Abstract
Study Objectives:
Controllable stress, modeled by escapable shock (ES), can produce significant alterations in post-stress sleep, including increased rapid eye movement (REM) sleep. Recent work has demonstrated that post-stress sleep may be influenced by stressor predictability, modeled by predictive auditory cues. In this study, we trained mice with ES, either signaled (SES) or unsignaled (UES) by auditory cues, and investigated the effects of predictability on escape learning and sleep associated with ES.
Design:
Adult male BALB/cJ mice were implanted for recording electroencephalography and activity via telemetry. After the mice recovered from surgery, baseline sleep recordings were obtained. The mice were then randomly assigned to SES and UES conditions. Both groups had control over the duration of footshocks (0.5 mA; 5.0 sec maximum duration) by moving to the non-occupied chamber in a shuttlebox. SES mice were presented tones (90 dB, 2 kHz, 10 sec maximum duration) that started 5.0 sec prior to and co-terminated with footshocks. UES mice were presented identical tones that were not synchronized to shock presentation. ES training continued for 2 consecutive days (EST1 and EST2) with 20 footshock presentations (1 min inter-stimulus intervals). Seven days after EST2, the animals were re-exposed to the training chamber (context) alone for 30 min.
Measurements and Results:
Escape latency was used to determine successful or unsuccessful escape learning. Sleep was scored for 20 h for baseline and on each treatment day. Freezing in the training context was scored as a behavioral index of fear. Nine of 14 SES mice successfully learned escape (SESl), and 5 failed to learn escape (SESf). Compared with baseline, SESl mice, but not SESf mice, showed significantly increased post-shock REM. All UES mice learned escape and showed enhanced post-shock REM. Freezing and sleep did not differ among groups on the context re-exposure day.
Conclusions:
The results indicate that information available in a stressful situation can affect an animal's ability to learn an appropriate response and post-stress sleep.
Citation:
Machida M; Yang L; Wellman LL; Sanford LD. Effects of stressor predictability on escape learning and sleep in mice. SLEEP 2013;36(3):421-430.
Keywords: Controllability, escape learning, mice, predictability, rapid eye movement (REM) sleep, sleep, stress
INTRODUCTION
Stress has long been associated with negative health outcomes. However, successful coping can reduce psychological distress, and thus, favorably influence health outcomes.1 Several factors can affect the ability to cope with a stressful challenge, including individual resilience,2 and specific stressor characteristics including controllability, predictability, intensity, and duration.3 For example, lack of stressor controllability has been suggested to be a factor in the development of anxiety disorders, including posttraumatic stress disorder (PTSD).4,5 Indeed, uncontrollable stress figures prominently in hypotheses regarding the negative outcomes of stress, whereas controllable stress is typically associated with adaptive coping and neutral or positive outcomes.4–6 Predictability also plays important roles in the effects of stress. In humans, poor predictability is associated with increased pain, fear, and physiologic arousal,5,7–10 whereas work in animals suggests that predictable stress may be less anxiogenic than unpredictable stress based on findings that animals prefer signaled over unsignaled shocks.11–13
Stress also can produce disturbances in sleep (e.g., insomnia14) and studies in animals have found significant alterations in sleep in a variety of stress paradigms.15 In mice, we have found that uncontrollable stress (modeled by inescapable shock [IS])) is followed by significant decreases in sleep, whereas controllable stress (modeled by escapable shock [ES]) is followed by significant increases in sleep.16 Stressful memories associated with IS and ES produced alterations in sleep similar to those produced by the original stressor.16 Rapid eye movement (REM) sleep is the state most altered with both IS and ES. Enhanced REM has also been reported to occur after training with avoidable shock, another controllable stressor.17–20
Recently we extended our studies of the effects of stress on sleep to examine the effects of a predictive auditory cue presented prior to onset of ES and IS.21 We trained yoked pairs of mice either with signaled escapable shock (SES) or signaled inescapable shock (SIS). Predictive auditory cues presented prior to shock in the SES or SIS conditions attenuated the post-stress changes in sleep compared with the increases and decreases found after ES or IS alone. Later presentation of the auditory cue alone produced similar short-term reductions in REM and non-rapid eye movement (NREM) sleep in both SES and SIS trained mice. These results suggest that there are possible interactions between predictability and controllability in meditating the effects of stress on sleep, or that the shock-associated cues may be processed differently from shock-associated contextual information.
To assess the possibility that predictability and controllability interact, we tested the hypotheses that SES and unsignaled escapable shock (UES) would differentially affect post-stress sleep. We trained pairs of BALB/cJ (C) mice, a stress-vulnerable strain,22 either with SES or UES. In the SES condition, auditory cues were presented prior to shock onset whereas in the UES condition, identical auditory cues were presented except that the cue did not predict shock occurrence. This design enabled us to investigate the effects of predictability on alterations in post-stress sleep associated with stressor controllability, and to separately investigate the effects of predictive or non-predictive auditory cues on sleep. We also examined the role of escape learning on subsequent sleep, by comparing animals that successfully learned the escape response to mice that did not. Freezing during the context re-exposure period was evaluated across groups as a behavioral index of fear memory.
METHODS
Subjects
Adult male BALB/cJ mice were obtained from Jackson Laboratory, Bar Harbor, Maine. The mice weighed 20-25 g at arrival. Animals were individually housed with food and water available ad libitum. The mouse colony room was kept on a 12 h: 12 h light-dark cycle and ambient temperature was maintained at 24°C ± 1.5°C. Throughout the experimental procedures, measures were taken to minimize unnecessary pain and discomfort of the animals. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol #08-007).
