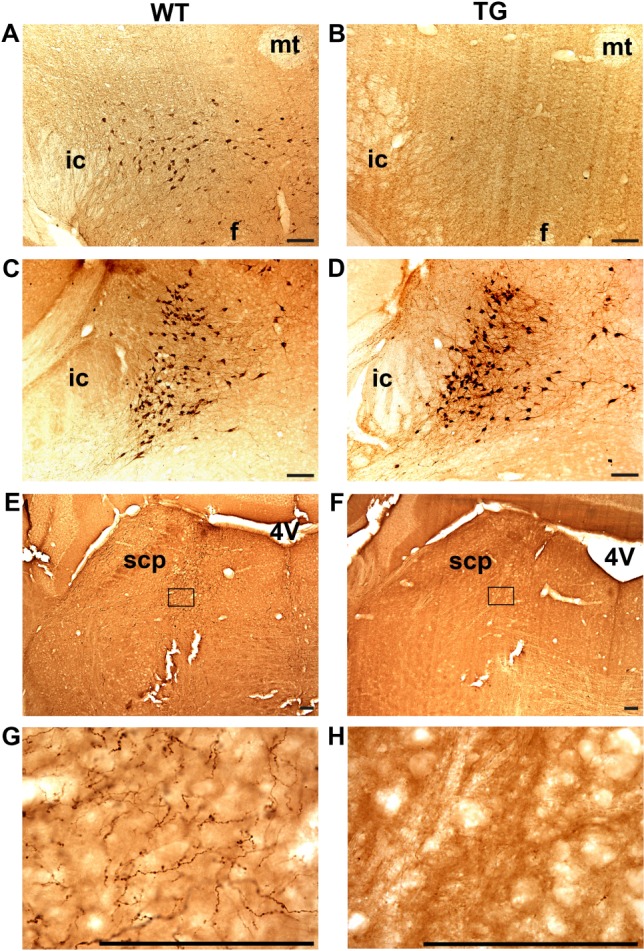
Selective ablation of hypocretin (Hcrt) neurons in orexin/ataxin-3 transgenic(TG) mice. Matched brain sections from representative wild-type (WT, left panels) and TG (right panels) mice stained with anti-Hcrt2 (A, B) and anti-MCH (C, D) antisera at the tuberal region of the hypothalamus. Whereas Hcrt-IR fibers are readily evident in the locus coeruleus region of WT mice (E, G) few if any fibers are present in the same region in TG mice (F, H). G, H are enlargements of the boxed areas in E, F. f, fornix; ic, internal capsule; mt, mammillothalamic tract; scp, superior cerebellar peduncle; 4V, fourth ventricle. Bars = 100 μm.
Cataplexy
All TG mice exhibited episodes of cataplexy that were electrophysiologically and behaviorally distinct from wake, NREM, and REM sleep (Figure 2 and Video 1 in supplemental material). ALM exacerbated cataplexy in TG mice in a dose-related manner. The amount of time spent in cataplexy, the number of cataplexy episodes, and the duration of cataplexy bouts over the 12-h dark period were assessed in TG mice after the administration of ALM (30, 100, or 300 mg/kg), QNP (0.5 mg/kg), or VEH by one-way RM-ANOVA (Figure 3A-C). Figure 3 illustrates that ALM approximately doubled the total amount of time spent in cataplexy (F4,44 = 7.51, P < 0.001) and the number of cataplexy bouts (F4,44 = 6.08, P < 0.001), but did not influence the duration of cataplexy episodes (F4,43 = 0.44, not significant) The time course of cataplexy bout occurrence during the dark period (Figure 3D) was evaluated by two-way RM-ANOVA. An interaction between factors “drug condition” and “time” revealed a complex dose × time effect of ALM on cataplexy (F44,539 = 2.70, P < 0.001). ALM (100 mg/kg) significantly increased the number of cataplexy bouts by 6 h post-treatment, whereas the promotion of cataplexy by ALM (300 mg/kg) only reached significance 11-12 h after treatment. In contrast to orexin ligand knockout mice15, QNP (0.5 mg/kg) had no significant effect on cataplexy at any time point.
Figure 2.
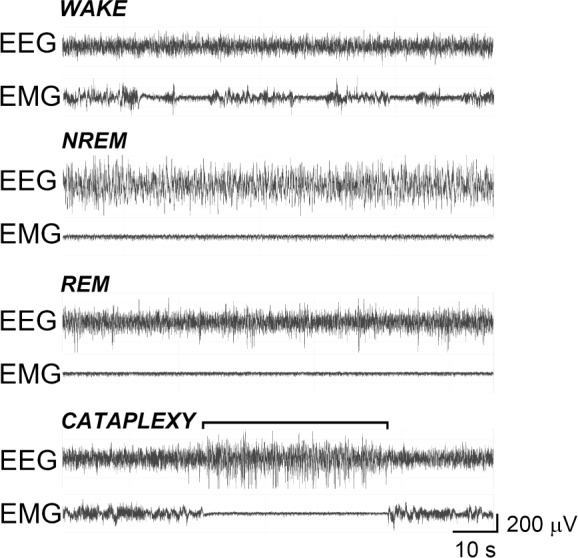
Orexin/ataxin-3 transgenic (TG) mice exhibited episodes of cataplexy distinct from wake, nonrapid eye movement (NREM) and rapid eye movement (REM) sleep. Representative electroencephalograph (EEG) and electromyography (EMG) traces from a single TG mouse during wake, NREM sleep, REM sleep, and cataplexy (bracket). Immobility during cataplexy was confirmed by video (see Video 1 in supplemental material). Four cataplexy-like events were recorded in wild-type (WT) mice after almorexant (ALM); see Results for details (also see Video 2 in supplemental material). Videos show five-times real-time speed, vertical stationary line demarcates synchronous time point of electrophysiologic signals and videographed behavior, vertical moving lines separate 10-sec epochs. Traces on videos (in order from top to bottom): locomotor activity counts, EEG, EMG, and EEG periodogram (0-25 Hz per 10-sec epoch).
Figure 3.
