Tumor necrosis factor-α (TNF)-induced apoptotic activation of caspase-8 requires internalization of its receptor. This study shows that constitutively activated β-catenin is required to facilitate the lysosomal delivery of internalized TNF, the inhibition of caspase-8 activation, and the suppression of apoptosis in colon cancer cells.
Abstract
The Wnt/β-catenin pathway is constitutively activated in more than 90% of human colorectal cancer. Activated β-catenin stimulates cell proliferation and survival, however, its antiapoptotic mechanisms are not fully understood. We show here that activated β-catenin is required to suppress caspase-8 activation, but only in colon cancer cells that are resistant to tumor necrosis factor-α (TNF)-induced apoptosis. We found that lysosomal delivery of internalized TNF occurred at a faster pace in apoptosis-resistant than in apoptosis-sensitive colon cancer cells. Retardation of endosomal trafficking through vacuolar ATPase (V-ATPase) inhibition enhanced caspase-8 activation in apoptosis-resistant but not apoptosis-sensitive cells. Interestingly, knockdown of β-catenin also prolonged TNF association with the early endosome and enhanced caspase-8 activation in apoptosis-resistant but not apoptosis-sensitive colon cancer cells. In a mouse model of inflammation-associated colon tumors, we found nuclear expression of β-catenin, resistance to TNF-induced apoptosis, and reactivation of apoptosis in vivo after cotreatment of TNF with a V-ATPase inhibitor. Together these results suggest that activated β-catenin can facilitate endosomal trafficking of internalized TNF to suppress caspase-8 activation in colon cancer cells.
INTRODUCTION
Tumor necrosis factor-α (TNF) is an inflammatory cytokine that orchestrates systemic physiological responses to infections and injuries. TNF interacts with its ubiquitously expressed type-1 receptor (TNFR1) to activate a large number of intracelllular signaling pathways, including that of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB; Karin and Lin, 2002), the mitogen-activated protein kinases (Kant et al., 2011), the NADPH oxidase 1 (NOX1) (Kim et al., 2007), the sphigomyelinases (Adam-Klages et al., 1998), and caspase-8 (Ashkenazi and Dixit, 1999). Interestingly, the same set of adaptor proteins, including TRADD, RIPK1, TRAF2, and cIAP are required for the activation of NF-κB and caspase-8 (Muppidi et al., 2004). Whereas NF-κB activation occurs at the plasma membrane, caspase-8 activation is mediated by a soluble adaptor complex (Micheau and Tschopp, 2003; Muppidi et al., 2004). Furthermore, the juxtamembrane region of TNFR1 contains a YXXW motif that is required for receptor endocytosis and the activation of caspase-8 (Schneider-Brachert et al., 2004).
Caspase-8 is an apical caspase with two death effector domains (DED) that interact with the DED of Fas-associated protein with death domain (FADD; Bender et al., 2005; Thorburn, 2004). In the soluble complex stimulated by TNF/TNFR1, FADD is recruited through death domain (DD) interaction with RIPK1 and TRADD (Bender et al., 2005). FADD also binds to the DED of FLICE inhibitory protein (FLIP; van Raam and Salvesen, 2012). Dimerization with FLIP or self-dimerization is sufficient to activate caspase-8 activity; in other words, caspase-8 does not have to be processed to have catalytic activity. (Boatright et al., 2003; Boatright and Salvesen, 2003). The activated FLIP–caspase-8 heterodimer cleaves RIPKI, RIPK3, and CYLD to inhibit TNF-induced necrosis (Green et al., 2011). Mutation of the self-cleavage site in caspase-8 does not interfere with its antinecrosis function but abolishes its proapoptotic function (Kang et al., 2008), suggesting that formation of caspase-8 homodimer and subsequent processing to the mature L2S2 soluble caspase-8 is necessary for the activation of extrinsic apoptosis.
Under chronic inflammation, which is a risk factor for colorectal cancer (CRC), TNF-dependent activation of NF-κB can contribute to tumor growth and survival (Karin, 2009). Because caspase-8 inhibits TNF-induced necrosis (Green et al., 2011), cancer cells can retain the caspase-8–dependent antinecrosis pathway to enhance survival under chronic inflammation. However, this will necessitate activation of tumor mechanisms that can selectively disengage the proapoptotic activation of caspase-8. It is generally accepted that activated NF-κB can up-regulate the expression of antiapoptotic factors, such as FLIP and the inhibitors of apoptosis (IAPs) to block TNF-induced apoptosis (Karin and Lin, 2002; Ozturk et al., 2012). However, because the activation of NF-κB and caspase-8 is mediated by the same set of adaptor proteins (Muppidi et al., 2004), and because the expression of NF-κB–dependent survival factors requires time for transcription and translation, cancer cells are likely to activate additional mechanisms to selectively inhibit the apoptotic activation of caspase-8.
The canonical Wnt signaling cascade plays a crucial role in intestinal crypt proliferation and homeostasis (Clevers, 2006; Medema and Vermeulen, 2011). The central role of canonical Wnt signaling is to stabilize the cytoplasmic β-catenin protein and to stimulate its transcription regulatory function (Clevers, 2006). The recent data from the Cancer Genome Atlas Network (2012) show that β-catenin is constitutively activated in more than 90% of CRC by genetic and epigenetic alterations in a number of genes involved in the Wnt pathway. On the other hand, a recent histological survey on CRC cases showed that, among 724 tumors, only 323 (47%) expressed nuclear β-catenin (Morikawa et al., 2011). Although activated β-catenin must enter the nucleus to regulate gene expression, its constitutive nuclear localization requires additional factors that have not been fully elucidated (Bowman and Nusse, 2011). A previous study has shown that activated β-catenin can suppress TNF-induced apoptosis in head and neck cancer cells without affecting the expression levels of FLIP or other known components of the TNF apoptotic signaling pathways (Yang et al., 2006). Because chronic inflammation is a major risk factor in human CRC, and because β-catenin is constitutively activated in virtually all human CRC, we investigated whether and how β-catenin may suppress TNF-induced apoptosis in colon cancer cells.
