Abstract
Introduction:
Smoking is a major risk factor for a variety of diseases. Electronic cigarettes are battery-operated devices that deliver nicotine to the lungs by evaporation of a liquid. Chronic idiopathic neutrophilia is a condition characterized by elevated white blood cell and neutrophil counts without any underlying disease; smoking has been implicated as a potential cause.
Case Presentation:
A male Caucasian patient, born in 1977, presented in September 2005 with asymptomatic elevation of white blood cell and neutrophil count, and mildly-elevated C-reactive protein levels. He was a smoker since 1996 and was treated with 20 mg/day of simvastatin since 2003 due to hyperlipidemia. Clinical examination, and laboratory and imaging investigations ruled out any infectious, haematological, rheumatological, or endocrine conditions. He was followed-up regularly and was advised to stop smoking. He had 2 unsuccessful attempts to quit smoking; one was unassisted and the second was performed with the use of both varenicline and nicotine replacement therapy (patches). During the subsequent 6.5 years, his leukocyte and C-reactive protein levels were repeatedly elevated; the condition was consistent with chronic idiopathic neutrophilia. In February 2012, he started using electronic cigarettes and he managed to quit smoking within 10 days. After 6 months, laboratory examination showed normalized leukocyte count and C-reactive protein levels, confirmed immediately by a second laboratory and by repeated tests after 1 and 2 months.
Conclusion:
Smoking cessation with the use of electronic cigarette led to reversal of chronic idiopathic neutrophilia. The daily use of electronic cigarette may help preserve the beneficial effects of smoking cessation.
Keywords: electronic cigarette, smoking, chronic idiopathic neutrophilia, inflammation, smoking cessation
Introduction
Cigarette smoking is a major cause of disease, affecting several systems in the human body.1,2 Although reducing cigarette consumption does not improve prognosis,3 smoking cessation has important beneficial socioeconomic and health-related implications.4,5 However, quitting smoking is a very difficult task. Smokers that try to quit without any medical aid or treatment have extremely low success rate.6 Although several pharmaceutical products are available for smoking cessation, long term quit-rates are relatively low.7 Therefore, tobacco harm reduction strategies and products have been developed, with the main goal to reduce the amount of harmful substances administered to the human body.8
Electronic cigarettes have been introduced to the market in recent years as an alternative to smoking. They are hand-held electronic nicotine-delivery devices consisting of a battery, a cartridge containing liquid, and an electrical resistance that is heated through the battery power and evaporates the liquid. The do not contain tobacco and there is no combustion involved. They deal with the chemical addiction by delivering nicotine to the lungs and consequently to the circulation. Although millions of people use them all over the world, there is lack of clinical evidence on their efficacy in reversing smoking-related disease and conditions.
Chronic idiopathic neutrophilia (CIN) is a condition characterized by asymptomatic elevation of white blood cells (WBCs) and neutrophil count that persists for years without any underlying disease.9 Smoking has been implicated as a cause of this condition,9,10 and leukocyte count is a predictor of future cardiovascular events.11,12
To the best of our knowledge, we report for the first time a case study of a subject with CIN that was reversed by smoking cessation with the daily use of electronic cigarettes. Written informed consent was obtained from the patient for presenting this case report.
Case Presentation
A male Caucasian, born in 1977, was presented in September 2005 with an elevated WBC count found during a routine check-up. At this time, he had been a smoker since 1996 (9 pack-years at the time of presentation). He had a positive family history of premature coronary heart disease and hyperlipidemia, which was treated with simvastatin at 20 mg/day since 2003. Complete blood count tests performed 9 and 18 months earlier were normal (WBC: 8900–9700/μL, neutrophils: 4183–4462/μL, lymphocytes: 4005–4268/μL, eosinophils: 89–194/μL, basophils: 623–776/μL). At presentation, his WBC count was 14,600/μL (8614/μL neutrophils, 5256/μL lymphocytes, 292/μL eosinophils and 438/μL basophils). Hematocrit (45.2%) and platelet count (305,000/μL) were within normal range. Blood smear was normal. The test was repeated twice in a different laboratory with similar results. He was completely asymptomatic, had no history of recent infections or trauma and reported no fever. He had no changes in body weight or appetite over the past months; his body mass index (BMI) was 27.7 kg/m2 at presentation. Clinical examination was normal and he was afebrile. Routine laboratory examinations did not reveal any renal or liver dysfunction. Thyroid hormones were within normal range, as were serum cortisol levels. He did not report any recent intake of steroid drugs. C-reactive protein was elevated at 14 mg/L (normal range < 5 mg/L). Rheumatologic and infectious disease work-up (including ANA, anti-dsDNA, Le-test, Ra-test, ASTO, CMV and EBV antibodies, Wright test and Widal reaction) were all negative for disease. Chest x-ray, echocardiogram and upper abdominal ultrasound were normal. Spleen and liver size were within normal limits. A CT-scan of thorax and abdomen were also normal.