Surgery
After at least one week of acclimation to the colony room, the mice were implanted intraperitoneally with telemetry transmitters (ETA10-F20, DataSciences, St. Paul, MN) for recording electroencephalography (EEG) and activity to monitor sleep and wakefulness as previously described.23 These transmitters can reduce spontaneous activity in mice,23 but do not interfere with their ability to escape shock in the ES condition. EEG leads from the transmitter body were led subcutaneously to the head, and the free ends were placed into holes (one in the left anterior quadrant and one in the right posterior quadrant around bregma) drilled in the dorsal skull to allow recording cortical EEG. Before surgery, animals received potassium penicillin (100 IU/g weight), dexamethasone (0.005 mg/g weight), and gentamicin (0.005 mg/g weight) subcutaneously. All surgery was conducted under isofluorane (as inhalant: 5% induction; 2% maintenance) anesthesia. Ibuprofen (30 mg/kg, oral) was continuously available in each animal's drinking water, for 24-48 h preoperatively and for a minimum of 72 h postoperatively, to alleviate potential postoperative pain. The animals were given at least three weeks for post-surgery recovery, and kept undisturbed except for weekly bedding changes.
SES and UES Training Procedures
The mice were randomly assigned to either SES or UES conditions. Training was conducted in a shuttlebox (Model E10-15SC, Coulbourn Instruments, Whitehall, PA) consisting of two chambers divided by a guillotine door. Opening and closing of the guillotine door, as well as the tone (90 dB, 2 kHz, 10 sec maximum) and footshock (0.5 mA; 5.0 sec maximum) administration were controlled by Coulbourn Graphic State (GS) software (version 2.1). Electric footshock was produced via Coulbourn Precision Regulated Animal Shockers and administered via grid floors of a shuttlebox. Training started between the third and fourth h after lights on. The entire training procedure was of approximately 30 min duration. For the first 5 min (pre-shock period), the mice were allowed to freely explore the shuttlebox followed by presentation of 20 shocks. When footshock was administered, the door opened simultaneously and mice were able to move from one chamber to the other unoccupied chamber in a shuttlebox. The movement caused interruption of photo-beam sensors that was detected by GS software that terminated shock presentation. The footshock was escapable, but not avoidable, for movement prior to footshock onset did not prevent shock presentation. The door closed 5 sec after shock onset, and remained closed until the next footshock was presented after a 1 min interval. After the final shock, the animals remained undisturbed in the shuttlebox for 5 additional min (post-shock period) and then were returned to their home cages.
In the SES condition, the auditory tone was presented to the animals 5 sec prior to shock onset as a “signal,” which co-terminated with footshock. UES mice were trained in pairs with SES mice, and thus identical tones were presented, but were not synchronized to or predictive of shock presentation. Training for both SES and UES consisted of 2 consecutive days (EST1 and EST2, 24 h interval) each with 20 footshocks. Seven days after EST2, the animals were re-exposed to context alone (training chamber with no shock presented) for 30 min. Training and testing was conducted at the same circadian time across days.
Determination of Escape Learning
GS software recorded the time at shock onset as well as the time of shock termination. Subtracting these two time values showed the duration of shock a mouse experienced on each shock trial and was used as a measure of escape latency. Summing escape latency across trials provided an estimate of the total shock duration each mouse received on EST1 and EST2. Decreased total shock on the second training day (EST2) was considered as evidence that a mouse had learned the escape response.
Scoring of Freezing
The training and test sessions were videotaped for subsequent scoring of freezing, which is defined as a rigid posture with the complete absence of visible movement except for respiration24,25 and was assessed as a measure of fear. Freezing was scored by a trained observer during the 5 min pre-shock period on EST1 and for the entire 30 min on the context re-exposure day. The percentage time spent in freezing was calculated as FT%: freezing time / observed time × 100% for each animal.
Sleep Recording and Determination of Sleep State
Sleep and wakefulness of the animals were monitored in the colony room. Recording started at the fifth h after lights on. The transmitters for the mice in their home cages were activated with a magnetic switch and were placed on a telemetry receiver (Model RPC-1, Data Sciences, St. Paul, MN). When the animals were not on study, the transmitters were inactivated. Signals from the transmitter were detected by the receiver, and were processed and saved by Data Sciences software for subsequent visual scoring. Sleep and wakefulness were determined by a trained observer in 10-sec epochs using SleepWave software (Biosoft Studio, Hershey, PA). Each epoch was scored either as state of sleep (NREM or REM) or wakefulness (active wakefulness [AW], or quiet wakefulness [QW]), based on EEG and gross whole body activity as previously described.23 Sleep latency was calculated from the start of recording to the first episode of REM (defined as more than two consecutive epochs, > 20 sec) or NREM (defined as more than six consecutive epochs, > 1 min). Twenty hour uninterrupted sleep recordings were collected for baseline and after treatments on EST1, EST2, and Context.
Data Analyses
Data were analyzed using SigmaStat V2.03 (SPSS, Inc., Chicago, IL). Training effects across days was analyzed using the paired t-test. Group effects, treatment effects, and time effect were examined using analysis of variance (ANOVA) procedures. When appropriate, post hoc comparisons among means were conducted using Tukey tests. In case the equal variance test failed, data were analyzed by Kruskal-Wallis ANOVA on ranks followed by Dunn method for pairwise comparisons. Differences were considered significant at P < 0.05.
RESULTS
Escape Latencies and Escape Learning
Mice that received UES training showed a significant reduction in average escape latency across trials across training days (EST1: 2.13 ± 0.91 sec; EST2: 0.86 ± 0.18 sec; t9 = 4.36, P = 0.002). Compared with EST1, reductions on EST2 were significant in 11 of 20 trials (Figure 1A). By comparison, SES mice that were presented identical but predictive auditory cues prior to shock did not show significant reductions in escape latency across training days (Figure 1B).
