Abstract
The prevalence of obesity continues to rise throughout the world. Increasingly, bariatric surgery is used for those with morbid obesity as a pivotal approach to achieve weight loss. Along with substantial weight loss, malabsorption of essential vitamins, minerals, and drugs also occurs. Therefore, more than ever, a better understanding of the physiology and mechanisms by which these deficiencies occur is essential.
We review the normal physiology of vitamin, mineral, and drug absorption. This is followed by a description of currently performed bariatric surgeries in the United States. A detailed review of specific nutrient and mineral deficiency states is presented, based on the most significant studies published in the last two decades. Of note, screening and supplementation recommendations have been included. Drug absorption data after these procedures is presented and discussed. Studies were identified by searching the Cochrane Registry and MEDLINE using relevant search terms, as well as through review of the reference section of included manuscripts.
Conclusions
Bariatric surgery can be effectively used to achieve sustainable weight-loss in morbidly obese patients. It simultaneously brings forth important functional consequences on nutrient deficiencies and drug absorption that clinician’s must be aware of. Further prospective, randomized research on specific procedures and deficiencies is required.
Keywords: bariatric, gastric bypass, drug malabsorption, vitamin deficiency
Introduction
In United States obesity, defined as BMI ≥ 30 Kg/m2, is increasing in prevalence. The prevalence for adults has increased from 15% in 1980 to 33.4% in 2004. In 1980, 6% of children and adolescents were obese and by 2008 over 30% of the population between the ages of 2 through 19 years were at or above the 85th percentile of BMI for age.[1,2]The prevalence of a BMI ≥ 40 has quadrupled from 1:200 in 1986 to 1:50 in the year 2000. The prevalence of individuals with a BMI ≥ 50 has increased even more from 1:2,000 to 1:400. [2]
Obesity is highly associated with increased morbidity and early mortality.[3] Chronic diseases such as diabetes mellitus, cardiovascular disease, dyslipidemia, hypertension, non-alcoholic fatty liver disease, numerous cancers, musculoskeletal disorders, and other disabilities have repeatedly been shown to be associated with obesity, with significant improvement of outcomes after minimal weight loss.[4] Minimal weight loss of 5–10% has been associated with marked reductions in chronic diseases, including reductions in the incidence of diabetes, metabolic syndrome, nonalcoholic fatty liver disease, and cardiovascular risk factors.[5,6]Unfortunately, traditional therapies including dietary modifications, as well as medical therapy with and without psychological support are relatively ineffective to treat obesity in the long run.[7]
Surgery for weight loss in morbid obesity is considered to be the most effective therapy.[8,9]Over the last 20 years, there has been an increasing body of literature describing different surgical techniques such as intragastric balloons, sleeve gastrectomy, Roux-en-Y Gastric Bypass (RYGBP), gastroplasty, and gastric banding.[10,11] Pope et al reported that the total bariatric surgical procedures in the United States increased from 4,925 in 1990 to 12,541 in 1997.[12] Santry and colleagues reported an increase in bariatric surgical procedures from 13,365 in 1998 to 72,177 in 2002 based on their pooled data from the National Inpatient Survey.[13] By 2006 the number of bariatric surgeries was reported to be 113,000 cases per annum, representing an over 50% increase in the number of procedures performed.[14] According to the American Society for Metabolic and Bariatric Surgery the number of bariatric surgeries performed in 2009 was approximately 220,000. (ASMBS.org, accessed April 2012)
The presence of nutritional deficiencies in obese patients before and after bariatric surgery has been recognized for several decades.[15] Likewise, pharmacokinetics abnormalities for a wide array of medications have been described either due to decreased absorption, increased first pass metabolism or other mechanisms in these patients.[16] As the obesity epidemic continues to expand in the Western world and the number of bariatric surgeries parallels this trend, a better understanding of its impact on nutrient and drug absorption is paramount. The purpose of this article is to provide a comprehensive review of the physiologic mechanisms of nutrients and drug absorption, emphasizing those aspects involved in drug pharmacokinetics.
Nutrient absorption: macronutrients, micronutrients, vitamins, minerals
The majority of micronutrient and trace element absorption occurs in the duodenum and proximal jejunum. Under normal conditions 92–97% of the diet is digested and absorbed. Water, monosaccharides, amino acids, vitamins, and minerals are absorbed primarily in their original forms. Conversely, disaccharides, polysaccharides, peptides and lipids are digested prior to absorption. Digestive enzymes are synthesized by specialized cells in the mouth, stomach, pancreas, and small intestine. Hydrochloric acid, bile, and sodium bicarbonate facilitate enzymatic activity.[17]
Digestion begins in the mouth, where lipase-containing oropharyngeal secretions digest minimal amounts of fat.[17] Starches composed of linear or branched glucose molecules are hydrolyzed into maltose and shorter polysaccharides by the neutral to slightly alkaline salivary enzyme amylase.[18]Amylase activity ceases when it comes into contact with the low pH of the stomach.[18] The food bolus undergoes triturition into 1 mm particles, which are mixed with hydrochloric acid, gastric lipase, mucus, intrinsic factor, gastrin, and proteases.[17] The stomach empties completely within 1–4 hours by starting with carbohydrates which are followed by protein, fat, and fibrous foods. Liquid emptying occurs before solids, and low-calorie foods before concentrated ones; this becomes relevant in advising patients after gastrointestinal surgery.
The small intestine has an absorptive surface area of approximately 250 m2.[19] Some nutrients may share the same carrier and thus compete for absorption.[19] Carrier systems can also become saturated, thus slowing the absorption of a nutrient (e.g. intrinsic factor for the absorption of B12).[19] The duodenum and proximal jejunum are the primary sites of small bowel digestion and absorption, thus nutrient absorption is largely completed once food reaches the mid- jejunum.[19] Starch digestion is resumed in the small intestine, where its molecules are reduced to simple sugars (oligosaccharides and disaccharides) by pancreatic amylase.[18,20] These sugars are then broken down further into monosaccharides by specific disaccharidases enzymes lining the brush border.[20] Cholecystokinin is released in response to the presence of protein and fat in the proximal duodenum and serves to stimulate the release of bile from the gallbladder and lipase from the pancreas.[19] The digestion of protein is completed in the duodenum by pancreatic proteolytic enzymes in the brush border of the small intestine.[19,21] Dipeptides and tripeptides are absorbed by specific transporters as are individual amino acids.[19] Triglycerides are digested into fatty acids and monoglycerides by pancreatic lipase in the small intestine. Cholesterol and cholesterol esters combine with bile salts to create micelles.[19] The distal ileum serves to absorb the vitamin B12/intrinsic factor complex in addition to 95% of bile acids which are then shuttled back to the liver for reuse.[19]
Minerals (e.g. iron, calcium), vitamins (e.g. folate), trace elements (e.g. selenium) and most of the remaining luminal fluid water are absorbed before reaching the colon. Most vitamins enter the bloodstream by passive diffusion in their original form.[18] Mineral absorption is more complicated and is absorbed into the body in three stages: the intraluminal stage, which involves the chemical interactions that occur in the stomach and intestines; the translocation stage, which involves passage across the membrane into the intestinal mucosal cells ; and the mobilization stage, in which minerals are either stored within the cells or are carried across the basolateral surfaces of the intestinal cells into the bloodstream.[18] Similar to amino acid and monosaccharide absorption, minerals require the presence of specific carriers and utilize ATP for active transport.