He was invited for a repeat complete blood count after 2 months, with WBC reaching 21,000/μL (neutrophils: 14,280/μL, lymphocytes: 5250/μL, eosinophils: 630/μL, basophils: 840/μL). Once again he was asymptomatic and with no signs of infection or any other inflammatory condition. He was instructed to stop intake of simvastatin and repeat the examination in another 2 months. In January 2006, leukocytosis was still present (WBC: 17,900/μL, neutrophils: 11635/μL, lymphocytes: 5012/μL, eosinophils: 537/μL, basophils: 716/μL). He was prescribed atorvastatin at 20 mg/day because of elevated LDL levels. The diagnosis of CIN was suspected and he was offered a bone marrow aspiration biopsy to rule out other conditions. He refused the exam and was scheduled for routine follow-up. He was also advised to stop smoking.
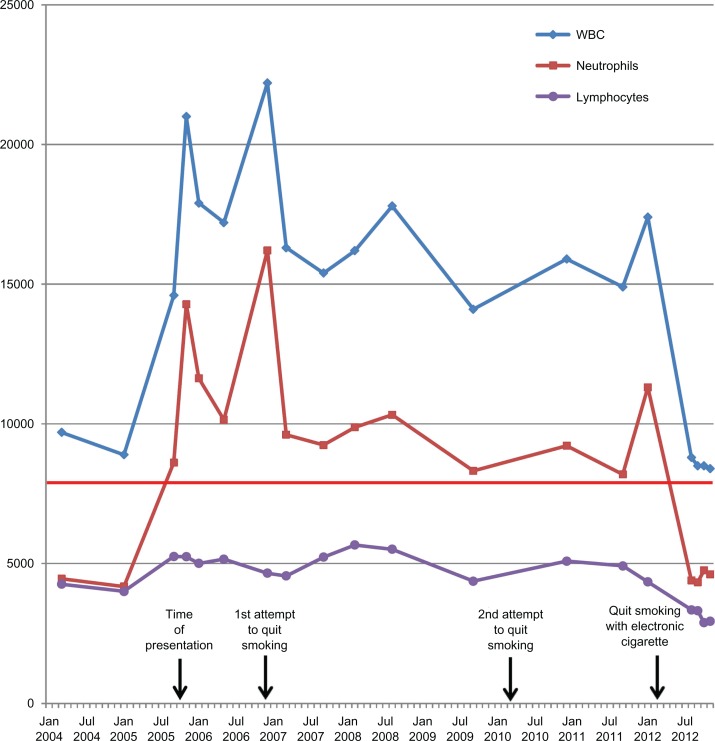
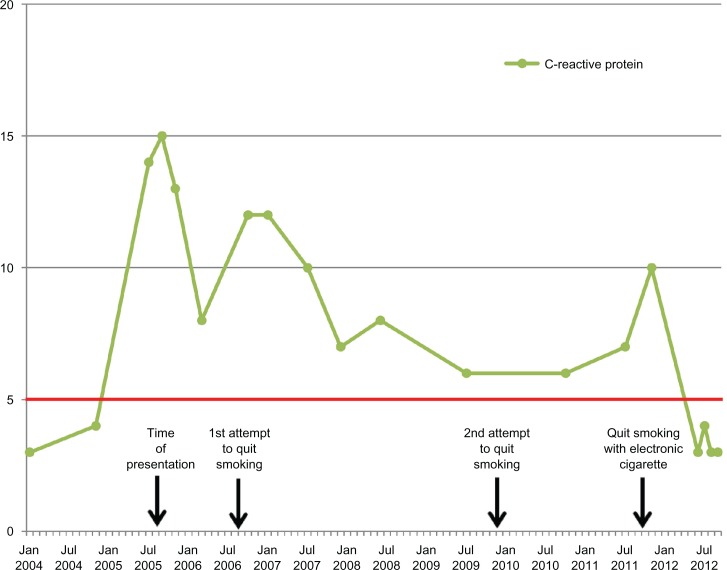
Figure 1 displays all WBC counts over the course of 7 years since presentation. All results were consistent with CIN, and mild elevations in C-reactive protein were also noted (Fig. 2). He did not receive any other medications during this period besides antipyretics for 2 episodes of common cold; all laboratory examinations were performed at least 10 weeks far from the common cold episodes. He had two unsuccessful attempts to quit smoking, one without any medical treatment in 2006 and one with varenicline plus nicotine patches in 2010. A change in statin prescription from atorvastatin (20 mg/day) to rosuvastatin (20 mg/day) was done in May 2010, but no difference was observed in WBC count in subsequent measurements.