Figure 1.
Shock escape latency and total shock durations received by mice trained with signaled escapable shock (SES, n = 14) and unsignaled escapable shock (UES, n = 10) across two training days (EST1, EST2). (A) Average shock escape latency for UES mice across 20 individual shock trials during EST1 and EST2 (*P < 0.05 between EST1 and EST2). (B) Average shock escape latency for all SES mice across 20 individual shock trials during EST1 and EST2. (C) Average shock escape latency for SES failed (SESf), SES learned (SESl), and UES mice during EST1 presented in four-trial blocks (*P < 0.05 between SESf and SESl). (D) Average shock escape latency for SESl, SESf, and UES mice during EST2 presented in four-trial blocks (*P < 0.05 between SESf and SESl). (E) Total shock duration presented to UES, SES collectively and separately as SESf and SESl during EST1 and EST2 (*P < 0.05 between EST1 and EST2). Error bars are ± SEM.
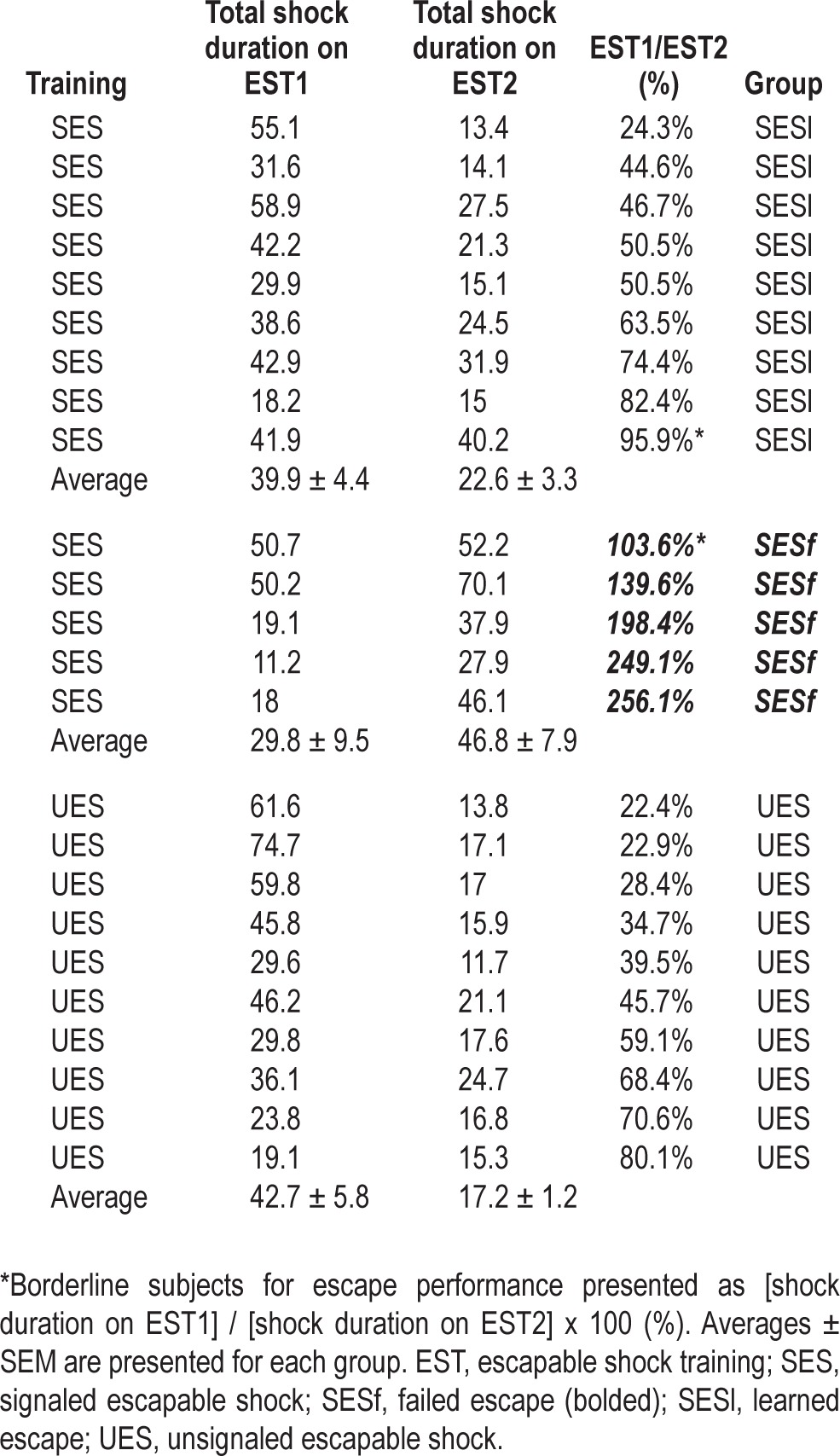
SES training resulted in greater individual variability in escape latencies (3.51-0.67 sec, range = 2.84 sec) compared with the UES trained mice (1.24-0.59 sec, range = 0.65 sec). Table 1 shows total shock duration received by each animal on EST1 and EST2. As improved escape latencies (percentage change from EST1 to EST2) indicate, no UES animals failed to learn escape. However, using this criterion, five of 14 mice failed to learn escape (SESf, bold italic), whereas nine of 14 SES mice successfully learned escape (SESl). We therefore treated SESl and SESf mice as separate groups for subsequent analyses of sleep. For two borderline SES mice (95.9% and 103.6% shock durations on EST2 compared to EST1), escape performance was closely compared with the averaged performance of learned or failed animals in four-trial blocks. This resulted in one borderline case being categorized as SESl and one as SESf.