The large intestine is the site of absorption for water, salts, and the vitamins created by bacterial action within the organ.[18] These specific vitamins are synthesized when colon bacteria digest materials that have not already been absorbed before they reach the large intestine; vitamins K and B12, thiamin, and riboflavin are included in this group.[18]
Mechanisms of Drug Absorption
Oral drug absorption is defined as the penetration of a molecule across the intestinal membrane and the appearance of that molecule in an unchanged form in the blood draining the gastrointestinal tract. Drug molecules must penetrate a comparatively protracted distance through the apical cell surface of enterocytes, across the cytosol and basal membrane to finally reach the capillary cell and membrane of capillary vessels.[22] The movement across a heterogeneous lipid bilayer and aqueous media is a highly complex process, which mechanistically may be determined by a number of parallel processes, some biologically passive and some active.[23] Utilizing the physiochemical properties of individual drugs or drug classes, as well as the distribution of intestinal drug transporters could potentially help predict the effect of bariatric surgeries on drug absorption.[24]
The majority of oral drugs use a transcellular pathway via passive diffusion.[25] When molecules are introduced on one side of a permeable membrane they move to the opposite side having the lower concentration. This process is governed by Fick’s first law of diffusion, which postulates a first-order kinetic reaction based on the product of permeability and aqueous solubility.[26] Factors influencing the permeability of any substance are the surface area of the membrane, the diffusion coefficient of the drug through the membrane, and the lipophilicity of the drug.[27]
Lipophilicity is also known as the oil-water partition coefficient. This represents the ability of a molecule to partition from an aqueous to a lipid phase allowing it to pass freely across the bilipid layer. Solubility on the other hand is a thermodynamic concept generally determined by the intermolecular forces favoring the solid phase versus the intermolecular forces favoring the solute–solvent phase.[23] If the intermolecular forces (e.g. London, dipole-dipole, or dipole-induced) between the solute-lipid interactions are greater than the solute-water forces, drug diffusion will be favored. Therefore, for some drugs the rate-limiting step for membrane passive transport will be the movement of the drug through the membrane, while for others the limiting step will be the diffusion through the aqueous layer.
Another factor affecting the permeability and solubility of drugs is the degree of ionization of molecules which is determined by the pKa of the drug and the pH of the fluid in which it is dissolved.[28] Thus, non-ionized forms of a molecule such as weak acids under acidic pH or weak bases under alkaline pH would be expected to be better absorbed in the stomach and intestine, respectively. This premise is known as the pH-partition hypothesis and although its influence on drug dissolution and permeability is well accepted, most drugs are best absorbed from the small intestine as a result of the large surface area.[22]
In addition to passive diffusion, facilitated and active transport play an important role in the absorption of a number of clinically relevant drugs.[29,30] Facilitative transporters assist in the passage of a solute down an electrochemical gradient, whereas active transporters move solutes against such gradient and are usually coupled to mechanisms of energy production.[28] For example, some anticancer medications (e.g. methotrexate), and other analogues of natural occurring metabolites such as amino-penicillins or amino-cephalosporins, undergo carrier-uptake transport.[31–33] The distribution of metformin to the liver and small intestine of genetically modified mice or knockouts such as OCT1 (−/−) is lower than those OCT1 (+/+) suggesting that OCT1 is responsible for this drug’s intestinal uptake.[34] Similarly, hOCT1 also expressed in the liver and small intestine plays an important role in the absorption of ranitidine and famotidine.[35] Other drugs and their respective transporters are included in Table 1.
Table 1.
| Transporter | Drugs |
|---|---|
| Lipid/ Bile Acid Transporters | Fatty acids, lipophilic drugs (?) |
| Organic Anionic Transporters | Salycilic acid, Statins (e.g. simvastatin, atorvastatin), NSAIDs, fluoroquinolones |
| Organic Cationic transporters | Dopamine, Verapamil, Cimetidine, Famotidine, Biguanides |
| Nucleoside Transporter | Antivirals and anticancer compounds |
| Intestinal Dipeptide Transporter | B-lactams, ACEI®, thrombin inhibitors |
| Aminoacid Transporters | Gabapenti, baclofen |
| Vitamin Transporters | Valproic acid, salicylic acid, methotrexate |
| P-glycoprotein | Vincristine, paclitaxel, cyclosporin, ciprofloxacin, vinblastine |
ACEI Angiotensin converting enzyme inhibitors.
Transport proteins have also been implicated in efflux processes across the gastrointestinal membrane counteracting drug absorption and participating in drug resistance.[28] One of the most widely studied proteins is the P-glycoprotein pump (P-gp), member of the ATP-binding cassette (ABC) transporter family. This protein serves as a true barrier to absorption of several important and different drugs including cyclosporine, tacrolimus, saquinavir, indinavir, paclitaxel, and vinblastine.[36] Its activity progressively increases through the intestine and there is evidence to suggest that substrate affinity may vary depending on the intestinal site.[37]
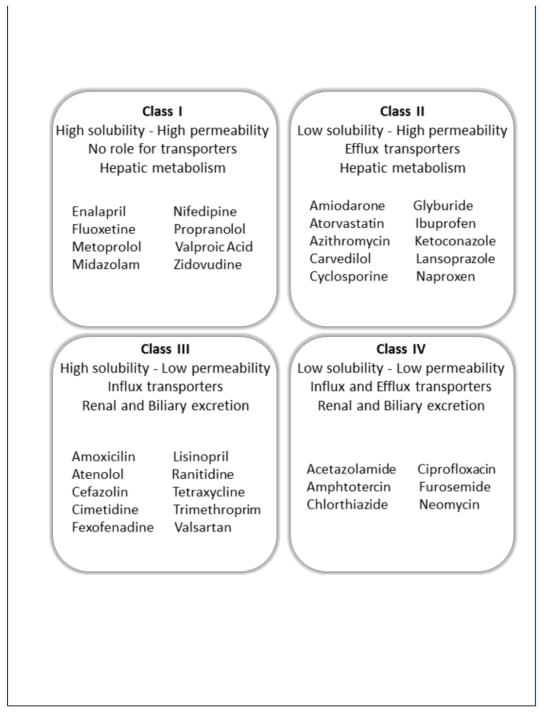
Based on the principles of passive diffusion and active transport, drugs can be classified according to the Drug Disposition Classification System (DDCS). This is a descriptive and predictive tool currently used by researchers and the Food and Drug Administration (FDA) to anticipate drug absorption and establish regulatory standards for new drug development.[38,39] This system classifies drugs in terms of their aqueous solubility and permeability, presence of gut transporters (influx and efflux carriers), mechanism of elimination and the anticipated effect of food on drug absorption.[23] Class I drugs exhibit high solubility and permeability allowing enough concentration of the compound in the gut lumen and also across the cellular membrane. Such drugs are readily available in the outer and inner site of the apical membrane, which would saturate any influx or efflux transporters; thus making the role of transporters minimal. Class II drugs have typically low solubility and high permeability. These types of drugs cross the cellular membrane easily without any role for uptake transporters. However, their low solubility would limit their dissolution inside the enterocytes preventing the saturation of efflux transporters. Class III drugs are characterized by high solubility, but low permeability and therefore their absorption will be rate-limited by their capacity of crossing the cell membrane. Uptake transporters have a very important role helping overcome poor permeability of these drugs. Finally Class IV drugs have low solubility, low permeability, and therefore usually low oral bioavailability. For these drugs to access the cells, they must rely on protein transporters.[27,38,39] Figure 1 shows a schematic view of the DDCS, as well as examples of drugs that belong to each of these classes.
Figure 1.
Biopharmaceutical Drug Disposition Classification System (BDDCS)39,123
Types of Bariatric Surgeries
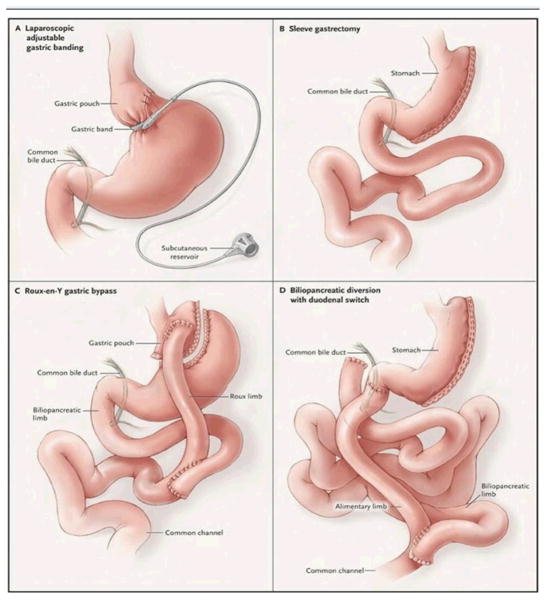
Bariatric surgery achieves weight loss by altering the anatomy of the gastrointestinal tract (Figure 2). Classically, two mechanisms are responsible for decreasing energy intake and consequently produce weight reduction. First, this is achieved by limiting the volume of food intake while diminishing the stomach’s capacity. These procedures are commonly referred to as restrictive. A second mechanism is by inducing malabsorption by surgically bypassing regions of the small intestine and diverting biliopancreatic secretions, which limit nutrient absorption. These are known as malabsorptive. Depending on the procedure, weight loss may occur as a result of either mechanisms or frequently both. Ultimately, the goal is to achieve optimal weight loss while minimizing surgical morbidity and nutritional deficiency.