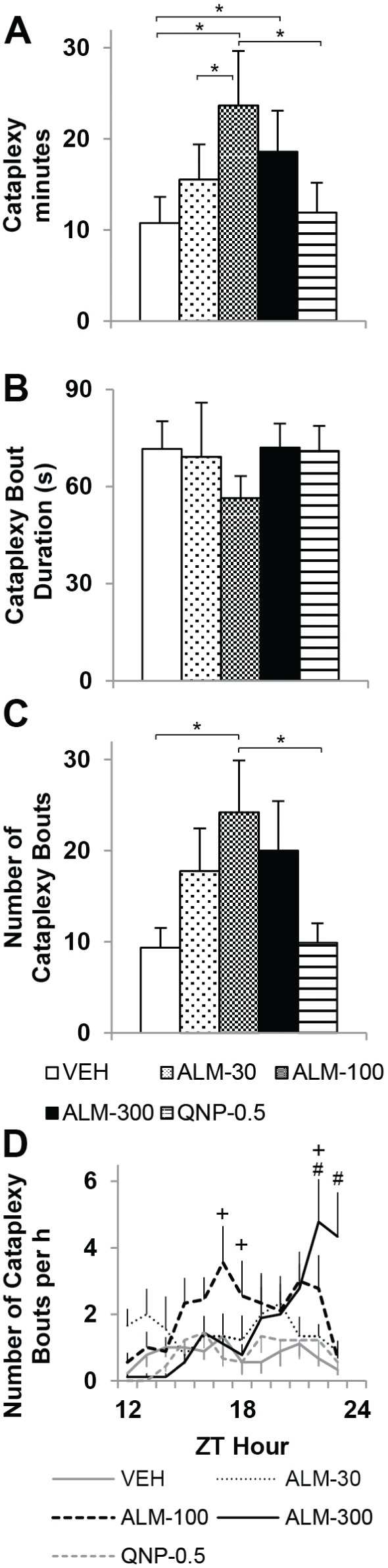
Almorexant (ALM) increased cataplexy in orexin/ataxin-3 transgenic (TG) mice. Time spent in cataplexy (A), mean cataplexy bout duration (B), and number of cataplexy bouts (C) per 12-h dark period after dosing with ALM (30, 100, or 300 mg/kg), quinpirole (QNP, 0.5 mg/kg) or vehicle (VEH). One-way repeated-measures analysis of variance and post hoc Bonferroni t tests: *P < 0.05. (D) Time course of cataplexy bouts over the 12-h dark period. Two-way repeated-measures analysis of variance and post hoc Bonferroni t tests: P < 0.05 +ALM-100 versus vehicle (VEH), #ALM-300 versus VEH. Data represent the mean ± standard error of the mean; n = 9. ZT, Zeitgeber time.
Although the TG mice as a group exhibited 16.2 episodes of cataplexy per 12-h recording (731 episodes in 540 h of recording time), the WT group very rarely showed behavior that could be classified as cataplexy-like—only 0.042 incidents per recording (four incidents in 1,140 h of recording time). Cataplexy-like events (Video 2 in supplemental material) occurred in two of the 10 WT mice, lasted 20-30 sec each, and always after wheel-running activity. One event occurred within the first h after dosing with ALM (100 mg/kg); the other three events occurred 7-11 h after ALM (300 mg/kg).
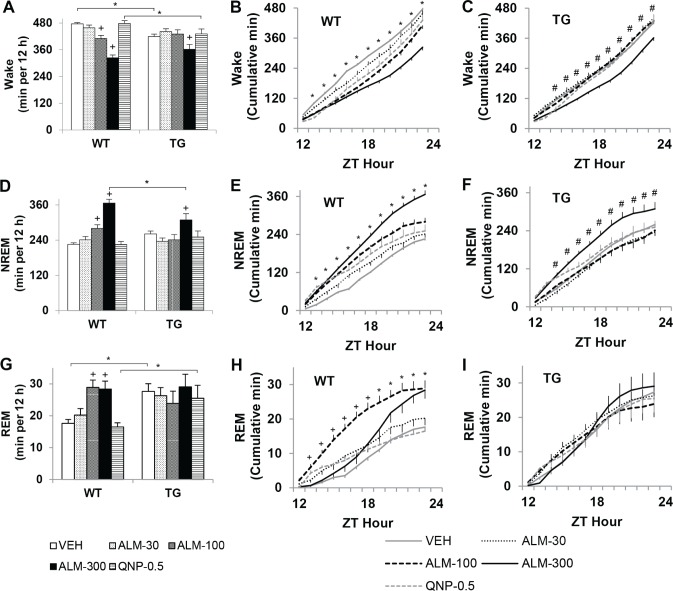
NREM Sleep and Wakefulness
ALM promoted NREM sleep and was more potent in WT mice with an intact Hcrt system than in TG mice (Figure 4). Genotypes were compared on the total amount of time spent awake and in NREM sleep during the 12 h after dosing using two-way mixed-model ANOVA (Figure 4A, D). Under control conditions, TG mice spent less time awake during their active period than WT mice (F4,94 = 8.41, P < 0.001). WT mice exhibited a dose-related reduction in wakefulness and a concomitant increase in NREM sleep after ALM treatment (F4,94 = 8.89, P < 0.001). Only ALM (300 mg/kg) increased sleep and decreased wakefulness relative to VEH treatment in TG mice. ALM was more potent in WT than in TG mice: ALM (100 mg/kg) did not affect sleep/wake in TG mice but promoted NREM sleep in WT mice; ALM (300 mg/kg) significantly increased NREM sleep in WT mice beyond the level observed in TG mice. QNP did not significantly affect the amount of wakefulness or NREM sleep in any of the animals.
Figure 4.
Almorexant (ALM) dose-dependently promoted nonrapid eye movement (NREM) sleep and was more potent in wild-type (WT) than orexin/ataxin-3 transgenic (TG) mice. Almorexant enhanced REM sleep in a dose × time-dependent manner. Time spent awake (A-C), in NREM (D-F), and REM sleep (G-I) over the 12-h dark period in WT (n = 10) and TG (n = 9) mice after treatment with ALM (30, 100, or 300 mg/kg), quinpirole (QNP, 0.5 mg/kg), or vehicle (VEH). For 12-h binned data (A, D, and G), two-way mixed-model analysis of variance and post hoc Bonferroni t tests: on between-subjects factor “genotype,” *P < 0.05; on within-subjects factor “drug condition,” P < 0.05 +versus VEH. For cumulative time series data (B, C, E, F, H, I), two-way repeated-measures analysis of variance and post hoc Bonferroni t tests (P < 0.05 versus VEH) on factor “drug condition” at each h: *ALM-100 and ALM-300, +ALM-100, #ALM-300. Data represent the mean ± SEM. ZT, Zeitgeber time.
The time course of the hypnotic effects of ALM (100 and 300 mg/kg) versus VEH over the 12-h dark period were analyzed with two-way RM-ANOVA on cumulative data for WT and TG mice (Figure 4B, C, E, F). WT mice gained more NREM sleep over a longer time period after ALM (100 and 300 mg/ kg) compared with VEH, F22,359 = 28.05, P < 0.001. A similar time course was reflected in the cumulative wakefulness data (F22,359 = 28.28, P < 0.001). By contrast, TG mice only accumulated more NREM sleep compared with VEH after ALM (300 mg/kg), F22,323 = 6.74, P < 0.001, presumably reflecting overall reduced Hcrt tone in this genotype. The rate of NREM sleep accumulation leveled off 9 h after ALM (300 mg/kg) in TG mice, after which increased wakefulness became apparent.