Table 1.
Total shock duration in sec received by each animal during EST1 and EST2 and the percentage change across training days
For each training day, we examined performance across the 20 trials in five blocks of four trials. This analysis revealed that SESf mice showed significantly different escape responses from SESl and UES mice. SESf mice initially responded to shock more rapidly on EST1 (Trial 1-4; F(2,21) = 3.558, P < 0.05) followed by performance similar to SESl and UES mice on later trial blocks (Figure 1C). However, on EST2 (Figure 1D), the escape latency of SESf mice became significantly longer (Trial 5-8; F(2,21) = 12.635, P < 0.001, Trial 9-12; H = 11.107, P = 0.004, Trial 13-16; H = 10.043, P = 0.007), but returned to levels seen with the SESl and UES mice on Trial 17-20.
Subsequent analysis of the total shock received on EST1 and EST2 revealed that shock was reduced on EST2 for both UES (H = 12.632, P ≤ 0.001) and SESl (F(1,16) = 11.146, P = 0.004) (Figure 1E). However, total shock for the SESf mice was significantly increased on EST2 compared to EST1.
Freezing in Response to Context Re-exposure
Freezing was evaluated for the pre-shock period (PS; 5 min) as baseline activity and also during the context re-exposure period (30 min). During the PS period, no freezing was observed in any of the animals and behavior did not differ across groups. In contrast, during context re-exposure, all the mice showed significantly enhanced freezing compared with PS. However, there was no significant difference among groups either in total freezing (Figure 2A) or during any of the 5-min blocks (Figure 2B).
Figure 2.
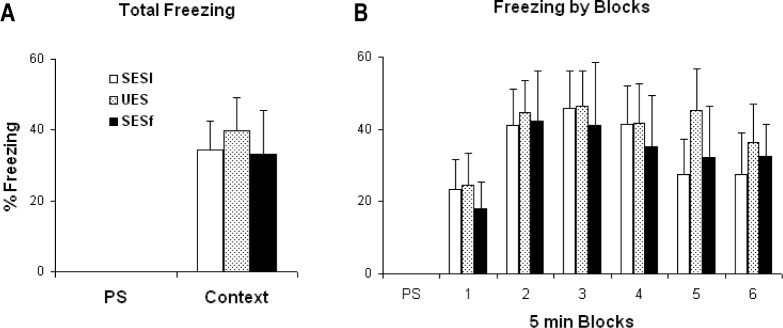
Behavioral freezing exhibited during re-exposure to the shock context alone. (A) Percent time freezing plotted for the 5-min pre-shock period (PS) and for the entire 30-min context re-exposure after the mice had two shock training sessions. (B) Percent time freezing plotted for PS and in 5-min blocks across context re-exposure. Freezing did not significantly differ among groups.
Effects of SES and UES on NREM Sleep, Total Sleep Time, and Wakefulness
Based on escape learning, the UES, SESl, and SESf mice were treated as separate groups for analyses of the sleep data. Baseline sleep did not differ across groups.
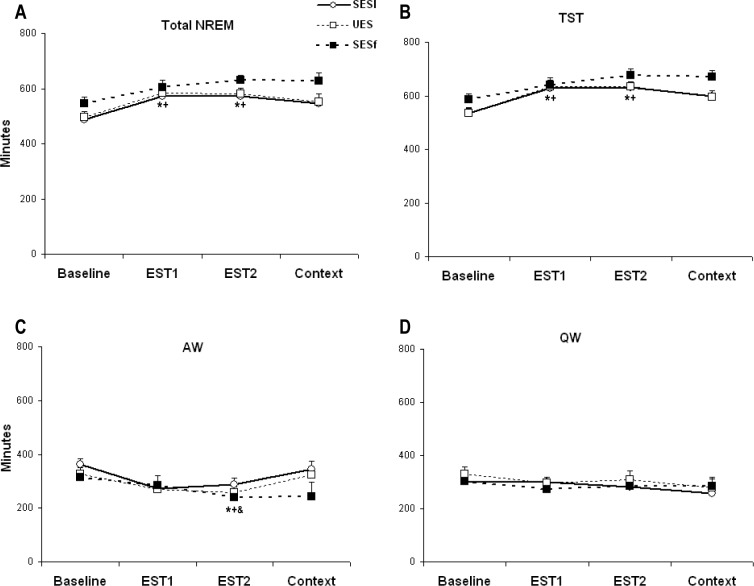
The analyses of total NREM (Figure 3A) revealed significant group (F(2,92) = 5.525, P = 0.006) and treatment (F(3,91) = 8.790, P < 0.001) effects. In general, SESf mice showed greater NREM compared with the SESl (P = 0.008, Tukey) and UES (P = 0.010, Tukey) mice. NREM did not differ between the SESl and UES groups. Specifically SESl and UES but not SESf mice showed significant increases of NREM on EST1 and EST2 compared with baseline. Sleep on the context-alone day did not significantly differ from baseline in any of the groups. There were no significant differences in NREM latency among groups.
Figure 3.
Selected sleep and waking parameters plotted as 20-h totals for Baseline, EST1, EST2, and the context test day (Context). (A) Total non-rapid eye movement (NREM). (B) Total sleep time (TST). (C) Active wakefulness (AW). (D) Quiet wakefulness (QW). SESf, failed escape; SESl, learned escape; UES, unsignaled escapable shock. *P < 0.05 for SESl compared with baseline; +P < 0.05, UES compared with baseline; &P < 0.05, SESf compared with baseline. Error bars are ± SEM.