Figure 2.
Bariatric Surgery Procedures. With permission from DeMaria EJ.45
A number of procedures including the Jejuno-Ileal Bypass (JIB) have been relegated to history. Despite dramatic weight loss, JIB was often associated with serious and sometimes life-threatening complications.[40] Some of these adverse events included severe electrolyte imbalances secondary to renal and liver failure.[28,41] Interestingly, most of the available literature on drug absorption and bariatric surgeries is based on this outdated operation, leaving a gap in our understanding of the effect of modern procedures on nutrient absorption and drug pharmacokinetics.[42]
Laparoscopic Adjustable Gastric Banding (LAGB)
The LAGB technique is based in a synthetic band that is placed just distal to the gastroesophageal junction, creating a gastric pouch approximately 20 to 30 cc in size. The procedure works on the principle of restriction of oral intake by limiting the volume of the proximal stomach.[43] The gastric band can be inflated or deflated to alter the degree of constriction around the upper portion of the stomach; this is accomplished by either the infusion or aspiration of saline solution to and from an external port connected to the band.[44] Several different types of adjustable bands exist and are used in Europe. In the US the LAP-BAND® (Allergan, Inc. Irvine CA) and the Realize Band® (Ethicon-Endo Surgery, Somerville, NJ) are FDA approved for use in the U.S.[45] A review by Franco et al in 2011 listed the % excess weight loss at 4–5 years post-operatively as 45.4%–47.5%.[46]
Sleeve Gastrectomy (SG)
This procedure involves a vertically stapling of the stomach, preserving the antrum and pylorus. In essence, a partial gastrectomy is performed removing the greater curvature creating a tubular gastric conduit with restriction of caloric intake. The staple line is generally started approximately 6 cm proximal to the pylorus and continued parallel to the lesser curvature until the angle of His is reached. Although SG has been classically classified as a strictly restrictive procedure, more recently it has been proposed that the resection of the greater curvature of the stomach may have an important impact in intestinal hormone regulation. Pancreatic peptides such as peptide YY may be increased after this procedure, and blood ghrelin levels decreased favoring an overall state of satiety.[47] SG is usually indicated for patients deemed to be at high risk for definitive bariatric surgery. The SG allows for initial weight loss and improvement in associated medical co-morbidities, and usually could be followed by a second stage malabsorptive procedure.[47] Nevertheless, over the past several years there has been interest in using SG as stand-alone definitive bariatric surgery. In one series of 163 patients, a loss of 60% of excess body weight occurred at two years after laparoscopic SG.[44]
Roux-en-Y Gastric Bypass (RYGBP)
Roux-en-Y gastric bypass is the most commonly performed bariatric operation in the United States and is considered both a restrictive and malabsorptive procedure. It can be performed in a laparoscopic or open technique. The restrictive aspect of this operation entails creating a 20 to 30 cc gastric pouch just below the gastroesophageal junction which is divided from the remainder of the stomach (Figure 1).[45,48] The proximal jejunum and its mesentery are divided 30 to 40 cm distal to the ligament of Treitz; the distal jejunal limb from this division becomes the Roux limb and is anastomosed to the new gastric pouch. The proximal biliopancreatic limb of the jejunum, which carries gastric and biliopancreatic secretions, is anastomosed to the Roux limb anywhere from 80 to 120 cm beyond the gastrojejunal anastomosis. This alteration of jejunal anatomy encourages malabsorption by preventing the mixing of food and digestive enzymes as food traverses the Roux limb.[43,49,50] The average excess body weight loss at 3 years is approximately 67% across studies.[46]
Biliopancreatic Diversion with Duodenal Switch (BPD/DS)
This procedure is both restrictive and malabsorptive. First a vertical sleeve gastrectomy of approximately 100 to 150 cc is created.[51] The duodenum is then transected in the first portion, preserving the pylorus, and a duodenal ileostomy is created resulting in a 150 cm alimentary limb and a common channel of 100 cm. The alimentary limb is connected to the duodenum while the biliopancreatic limb is connected to the ileum.[52,53] This procedure has shown to be especially effective in patients known to be super obese with BMI over 50 Kg/m2.[48] Buchwald et al reported an average weight loss of 70%.[46]
Specific Nutrient Deficiencies and Related Complications
Morbidly obese patients have increasingly been recognized to have pre-existing nutritional deficiencies before undergoing any bariatric surgery.[15] The cause is not totally understood, although it is likely related to a higher intake of high calorie processed foods with poor nutritional value.[54] Also, fat soluble vitamins (such as vitamin D) may become sequestered in adipose tissue in obese individuals. In addition, the adipose tissue functioning as an active endocrine organ may increase the production of inflammatory cytokines (i.e TNF-α, IL-6, leptin, hepcidin, and the siderophore lipocalin-2) [55] and down-regulate the synthesis and secretion of anti-inflammatory adipokines, particularly adiponectin.[56–58] Overall this effect contributes to a low-grade, chronic inflammatory state disturbing the absorption and the metabolism of certain nutrients.[56]
Post-operatively, bariatric surgery patients are at increased risk of developing nutrient deficiencies because of vomiting, decreased food intake, food intolerance, reduction of gastric secretions, and bypass of absorption surface areas.[59] The latter is perhaps the most important factor in causing nutritional deficiencies; thus procedures such as BPD, BPD/DS, and RYBG carry the most significant risk. Not surprisingly, the risk of developing proteins, vitamins and oligoelement deficits seems to be proportional to the length of bypassed proximal intestine.[60] In the case of restrictive procedures, micronutrient absorption is preserved, but daily intake is significantly restricted having less impact in nutrient absorption overall.[61]
Some authors have recommended monitoring the overall nutritional status of patients by using surrogates such as total protein, albumin or pre-albumin, and cholesterol.[62,63] Regular screening for metabolic bone disease and deficiencies of iron, thiamine, B12, calcium, folate, and Vitamin A and D are also necessary.[59,62] Folate measurements and iron levels are especially important in women of childbearing age and pre-menopausal woman.[64,65] Nevertheless no single measurement is of consistent value in individual patients, but rather a global assessment is recommended.[66,67] Many authors recommend monitoring patients every three months in the first year after surgery, every six months in the second year, and every 6–12 months starting in the third year in order to identify and treat nutritional deficits early.[59,62]
A combined joint statement by the American Association of Clinical Endocrinologist (AACE), the Obesity Society (TOS), and the American Society for Metabolic and Bariatric Surgery (ASMBS) [68] recommends that all patients should undergo appropriate nutritional evaluation, including selective micronutrient measurements before and after any bariatric surgical procedure (Category of Recommendation: Grade C). The extent of such evaluation should be guided by the type of surgical procedure performed, even in the absence of other post operative complications such as caloric or nutritional restriction, vomiting or diarrhea. Similarly, the recommended nutrient supplementation regimen varies according to the surgical procedure performed. After RGYBP, supplementation with a multivitamin–mineral preparation, iron, vitamin B12, and calcium with vitamin D is common; whereas after BPD or BPD/DS, routine supplementation regimens recommended include a multivitamin–mineral preparation, iron, vitamin B 12, calcium, and fat-soluble vitamins. Nevertheless, some of these recommendations may be based on observational research and expert opinions, and not necessarily based on prospective, randomized trials on specific surveillance protocols or optimal types and amounts of supplementation.[69]Table 2 summarizes the expected nutrient deficiencies for each of the most common bariatric procedures, as well as the recommended doses and route of supplementation.