RESULTS
V-ATPase inhibitors enhanced TNF-induced apoptosis in colon cancer cells expressing nuclear β-catenin
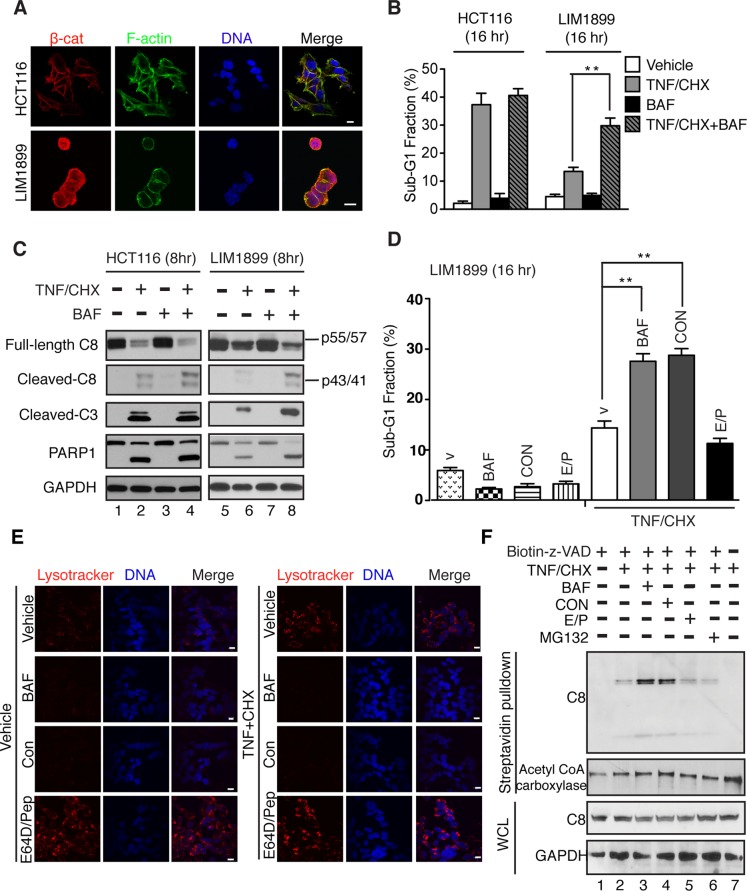
We identified two human colon cancer cell lines, HCT116 and LIM1899, that contain similar β-catenin codon-45 mutations (Sekine et al., 2002; Zhang et al., 2009) but exhibit different sensitivity to TNF-induced DNA fragmentation (Figure 1). The codon-45–mutant β-catenin is localized to the cytoplasm and the nucleus of LIM1899 cells (Figure 1A). However, stable nuclear localization was not observed with the codon-45–mutant β-catenin in HCT116 cells (Figure 1A). When treated with recombinant human TNF and cycloheximide (CHX) to inhibit NF-κB, HCT116 cells underwent DNA fragmentation at a faster rate and to a greater extent than did LIM1899 cells (Figure 1B). The induction of DNA fragmentation in LIM1899 and HCT116 cells was completely abolished by the pan-caspase inhibitor, zVADfmk, but was unaffected by the antioxidant butylated-hydroxyanisole (BHA), suggesting TNF/CHX activates caspase-dependent apoptosis rather than reactive oxygen species (ROS)-dependent necrosis in these colon cancer cells. Consistently TNF/CHX-induced cleavage of caspase-8, caspase-3, and poly(ADP-ribose) polymerase-1 (PARP1) occurred at a faster rate and was more efficient in HCT116 than in LIM1899 cells (Figure 1C, compare lanes 2 and 6).
FIGURE 1:
Selective sensitization to TNF-induced apoptosis by V-ATPase inhibitors in colon cancer cells expressing nuclear β-catenin. (A) Subcellular localization of β-catenin in HCT116 and LIM1899 cells: β-catenin (red), F-actin (green), and DNA (blue). Scale bars: 10 μm. (B) Sub-G1 fractions of cell populations treated with vehicle, human TNF-α (10 ng/ml) plus CHX (2.5 μg/ml), and/or BAF (200 nM), as indicated. (C) Effect of BAF on TNF/CHX-induced protein cleavage determined by immunoblotting of whole lysates from the indicated cells with the indicated treatments: PARP1; C8, caspase-8; C3, caspase-3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (D) Sub-G1 fractions of LIM1899 cells treated with TNF/CHX, BAF (200 nM), CON (100 nM), EP (10 μg/ml each), as indicated. (E) LysoTracker (200 nM) staining (15 min) of LIM1899 cells following treatment (1 h) with the indicated drugs. Scale bar: 10 μm. (F) In vivo biotinylation of activated caspase-8 in LIM1899 cells after the indicated treatments. The naturally biotinylated acetyl-CoA carboxylase was probed as a pull-down control.