Virtually identical directional changes were found in the analyses of total sleep time (TST; Figure 3B) with significant group (F(2,92) = 4.408, P = 0.015) and treatment (F(3,91) = 10.758, P < 0.001) effects. Mice in the SESf group showed greater TST compared with the SESl (P = 0.025, Tukey) and UES (P = 0.020, Tukey) groups. TST did not differ between the SESl and UES groups. Both SESl and UES mice showed significant increases of TST on EST1 and EST2 compared with baseline; however, in contrast, SESf mice did not show increases in TST compared with baseline. None of the groups showed significantly increased TST after context-alone exposure.
Wakefulness was separated into AW (Figure 3C) and QW (Figure 3D) for analyses. The analysis for AW revealed a significant treatment effect (F(3,91) = 3.224, P = 0.027). In general, the mice were less active after shock training compared with baseline, which was significant on EST2 compared with baseline. By comparison, QW did not significantly differ across treatments or groups.
Effects of SES and UES on REM Sleep
Baseline REM parameters (total REM, REM episode counts and duration, and REM percentage) that we examined did not differ across groups. However, shock training altered REM sleep. The analysis for total REM revealed significant group (F(2,92) = 6.924, P = 0.002) and treatment (F(3,91) = 2.750, P = 0.048) effects (Figure 4A). Group differences were the most significant between SESl and SESf animals (P = 0.002), but also between SESl and UES animals (P = 0.028). When the two SES groups (SESf and SESl) alone were compared, their total REM significantly differed on EST1 (F(1,53) = 14.297, P = 0.004) and on EST2 (P = 0.044), but not on the context day. Treatment effects were most notable in SESl animals and ANOVA revealed that total REM of SESl mice was significantly increased on EST1 (F(1,16) = 6.384, P = 0.022) and on EST2 (F(1,16) = 7.078, P = 0.017) compared with baseline. SESf mice did not show any post-shock increases in REM. UES mice also showed increased post-shock REM, although the increase was only significant on EST2. Although there was a tendency toward increased latency after the first training session, there were no significant differences in REM latency among groups.
Figure 4.

Selected REM parameters plotted at 20-hr totals for Baseline, EST1, EST2, and Context. (A) Total time spent for REM. (B) Number of REM episodes. Error bars are ± SEM. SESf, failed escape; SESl, learned escape; UES, unsignaled escapable shock. *P < 0.05 for SESl compared with baseline; +P < 0.05, UES compared to baseline. #P < 0.05 for comparisons between SESl and SESf.
The numbers of REM episodes were significantly different between SESf and SESl (F(2,92) = 4.375, P = 0.016) (Figure 4B), but not the duration (not shown). Thus, the increase in post-shock REM found in SESl mice appeared to be due to the increase of the number of REM episodes.
Because we did not see any treatment effects in response to the context re-exposure in any groups, we evaluated the hourly response during the first 4 h of recording for potential differences in REM percentage (REM/TST) (Figure 5A [SESl], B [SESf], and C [UES]). There was no difference in baseline sleep amounts or time course during this period; however, we found significant group (F(11,80) = 3.472, P = 0.036) and treatment (F(11,80) = 3.431, P = 0.021) effects. Pairwise multiple comparison revealed that, compared to baseline, REM percentage was significantly reduced during h 1 for UES mice (P = 0.008), but not for SESl or SESf mice. NREM did not significantly differ across groups (data not shown).
Figure 5.
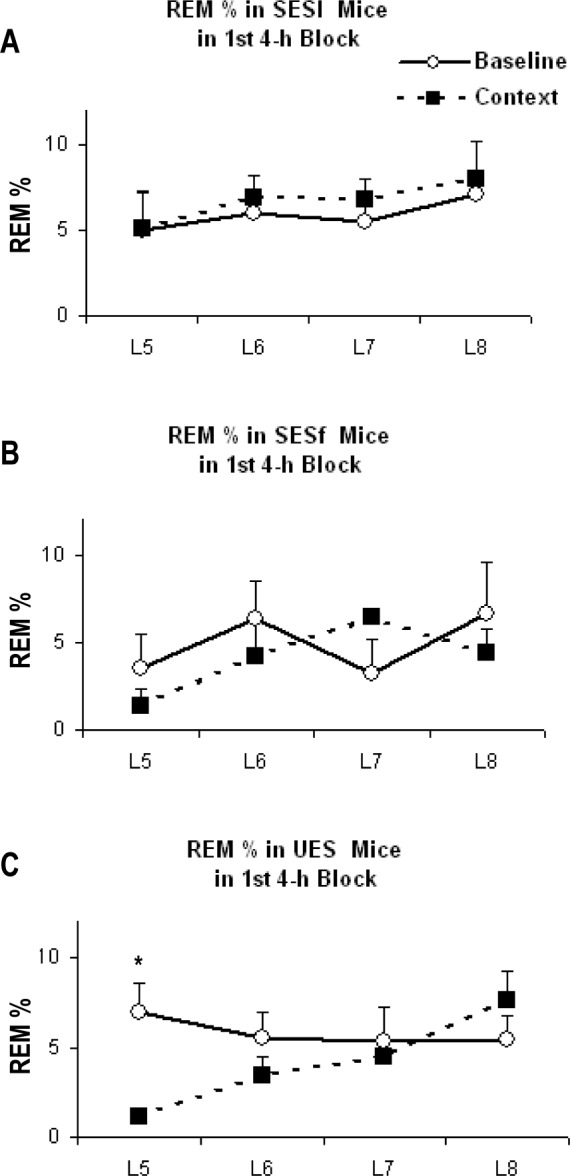
REM percentage plotted for the first 4 h (the fifth to eighth h after lights on) of Baseline and Context in (A) SESl, (B) SESf, and (C) UES mice. Error bars are ± SEM. *P < 0.05 compared with baseline. SESf, failed escape; SESl, learned escape; UES, unsignaled escapable shock.