Table 2.
Pre and post-operative vitamin deficiencies, screening and supplementation 69
| Vitamin/Mineral | Screening | Preoperative Deficiency | LAGBY | RYGBδ | BPD/DSΔ |
|---|---|---|---|---|---|
| B1(Thiamine) | Serum thiamin | 15–29%, more common in African Americans and Hispanics | Rare, but it may occur with all procedures. Consider daily supplementation in first 6m | ||
| B6(Pyidoxine) | Pyidoxal-5′-phosphate | Unknown | Rare | Rare | Rare |
| B12 (Cobalamin) | Serum B12 | 10–13%, may occur in elderly patients and those taking H2 and PPIs | _ | Common (12–33%) IM 1000 μg/mo or PO 350–500 μg/d Begin 0–3 mon after surgery | _ |
| Folate | RBC folate | Uncommon | Rare | Rare | Rare |
| Iron | Ferritin Fe/TIBC | 9–16% of adult women in general population are deficient | _ | Common (20–49%). Menstruating women and super obese patients (≥50%). Minimum addition 18–27 mg/d elemental. May add Vit C supplement Begin on day 1 after surgery | Minimum addition 18–27 mg/d elemental. May add Vit C supplement Begin on day 1 after surgery. |
| Vitamin A | Plasma Retinol | Uncommon | Rare | Rare | Common (50%) after 1y Up to (70%) at 4 y. PO 10.000 IU/d. Begin 2–4 w after |
| Calcium | Serum calcium | Common | _ | Common (≥50%) after 1y PO Calcium Citrate 1500–1700 mg/d | |
| Vitamin D | 25(OH)D | 60–70% | Occasionally. May give 1000 IU/d. Begin 2–4 w after | Common after 1y. PO 2000 IU/d. Begin 2–4 w after | |
| Vitamin E | α-Tocopherol | Uncommon | Rare | Rare | Rare |
| Vitamin K | PT | Uncommon | _ | _ | Common after 1 y. PO 300 μg/d |
| ZincΛ | Plasma Zinc | Uncommon | _ | Occasionally | Common after 1 y. |
| Protein | Serum albumin, pre-albumin, total protein | Uncommon | Occasionally (10–20%) | Occasionally (10%) | Occasionally (10–20%) |
A high-potency vitamin containing 100% and 200%δΔ of daily value for at least 2/3 of nutrients is recommended daily.
Patients should be cautioned that exogenous zinc consumption may impair copper absorption.
LAGB Laparoscopic adjustable gastric band, RYGB Roux-en-Y gastric bypass, BPD/DS biliopancreatic diversion/duodenal switch, Fe Iron, TIBC Total Iron Binding Capacity, IM Intramuscular, PO By mouth, Vit Vitamin, IU International Units
Iron
Post-operative iron deficiency has been commonly reported in multiple series, with reports ranging from 20% to 49%.[70,71] There are several mechanisms by which bariatric surgery can lead to iron deficiency. First, post-bariatric surgery patients have reduced iron intake secondary to a considerable reduction of their meat intake. Ruz et al.[72] similarly to Kushner [73], showed a net reduction of almost 50% of the total amount of meat per day consumption, suggesting a low tolerance to red meats. Second, most if not all bariatric surgery procedures reduce gastric capacity and consequently hydrochloric acid production and volume. This has important implications in the conversion of Fe3+ into the more absorbable Fe2+ ion, limiting the release of iron from the structural proteins, and also reducing its affinity by its specific co-transporters, mainly DMT1.[72] Third, these operations result in reduction of the total absorption surface area. Iron deficiency is especially prevalent in RYBP, precisely due to reduction of gastric capacity [73] in addition to bypass of the duodenum and proximal jejunum, primary sites of iron absorption.[74] Lastly, peri-operative iron deficiency anemia occurs in approximately 9–16% of bariatric surgery patients, which may account for some of the post-operative deficit.[69] This has been associated with increased postoperative morbidity and mortality, and decreased quality of life.[75]
Screening for iron deficiency can be done by assessing serum ferritin levels, however the lack of specificity of this test favors the use of serum iron along with the total iron binding capacity as preferred methods. These should be measured at least 6 months after surgery. Lifelong monitoring for deficiency is required, and in some cases, despite appropriate supplementation and addition of Vitamin C to enhance absorption,[76] intravenous doses of iron gluconate are required.[77] Concomitant copper deficiency must also be sought as this represents a rare cause of iron deficiency.[59] More attention and cautious screening should be done in specific patient populations, such as young fertile women [78] and patients with previous history of peptic ulcer disease. A retrospective analysis of 206 patients undergoing RYGB showed that these two factors were significantly associated with the development of iron deficient anemia despite standard iron supplementation.[79] This may indicate that additional iron supplementation and screening may be required in certain patients after RYGB surgery.
Vitamin D and Calcium
Vitamin D is a lipid soluble vitamin with two essential functions: to optimize bone mineralization and to maintain calcium homeostasis. Prolonged deficiency of vitamin D leads to osteopenia, osteoporosis, and hypocalcemia. Although an obese state was previously believed to be protective against bone loss, studies have shown that rates of vitamin D deficiency average 60% in obese individuals prior to gastric bypass surgery [80,81]. The main mechanism by which this is believed to occur is secondary to enhanced uptake and clearance of the vitamin by adipose tissue with concomitant decreased bioavailability of 1,25-dihydroxycholecalferol.[82,83] Interestingly, a prospective cohort study by Lin et al. [84] serially measured the levels of 25-hydroxyvitamin D after RYGB showing an acute and transient increase in its systemic concentrations during the first month (p<0.004). This was followed by a decreasing trend during the remaining 23-months. These findings suggest increased storage and sequestration of Vitamin D by adipose tissue with its concomitant release during initial weight loss.
Calcium and vitamin D deficiency after bariatric surgery has been extensively documented.[85,86] Multiple prospective case series after RYGB and BPD/DS estimate that over 50% of post-operative patients develop low levels of vitamin D and 25–50% develop hypocalcemia. Slater et al. demonstrated a progressive increase in the incidence and severity of these deficits with time after BPD/DS.[86]
Due to an increased risk of developing metabolic bone disease in post-bariatric surgery patients, lifelong prophylaxis with oral calcium and vitamin D supplementation is strongly recommended.[69,87] Although debate exists with regard to which form of oral calcium should be prescribed, a meta-analysis published by Sakhaee et al. showed that calcium citrate is significantly better absorbed than its carbonated form.[88] Multiple studies have also shown that standard doses of calcium and oral vitamin D supplementation are not necessarily sufficient in the setting of post-bariatric surgery patients.[85,86] Therefore, additional supplementation other than the regular daily multi-vitamin should be administered as standard practice (Table 2).
B12 and Folate
Vitamin B12 and folate are typically evaluated together, as deficiencies of either can lead to macrocytic anemia. In addition, isolated and long-standing deficits of B12 can lead to irreversible neurologic sequelae. The main mechanism by which bariatric surgery patients develop vitamin B12 deficiency is associated to a reduced production of intrinsic factor by limited number of parietal cells with consequent decrease in cobalamin-intrinsic factor complex formation and absorption.[89,90] Purely restrictive operations do not cause a significant deficiency states of either nutrient [78] however, for mixed procedures such as RYGBP about one third develop vitamin B12 deficiency. [91–96] When comparing RYGBP and BPD/DS, there does not seem to be a significant difference of vitamin B12 deficiency occurrence, but for both procedures vitamin B12 along with iron deficiency continue to be the most frequently encountered.[97] Moreover, a two year retrospective follow up by Gasteyger et al.[98] pointed that vitamin B12 was the most frequently prescribed supplement among all nutrients after RYGBP. Based on multiple prospective studies, the recommends that daily oral supplementation in appropriate dosages (Table 2) should be enough to overcome existing deficits after such procedures.[69]
Similarly, folate deficiency occurs commonly after bariatric surgery procedures with reports suggesting rates as high as 45% after RYCBP.[99,100] This deficiency occurs due to bypass of proximal portions of the small intestine, which represent the main site of absorption. Screening tests include erythrocyte folate, as well as homocysteine levels with generally agreed doses of supplementation (Table 2).