We previously found (Huang et al., 2007) that TNF-induced caspase-8 activation can be enhanced by pharmacological inhibition or genetic ablation of the vacuolar ATPase (V-ATPase) (Inoue et al., 2005; Toei et al., 2010) in cells resistant to apoptosis due to the preservation of RB. We therefore tested the effect of bafilomycin A1 (BAF), a V-ATPase inhibitor (Gagliardi et al., 1999), on TNF/CHX-induced apoptosis in HCT116 and LIM1899 cells. With the apoptosis-sensitive HCT116 cells, cotreatment with BAF had no effect on TNF/CHX-induced DNA fragmentation or protein cleavage (Figure 1, B and C). However, with LIM1899 cells, BAF enhanced TNF/CHX-induced DNA fragmentation and caspase-8 activation (Figure 1, B and C). By inhibiting the V-ATPase, which acidifies the endosomes and lysosomes, BAF can inhibit endosomal trafficking and the lysosomal degradative function (Hurtado-Lorenzo et al., 2006). We therefore compared the apoptosis-enhancing effect of BAF with the lysosomal protease inhibitors E64D/pepstatin (EP) and found that BAF or concanamycin (CON), another V-ATPase inhibitor (Drose and Altendorf, 1997), enhanced TNF/CHX-induced DNA fragmentation to a similar extent, while EP did not enhance the apoptotic response (Figure 1D). As would be expected, BAF and CON, but not EP, eliminated LysoTracker staining of LIM1899 cells, and this effect was not altered by treatment with TNF/CHX (Figure 1E). To further demonstrate that BAF and CON enhanced caspase-8 activation, we adopted the method of Tu et al. (2006) to covalently tag the activated caspase-8 in vivo with a suicide substrate (biotin-VADfmk). None of the inhibitors, BAF, CON, EP, or MG132 alone, activated the in vivo biotinylation of caspase-8 (Supplemental Figure S1). Treatment of LIM1899 cells with TNF/CHX generated a low level of biotinylated caspase-8 (Figure 1F, lane 2), which was significantly increased by cotreatment with BAF or CON (Figure 1F, lanes 3 and 4). By contrast, the lysosomal protease inhibitors (EP) or the proteosomal inhibitor (MG132) did not increase the levels of biotinylated caspase-8 (Figure 1F, lanes 5 and 6). These results suggest that BAF and CON are likely to enhance TNF-induced caspase activation by inhibiting endosomal trafficking rather than lysosomal degradation.
Knockdown of β-catenin enhanced caspase-8 activation in apoptosis-resistant but not apoptosis-sensitive colon cancer cells
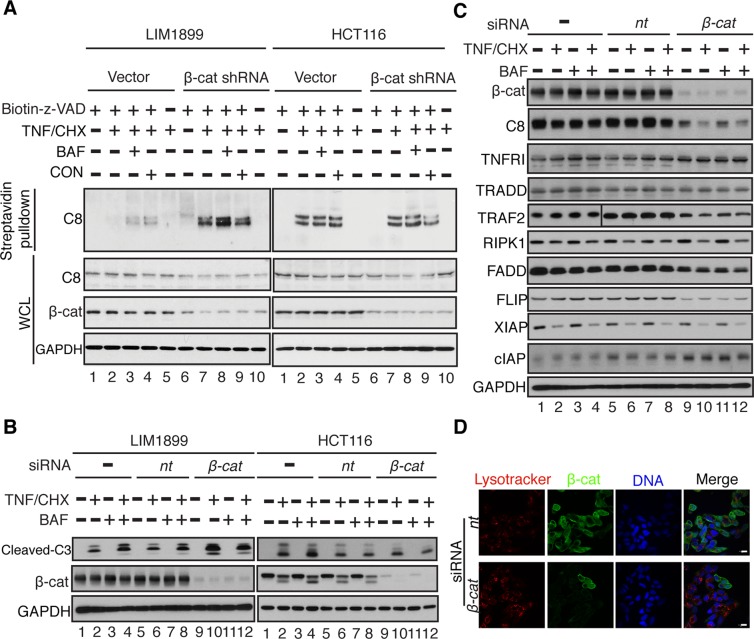
To determine whether activated β-catenin is required to suppress TNF-induced caspase-8 activation, we knocked down its expression with lentiviral-mediated expression of an short hairpin RNA (shRNA) in LIM1899 and HCT116 cells (Figure 2A). We could stably maintain β-catenin–knockdown HCT116 cells in culture, consistent with the previous conclusion that this colon cancer cell line is not dependent on β-catenin for proliferation or survival (Sekine et al., 2002). However, β-catenin–knockdown LIM1899 cells could only be maintained for three to five passages, suggesting LIM1899 cells are dependent on β-catenin for proliferation and survival. Using the in vivo biotinylation assay, we showed that β-catenin knockdown significantly enhanced TNF/CHX-induced caspase-8 activation in LIM1899 cells (Figure 2A, left panels, compare lanes 2 and 7). By contrast, β-catenin knockdown did not affect TNF-induced caspase-8 activation in HCT116 cells either in the absence or the presence of BAF or CON (Figure 2A, right panels, compare lanes 2–4 with lanes 7–9). We also examined the cleavage of caspase-3 in LIM1899 and HCT116 cells transfected with nontarget (nt) or β-catenin–targeted small interfering RNA (siRNA; Figure 2B). Cotreatment with TNF/CHX and BAF enhanced cleavage of caspase-3 in the parental and the nt-siRNA–transfected LIM1899 cells (Figure 2B, left panel, compare lanes 2 and 4 with lanes 6 and 8). Caspase-3 cleavage was stimulated by the knockdown of β-catenin and not further enhanced by BAF (Figure 2B, left panel, compare lane 10 to lanes 2, 6, and 12). Again, the knockdown of β-catenin did not enhance TNF/CHX-induced caspase-3 cleavage in HCT116 cells (Figure 2B, right panels). In the apoptosis-sensitive HCT116 cells, TNF/CHX treatment induced the cleavage of β-catenin, which has been shown to be a substrate of caspase, and its cleavage associated with apoptosis (Senthivinayagam et al., 2009). Consistent with reduced caspase activation, TNF/CHX treatment did not induce β-catenin cleavage in the apoptosis-resistant LIM1899 cells (Figure 2B). While BAF enhanced TNF-induced caspase-8 activation (Figure 1, C and F), it did not stimulate β-catenin cleavage (Figure 2B). These results suggest that BAF could enhance caspase-8 activation and DNA fragmentation independent of β-catenin cleavage.