DISCUSSION
Effects of Predictive and Non-predictive Auditory Cues
The first major finding of this study was that presentation of auditory information, predictive or not, during successful ES training blunted the effect of controllability on post-stress REM sleep. That is, mice successfully trained with SESl or UES showed enhanced REM after training that was directionally similar, but reduced in magnitude, in comparison with that seen after ES training without auditory cues.16 Moreover, the presence of an auditory cue during training diminished subsequent changes in REM that occur in mice presented ES context alone.16 That is, REM was not increased above baseline levels when the mice were re-exposed to training context alone 7 days after SES/UES training.
The findings of attenuated increases in REM are in concordance with our previous study reporting on the effects of predictable (auditory cued) controllable (SES), and uncontrollable stress (SIS) on REM sleep.21 Auditory stimuli that predict the onset of footshock that cannot be avoided may produce complex effects on stress-related learning that also affects post-stress sleep. For example, BALB/cJ mice trained with SES showed significantly increased REM after shock training compared with mice trained with SIS, whereas SIS mice showed significantly increased NREM after shock training. However, both groups showed reduced REM and similar stress-induced increases in temperature and freezing after presentation of the predictive cue alone. In that study, predictable auditory cues presented during training attenuated increases in REM compared with training in the context alone. Presentation of the predictive cues alone 7 days after training produced an initial reduction in REM similar to that seen with cues associated with inescapable shock, thereby suggesting that the cue and contextual information had competing influences on post-stress sleep.
Although predictability is typically modeled by an explicit, temporally-related warning signal, predictability may also be implicit in the timing of events.9 Thus, shocks in our UES paradigm were also predictable to a certain degree because they were presented at fixed intervals. In addition, the ability of the mice to behaviorally control the shock made its termination predictable and the presentation of shock in the same chamber each time made it situationally predictable. Therefore, the results of this study should be viewed with the understanding that each condition in this paradigm had some element of predictability for the animal, although the auditory cue varied with respect to whether it specifically signaled the onset of shock.
Predictive Cues and Learning
One of the secondary findings in this study was that predictive auditory information appeared to be able to adversely affect performance across training sessions. Of the 14 mice receiving identical SES training, nine successfully learned escape (SESl) as indicated by improved performance across days and five did not (SESf). In contrast, none of the mice failed to improve in escape learning across training days when identical, but non-predictive, auditory cues were presented during training (UES, n = 10).
Interestingly, the SESf mice had shorter escape latencies when shock training began on EST1; however, their performance deteriorated over time and was worse during much of EST2, but returned to levels equivalent to the SESl and UES groups by the end of EST2. That is, although the SESf mice had poorer overall performance, they also had better initial performance and similar end-of-training performance. Thus, although it may be tempting to suggest that predictive auditory information has the potential to simply interfere with learning the simple escape task, the situation may be more complex. Variations between the SESl and SESf mice also may involve differences in emotion and alternate response strategies elicited by the predictive cue that could affect performance.
Post-shock sleep differences in REM were also related to performance on the escape task and were different for the SESl and SESf mice. SESl mice showed enhanced REM on EST1 and EST2, whereas the SESf mice did not show significantly enhanced REM on either training day. SESl mice also showed significant increases in NREM and TST on the training days compared with baseline, whereas the SESf mice did not. Thus, the results from the SESl and SESf mice show a positive relationship between the amount of REM and subsequent day performance.
There have long been arguments that REM plays a role in memory consolidation; some studies26,27 present alternative views. At a correlative level, our data appear to support a role for enhanced REM in the consolidation of successful escape learning. However, it is important to note that, in the paradigm we used, the association between the shock stimulus and the appropriate behavioral response is very simple and, as we have indicated before, the increases in REM that can occur with simple variants of the paradigm (i.e., without auditory cues) seem disproportionately large for learning a relatively simple set of associations and stimulus-response behaviors.16 In fact, learning in the SES-UES paradigm, which shows less pronounced increases in REM, may be more complex because the mice must incorporate the auditory information into their responses and must discriminate among more types of useful and non-useful information.
There have been recent suggestions that REM plays a role in “decoupling” memory from its emotional charge28–30 and that intact REM may aid in the processing of the memory for trauma.31,32 Based on our work with animals, we also have suggested that REM may play an adaptive function in recovery from stress.33 However, as learning an appropriate coping response can lessen the negative effect of stress, it is likely that teasing apart the possible role of REM in learning, emotion, and adaptive response to stress will be difficult.
Suggestions that REM may be important for emotional processing also may appear to be at odds with the prevailing view that predictable shock is less anxiogenic than unpredictable shock, based on findings that animals prefer signaled over unsignaled shocks.11–13 However, studies have also reported that predictable shock is associated with an increase in ulcer development,34,35 greater weight loss,36,37 and increased susceptibility to disease.38 Indeed, the preference for predictability is likely affected by several experimental parameters, including shock intensity, signal duration, inter-shock intervals, amount of training, and the dependability of shock-free periods.39 Studies in humans also suggest that the effects of predictability varies with individual preferences for information.8
Escape and Freezing
This study found no advantage of predictive over non-predictive auditory cues in improving escape performance in the simple paradigm we used. There was no significant difference in the SESl and UES mice in amount of shock received on EST2 or in improvement on EST2 compared with EST1. There also were no significant between-group differences in post-training sleep, although there were some variations in the temporal distribution of REM in the post-stress period. This type of difference has been previously noted in the “windows” of enhanced REM observed after training with avoidable shock.17,19,20
Behavioral freezing is a commonly used measure of fear memory in rodents, with greater percentage of freezing (FT%) being interpreted as indicating stronger fear reactions.24,40 Mice trained with either SES or UES showed virtually equivalent increases in freezing in response to context alone, indicating that they associated contextual information with shock. In other studies, we have demonstrated that freezing is similar in mice trained with other variants of ES and IS.16,41 Moreover, plasma corticosterone, a marker of stress-induced activation of the hypothalamo-pituitary-adrenal axis, is similarly increased by ES and IS relative to non-shocked mice (unpublished data) and rats.42 These findings suggest that, with the extensive training paradigms we use, fear behavior and the peripheral stress response do not distinguish whether a stressor is controllable or uncontrollable. In the current and previous studies we also found a dissociation of post-stress sleep from freezing behavior.16,21 Thus, although stressful experiences almost always produce alterations in subsequent sleep, behavioral indices of fear in wakefulness alone do not predict the direction of those changes.