Vitamin A, E, K, Zinc
Fat malabsorption induced by biliopancreatic diversion and other mixed procedures may cause important deficiency of liposoluble vitamins. Although rare, bariatric surgery patients with vitamin A deficiency can develop ophthalmologic complications such as night blindness or ocular xerosis.[101,102] Slater et al.[85] estimated that the prevalence of vitamin A deficiency after BPD/DS was 52% among a cohort of 170 subjects after the first year of surgery, increasing to 69% four years after the procedure. Vitamin K deficiency may lead to alterations in clotting factor levels and the process of chondrogenesis during fetal development.[103–104] Vitamin K levels were low in 51% of the cohort described by Slater one year after surgery and in 68% four years after the procedure. For vitamin E, low levels persisted four years after surgery in up to 50% of patients. Pre and post-operative evaluation of fat soluble vitamins, as well as routine supplementation is recommended (Table 2).[69]
Protein
Protein is absorbed in the small bowel as individual amino acids via specific and non-specific transporters, and in the form of di- and tri-peptides. Intake of protein during the immediate postoperative period is necessary to prevent the loss of lean body mass and maintain a positive nitrogen balance. However, tolerance, compliance, and malabsorption are hurdles.[60] Protein deficiency, characterized by hypoalbuminemia, edema, asthenia, and alopecia, is a potential side effect of bariatric procedures. Nevertheless, the results of studies looking at the incidence of protein deficiencies after these surgeries have been equivocal.
Some authors have argued that restrictive procedures can produce a significant decrease in protein intake due to meat intolerance.[72,105] One study [106] evaluating the intestinal absorption of albumin and nitrogen after BPD showed levels of 73% and 57%, respectively. The investigators concluded that BPD subjects had a loss of endogenous nitrogen by a mean of 4.9 g/day. This loss is thought to play a significant role in the development of protein deficiency after BPD, especially during the early postoperative period when restricted food intake may cause a negative balance of both calories and protein. In contrast, Scroubis et al. [107] after a two year follow up of a prospective cohort, showed a minimal risk of protein deficiency in patients undergoing both RYBPD (n=65, 1.5%) and BPD (n=65, 9.2%).
If protein deficiency is encountered, dietary counseling and protein supplementation are usually successful measures.[108] Monitoring of protein deficiency requires frequent assessment of anthropologic measurements of muscle lean mass, as well as biochemical surrogates such as total protein, albumin and pre-albumin levels. Serum albumin levels have a low sensitivity and specificity to detect changes in nutrition intake due to its long half-life (over 20 days) and large body reserve.[67] For this reason, pre-albumin and a comprehensive evaluation of the patient’s clinical condition (e.g. wound healing, presence of edema) may be required for the assessment of patient’s protein status.
Drug absorption and bariatric surgery
The absorption, first-pass metabolism, volume of distribution, and half-life of drugs may all be altered after bariatric surgery in a drug-dependent manner.[109] Unfortunately there are no well-controlled randomized prospective data, however, in vitro data and evidence from case reports and case series do provide some insight into drug absorption after these procedures.[42]
Evidence for decreased drug absorption after bariatric surgery
Seaman et al. developed an in vitro drug dissolution model to approximate the gastrointestinal environment of the preoperative and post Roux-en-y gastric bypass states to better understand dosing of psychiatric drugs.[16] After a chemical recreation (accounting for pH, temperature, peristalsis, transit time, but no biliary salts or pancreatic secretions), the dissolution of 22 common psychiatric medications was measured by calculating the median weights of the dissolved portions of the drugs. Ten of twenty-two medications studied including fluoxetine, sertraline, clonazepam, quetiapine and risperidone showed significantly more dissolution under normal conditions (p <0.04). Except for bupropion (p <0.04) and lithium (p <0.05), which showed increased dissolution in the post-surgical medium, the remaining medications showed no considerable change. Although the isolated solubility of these medications does not predict its absorption, it provides qualitative information about its potential behavior after surgery.
Phenytoin absorption was demonstrated to be diminished in patients undergoing jejunoileal bypass. One study showed significantly lower levels of the drug in 7 surgical patients compared to 9 healthy volunteers.[110] Peak plasma concentrations and area under the curve (AUC) at all measured times were significantly decreased after bypass (p<0.005). Case reports demonstrate that increased maintenance doses of phenytoin are required in patients after jejunoileal bypass surgery.[111] Not surprisingly, reversal of jejunoileal bypass leads to a doubling of plasma phenytoin levels.[111]
Prince et al. studied the absorption of erythromycin in 7 adult patients before and after Roux-en-y gastric bypass surgery.[112] Their results showed an 85% prolongation in the time to reach peak drug concentration and a 50% decrease in peak drug concentration after surgery. Mean weight corrected AUC was reduced 41% after the procedure (p=0.05), with two patients having no detectable levels of the drug in serum postoperatively. Similarly, Kampmann et al. showed decreased absorption of ampicillin after an intestinal shunt procedure in six patients.[113] In addition, a case report suggested that the absorption of amoxicillin and macrodantin in a pregnant female who had undergone Roux-en-y gastric bypass was reduced. This patient was diagnosed with a urinary tract infection and, despite demonstrated bacterial sensitivity to these antibiotics, she did not improve after treatment.[114]
Two case series have suggested that the absorption of immunosuppressive drugs is reduced as a result of bariatric surgery.[115,116]Chenhsu et al. described a patient with decompensated cirrhosis as a complication of jejunoileal bypass who subsequently underwent liver transplant.[115] This patient required cyclosporine after transplantation. Cyclosporine levels were measured and compared to controls undergoing liver transplant without a history of bariatric surgery. This patient required twice the dose of cyclosporine compared to the control group to achieve therapeutic levels. The authors accounted for the bile-dependent dispersion step for the absorption of cyclosporine (high permeability but very low aqueous solubility- DDCS Class II) by using a micro-emulsified form of the medication. Reversal of gastrointestinal bypass has been reported to increase the concentration of tacrolimus with a similar dosing regimen as prior to surgery.[116]
With contraceptive hormone therapy, pharmacokinetic studies have reported decreased absorption levels following jejunoileal bypass.[117,118] For instance, Victor et al., reported reduced plasma levels of two different commonly prescribed progestins (levonorgestrel and norethisterone) in a group of seven patients following surgery compared to normal weight controls, and in non-operated morbidly obese patients.[118] However the only statistical significant difference was found for levonorgestrel, and no clear weight adjusted calculations were presented. Similarly, one case series reported three cases of tamoxifen malabsorption with consistent sub-therapeutic levels at standard doses in patients with history of Roux-en-Y gastric bypass.[119]
Case reports have demonstrated decreased absorption of warfarin, anti-tuberculosis medications, and thyroid hormones after bariatric surgery.[97,120]
Evidence for unchanged or enhanced drug absorption after bypass surgery
Marcus et al. studied the bioavailability of digoxin in six patients before and after jejunoileal bypass.[121] After adjusting for differences in body weight, the average ratio of the AUC approached 1.0. Following a similar study design, Terry et al. looked at the absorption of acetaminophen and penicillin in a group of eight patients before and after jejunoileal bypass.[122] For acetaminophen, none of the pharmacokinetic parameters including AUC, time to peak concentrations, and urinary recovery were altered. In the case of penicillin, increased absorption was reported. No statistical analysis or power of the study was provided in either of these cases.