FIGURE 2:
Knockdown of β-catenin–enhanced caspase-8 activation in LIM1899 but not HCT116 cells. (A) In vivo biotinylation of activated caspase-8 in cells stably transduced with pLKO (vector) or β-catenin shRNA lentivirus and treated as indicated and described in Materials and Methods. (B) β-catenin (β-cat) and cleaved caspase-3 (C3) were detected by immunoblotting of total lysates from cells transfected with the indicated siRNA (nt; β-cat, β-catenin) at 8 h after the indicated treatment. (C) Levels of caspase-8, TNFRI, TRADD, TRAF2, RIPK1, FADD, FLIP, XIAP, and cIAP in total lysates of LIM1899 cells transfected with the indicated siRNA (nt, β-cat) at 8 h after the indicated treatment. (D) LysoTracker (red) and β-catenin (β-cat; green) staining of LIM1899 cells transfected with the indicated siRNA (nt, β-cat). Scale bars: 10 μm.
We examined the expression of caspase-8, TNFR1, TRADD, TRAF2, RIPK1, FADD, FLIP, XIAP, and cIAP in LIM1899 cells before and after the knockdown of β-catenin but did not find any changes that could readily account for the enhanced activation of caspase-8 (Figure 2C). We observed a two- to threefold reduction in the levels of caspase-8, FADD, and FLIP in β-catenin–knockdown relative to nt-siRNA–transfected LIM1899 cells (Figure 2C), but a five- to sixfold increase in caspase-8 activity (Figure 2A); thus the coordinated reduction in the levels of caspase-8, FADD, and FLIP was not an important determinant of caspase-8 activation. The knockdown of β-catenin also did not interfere with the acidification of intracellular organelles (Figure 2D). Nevertheless, when β-catenin was knocked down, BAF no longer had an enhancing effect on TNF-induced caspase-8 activation (Figure 2A). These results show that activated β-catenin is required to suppress caspase-8 activation and is likely to act at a point upstream of BAF in the endosomal pathway.
Subcellular localization of internalized TNF
Previous studies have shown that TNFR1 endocytosis is required for the activation of caspase-8 (Schneider-Brachert et al., 2004). To determine whether TNF is internalized in the apoptosis-resistant LIM1899 cells, we developed an image-based assay to track the subcellular localization of biotin-TNF following a synchronized induction of endocytosis by temperature shift (Figure S2). Cells were preincubated with biotin-TNF and streptavidin at 4°C, warmed to 37°C to induce internalization, and then fixed at different time points up to 60 min after temperature shift. As controls, we showed that streptavidin–biotin–soybean trypsin inhibitor or streptavidin alone were not internalized upon warming (Figure S2). We established that EEA1, a marker for early endosome, and V0D1, a membrane component of the V0 complex of V-ATPase, do not significantly colocalize throughout the 60-min warming time course in the presence or absence of BAF (Figure S2). We therefore examined the colocalization of internalized biotin-TNF with EEA1 and V0D1.
β-Catenin–dependent endosomal trafficking of internalized TNF in apoptosis-resistant but not apoptosis-sensitive cells
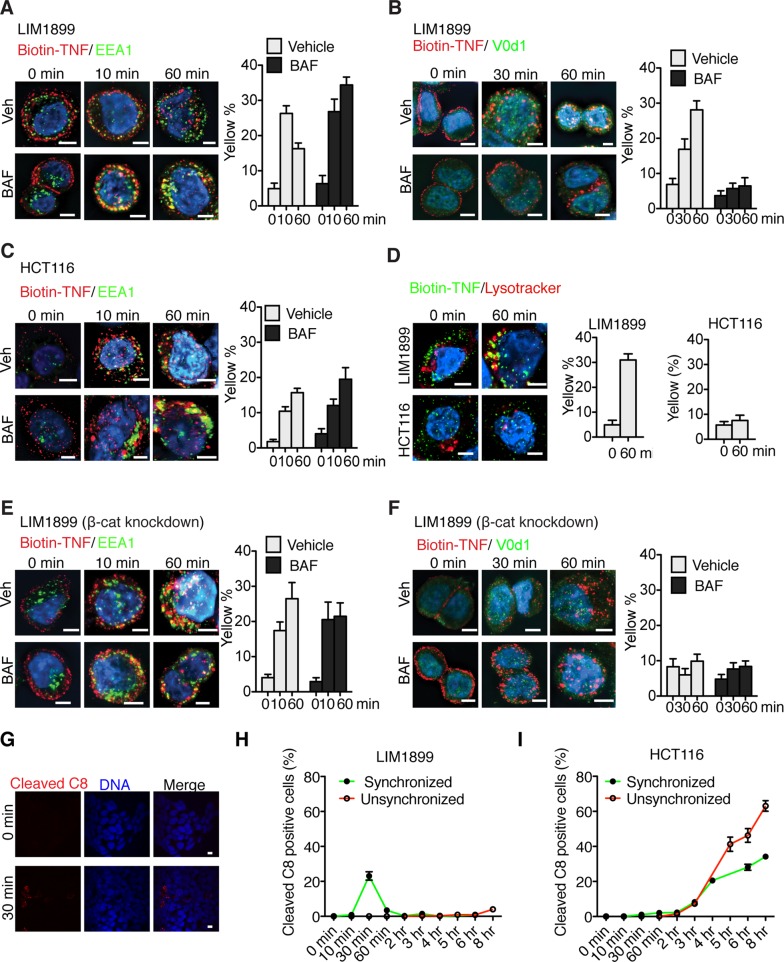