Surprisingly, ALM at the low dose (30 mg/kg) briefly induced active wakefulness in TG mice. The latency to NREM was assessed in WT and TG mice during the first h after dosing with ALM (30, 100, and 300 mg/kg), QNP (0.5 mg/kg), or VEH using two-way mixed-model ANOVA (Figure 5). After VEH treatment, TG mice exhibited reduced latency to NREM sleep compared with WT mice (Figure 5A), F4,94 = 3.24, P = 0.017. In WT mice, ALM (100 and 300 mg/kg) shortened NREM sleep latency to the levels observed in TG mice. Paradoxically, ALM (30 mg/kg) enhanced wakefulness in TG mice—NREM sleep latency increased 3.5-fold, comparable to the level observed in WT mice after VEH. Behavioral activation after ALM (30 mg/ kg) was also evident in wheel-running and LMA in TG mice (Figure 5B, C). The number of wheel-running bouts was reduced in both genotypes after ALM (300 mg/kg) and QNP (0.5 mg/kg), but not after 30 mg/kg ALM (F4,94 = 15.5, P < 0.001; main effect for factor “drug condition”). LMA increased in TG mice after ALM (30 mg/kg) compared with VEH and WT mice (F4,94 = 5.83, P < 0.001).
Figure 5.
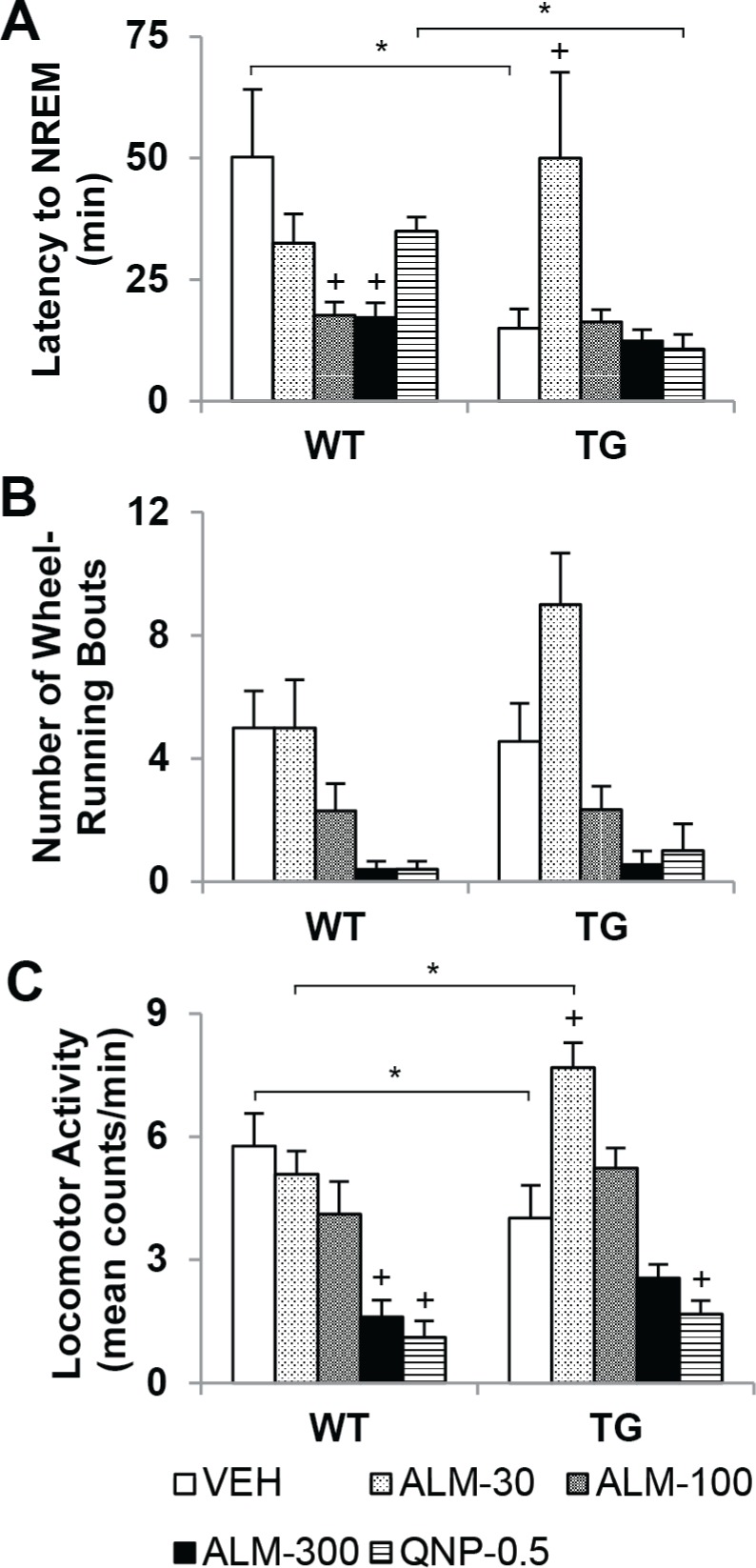
Almorexant (ALM) at a low dose (30 mg/kg) induced paradoxical behavioral activation in orexin/ataxin-3 transgenic (TG) mice. Latency to nonrapid eye movement (NREM) sleep (A), number of wheel-running bouts (B) and locomotor activity (C) during the fi rst h after dosing with ALM (30, 100, or 300 mg/kg), quinpirole (QNP, 0.5 mg/kg), or vehicle (VEH) in wild type (WT, n = 10) and TG (n = 9) mice. Two-way mixed-model analysis of variance and post hoc Bonferroni t tests: on between-subjects factor “genotype,” *P < 0.05; on within-subjects factor “drug condition,” P < 0.05 +versus VEH (main effect in number of wheel-running bouts for ALM-300 and QNP). Data represent the mean ± standard error of the mean.
REM Sleep
ALM enhanced REM sleep in a dose × time-dependent manner in WT mice only. Total REM sleep time across the 12-h period after dosing was compared between TG and WT mice using two-way mixed-model ANOVA (Figure 4G). After vehicle treatment, TG mice spent more time in REM sleep than WT mice (F4,94 = 5.36, P < 0.001). In WT mice, ALM (100 and 300 mg/kg) robustly elevated REM sleep above control levels; this increase in REM sleep did not surpass the amount of REM sleep observed in TG mice. A striking difference was evident in the time course of REM sleep accumulation after ALM (100 mg/kg) versus ALM (300 mg/kg) in WT mice (Figure 4H; two-way RM-ANOVA: F22,359 = 74.6, P < 0.001). After ALM (100 mg/kg), REM sleep accumulated rapidly for 8 h and then abated, whereas after ALM (300 mg/kg), REM sleep gains over VEH levels were not apparent until 8 h after dosing and continued until the end of the recording. No significant differences in REM sleep were found across drug conditions in TG mice, nor after QNP in either genotype.