Figure 1.
White blood cell, neutrophil and lymphocyte count over the course of 7 years follow-up of the patient.
Notes: Time of presentation and two unsuccessful attempts of smoking cessation are indicated with arrows. The time of initiation of electronic cigarette, leading to smoking cessation, is also indicated by an arrow. Normal value for neutrophils is less than 8,000/μL (horizontal red line).
Figure 2.
C-reactive protein levels over the course of 7 years follow-up of the patient.
Notes: Time of presentation and two unsuccessful attempts of smoking cessation are indicated with arrows. The time of initiation of electronic cigarette, leading to smoking cessation, is also indicated by an arrow. Normal reference value for C-reactive protein is less than 5 mg/L (horizontal red line).
In February 2012 he managed to quit smoking by the use of electronic cigarettes. It should be mentioned that use of electronic cigarettes was a personal choice of the patient; no advice or recommendation to use it was provided by the physicians, since it has not been approved as a smoking cessation method. He reported complete smoking cessation after 10 days of using the device. One month earlier, his complete blood count was consistent with CIN. In August 2012, routine follow-up showed no leukocytosis (WBC: 8800/μL, neutrophils: 4400/μL, lymphocytes: 3344/μL, eosinophils: 352/μL, basophils: 704/μL). C-reactive protein levels were also normalized. His BMI was 28.4 kg/m2. The test was repeated in 2 different laboratories, with similar results. Further tests, 1, 2 and 3 months later revealed no leukocytosis. During this period, he was using the electronic cigarette daily, consuming liquid with nicotine concentration of 9 mg/mL. Smoking abstinence was confirmed during his last three visits by measuring carbon monoxide in exhaled breath; it was within normal limits (4 ppm).
Discussion
To the best of our knowledge, this is the first study which reports that smoking cessation with the use of electronic cigarette leads to reversal of chronic idiopathic neutrophilia. The most important message is that despite the daily use of electronic cigarette by this patient, the beneficial effects of smoking cessation were maintained.
Electronic cigarettes were invented in 2003, with awareness and use increasing significantly over the past 3 years.13 They have been introduced to the market as tobacco harm-reduction products and they may have a unique role in this field. They work by evaporating a nicotine-containing liquid, which is subsequently inhaled by the user. In addition to nicotine, the constituents of liquids used for evaporation are limited to propylene glycol, glycerol, and flavorings. Since they deliver nicotine and at the same time resemble the act of smoking by production of visible vapor, they deal with both the chemical (nicotine delivery) and behavioral components of cigarette addiction.14 A non-randomized study by Polosa et al15 and an internet survey by Siegel et al16 suggested that they may be effective as a smoking cessation tool.