In the apoptosis-resistant LIM1899 cells, biotin-TNF was colocalized with TNFR1 and internalized upon warming to 37°C. The internalized TNF/TNFR1 colocalized with EEA1 at 10 min after warming, but this colocalization was reduced at 60 min (Figure 3A). Treatment with BAF did not alter the initial colocalization at 10 min but stabilized the biotin-TNF/EEA1 association for up to 60 min (Figure 3A). Internalized biotin-TNF also colocalized with V0D1, albeit with a slower kinetics than that of EEA1 (Figure 3B). Treatment with BAF abolished this colocalization (Figure 3B) consistent with the fact that V-ATPase is required for endosomal trafficking (Hurtado-Lorenzo et al., 2006). In the apoptosis-sensitive HCT116 cells, biotin-TNF was also internalized; however, its colocalization with EEA1 was maintained for 60 min and was not affected by BAF treatment (Figure 3C). Biotin-TNF did not colocalize with V0D1 in HCT116 cells (Figure S3). We also examined the colocalization of biotin-TNF with LysoTracker, which reacts with V-ATPase–acidified organelles (Figure 1E) and found colocalization of biotin-TNF with LysoTracker in LIM1899 but not HCT116 cells at 60 min after warming (Figure 3D). Together these results suggest that TNF/TNFR1 is internalized in colon cancer cells; however, the internalized TNF appeared to move through the endosomal pathway toward the lysosome more efficiently in the apoptosis-resistant LIM1899 than in the apoptosis-sensitive HCT116 cells.
FIGURE 3:
Effects of BAF, β-catenin knockdown on subcellular distribution of internalized TNF and effect of synchronized endocytosis on TNF-induced caspase-8 cleavage in LIM1899 cells. (A) Colocalization of biotin-TNF with EEA1 in LIM1899 cells (see Figure S2 and Materials and Methods). Scale bars: 10 μm. (B) Colocalization of biotin-TNF with V0D1 in LIM1899 cells. Scale bars: 10 μm. (C) Colocalization of biotin-TNF with EEA1 in HCT116 cells. Scale bars: 10 μm. (D) Colocalization of biotin-TNF with LysoTracker in LIM1899 and HCT116 cells. Scale bars: 10 μm. (E and F) LIM1899 cells were transfected with β-catenin siRNA and labeled with biotin-TNF as in (A) and (B) at 48-h posttransfection. (G) Detection of cleaved caspase-8 in TNF/CHX-treated cells. Scale bars: 10 μm. (H and I) LIM1899 (H) and HCT116 (I) cells were incubated with TNF/CHX at 37°C (unsynchronized) or for 45 min at 4°C and then warmed up to 37°C (synchronized). Cleaved caspase-8 was detected by immunofluorescence. Values shown are mean ± SD from five fields with at least 1000 cells counted for each sample.
We then examined the endocytic trafficking of biotin-TNF in β-catenin–knockdown LIM1899 cells and found that the colocalization of biotin-TNF with EEA1 was prolonged, and the effect of BAF on biotin-TNF/EEA1 colocalization was no longer observed (Figure 3E). In β-catenin–knockdown LIM1899 cells, the internalized biotin-TNF did not colocalize with V0D1 (Figure 3F). As shown in Figure 2D, β-catenin knockdown did not abolish V-ATPase activity; however, it exerted an effect similar to that of BAF. These results suggest that trafficking of internalized TNF/TNFR1 through the endosomes can be regulated by β-catenin. The faster movement of internalized TNF/TNFR1 down the endocytic pathway toward the lysosome in the apoptosis-resistant LIM1899 cells requires nuclear expression of β-catenin and is associated with reduced activation of caspase-8.
To determine whether nuclear β-catenin can also regulate the down-regulation of other cell surface receptors, we stimulated HCT116 and LIM1899 cells with EGF at 4°C and measured the levels of phospho-EGFR (epidermal growth factor receptor) at 10, 30, and 60 min after the synchronous induction of endocytosis by warming to 37°C. We found that the decay of phospho-EGFR upon warming to 37°C followed a similar kinetics in HCT116 and LIM1899 cells (Figure S4). Furthermore, we showed that the decay of phospho-EGFR was inhibited by BAF in both cell lines (Figure S4). This result suggests that nuclear β-catenin selectively stimulates the endosomal trafficking of TNF/TNFR1 in colon cancer cells to inhibit apoptotic signaling.