Overall, ALM (100 mg/kg) was found to promote REM sleep by increasing the likelihood of its occurrence in WT mice. REM sleep architecture was evaluated over the dark period after dosing in TG and WT mice using two-way mixed-model ANOVA (Figure 6, Table 1). The latency to REM sleep (Figure 6A) was reduced after ALM (100 mg/kg) in both geno-types (F4,94 = 6.86, P < 0.001; main effect for factor “drug condition”). The time interval between REM bouts (Figure 6B) decreased only in WT mice after ALM (100 mg/kg) compared with VEH and TG mice (F4,94 = 5.10, P < 0.001). In addition, the ratio of REM to NREM sleep (Figure 6C) only increased in WT mice after ALM (100 mg/kg), reaching the level observed in TG mice (F4,94 = 2.83, P = 0.031). Under control conditions, the REM:NREM ratio was elevated in TG mice relative to WT. In WT mice, ALM (100 and 300 mg/kg) increased the number of REM sleep bouts (Table 1, F4,94 = 4.13, P = 0.005) and ALM (100 mg/kg) lengthened the duration of REM sleep episodes (F4,94 = 2.94, P = 0.026).
Figure 6.
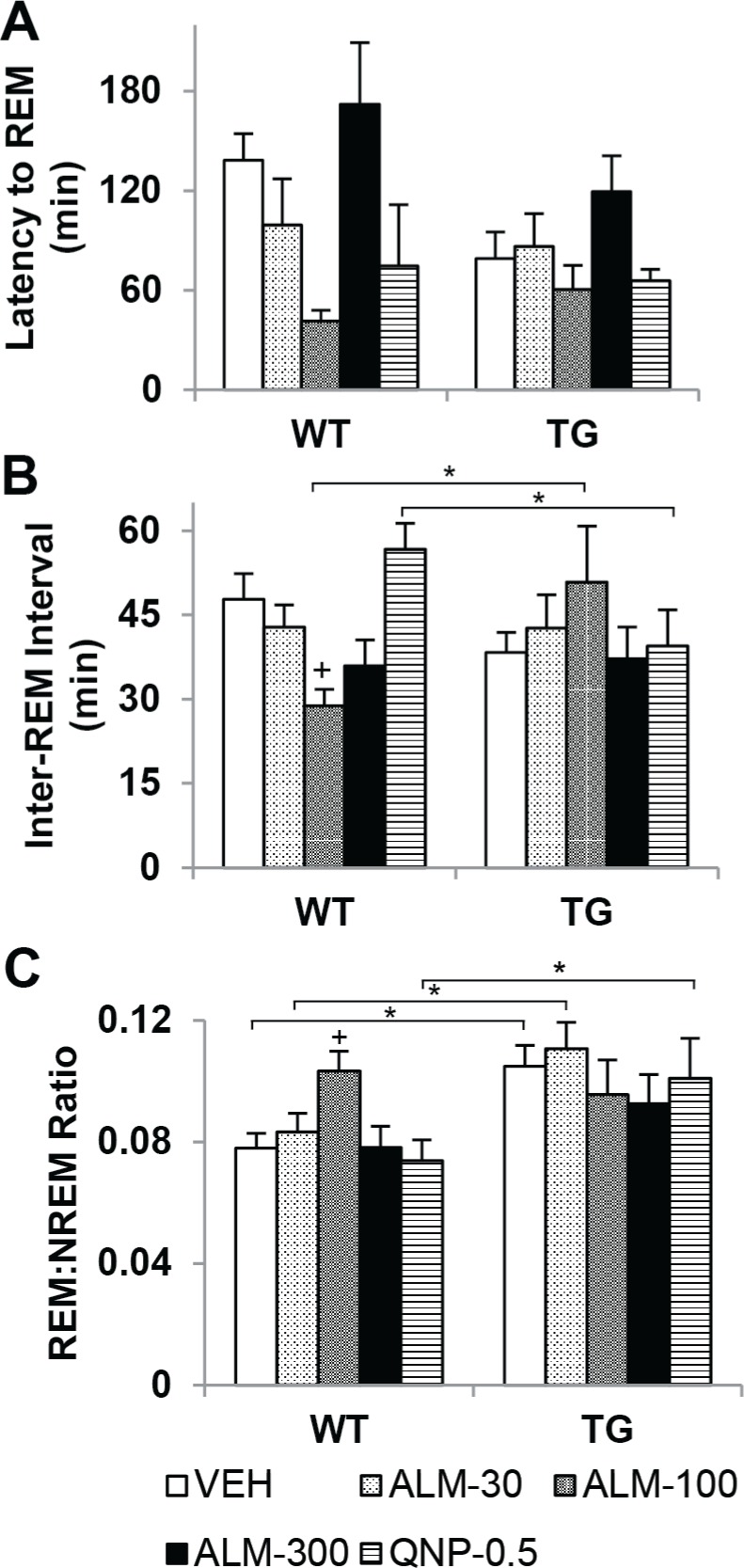
Almorexant (ALM, 100 mg/kg) promoted rapid eye movement (REM) sleep in wild-type (WT) mice. Latency to REM sleep after dosing (A), time interval between REM sleep bouts (B), and ratio of REM to nonrapid eye movement sleep (C) in WT (n = 10) and TG (orexin/ataxin-3 transgenic, n = 9) mice per 12-h period after dosing with ALM (30, 100, or 300 mg/kg), quinpirole (QNP, 0.5 mg/kg), or vehicle (VEH). Two-way mixed-model analysis of variance and post hoc Bonferroni t tests: on between-subjects factor “genotype,” *P < 0.05; on within-subjects factor “drug condition,” P < 0.05 +versus VEH (main effect in latency to REM for ALM-100). Data represent the mean ± standard error of the mean.