Skottheim et al. evaluated the effect of RYGBP and biliopancreatic diversion on the bioavailability of atorvastatin.[124,125] Their studies were based on the recognition of inter-individual variability of enzymatic expression and consequently, differences in first-pass metabolism as a potential effect in drug bioavailability. From 12 morbidly obese patients undergoing gastric bypass, and 10 patients undergoing BPD/DS, pharmacokinetic measurements were obtained as well as genotypic profiling for CYP3AC and ABCB1. Those patients with the lowest systemic exposure (high enzymatic expression) prior to surgery showed a median 1.2-fold increase in AUC of the drug (range of 0.8–2.3, P=0.03) and a 2-fold increase after BPD (range 1.0–4.2, P = 0.001).[124,125] Since CYP enzymatic content is greater in the proximal portions of the small intestine, bypass of this segment should cause an important reduction in intestinal metabolic activity. Therefore, this may increase the bioavailability of certain drugs as in this case atorvastatin.
Despite small samples and uncontrolled times of administration of the medication among subjects, the studies in this section provide some insight into our understanding of the complexity of drug pharmacokinetics.
Conclusions
As the world epidemic of obesity continues to expand, the performance of bariatric surgery has exponentially increased during the last two decades. Most commonly gastric bypass surgery is performed with the intention, in part, to induce caloric malabsorption. As a consequence those vitamins, minerals, and drugs that require an intact small bowel are similarly malabsorbed. Many of these deficiencies, particularly of dietary nutrients are anticipated and proactively supplemented either orally or parenterally. However, little is known about the absorption of most drugs in this setting and monitoring drug levels is not an option. Patient providers must monitor the effects of any enterally administered drug to determine whether the anticipated effects of the drug are seen. If there is a discrepancy, then malabsorption must be suspected. If available, alternative drugs less dependent on proximal small bowel function can be tried. Creative solutions such as transdermal and parenteral administration should be sought.
References
- 1.Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinology & Metabolism Clinics of North America. 2003;32:741–760. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM. Prevalence of High Body Mass Index in US Children and Adolescents, 2007–2008. JAMA. 2010;303:242–247. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.National institutes of health. Clinical guidelines on the identification, evaluation, and treatment of over- weight and obesity in adults: the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 5.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 6.Mattar SS, Velcu LM, Rabinovitz M, Demetris AJ. Surgically-Induced Weight Loss Significantly Improves Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome. Annals of surgery. 2005;242:610–620. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North American Association for the Study of Obesity and the National Heart, Lung, and Blood Institute. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Vol. 200. Bethesda, Md: National Institutes of Health; NIH publication 00–4084. [Google Scholar]
- 8.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31:1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950–2000. Obes Surg. 2002;12:705–717. doi: 10.1381/096089202321019747. [DOI] [PubMed] [Google Scholar]
- 11.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 12.Pope GD, Birkmeyer JD, Finlayson SR. National trends in utilization and in-hospital outcomes of bariatric surgery. J Gastrointest Surg. 2002;6:855–60. doi: 10.1016/s1091-255x(02)00085-9. discussion 861. [DOI] [PubMed] [Google Scholar]
- 13.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 14.Livingston EH. The incidence of bariatric surgery has plateaued in the U. S Am J Surg. 2010;200:378–385. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009;56:1105–1121. doi: 10.1016/j.pcl.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman JS, Bowers SP, Dixon P, Schindler L. Dissolution of common psychiatric medications in a Roux-en-Y gastric bypass model. Psychosomatics. 2005;46:250–253. doi: 10.1176/appi.psy.46.3.250. [DOI] [PubMed] [Google Scholar]
- 17.Mirmiran P, Hosseini-Esfahanil F, Jessri M, Mahan LK, Shiva N, Azizis F. Does dietary intake by Tehranian adults align with the 2005 dietary guidelines for Americans? Observations from the Tehran lipid and glucose study. J Health Popul Nutr. 2011;29:39–52. doi: 10.3329/jhpn.v29i1.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 1992;55:299S–308S. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]
- 20.Levin RJ. Digestion and absorption of carbohydrates--from molecules and membranes to humans. Am J Clin Nutr. 1994;59:690S–698S. doi: 10.1093/ajcn/59.3.690S. [DOI] [PubMed] [Google Scholar]
- 21.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schanker LS. On the mechanism of absorption of drugs from the gastrointestinal tract. J Med Pharm Chem. 1960;2:343–359. doi: 10.1021/jm50011a001. [DOI] [PubMed] [Google Scholar]
- 23.Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60:717–733. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011 Dec 1;68(23):2241–7. doi: 10.2146/ajhp100630. [DOI] [PubMed] [Google Scholar]
- 25.Stenberg P, Luthman K, Artursson P. Virtual screening of intestinal drug permeability. J Control Release. 2000;65:231–243. doi: 10.1016/s0168-3659(99)00239-4. [DOI] [PubMed] [Google Scholar]
- 26.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 27.Dearden JC. Partitioning and lipophilicity in quantitative structure-activity relationships. Environ Health Perspect. 1985;61:203–228. doi: 10.1289/ehp.8561203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 29.Ciarimboli G. Organic cation transporters. Xenobiotica. 2008;38:936–971. doi: 10.1080/00498250701882482. [DOI] [PubMed] [Google Scholar]
- 30.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman J. Methotrexate transport in the human intestine. Evidence for heterogeneity. Biochem Pharmacol. 1992;43:2377–2383. doi: 10.1016/0006-2952(92)90316-b. [DOI] [PubMed] [Google Scholar]
- 32.Duverne C, Bouten A, Deslandes A, et al. Modification of cefixime bioavailability by nifedipine in humans: involvement of the dipeptide carrier system. Antimicrob Agents Chemother. 1992;36:2462–2467. doi: 10.1128/aac.36.11.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westphal JF, Trouvin JH, Deslandes A, Carbon C. Nifedipine enhances amoxicillin absorption kinetics and bioavailability in humans. J Pharmacol Exp Ther. 1990;255:312–317. [PubMed] [Google Scholar]
- 34.Wang DS, Kusuhara H, Kato Y, Jonker JW, Schinkel AH, Sugiyama Y. Involvement of organic cationtransporter 1 in the lactic acidosis caused by metformin. Mol Pharmacol. 2003;63:844–848. doi: 10.1124/mol.63.4.844. [DOI] [PubMed] [Google Scholar]
- 35.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005;315:1288–1297. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- 36.Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20:1595–1599. doi: 10.1023/a:1026183200740. [DOI] [PubMed] [Google Scholar]
- 37.Saitoh H, Aungst BJ. Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm Res. 1995;12:1304–1310. doi: 10.1023/a:1016217505990. [DOI] [PubMed] [Google Scholar]
- 38.Benet LZ, Zia-Amirhosseini P. Basic principles of pharmacokinetics. Toxicol Pathol. 1995;23:115–123. doi: 10.1177/019262339502300203. [DOI] [PubMed] [Google Scholar]
- 39.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 40.Requarth JA, Burchard KW, Colacchio TA, Stukel TA. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995 Mar;130:318–25. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 41.Mole DR, Tomson CR, Mortensen N, Winearls CG. Renal complications of jejuno-ileal bypass for obesity. QJM. 200;94:69–77. doi: 10.1093/qjmed/94.2.69. [DOI] [PubMed] [Google Scholar]