Transient versus sustained caspase-8 cleavage in apoptosis-sensitive versus apoptosis-resistant colon cancer cells
Although oligomerization is sufficient to activate caspase-8 without cleavage, caspase-8 cleavage is required for the induction of apoptosis (van Raam and Salvesen, 2012). We therefore examined TNF-induced caspase-8 cleavage using a commercial antibody specific for cleaved caspase-8 (∆C8), which reacted with cells only following TNF/CHX treatment (Figure 3G). With unsynchronized (37°C) LIM1899 cells, very few ∆C8-positive cells were detected over an 8-h period after TNF/CHX addition (Figure 3H). A transient burst of ∆C8-positive cells was observed under conditions of synchronized endocytosis, with the peak at 30 min (Figure 3H). These results showed that caspase-8 cleavage can occur in the apoptosis-resistant LIM1899 cells, however, this apoptotic signaling mechanism is inefficient (not observed at 37°C) and unstable (decayed within 60 min after synchronized endocytosis). In the apoptosis-sensitive HCT116 cells, TNF/CHX did not induce ∆C8-positive cells until after 3 h of incubation. Synchronization of endocytosis did not affect the formation of ∆C8 in HCT116 cells (Figure 3I), suggesting that the efficient and stable cleavage of caspase-8 in apoptosis-sensitive cells does not occur immediately upon receptor endocytosis but occurs with a lag time that is likely to involve the remodeling and the dissociation of the adaptor complex from the internalized receptor (Micheau and Tschopp, 2003).
V-ATPase inhibitor enhanced TNF-induced apoptosis in mouse colon tumors expressing nuclear β-catenin
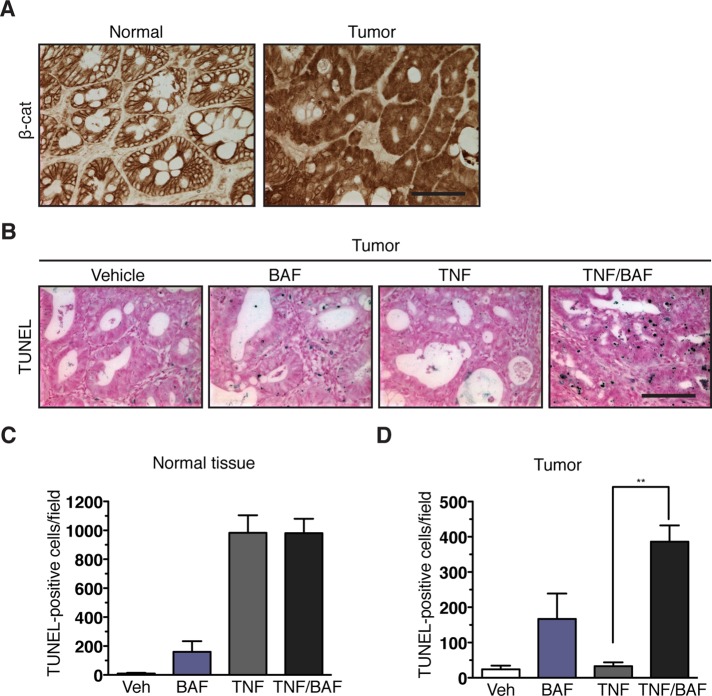
To determine whether V-ATPase inhibition can also reactivate TNF-induced apoptosis in colon tumor cells in vivo, we induced colon tumors in mice using the azoxymethane–dextran sulfate sodium (AOM-DSS) carcinogenesis protocol, in which a single injection of the carcinogen AOM is followed by 1 wk of feeding with DSS in the drinking water to induce colorectal inflammation, ulceration, and tissue regeneration (Tanaka et al., 2003). We found that AOM-DSS–induced colon tumors acquire resistance to TNF-induced apoptosis, and this phenotype is associated with the nuclear expression of β-catenin (Figure 4). We injected tumor-bearing mice with TNF or BAF alone and in combination and quantified terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL)-positive cells in the tumor and the adjacent colonic tissues. In the normal colonic tissues, β-catenin is localized to the epithelial cell–cell junction (Figure 4A, left panel). In AOM-DSS–induced tumors, β-catenin is localized to the nucleus, due to exon-3 mutations that inactivate GSK3β phosphorylation sites (Greten et al., 2004). Treatment with BAF alone induced a low level of TUNEL-positive cells similar to that seen in the normal and the tumor tissues (Figure 4, C and D). Treatment with TNF alone induced a high level of TUNEL-positive cells in the normal but not the tumor tissues (Figure 4, B–D). In the normal tissues, cotreatment with BAF did not enhance the apoptotic response to TNF (Figure 4C). However, in the tumor tissues, cotreatment with TNF and BAF induced a high level of apoptotic response that was significantly above the additive effect of these two agents (Figure 4D). These results show that BAF also enhanced TNF-induced apoptosis in vivo in apoptosis-resistant colonic tumor cells that express nuclear β-catenin.
FIGURE 4:
BAF, a V-ATPase inhibitor, reactivates TNF-induced apoptosis in mouse colonic tumors. (A) Representative β-catenin staining in adjacent normal and colonic tumor tissue sections. Scale bar: 50 μm. (B) Representative TUNEL staining (dark blue dots) of colon tumor tissue sections from the indicated mouse treated with PBS (Vehicle), BAF, murine TNF-α (TNF) or TNF plus BAF (TNF/BAF). Nuclei were counterstained with TACS Fast Red. Scale bar: 50 μm. (C and D) Quantification of TUNEL-positive cells (mean and SD from 25 fields at 200× magnification) from at least three mice per treatment. **, p < 0.01 by Student's t test.