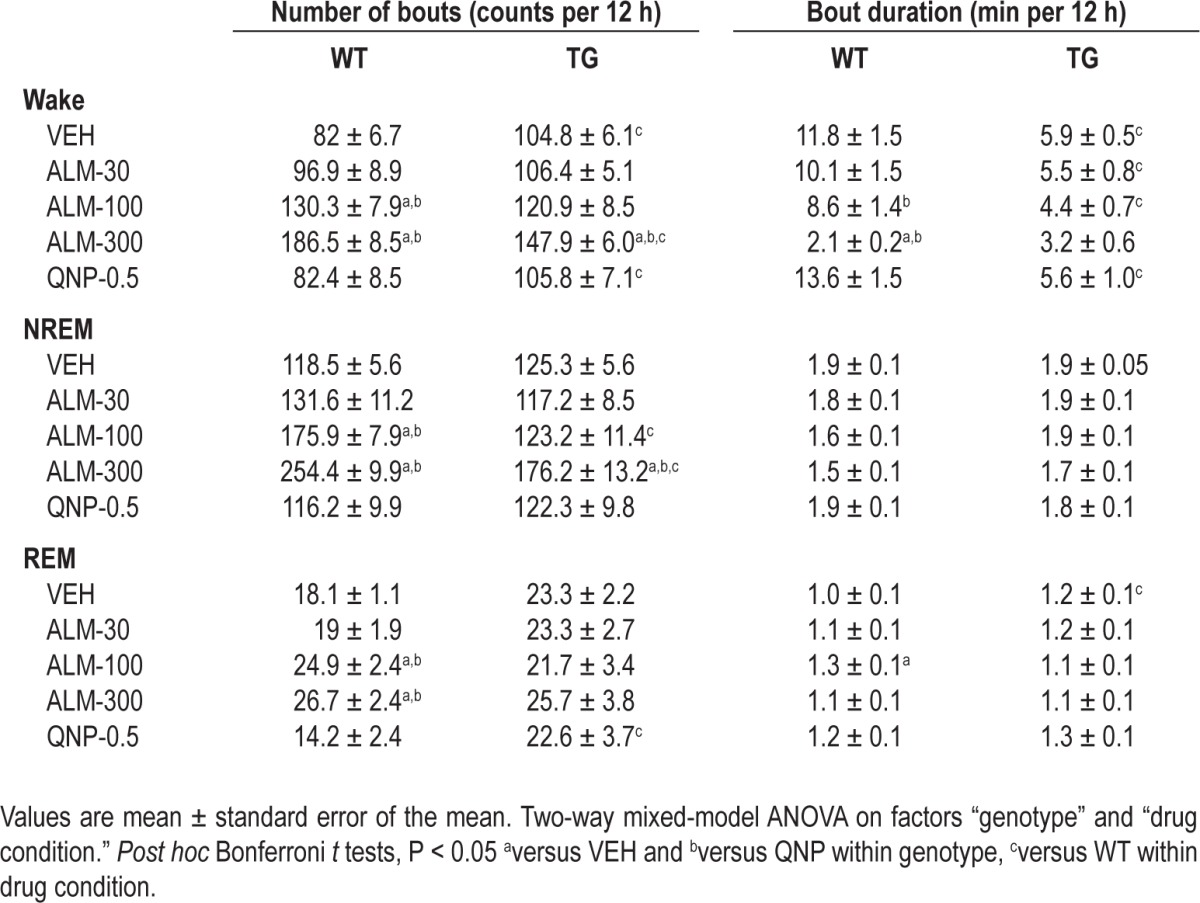
Table 1.
Measures of sleep consolidation in orexin/ataxin-3 transgenic (TG, n = 9) and wild-type (WT, n = 10) mice after almorexant (ALM; 30, 100, 300 mg/kg), quinpirole (QNP; 0.5 mg/kg) or vehicle (VEH) during the dark period
Sleep Consolidation and Intensity
Indices of sleep consolidation (Table 1) and intensity (Figure 7) were compared between genotypes during the 12 h after dosing using two-way mixed-model ANOVA. Although ALM promoted NREM sleep (Figure 4), arousal states under the influence of ALM were highly fragmented with an increased number of bouts of wakefulness (F4,94 = 9.73, P < 0.001), NREM (F4,94 = 12.84, P < 0.001) as well as REM sleep (F4,94 = 4.13, P = 0.005). After ALM (300 mg/kg), WT mice exhibited more wake and NREM sleep bouts than TG mice, despite the greater number of bouts in TG mice versus WT mice after VEH treatment. Wake bout duration was also reduced after ALM (100 and 300 mg/kg) in WT mice, and the reduction in wake bout duration reached the low levels observed in TG mice at the high dose of ALM (F4,94 = 5.38, P < 0.001).
Figure 7.
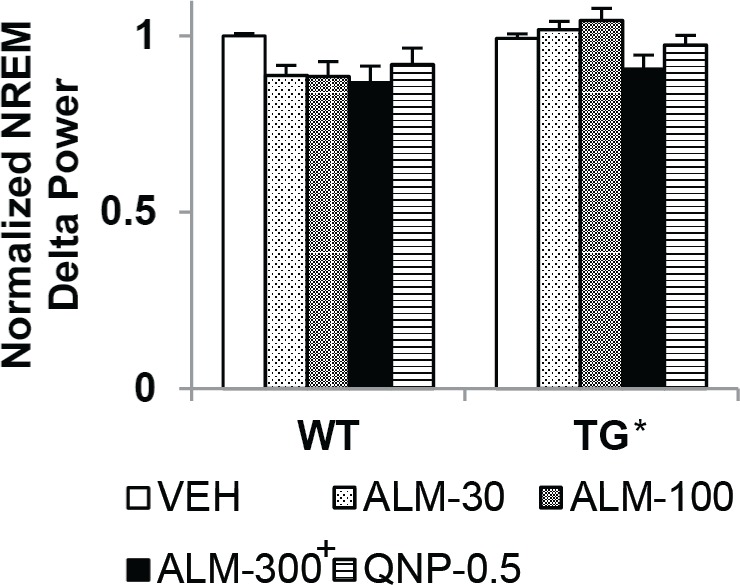
Almorexant (ALM, 300 mg/kg) reduced nonrapid eye movement (NREM) delta power similarly in both wild-type (WT) and orexin/ataxin-3 transgenic (TG) mice. Overall, TG mice had higher NREM delta power than WT mice, regardless of drug condition. Normalized NREM delta power in WT and TG mice per 12-h period after dosing with ALM (30, 100, 300 mg/kg), quinpirole (QNP, 0.5 mg/kg) or vehicle (VEH). Two-way mixed-model analysis of variance and post hoc Bonferroni t tests: main effect on between-subjects factor “genotype,” *P < 0.05; main effect on within-subjects factor “drug condition,” P < 0.05 +versus VEH. Data represent the mean ± standard error of the mean.
Remarkably, despite the increased fragmentation of all states and the effects on the duration of wake and REM sleep bouts, neither drug condition nor genotype affected NREM bout duration (F4,94 = 2.03, not significant). Sleep intensity, as indexed by NREM delta power (Figure 7), was elevated in TG versus WT mice regardless of drug condition (main effect on between-subjects factor “genotype,” F1,94 = 16.08, P < 0.001), and was reduced by ALM (300 mg/kg) regardless of geno-type (main effect on within-subjects factor “drug condition,” F4,94 = 3.67, P = 0.008).