- 42.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11:41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 43.Fontana MA, Wohlgemuth SD. The surgical treatment of metabolic disease and morbid obesity. Gastroenterol Clin North Am. 2010;39:125–133. doi: 10.1016/j.gtc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Nocca D, Krawczykowsky D, Bomans B, et al. A prospective multicenter study of 163 sleeve gastrectomies: results at 1 and 2 years. Obes Surg. 2008;18:560–565. doi: 10.1007/s11695-007-9288-7. [DOI] [PubMed] [Google Scholar]
- 45.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 46.Franco JVA, Ruiz PA, Palermo M, et al. A Review of Studies Comparing Three Laparoscopic Procedures in Bariatric Surgery: Sleeve Gastrectomy, Roux-en-Y Gastric Bypass and Adjustable Gastric Banding. Obesity Surgery. 2011;21:1458–1468. doi: 10.1007/s11695-011-0390-5. [DOI] [PubMed] [Google Scholar]
- 47.Gumbs AA, Gagner M, Dakin G, Pomp A. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17:962–969. doi: 10.1007/s11695-007-9151-x. [DOI] [PubMed] [Google Scholar]
- 48.Ward M, Prachand V. Surgical treatment of obesity. Gastrointest Endosc. 2009;70:985–990. doi: 10.1016/j.gie.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Endocrinol Metab Clin North Am. 2008;37:943–964. doi: 10.1016/j.ecl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Truesdell DD, Whitney EN, Acosta PB. Nutrients in vegetarian foods. J Am Diet Assoc. 1984;84:28–35. [PubMed] [Google Scholar]
- 51.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–282. doi: 10.1381/096089298765554476. [DOI] [PubMed] [Google Scholar]
- 52.Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–954. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 53.DeMeester TR, Fuchs KH, Ball CS, Albertucci M, Smyrk TC, Marcus JN. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–426. doi: 10.1097/00000658-198710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kant AK. Consumption of energy-dense, nutrient-poor foods by adult Americans: nutritional and health implications. The third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2000;72:929–936. doi: 10.1093/ajcn/72.4.929. [DOI] [PubMed] [Google Scholar]
- 55.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–2296. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 56.O’Rourke RW. Inflammation in obesity-related diseases. Surgery. 2009;145:255–259. doi: 10.1016/j.surg.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morinigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. 2007;30:1906–1908. doi: 10.2337/dc07-0189. [DOI] [PubMed] [Google Scholar]
- 58.Munoz M, Mazure RA, Culebras JM. Obesity and the immune system. Nutr Hosp. 2004;19:319–324. [PubMed] [Google Scholar]
- 59.Schweiger C, Keidar A. Nutritional deficiencies in bariatric surgery patients: prevention, diagnosis and treatment. Harefuah. 2010;149:715–20. 748. [PubMed] [Google Scholar]
- 60.Gracia JA, Martinez M, Aguilella V. Postoperative morbidity of biliopancreatic diversion depending on common limb length. Obes Syrg. 2007;17:1306–1311. doi: 10.1007/s11695-007-9233-9. [DOI] [PubMed] [Google Scholar]
- 61.Xanthakosa SA, Thomas IH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–96. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 62.Rudnicki SA. Prevention and treatment of peripheral neuropathy after bariatric surgery. Curr Treat Options Neurol. 2010;12:29–36. doi: 10.1007/s11940-009-0052-2. [DOI] [PubMed] [Google Scholar]
- 63.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 64.Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10:1033–1037. doi: 10.1016/j.gassur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 66.Seres DS. Surrogate nutrition markers, malnutrition, and adequacy of nutrition support. Nutr Clin Pract. 2005;20:308–313. doi: 10.1177/0115426505020003308. [DOI] [PubMed] [Google Scholar]
- 67.Fuhrman MP. The albumin-nutrition connection: separating myth from fact. Nutrition. 2002;18:199–200. doi: 10.1016/s0899-9007(01)00729-8. [DOI] [PubMed] [Google Scholar]
- 68.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Spitz AF, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 2009 Apr;17(Suppl 1):S1–70. doi: 10.1038/oby.2009.28. [DOI] [PubMed] [Google Scholar]
- 69.Aills L, Blankenship J, Buffington C. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4:S73–108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Amaral JF, Thompson WR, Caldwell MD, Martin HF, Randall HT. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201:186–93. doi: 10.1097/00000658-198502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halverson JD, Zuckerman GR, Koehler RE, et al. Gastric bypass for morbid obesity: a medical-surgical assessment. Ann Surg. 1981;194:152–60. doi: 10.1097/00000658-198108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruz M, Carrasco F, Rojas P, Codoceo J. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–532. doi: 10.3945/ajcn.2009.27699. [DOI] [PubMed] [Google Scholar]
- 73.Kushner RF. Micronutrient deficiencies and bariatric surgery. Curr Opin Endocrinol Diabetes Obes. 2006;13:405–411. [Google Scholar]
- 74.Davies DJ, Baxter JM, Baxter JN. Nutritional deficiencies after bariatric surgery. Obes Surg. 2007;17:1150–1158. doi: 10.1007/s11695-007-9208-x. [DOI] [PubMed] [Google Scholar]
- 75.Avgerinos DV, Llaguna OH, Seigerman M, Lefkowitz AJ, Leitman IM. Incidence and risk factors for the development of anemia following gastric bypass surgery. World J Gastroenterol. 2010;16:1867–1870. doi: 10.3748/wjg.v16.i15.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhode BM, Shustik C, Christou NV, et al. Iron absorption and therapy after gastric bypass. Obes Surg. 1999;9:17–21. doi: 10.1381/096089299765553656. [DOI] [PubMed] [Google Scholar]
- 77.Bal B, Koch TR, Finelli FC, Sarr MG. Managing medical and surgical disorders after divided Roux-en-Y gastric bypass surgery. Nat Rev Gastroenterol Hepatol. 2010;7:320–334. doi: 10.1038/nrgastro.2010.60. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004 Sep;7(5):569–75. doi: 10.1097/00075197-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Avgerinos DV, Llaguna OH, Seigerman M, Lefkowitz AJ. Incidence and risk factors for the development of anemia following gastric bypass surgery. World J Gastroenterol. 2010;16(15):1867–1870. doi: 10.3748/wjg.v16.i15.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldner WS, Stoner JA, Thompson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 81.Gemmel K, Santry HP, Prachand VN, Alverdy JC. Vitamin D deficiency in preoperative bariatric surgery patients. Surg Obes Relat Dis. 2009;5:54–59. doi: 10.1016/j.soard.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Brouwer DA, van Beek J, Ferwerda H, et al. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr. 1998;79:527–532. doi: 10.1079/bjn19980091. [DOI] [PubMed] [Google Scholar]
- 83.Bell NH, Epstein S, Shary J, Greene V, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D–endocrine system in obese subjects. J Clin Invest. 1985;76:370–3. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin E, Armstrong-Moore D, Liang Z, Sweeney JF, Torres WE, Ziegler TR. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring) 2011 Mar;19(3):588–94. doi: 10.1038/oby.2010.239. Epub 2010 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slater GH, Ren CJ, Seigel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Newbury L, Dolan K, Hatzifotis M, Fielding G. Calcium and vitamin D depletion and elevated parathyroid hormone following bilio- pancreatic diversion. Obes Surg. 2003;13:893–5. doi: 10.1381/096089203322618722. [DOI] [PubMed] [Google Scholar]
- 87.Ducloux R, Nobecourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21:556–560. doi: 10.1007/s11695-010-0352-3. [DOI] [PubMed] [Google Scholar]