DISCUSSION
This study shows that activated β-catenin is required to suppress TNF-induced caspase-8 activation in apoptosis-resistant colon cancer cells. Furthermore, this antiapoptotic effect of β-catenin is cell context–dependent and associates with its nuclear expression. It is conceivable that the constitutive nuclear localization of β-catenin may allow it to interact with low-affinity promoters that regulate gene products involved in the lysosomal delivery of internalized TNF. Alternatively, nuclear β-catenin may suppress caspase-8 by a nontranscriptional mechanism, for example, by the nuclear sequestration of a factor that retards endosomal trafficking of TNF/TNFR1 and stimulates caspase-8 activation. Previous studies have identified the up-regulation of FLIP as a principal mechanism to suppress TNF-induced apoptosis. In head and neck cancer cells (Yang et al., 2006) and LIM1899 cells (Figure 2), knockdown of β-catenin did not alter the relative levels of FLIP and caspase-8, suggesting that β-catenin does not suppress caspase-8 activation through FLIP. Previous studies have shown that impairment of TNFR1 internalization by an adenoviral protein can suppress apoptosis (Schneider-Brachert et al., 2006). With the apoptosis-resistant LIM1899 colon cancer cells, the biotin-TNF/TNFR1 is efficiently internalized and targeted to the lysosome (Figure 3), suggesting that β-catenin does not act through the suppression of receptor endocytosis. Instead, this study suggests that the endosomal trafficking of internalized TNF/TNFR1 is a target of regulation by activated β-catenin in apoptosis-resistant colon cancer cells.
Receptor down-regulation via endosomal delivery to the lysosome is a common mechanism for extinguishing receptor-mediated signaling. In the apoptosis-resistant LIM1899 cells, internalized TNF associated with the EEA1 endosome, the V-ATPase endosome, and the lysosome within the first hour following synchronized induction of endocytosis. The knockdown of β-catenin stabilized the association of TNF/TNFR1 with the EEA1 endosome and enhanced the apoptotic activation of caspase-8. We could also enforce the association of internalized TNF with the EEA1 endosome by inhibiting V-ATPase with BAF to enhance caspase-8 activation. Because β-catenin knockdown had the same effect as BAF and abolished the apoptosis-enhancing effect of BAF, these results strongly suggest that lysosomal targeting of TNF/TNFR1 is a mechanism for extinguishing apoptotic signaling and that nuclear β-catenin can stimulate TNFR1 down-regulation to suppress caspase-8 activation. A recent report suggested that β-catenin is required for Wnt to stimulate the sequestration of GSK3β in multivesicular endosomes (Taelman et al., 2010). Whether the β-catenin–dependent endosomal trafficking of TNF/TNFR1 and GSK3β involve similar or distinct pathways is of interest and requires further investigation.
Activation of caspase-8 by TNF requires not only internalization of the activated TNFR1 but also the dissociation of the TRADD-RIP1-TRAF2-cIAP signaling complex (Micheau and Tschopp, 2003; Muppidi et al., 2004). We found in apoptosis-sensitive HCT116 cells that internalized TNF associated with the EEA1 endosome but did not reach the lysosome for at least 60 min. Although the prolonged association of TNF/TNFR1 with EEA1 endosome correlates with enhanced activation of caspase-8, expansion or contraction of the EEA1 endosome, achieved by the ectopic expression of dominant-active or dominant-negative Rab5, had no effect on caspase-8 cleavage in HCT116 cells (Figure S5). This finding and the fact that caspase-8 is activated by a soluble TRADD-RIP1-TRAF2-cIAP complex (Micheau and Tschopp, 2003) suggest that reduced endosomal trafficking of TNF/TNFR1 most likely contributes to caspase-8 activation by allowing time for the remodeling and dissociation of the TRADD-RIP1-TRAF2-cIPA signaling complex from the internalized TNF/TNFR1.
MATERIALS AND METHODS
Mice
For the AOM-DSS protocol, 6- to 7-wk-old C57BL/6 male mice were given one intraperitoneal (i.p.) injection with 12.5 mg/kg AOM (Sigma-Aldrich, St. Louis, MO). A week later, DSS salt (3%, 36–50 kDa; ICN Biomedicals, Costa Mesa, CA) was given in the drinking water for 7 d, followed by regular water for 19 wk. For apoptosis induction, tumor-bearing mice were injected i.p. with murine recombinant TNF-α (104 U in phosphate-buffered saline [PBS] containing 2% fetal bovine serum [FBS]; PeproTech, Rocky Hill, NJ), BAF (25 μg/kg body weight; Sigma-Aldrich), or PBS with 2% FBS, and killed at 24 h, after which their colons were collected, cleaned, fixed in 4% paraformaldehyde (PFA) overnight, and paraffin-embedded. All animal studies were conducted under protocols approved by the University of California at San Diego institutional animal care and use committee.
Immunohistochemistry and TUNEL assay
Paraffin-embedded colon tissues in “Swiss rolls” were sectioned (5 μm) stepwise (200 μm). Sections were deparaffinized, rehydrated, and boiled in 10 mM sodium citrate buffer (pH 6.0) for 10 min to retrieve antigen. Tissues were blocked with 5% bovine serum albumin (BSA) and 0.25% Triton X-100 in PBS for 30 min and incubated with primary anti–β-catenin (BD Biosciences, Franklin Lakes, NJ) in PBS with 1% BSA at 4°C for 16 h. Immunohistochemistry was then carried out with DAKO LSAB+ System-HRP according to the manufacturer's protocol. TUNEL assay (TACS-XL in situ apoptosis detection kit) was performed according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) in 5-μm-thick paraffin-embedded colonic tissue sections. The percentage of TUNEL-positive nuclei in the epithelial layer was achieved by counting at least 1000 cells under the microscope at 400× magnification for each animal sample.