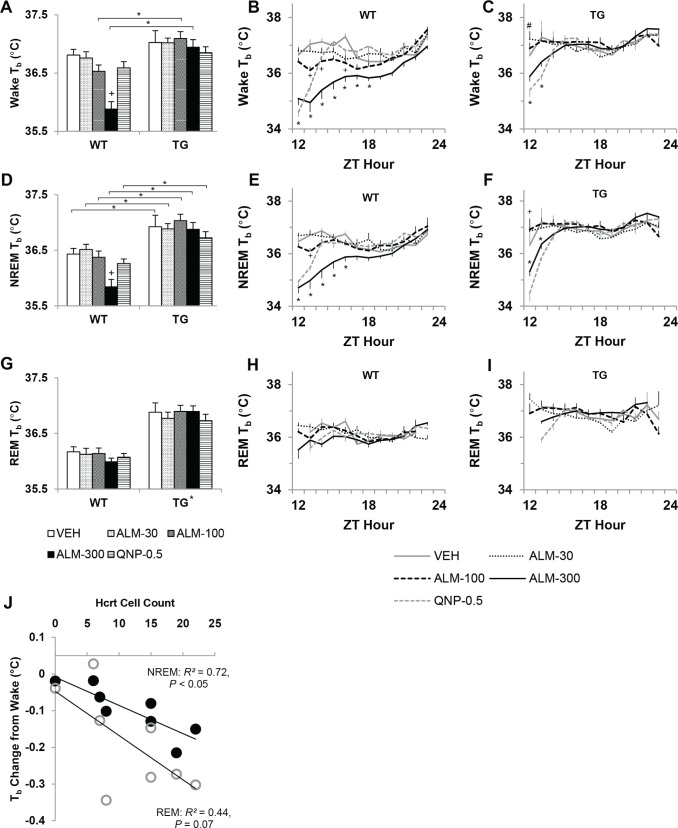
Core Body Temperature
The decrease in Tb that normally accompanies the transition from wakefulness to sleep was affected by the loss of Hcrt neurons (Figure 8). State-specific Tb over the 12-h period after dosing was compared between genotypes using two-way mixed-model ANOVA (Figure 8A, D, G). During wakefulness, TG mice exhibited a higher Tb than WT mice (main effect on between-subjects factor “genotype,” F1,94 = 11.38, P = 0.004), and this difference became more pronounced as the mice transitioned into NREM (F1,94 = 19.05, P < 0.001) and REM sleep (F1,94 = 33.96, P < 0.001). To evaluate the relationship between residual Hcrt and Tb, the thermolytic effect of sleep in TG mice was assessed in the VEH control condition by linear regression (Figure 8J). The change in Tb from wakefulness to NREM sleep positively correlated with the number of Hcrt cells that remained in TG mice (R2 = 0.72, P = 0.008). A trend was also evident in the relationship between the number of Hcrt cells and the change in Tb from wakefulness to REM sleep (R2 = 0.44, P = 0.073).
Figure 8.
Hypocretin (Hcrt) cell loss diminished thermolysis during sleep. Almorexant (ALM, 300 mg/kg) reduced core body temperature (Tb) in wild-type (WT) mice. Tb (mean ± standard error of the mean) during wakefulness (A-C), nonrapid eye movement (NREM) sleep (D-F) and rapid eye movement (REM) sleep (G-I) in WT (n = 10) and orexin/ataxin-3 transgenic (TG) mice (n = 9) per 12-h period post dosing with ALM (30, 100, or 300 mg/kg), quinpirole (QNP, 0.5 mg/kg), or vehicle (VEH). For 12-h binned data (A, D, and G), two-way mixed-model analysis of variance and post hoc Bonferroni t tests: on between-subjects factor “genotype,” *P < 0.05 (main effect in REM Tb); on within-subjects factor “drug condition,” P < 0.05 +versus VEH. For time series data (B, C, E, F, H, I), two-way repeated-measures analysis of variance and post hoc Bonferroni t tests (P < 0.05 versus VEH) on factor “drug condition” at each h: *ALM-300, +ALM-100, #ALM-30. Contrasts for QNP not shown for simplicity. (J) Correlation between number of Hcrt cells in TG mice and Tb change from wakefulness (mean per 12-h dark period) during NREM (closed circles) and REM sleep (open circles). ZT, Zeitgeber time.
Acute antagonism of Hcrt reduced Tb to a greater extent in WT mice than in TG mice (Figure 8A, D, G). In awake WT mice after ALM (300 mg/ kg), Tb fell 0.9°C (F4,94 = 10.03, P < 0.001). This reduction in Tb persisted during NREM sleep (F4,94 = 5.67, P < 0.001), but was not evident during REM sleep. The time course of state-specific Tb was analyzed within genotypes by two-way RM-ANOVA (Figure 8B, C, E, F, H, I). ALM (300 mg/kg) dramatically reduced Tb during wakefulness and NREM sleep for several hours in WT mice (F44,599 = 6.67, P < 0.001 and F44,563 = 6.08, P < 0.001, respectively). A milder, more sporadic reduction in Tb was observed after ALM (100 mg/kg). In TG mice, Tb decreased during wakefulness and NREM sleep to a lesser extent than in WT mice after 300 mg/kg of ALM (F44,537 = 4.49, P < 0.001 and F44,528 = 5.34, P < 0.001, respectively). In accordance with the unexpected increase in active wakefulness observed in TG mice after ALM (30 mg/kg), Tb increased 0.6°C during the first h after this low dose of ALM (F44,537 = 4.49, P < 0.001).
DISCUSSION
In the current study, we evaluated the hypnotic and cataplexy-promoting efficacy of ALM in the orexin/ataxin-3 model of narcolepsy. After dosing at ZT12, ALM exacerbated cataplexy in orexin/ataxin-3 mice and increased NREM sleep with heightened sleep/wake fragmentation. The Hcrt antagonist showed greater hypnotic potency in WT than in TG mice, as ALM (300 mg/kg) increased NREM sleep more in WT mice than in TG mice. ALM (100 mg/kg) conferred maximal promotion of cataplexy in TG mice and maximal promotion of REM sleep in WT mice. ALM (30 mg/kg) paradoxically enhanced wakefulness with elevated motor activity and increased Tb in TG mice for the first h after administration. In both genotypes, Tb decreased after acute Hcrt receptor blockade, but the reduction in Tb that normally accompanies the wake-to-sleep transition was blunted in TG mice. These complex dose- and genotype-dependent interactions underscore the importance of the effector mechanisms downstream of Hcrt receptors that regulate arousal state.