Neural Substrate Mediating the Effects of Stress-Related Cues and Contexts on Sleep
Together, the current study and previous work16,21 demonstrate that auditory information (predictive and non-predictive cues) presented in the training context can alter post-training and fear-conditioned changes in sleep without altering behavioral fear. This may involve an interaction between the associations made to the auditory cues and those made to the context. Auditory cues associated with both ES and IS training are followed by reductions in REM, whereas contexts associated with ES and IS are followed by directionally different alterations in REM.16,21 This suggests that these stimuli are processed differently during initial learning. One possible explanation is that the cued and contextual information may recruit overlapping but potentially competing neural substrates that result in different outcomes with respect to REM. This suggestion is consistent with variations in the neural circuitry underlying classic cued and contextual fear conditioning43–45 and stressor controllability.46,47
The amygdala is a central structure for both cued and contextual fear. In general, the lateral nucleus of the amygdala (LA) receives sensory information from modality-specific areas of thalamus and cortex48 and is responsible for the acquisition of cued conditioning.49 Contextual fear conditioning, on the other hand, requires the hippocampus as well as the amygdala,50 and damage to ventral hippocampal (CA1 and subiculum) projections to the basal amygdala (BA) or damage or inactivation of the BA itself interferes with contextual conditioning.51–54 The central nucleus of the amygdala (CNA) mediates the expression of fear responses elicited by both cued and contextual conditioned stimulus.55–57 BA and CNA also mediate fear and stress-induced alterations in REM.58–60
The ventromedial prefrontal cortex (vmPFC) is central to mediating the effects of stressor controllability and determining the consequences of stress and coping46,47,61,62 and is a putative site of neural plasticity underlying the lasting effects of experience with a controllable stressor.61 The prelimbic region (PL) of the vmPFC selectively responds to controllable stress and part of its influence is enacted through the serotonergic dorsal raphe nucleus (DRN).47,62 Given the putative inhibitory influence the serotonergic neurons in DRN have on REM,63 this PLDRN projection provides a potential pathway by which stressor controllability can influence REM. For example, in an intense wheel-turn controlled shock paradigm, IS activates DRN more than ES,47 which could suppress REM sleep. In line with this finding, IS but not ES upregulates neural activity indicated by Fos expression in mice DRN.41,64
The PL also robustly projects to the BA, but not to the LA.65 BA plays a role in regulating stress- and fear-induced alterations in REM.60 It also appears to be an important site for mediating the relationship between fear memory and sleep because a single microinjection into the BA of the corticotrophin-releasing factor antagonist, antalarmin, prior to IS training can block reductions in REM after shock and after context alone, but does not block freezing.60 Thus, projections from PL to BA provide another pathway by which stressor controllability can affect sleep, although the influence of BA is likely enacted via CNA.
Conceptions of the neural substrate thought to underlie conditioned fear are being revised in light of new research and older data that did not fit existing models.66 However, as with behavioral indices of fear, there appears to be sufficient evidence to suggest that distinct pathways may mediate the effects of explicit auditory cues and contexts on post-stress sleep. Evidence from studies on controllable stress and contextual fear suggests that the vmPFC likely plays a role in mediating the differential effects of controllable and uncontrollable stress on sleep, possibly via projections to the amygdala and/or DRN. LA-dependent auditory cues may interact with these pathways and alter the REM response to shock training and to subsequent fearful events. There is also evidence that CNA, a direct effector of the effects of fear and stress on REM, receives thalamic input and is a site of synaptic plasticity related to cued fear.66
CONCLUSION
Controllable and uncontrollable stress, modeled by ES and IS, can produce directionally different alterations in post-stress sleep16 and recent work has demonstrated that the changes in post-stress sleep may vary depending on whether or not the shock was signaled.21 The current study indicates that both predictive and non-predictive auditory cues may affect the learning and changes in sleep associated with controllable stress. The results also suggest that predictive auditory cues may have a negative effect on escape learning in some mice.
Although fear conditioning is an important model for understanding stress-based disorders, our results demonstrate that freezing, a typical fear behavior in wakefulness, can be quite similar across experimental conditions that produce substantial differences in post-stress sleep. It is becoming apparent that amounts and type of post-stress sleep can be highly influenced by situational variables including whether the stressor was controllable and/or predictable and what types of responses the animal learned. Examination of these factors and their interactions will be required to fully understand how stress influences sleep and the role that sleep may play in adaptive and non-adaptive responses to stress. Consideration of the linkages of the neural circuitry underlying fear and stressor control with those regulating sleep also will likely be required to fully understand the neurobiologic bases of fear- and stress-related disorders, many of which are linked to conditioning processes and are characterized by sleep disturbances.