The absence of tobacco and the lack of combustion are important features in the health-related profile of these products. Until recently, research on the composition, toxicology, and clinical effects of electronic cigarettes was scarce. Thus, FDA and WHO publicly expressed serious concerns about electronic cigarette use in 2009, recommending that their use should be avoided. Cahn and Siegel14 summarized several chemical analyses performed until 2011, showing that electronic cigarette liquid contents are far less harmful compared to tobacco.14 For nitrosamines they mentioned that, when present, the amount was 500-fold to 1400-fold reduced in electronic compared to tobacco cigarettes.14 Substances produced from combustion of tobacco cigarettes, like polycyclic aromatic hydrocarbons, were not present in any of the liquids tested. Although still inadequate, research on electronic cigarettes has progressed over the past year. During the 14th annual meeting of the Society for Research on Nicotine and Tobacco Europe, Romagna et al17 presented a cytotoxic study comparing electronic cigarette vapor with tobacco cigarette smoke; they found that vapor extract from 10 different commercially available liquids were not cytotoxic to cultured mammalian cells compared to significant cytotoxicity observed from tobacco smoke extract.17 Only three clinical studies on the effects of electronic cigarettes on human health have been performed. Vardavas et al18 found that 5 minute use of electronic cigarette produced a mild but significant elevation in pulmonary resistance.18 However, no comparison with the effects of tobacco cigarettes was performed. Flouris et al19 found that no elevation in WBC count was found after electronic cigarette use; in comparison, WBC and neutrophil counts were significantly elevated immediately after tobacco cigarette smoking.19 Farsalinos et al20 studied smokers and electronic cigarette users with echocardiography before and after smoking and electronic cigarette use respectively.20 Acute diastolic dysfunction was observed in smokers immediately after smoking 1 cigarette, while diastolic function was preserved after using the electronic cigarette for 7 minutes. Interestingly, although electronic cigarette users were previously heavy smokers, it took them on average only 2 days to quit smoking with the use of the device.
Despite all this data and the fact that no study has found that electronic cigarettes are more harmful when compared to tobacco cigarettes, it must be emphasized that research is still in its infancy. More studies are needed, especially clinical studies, on their long-term effects. It will take several years, however, before such studies are published as awareness and use of electronic cigarettes has increased only recently. Delay will also occur as the knowledge that smoking-related disease and the beneficial effects of smoking cessation take several years before becoming clinically evident. Until that time, research should focus on the pathophysiological mechanisms by which smoking causes disease and should proceed on both laboratory and clinical level. The crucial scientific question that should be addressed is whether electronic cigarettes are less harmful compared to tobacco cigarettes, since they should be marketed solely as a tobacco harm reduction product and not as a new habit for the general population. In any case, regulation and specific quality standards should be implemented as the use of non-pharmaceutical grade nicotine or other constituents may lead to the presence of toxic tobacco impurities in the liquids, which will be subsequently inhaled by the user.14
Although we cannot exclude that some constituents of electronic cigarette vapor may have had beneficial effects in reducing WBC count in our patient, the most probable explanation is that reversal of CIN was caused by smoking cessation itself. Smoking causes diseases by a variety of mechanisms, including inflammation.21 It causes a 20%–25% increase in peripheral blood leukocyte count22 in addition to elevated levels of inflammatory markers like C-reactive protein.23 CIN is an uncommon condition associated with greater elevation in WBCs and neutrophils than those observed in the majority of smokers. Smoking however has been implicated as a cause for the condition. The patient had persistently elevated WBC count and mildly elevated C-reactive protein levels, without any underlying disease. This may represent a state of low-grade inflammation, which is a risk factor for future cardiovascular disease.24 Although he was a smoker several years before CIN developed, we could not find any specific underlying cause for the development of the condition at the particular time of presentation. We know however that inflammatory markers have a temporal relationship to smoking,25 and this might explain the delay in CIN presentation. Cigarette smoking was suggested as a potential cause for this condition in our patient after an extensive diagnostic analysis excluded other possible conditions or intake of medications such as corticosteroids and lithium which are associated with neutrophilia.9 Despite the use of medically-approved methods, the patient failed to quit smoking. Finally, with the aid of electronic cigarettes, he was able to quit smoking in a timely manner. Five months later, CIN was reversed, although he was using the electronic cigarette on a daily basis.