Cataplexy Enhancement
Despite the loss of > 95% of Hcrt cells when evaluated at 28 wk of age, TG mice showed a surprisingly robust increase in cataplexy after ALM (Figure 3). These results indicate that the residual Hcrt tone in TG mice, which can be inferred from residual expression of the Hcrt precursor peptide in TG mice at 27 wk of age,10 suppressed cataplexy in the VEH condition. The magnitude of ALM-induced cataplexy was between that observed after systemic injections of QNP in Hcrt null mice15 and physostigmine in the double Hcrt receptor knockout model.16 Neither ALM, QNP,15 nor physostigmine16 affected the duration of cataplexy bouts, suggesting that the mechanism for cataplexy termination is independent of D2-like receptors, cholinergic transmission, and Hcrt. QNP (0.5 mg/kg) did not provoke cataplexy in our TG mice, in contrast to previous reports that this D2/D3 agonist increases cataplexy in dogs with narcolepsy29,30 and Hcrt null mice.15 Perhaps induction of cataplexy by QNP requires a large number of cell bodies in the posterior lateral hypothalamus that either produce Hcrt, as in the Hcrt2R-mutated dogs,31 or retain colocalized neurotransmitters such as glutamate or dynorphin,32 as in the Hcrt null mice.3 Altered sensitivity to dopaminergic agents between different murine models of narcolepsy has previously been demonstrated—TG mice were found to be less responsive to psychostimulant-induced hyperlocomotion than their Hcrt null or WT counterparts.33
The extended time period during which cataplexy was promoted after ALM treatment may offer clues about cataplexy induction. Cataplexy was increased after ALM (100 and 300 mg/ kg) from 6-12 h after dosing (Figure 3D), a time frame during which the Hcrt receptor 2 (HcrtR2) occupancy by ALM (30 mg/ kg) remains high in rats but Hcrt receptor 1 (HcrtR1) occupancy drops to less than 10%.27,34 To the extent that these receptor occupancy data extrapolate to mice, our observations suggest that antagonism of HcrtR2 contributes more to cataplexy induction than does blockade of HcrtR1. However, the higher doses of ALM induced NREM sleep in the TG mice for several h after dosing, precluding the possibility of cataplexy occurrence during that period. Nevertheless, the delayed but robust exacerbation of cataplexy after ALM in mice lacking Hcrt neurons contraindicates the use of Hcrt antagonists as hypnotic agents in human patients with narcolepsy and other patient populations with partial Hcrt cell loss.21–23
Sleep Promotion
As expected, ALM induced a dose-related increase in NREM sleep and a concomitant decrease in wakefulness (Figure 4A-F). However, the hypnotic effects of ALM were more potent in WT mice than in TG mice, presumably due to greater endogenous Hcrt tone in intact mice. Reduced hypnotic efficacy of ALM in TG mice may not be due to altered Hcrt receptor regulation, as Hcrt receptor levels remain stable for at least several mo after ligand deficiency.10,11 Our results complement previous findings9 that exogenous Hcrt is more potent in promoting arousal in TG mice than in WT control mice. The differential responsiveness could be caused in part by increased sensitivity of Hcrt-responsive pathways in TG mice, such as imbalanced cholinergic/monoaminergic networks downstream from Hcrt neurons.12,35,36
REM sleep appears to be particularly sensitive to the dose of ALM used and to the presence or absence of Hcrt-producing neurons. ALM promoted REM sleep most effectively in WT mice at 100 mg/kg (Figure 4G-I, Figure 6, Table 1)—the same dose that conferred maximal cataplexy expression in TG mice (Figure 3). However, ALM did not enhance REM sleep in TG mice at any of the doses tested in this study. It is unclear why Hcrt receptor antagonism in TG mice would be able to increase cataplexy but not REM sleep, given that the circuitry that underlies both states is hypothesized to have a high degree of overlap.12,37,38 Perhaps the circadian and/or homeostatic upper limit on REM sleep expression may already be maximal in TG mice during the dark period.
Another complex dose-by-genotype interaction is evident in the paradoxical wakefulness that was observed in TG mice during the first h after ALM (30 mg/kg). This low dose of ALM normalized NREM sleep latency in TG mice and stimulated active wakefulness (Figure 5) with increased Tb. Biphasic autonomic responses to low versus high doses of Hcrt1 have been described39; thus, low-level antagonism of low-level Hcrt tone could yield responses that are opposite to those observed with high levels. The biphasic response to Hcrt antagonism could be due to HcrtR1-mediated increase in dopamine release following ALM40 and altered dopamine sensitivity in TG mice.33 Follow-up studies in which low doses of ALM are tested during the light period (when endogenous Hcrt tone is low) might help further elucidate the paradoxical arousing effect observed here.
Overall, the hypnotic action of ALM rendered the sleep of WT mice to resemble, or even exceed, the highly fragmented sleep observed in TG mice. Frequent transitions between wakefulness, NREM, and REM sleep occurred after ALM treatment, as evidenced by increased bout numbers (Table 1), in agreement with prior reports.27,34,40 The fragmented sleep was not accompanied by reduced NREM bout duration; however, the extremely rapid arousal state transitions after the highest dose of ALM produced less intense sleep (Figure 7). Although ALM induced more frequent transitions between NREM sleep and wakefulness in WT mice compared with TG mice, REM sleep fragmentation (and REM sleep amount) in WT mice never exceeded the level observed in TG mice. These data suggest REM sleep pharmacodynamics are more directly dependent on Hcrt signalling, whereas NREM sleep and wakefulness can be further influenced by downstream effector mechanisms.
Thermoregulation
Acute antagonism of Hcrt in WT mice caused a transient, dose-dependent decrease in Tb during both wakefulness and NREM sleep. By contrast, chronic loss of Hcrt neurons was associated with increased Tb during NREM and REM sleep relative to WT mice, similar to human patients with narcolepsy.41 In TG mice, the thermolytic effect of sleep was diminished, and the degree of thermolysis in NREM sleep positively correlated with the number of Hcrt neurons that remained (Figure 8). Taken together, these data suggest that TG mice may compensate for the initial hypothermia associated with reduced Hcrt transmission with increased heat conservation mechanisms. Previous studies have raised the possibility that elevated Tb during sleep in Hcrt null mice could be due to impaired distal vasodilation.42 Peripheral heat loss in rats can be enhanced by intracerebroventricular microinjection of Hcrt143,44 and by stimulation of the posterior lateral hypothalamic area.45 Diminished thermolysis during sleep in TG mice is less likely due to sustained heat production because Hcrt1 has been shown to increase thermogenesis in brown adipose tissue.46 Our results on the acute versus chronic effects of Hcrt transmission blockade on thermoregulation suggest the hypothesis that a progression from reduced thermogenesis to hypothermia to compensatory heat conservation may accompany degeneration of the Hcrt neurons during the onset of narcolepsy.
Mechanism of Action
Our results on the promotion of cataplexy, sleep, and hypothermia with ALM are consistent with the hypothesis that these effects result from blockade of endogenous Hcrt tone. It is unlikely that ALM acts as an inverse agonist because it has no intrinsic effect on the spontaneous activity of neurons, at least for dopaminergic neurons of the ventral tegmental area.47 Although ALM is selective for Hcrt receptors, we cannot exclude the possibility that high doses of ALM exert hypnotic effects through off-target binding sites, including endocannabinoid receptors,18 which have been hypothesized to induce sleep by activating cholinergic neurons in the basal forebrain and pons.48 Interestingly, a HcrtR1 selective antagonist can alter the functionality of the endocannibinoid receptor CB1 via modulation of CB1/ HcrtR1 heterodimers49; perhaps ALM can similarly block CB1 signal transduction. The initial paradoxical wakefulness that was observed with a low dose of ALM in TG mice suggests that ALM may have partial agonist properties in the presence of low Hcrt tone. However, given that the selectivity profile of ALM indicates no meaningful stimulation of activity at the myriad of binding sites examined to date,18 it is unlikely that ALM acts as a partial agonist, at least at high doses.