ACKNOWLEDGMENT
This work was supported by NIH research grant MH64827.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. There was no off-label or investigational use of drugs.
REFERENCES
- 1.Penley JA, Tomaka J, Wiebe JS. The association of coping to physical and psychological health outcomes: a meta-analytic review. J Behav Med. 2002;25:551–603. doi: 10.1023/a:1020641400589. [DOI] [PubMed] [Google Scholar]
- 2.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–96. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 3.Natelson BH. Stress, hormones and disease. Physiol Behav. 2004;82:139–43. doi: 10.1016/j.physbeh.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Bolstad BR, Zinbarg RE. Sexual victimization, generalized perception of control, and posttraumatic stress disorder symptom severity. J Anxiety Disord. 1997;11:523–40. doi: 10.1016/s0887-6185(97)00028-5. [DOI] [PubMed] [Google Scholar]
- 5.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 6.Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur AZ. Stress of predictable and unpredictable shock. Psychol Bull. 1986;100:379–87. [PubMed] [Google Scholar]
- 8.Miller SM, Mangan CE. Interacting effects of information and coping style in adapting to gynecologic stress: should the doctor tell all? J Pers Soc Psychol. 1983;45:223–36. doi: 10.1037//0022-3514.45.1.223. [DOI] [PubMed] [Google Scholar]
- 9.Klein LC, Popke EJ, Grunberg NE. Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Exp Clin Psychopharmacol. 1997;5:99–106. doi: 10.1037//1064-1297.5.2.99. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Chapman CR, Kim B, et al. Predictability of painful stimulation modulates subjective and physiological responses. J Pain. 2009;11:239–46. doi: 10.1016/j.jpain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.French D, Palestine D, Leeb C. Preference for a warning in an unavoidable shock situation: replication and extension. Psychol Rep. 1972;30:72–4. [Google Scholar]
- 12.Miller RR, Daniel D, Berk AM. Successive reversals of a discriminated preference for signaled tailshock. Anim Learn Behav. 1974:271–4. [Google Scholar]
- 13.Badia P, Harsh J, Abbott B. Choosing between predictable and unpredictable shock conditions: data and theory. Psychol Bull. 1979;86:1107–31. [Google Scholar]
- 14.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 15.Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: Models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanford LD, Yang L. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33:621–30. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C, Kitahama K, Valatx JL, Jouvet M. Increased paradoxical sleep in mice during acquisition of a shock avoidance task. Brain Res. 1974;77:221–30. doi: 10.1016/0006-8993(74)90786-0. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–29. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Saha S, Prutzman SL, Mullins OJ, Mavanji V. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J Neurosci Res. 2005;80:727–37. doi: 10.1002/jnr.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C, Lapp L. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol Behav. 1986;36:1053–7. doi: 10.1016/0031-9384(86)90479-8. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Wellman LL, Ambrozewicz MA, Sanford LD. Effects of stressor predictability and controllability on sleep, temperature, and fear behavior in mice. Sleep (Abstract Supplement) 2011;34:759–71. doi: 10.5665/SLEEP.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep (Abstract Supplement) 2002;25:691–9. [PubMed] [Google Scholar]
- 24.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 26.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behav Brain Sci. 2000;23:867–76. doi: 10.1017/s0140525x00004003. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- 28.van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–32. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–42. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 32.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 34.Simpson CW, WIlson LGM, DiCara LV, Jarrett KJ, Carroll BJ. Stress-induced ulceration in adrenalectomized and normal rats. Bull Psychonomic Soc. 1975;6:189–91. [Google Scholar]
- 35.Glavin GB, Mikhail AA. Stress and ulcer etiology in the rat. Physiol Behav. 1976;16:135–9. doi: 10.1016/0031-9384(76)90296-1. [DOI] [PubMed] [Google Scholar]
- 36.Pare WP. Stress and consummatory behavior in the albino rat. Psychol Rep. 1965;16:399–405. doi: 10.2466/pr0.1965.16.2.399. [DOI] [PubMed] [Google Scholar]
- 37.Friedman SB, Ader R. Parameters relevant to the experimental production of “stress” in the mouse. Psychosom Med. 1965;27:27–30. doi: 10.1097/00006842-196501000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Friedman SB, Ader R, Glasgow LA. Effects of psychological stress in adult mice inoculated with coxsackie B viruses. Psychosom Med. 1965;27:361–8. doi: 10.1097/00006842-196507000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Badia P HJ, Abbott B. Choosing between predictable and unpredictable shock conditions: data and theory. Psychol Bull. 1979;86:1107–31. [Google Scholar]
- 40.Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–65. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Tang X, Sanford LD. Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol Behav. 2009;97:321–6. doi: 10.1016/j.physbeh.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–74. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- 43.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 44.Desmedt A, Garcia R, Jaffard R. Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. J Neurosci. 1998;18:480–7. doi: 10.1523/JNEUROSCI.18-01-00480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–8. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 47.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 48.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–9. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 51.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–64. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- 53.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–62. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 56.Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 57.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Wellman LL, Yang L, Ambrozewicz MA, Tang X, Sanford LD. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34:1539–49. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Yang L, Wellman LL, Tang X, Sanford LD. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32:888–96. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wellman LL, Ambrozewicz MA, Yang L, Sanford LD. Antagonizing corticotropin releasing factor 1 receptors (CRF1R) in the basolateral amygdala (BLA) attenuates the effect of footshock training on sleep in rats. Sleep. 2010;33:A44. [Google Scholar]
- 61.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–6. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steriade M, McCarley R. Brainstem control of wakefulness and sleep. New York: Plenum Press; 1990. [Google Scholar]
- 64.Grahn RE, Will MJ, Hammack SE, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 65.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]