- 88.Sakhaee K, Bhuket T, Adams-Huet B, Rao DS. Meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate. Am J Ther. 1999;6:313–21. doi: 10.1097/00045391-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Marcuard SP, Sinar DR, Swanson MS, Silverman JF, Levine JS. Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig Dis Sci. 1989;34:1238–1242. doi: 10.1007/BF01537272. [DOI] [PubMed] [Google Scholar]
- 90.Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39(2):315–20. doi: 10.1007/BF02090203. [DOI] [PubMed] [Google Scholar]
- 91.Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. doi: 10.1016/s1091-255x(01)00022-1. discussion 204–5. [DOI] [PubMed] [Google Scholar]
- 92.Ledoux S, Msika S, Moussa F, Larger E. Comparison of nutritional consequences of conventional therapy of obesity, adjustable gastric banding, and gastric bypass. Obes Surg. 2006 Aug;16(8):1041–9. doi: 10.1381/096089206778026415. [DOI] [PubMed] [Google Scholar]
- 93.Rhode BM, Arseneau P, Cooper BA, Katz M. Vitamin B-12 deficiency after gastric surgery for obesity. Am J Clin Nutr. 1996;63(1):103–9. doi: 10.1093/ajcn/63.1.103. [DOI] [PubMed] [Google Scholar]
- 94.Provenzale D, Reinhold RB, Golner B, Irwin V. Evidence for diminished B12 absorption after gastric bypass: oral supplementation does not prevent low plasma B12 levels in bypass patients. J Am Coll Nutr. 1992;11(1):29–35. doi: 10.1080/07315724.1992.10718193. [DOI] [PubMed] [Google Scholar]
- 95.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12(4):551–8. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 96.Gasteyger C, Suter M, Calmes JM, Gaillard RC, Giusti V. Changes in body composition, metabolic profile, and nutritional status 24 months after gastric banding. Obes Surg. 2006;16:243–50. doi: 10.1381/096089206776116381. [DOI] [PubMed] [Google Scholar]
- 97.Sobieraj DM, Wang F, Kirton OC. Warfarin resistance after total gastrectomy and Roux-en-Y esophagojejunostomy. Pharmacotherapy. 2008;28:1537–1541. doi: 10.1592/phco.28.12.1537. [DOI] [PubMed] [Google Scholar]
- 98.Griffiths TM, Thomas P, Campbell IA. Antituberculosis drug levels after jejunoileal bypass. Br J Dis Chest. 1982;76:286–289. [PubMed] [Google Scholar]
- 99.Mallory GN, Macgregor AM. Folate status following gastric bypass surgery (the Great Folate Mystery) Obes Surg. 1991;1:69–72. doi: 10.1381/096089291765561493. [DOI] [PubMed] [Google Scholar]
- 100.Boylan LM, Sugerman HJ, Driskell JA. Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988;88:579–85. [PubMed] [Google Scholar]
- 101.Hatizifotis M, Dolan K, Newbury L, Fielding G. Symptomatic vitamin A deficiency following biliopancreatic diversion. Obes Surg. 2003;13:655–657. doi: 10.1381/096089203322190916. [DOI] [PubMed] [Google Scholar]
- 102.Quaranta L, Nascimbeni G, Semeraro F, Quaranta CA. Severe corneoconjunctival xerosis after biliopancreatic bypass for obesity (Scopinaro’s operation) Am J Ophthalmol. 1994;118:817–818. doi: 10.1016/s0002-9394(14)72569-3. [DOI] [PubMed] [Google Scholar]
- 103.Van Mieghem T, Van Schoubroeck D, Depiere M, Debeer A. Fetal cerebral hemorrhage caused by vitamin K deficiency after complicated bariatric surgery. Obstet Gynecol. 2008;112(2 Pt 2):434–6. doi: 10.1097/AOG.0b013e3181649e7b. [DOI] [PubMed] [Google Scholar]
- 104.Kang L, Marty D, Pauli RM, Mendelsohn NJ, Prachand V. Chondrodysplasia punctata associated with malabsorption from bariatric procedures. Surg Obes Relat Dis. 2010;6(1):99–101. doi: 10.1016/j.soard.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 105.Moize V, Geliebter A, Gluck ME, Yahav E, Lorence M, Colarusso T, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003 Feb;13(1):23–8. doi: 10.1381/096089203321136548. [DOI] [PubMed] [Google Scholar]
- 106.Scopinaro N, Marinari GM, Pretolesi F, Papadia F, Murelli F, Marini P, et al. Energy and nitrogen absorption after biliopancreatic diversion. Obes Surg. 2000 Oct;10(5):436–41. doi: 10.1381/096089200321594309. [DOI] [PubMed] [Google Scholar]
- 107.Skroubis G, Anesidis S, Kehagias I, Mead N, Vagenas K, Kalfarentzos F. Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion in a non-superobese population: prospective comparison of the efficacy and the incidence of metabolic deficiencies. Obes Surg. 2006 Apr;16(4):488–95. doi: 10.1381/096089206776327251. [DOI] [PubMed] [Google Scholar]
- 108.Nanni G, Balduzzi GF, Capoluongo R, et al. Biliopancreatic diversion: clinical experience. Obes Surg. 1997;7:26–29. doi: 10.1381/096089297765556196. [DOI] [PubMed] [Google Scholar]
- 109.Malone M. Altered drug disposition in obesity and after bariatric surgery. Nutr Clin Pract. 2003;18:131–135. doi: 10.1177/0115426503018002131. [DOI] [PubMed] [Google Scholar]
- 110.Kennedy MC, Wade DN. Phenytoin absorption in patients with ileojejunal bypass. Br J Clin Pharmacol. 1979;7:515–518.108. doi: 10.1111/j.1365-2125.1979.tb00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peterson DI. Phenytoin absorption following jejunoileal bypass. Bull Clin Neurosci. 1983;48:148–149. [PubMed] [Google Scholar]
- 112.Prince RA, Pincheira JC, Mason EE, Printen KJ. J Clin Pharmacol. 1984;24:523–527. doi: 10.1002/j.1552-4604.1984.tb02762.x. [DOI] [PubMed] [Google Scholar]
- 113.Kampmann JP, Klein H, Lumholtz B, Molholm Hansen JE. Ampicillin and propylthiouracil pharmacokinetics in intestinal bypass patients followed up to a year after operation. Clin Pharmacokinet. 1984;9:168–176. doi: 10.2165/00003088-198409020-00004. [DOI] [PubMed] [Google Scholar]
- 114.Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20:310–313. doi: 10.3122/jabfm.2007.03.060177. [DOI] [PubMed] [Google Scholar]
- 115.Chenhsu RY, Wu Y, Katz D, Rayhill S. Dose-adjusted cyclosporine c2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther Drug Monit. 2003;25:665–670. doi: 10.1097/00007691-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Kelley M, Jain A, Kashyap R, et al. Change in oral absorption of tacrolimus in a liver transplant recipient after reversal of jejunoileal bypass: case report. Transplant Proc. 2005;37:3165–3167. doi: 10.1016/j.transproceed.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 117.Andersen AN, Lebech PE, Sorensen TI, Borggaard B. Sex hormone levels and intestinal absorption of estradiol and D-norgestrel in women following bypass surgery for morbid obesity. Int J Obes. 1982;6:91–96. [PubMed] [Google Scholar]
- 118.Victor A, Odlind V, Kral JG. Oral contraceptive absorption and sex hormone binding globulins in obese women: effects of jejunoileal bypass. Gastroenterol Clin North Am. 1987;16:483–491. [PubMed] [Google Scholar]
- 119.Wills SM, Zekman R, Bestul D, Kuwajerwala N, Decker D. Tamoxifen malabsorption after Roux-en-Y gastric bypass surgery: case series and review of the literature. Pharmacotherapy. 2010;30:217. doi: 10.1592/phco.30.2.217. [DOI] [PubMed] [Google Scholar]
- 120.Werbin N. Tuberculosis after jejuno-ileal bypass for morbid obesity. Postgrad Med J. 1981;57:252–253. doi: 10.1136/pgmj.57.666.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marcus FI, Quinn EJ, Horton H, et al. The effect of jejunoileal bypass on the pharmacokinetics of digoxin in man. Circulation. 1977;55:537–541. doi: 10.1161/01.cir.55.3.537. [DOI] [PubMed] [Google Scholar]
- 122.Terry SI, Gould JC, McManus JP, Prescott LF. Absorption of penicillin and paracetamol after small intestinal bypass surgery. Eur J Clin Pharmacol. 1982;23:245–248. doi: 10.1007/BF00547562. [DOI] [PubMed] [Google Scholar]
- 123.Food and Drug Administration. Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Food and Drug Administration; Rockville, MD: 2000. [Google Scholar]
- 124.Skottheim IB, Stormark K, Christensen H, Jakobsen GS. Significantly altered systemic exposure to atorvastatinacid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311–8. doi: 10.1038/clpt.2009.82. [DOI] [PubMed] [Google Scholar]
- 125.Skottheim IB, Stormark K, Christensen H, Jakobsen GS. Significant increase in systemic exposure of atorvastatin after biliopancreatic diversion with duodenal switch. Clin Pharmacol Ther. 2010 Jun;87(6):699–705. doi: 10.1038/clpt.2010.32. [DOI] [PubMed] [Google Scholar]