Implications
Our finding that cataplexy can be manipulated by direct antagonism of the receptors targeted by residual Hcrt neurons that remain in TG mice encourages the development of Hcrt agonists as narcolepsy therapeutic agents. First, the demonstration that cataplexy can be exacerbated by ALM even in mice with ≥ 95% Hcrt cell loss reveals that small amounts of Hcrt are sufficient to confer some protection against cataplexy. Second, pharmacologic enhancement of cataplexy with ALM in this model may be advantageous in anticataplexy efficacy testing, particularly given the long-lasting promotion of cataplexy we observed (Figure 3D). However, these same findings dictate cautious use of Hcrt antagonists in humans with narcolepsy and other populations with reduced Hcrt levels,21–24 as further Hcrt antagonism could lead to impaired ability to adapt to allostatic loads with negative consequences on metabolism, thermoregulation, motivation, and cardiovascular and endocrine function.2,39
ACKNOWLEDGMENTS
The authors thank Professor Takeshi Sakurai for graciously providing the orexin/ataxin-3 mice, Dr. Ling Jong for almorexant synthesis, and Mr. Alan Wilk and Ms. Kristy Silveira for technical assistance. This work was supported by NIH grant R01NS057464 and by Award Number W81XWH-09-2-0081 from the U.S. Army Medical Research Acquisition Activity to T.S.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Army Medical Research Acquisition Activity.
ABBREVIATIONS
- ALM
almorexant
- ANOVA
analysis of variance
- EEG
electroencephalograph
- EMG
electromyograph
- f
fornix
- Hcrt
hypocretin (orexin)
- Hcrt2
hypocretin-2 (orexin B)
- HcrtR1
hypocretin receptor type 1
- HcrtR2
hypocretin receptor type 2
- ic
internal capsule
- IP
intraperitoneally
- IRSI
inter-REM sleep interval
- LD12,12
12 h light period and 12 h dark period
- LMA
locomotor activity
- MCH
melanin-concentrating hormone
- mt
mammillothalamic tract
- NREM
non-rapid eye movement sleep
- NRD
EEG delta power (0.5-4 Hz) within NREM
- NRSL
NREM sleep latency
- PBS
phosphate buffered saline
- QNP
quinpirole
- REM
rapid eye movement sleep
- RM-ANOVA
repeated-measures ANOVA
- RSL
REM sleep latency
- scp
superior cerebellar peduncle
- SQ
subcutaneously
- Tb
core body temperature
- TG
orexin/ataxin-3 transgenic
- VEH
vehicle
- WT
wild type
- ZT
Zeitgeber time
- 4V
4th ventricle
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–62. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Sinton CM. Orexin/hypocretin plays a role in the response to physiological disequilibrium. Sleep Med Rev. 2011;15:197–207. doi: 10.1016/j.smrv.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 5.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 6.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypo-cretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 9.Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–54. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishima K, Fujiki N, Yoshida Y, et al. Hypocretin receptor expression in canine and murine narcolepsy models and in hypocretin-ligand deficient human narcolepsy. Sleep. 2008;31:1119–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 12.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 13.Beuckmann CT, Sinton CM, Williams SC, et al. Expression of a polyglutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–77. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–25. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess CR, Tse G, Gillis L, Peever JH. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33:1295–1304. doi: 10.1093/sleep/33.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalogiannis M, Hsu E, Willie JT, et al. Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One. 2011;6:e18697. doi: 10.1371/journal.pone.0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark EL, Baumann CR, Cano G, Scammell TE, Mochizuki T. Feeding-elicited cataplexy in orexin knockout mice. Neuroscience. 2009;161:970–7. doi: 10.1016/j.neuroscience.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–5. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 19.Cao M, Guilleminault C. Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr Neurol Neurosci Rep. 2011;11:227–34. doi: 10.1007/s11910-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 20.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexanta novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 21.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–9. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–85. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 23.Petersen A, Gil J, Maat-Schieman ML, et al. Orexin loss in Huntington's disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- 24.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–95. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koberstein R, Fischli W, Clozel M, Aissaoui H, Weller T. Substituted 1,2,3,4-tetrahydroisoquinoline derivatives. World Patent:WO2005118548. 2005
- 26.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morairty SR, Revel FG, Malherbe P, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7:e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Mignot E, Nishino S, Sharp LH, et al. Heterozygosity at the canarc-1 locus can confer susceptibility for narcolepsy: induction of cataplexy in heterozygous asymptomatic dogs after administration of a combination of drugs acting on monoaminergic and cholinergic systems. J Neurosci. 1993;13:1057–64. doi: 10.1523/JNEUROSCI.13-03-01057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid MS, Tafti M, Nishino S, Sampathkumaran R, Siegel JM, Mignot E. Local administration of dopaminergic drugs into the ventral teg-mental area modulates cataplexy in the narcoleptic canine. Brain Res. 1996;733:83–100. doi: 10.1016/0006-8993(96)00541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripley B, Fujiki N, Okura M, Mignot E, Nishino S. Hypocretin levels in sporadic and familial cases of canine narcolepsy. Neurobiol Dis. 2001;8:525–34. doi: 10.1006/nbdi.2001.0389. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, van den Pol AN. Differential target-dependent actions of coex-pressed inhibitory dynorphin and excitatory hypocretin/orexin neuropep-tides. J Neurosci. 2006;26:13037–47. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori T, Ito S, Kuwaki T, Yanagisawa M, Sakurai T, Sawaguchi T. Monoaminergic neuronal changes in orexin deficient mice. Neuropharmacology. 2010;58:826–32. doi: 10.1016/j.neuropharm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Morairty SR, Revel FG, Malherbe P, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7:e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalogiannis M, Grupke SL, Potter PE, et al. Narcoleptic orexin receptor knockout mice express enhanced cholinergic properties in laterodorsal tegmental neurons. Eur J Neurosci. 2010;32:130–42. doi: 10.1111/j.1460-9568.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishino S, Fujiki N, Ripley B, et al. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett. 2001;313:125–8. doi: 10.1016/s0304-3940(01)02270-4. [DOI] [PubMed] [Google Scholar]
- 37.Luppi PH, Clement O, Sapin E, et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15:153–63. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sSLEEP. J Neurosci. 2011;31:6518–26. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plazzi G, Moghadam KK, Maggi LS, et al. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011;15:187–96. doi: 10.1016/j.smrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–51. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 41.Mosko SS, Holowach JB, Sassin JF. The 24-hour rhythm of core temperature in narcolepsy. Sleep. 1983;6:137–46. doi: 10.1093/sleep/6.2.137. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R533–40. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balasko M, Szelenyi Z, Szekely M. Central thermoregulatory effects of neuropeptide Y and orexin A in rats. Acta Physiol Hung. 1999;86:219–22. [PubMed] [Google Scholar]
- 44.Szekely M, Petervari E, Balasko M, Hernadi I, Uzsoki B. Effects of orexins on energy balance and thermoregulation. Regul Pept. 2002;104:47–53. doi: 10.1016/s0167-0115(01)00348-2. [DOI] [PubMed] [Google Scholar]
- 45.Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. J Physiol. 1997;503:177–86. doi: 10.1111/j.1469-7793.1997.177bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adi-pose tissue thermogenesis. J Neurosci. 2011;31:15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76:618–31. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- 48.Murillo-Rodriguez E. The role of the CB1 receptor in the regulation of sSLEEP. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1420–7. doi: 10.1016/j.pnpbp.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–24. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.