Conclusion
In conclusion, we presented a case of a young smoker having CIN and low-grade inflammation which was reversed after smoking cessation. Electronic cigarette use was successful as a smoking-cessation tool, after two failures to quit smoking (one with the use of currently-approved pharmaceutical methods). The daily use of electronic cigarettes did not hinder the beneficial effects of smoking cessation in this patient. Undoubtedly, this case report is in no way conclusive about the effects of electronic cigarettes on health. However, it indicates that research on the potential efficacy and health consequences of electronic cigarettes as a tobacco harm reduction product should be intensified. Until that time, we cannot recommend their use, but physicians will face two important ethical dilemmas in daily practice. Should they advise patients who have managed to quit smoking by using electronic cigarettes (like our patient) to stop using them, with the risk of smoking relapse? And should patients who have repeatedly failed to quit smoking by currently approved methods, such as the patient in this study, be denied the possibility, however small it may be, to quit smoking by using electronic cigarettes?
Footnotes
Author Contributions
Conceived and designed the experiments: KF. Analysed the data: KF, GR. Wrote the first draft of the manuscript: KF. Contributed to the writing of the manuscript: GR. Agree with manuscript results and conclusions: KF, GR. Jointly developed the structure and arguments for the paper: KF, GR. Made critical revisions and approved final version: KF, GR. All authors reviewed and approved of the final manuscript.KF was involved in data acquisition, analysis and interpretation. GR was involved in data acquisition and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Authors disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Tonstad S, Johnston JA. Cardiovascular risks associated with smoking: a review for clinicians. Eur J Cardiovasc Prev Rehabil. 2006;13(4):507–14. doi: 10.1097/01.hjr.0000214609.06738.62. [DOI] [PubMed] [Google Scholar]
- 2.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–28. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tob Control. 2006;15(6):472–80. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightwood JM, Glantz SA. Short-term Economic and Health Benefits of Smoking Cessation. Circulation. 1997;96(4):1089–96. doi: 10.1161/01.cir.96.4.1089. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DH, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–6. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 7.Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodu B, Godshall WT. Tobacco harm reduction: an alternative cessation strategy for inveterate smokers. Harm Red J. 2006;3:37. doi: 10.1186/1477-7517-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weir AB, Lewis JB, Jr, Arteta-Bulos R. Chronic idiopathic neutrophilia: experience and recommendations. South Med J. 2011;104(7):499–504. doi: 10.1097/SMJ.0b013e31821ec7cc. [DOI] [PubMed] [Google Scholar]
- 10.Corre F, Lellouch J, Schwartz D. Smoking and leukocyte counts. Results of an epidemiological survey. Lancet. 1971;298(7725):632–4. [PubMed] [Google Scholar]
- 11.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290(23):1275–8. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 12.Imano H, Sato S, Kitamura A, et al. Leukocyte count is an independent predictor for risk of acute myocardial infarction in middle-aged Japanese men. Atherosclerosis. 2007;195(1):147–52. doi: 10.1016/j.atherosclerosis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. E-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–66. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward of a repeat of past mistakes? J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 15.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as smoking-cessation tool: results of an online survey. Am J Prev Med. 2011;40(4):472–5. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Romagna G, Allifranchini E, Bocchieto E, Todeshi S, Esposito M, Farsalinos K. Cytotoxicity of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-Life project): comparison with tobacco smoke extract [abstract]. 14th Annual Meeting of the Society for Research on Nicotine and Tobacco; Helsinki. 2012. [ http://www.srnteurope.org/assets/Abstract-Book-Final.pdf.] Poster RRP17. (Accessed Nov 2012). [Google Scholar]
- 18.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette. Chest. 2012;141(6):1400–6. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 19.Flouris AD, Poulianiti KP, Chorti MS, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50(10):3600–3. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Farsalinos K, Tsiapras D, Kyrzopoulos S, et al. Acute effects of using an electronic nicotine-delivery device (e-cigarette) on myocardial function: comparison with the effects of regular cigarettes [abstract] Eur Heart J. 2012;33(Suppl):203. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonçalves RB, Coletta RD, Silvério KG, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60(5):409–24. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 22.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Tracy RP, Psaty BM, Macy E, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17(10):2167–76. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. N Engl J Med. 1997;336(14):973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 25.Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the third national health and nutrition examination survey. PLoS Med. 2005;2:e160. doi: 10.1371/journal.pmed.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]