Cell culture
The human colon cancer cell line HCT116 (American Type Culture Collection, Manassas, VA) was maintained in DMEM medium supplemented with 10% FBS (HyClone, Rockford, IL). The LIM1899 cell line (Zhang et al., 2009) was maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and additives (10 μM thioglycerol [Sigma-Aldrich], 2.5 μg/ml insulin [Sigma-Aldrich], and 0.5 mg hydrocortisone [Sigma-Aldrich]).
β-Catenin knockdown
siRNA targeting β-catenin (s438) was from Ambion (Austin, TX). For transient gene silencing, ∼70% confluent cells were transfected with siRNA using Lipofectamine for HCT116 cells (Invitrogen), or Nucleofector electroporation for LIM1899 cells according to the manufacturer's instructions (Lonza, Basel, Switzerland). Cells were allowed to stabilize for 48 h before being used in the experiments. The lentiviral β-catenin shRNA plasmid (nucleotide: NM_001904; shRNA: TRCN0000003845; CCGGGCTTGGAATGAGACTGCTGATCTCGAGATCAGCAGTCTCATTCCAAGCTTTTT; Sigma-Aldrich) and packaging plasmids were transfected into 293FT cells using GeneTran (Biomiga, San Diego, CA) to produce lentiviral particles. Infection was with 8 μg/ml polybrene (Sigma-Aldrich), and selection with puromycin began at 48-h postinfection.
Biotin-TNF labeling and imaging
Cells (3 × 105 per coverslip) were incubated with 10 μl of biotin-TNF (2.5 ng/ml) and CHX (2.5 μg/ml) at 4°C for 45 min and then with 20 μl of streptavidin (5 ng/μl) for another 45 min at 4°C (Figure S2). When indicated, BAF (200 nM) was added with biotin-TNF and throughout the experiment. To induce endocytosis, the coverslips were transferred into wells of a 24-well plate with 37°C media and then fixed with 4% PFA at 10, 30, or 60 min. The 0-min samples were collected and fixed at 4°C. After fixation, cells were permeabilized with 0.3% Triton X-100 for 15 min at room temperature, blocked with 5% BSA for 30 min, and then stained with primary antibody for 2 h. at 37°C; this was followed by incubation with secondary antibody (donkey anti-rabbit Alexa Fluor 594, 1:300; and goat anti-mouse Alexa Fluor 488, 1:150) for 1 h at 37°C. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride. Fluorescence images were captured on a Zeiss fluorescence microscope (Jena, Germany) with Deconvolution/Image Pro Plus version 7.0 Imaging System (Media Cybernetics, Rockville, MD). Z-stack pictures for each color channel were taken using Scope-Pro and deconvolved using 3D Blind Deconvolution. The deconvolved Z-stacks were processed using maximum projection to generate one image.
Colocalization analysis
The colocalization of red and green signal in each deconvolved image was analyzed by Colocalizer Pro. The background was corrected by average contrast and fluorescence, and then the yellow% was calculated as the yellow pixels over the total pixels of each picture. At least six deconvolved images were analyzed for each sample.
In vivo labeling of activated caspase-8
Cells (2 × 107) were plated into 6-cm collagen-coated dishes overnight; 50 μM biotin-VADfmk was added for 2 h, followed by treatment with BAF (200 nM), CON (100 nM), EP (10 μg/ml), or MG132 (20 μM) for 3 h. TNF (10 ng/ml) and CHX (2.5 μg/ml) were then added for an additional 5 h. At the end of treatment, cells were washed in ice-cold PBS and lysed in 1.0 ml CHAPS lysis buffer (150 mM KCl, 50 mM HEPES, 0.1% CHAPS, pH 7.4). The lysates were centrifuged twice at 12,000 rpm at 4°C for 10 min. The lysates (1.0 mg) were incubated overnight at 4°C with 20 μl streptavidin-conjugated Sepharose beads (Pierce–Thermo Fisher Scientific, Rockford, IL). The beads were washed four times with 1.0 ml CHAPS buffer, and biotinylated proteins were eluted from the beads using 2X SDS sample buffer and by boiling for 15 min. The eluted proteins were probed for caspase-8 or acetyl-CoA decarboxylase by immunoblotting.
Antibodies, chemicals, immunoblotting, and immunofluorescence staining
See the Supplemental Material.
Statistical analysis
Prism (GraphPad, La Jolla, CA) programs were used to analyze the data and plot curves. Data are represented as mean ± SD. A two-tailed, unpaired Student's t test was used to determine statistical significance of the differences between data sets. A p value of < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
We thank Tony Burgess at the Ludwig Cancer Research Institute (Melbourne, Australia) for the generous gift of the LIM1899 cells. This work was supported by a grant from the National Cancer Institute, National Institute of Health (CA058320), to J.Y.J.W.
Abbreviations used:
- ∆C8
cleaved caspase-8
- AOM-DSS
azoxymethane–dextran sulfate sodium
- BAF
bafilomycin A1
- BHA
butylated-hydroxyanisole
- BSA
bovine serum albumin
- CHX
cycloheximide
- CON
concanamycin
- CRC
colorectal cancer
- DED
death effector domains
- EP
E64D/pepstatin
- FBS
fetal bovine serum
- i.p.
intraperitoneally
- nt
nontarget
- PARP1
poly(ADP-ribose) polymerase-1
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TNF-α
tumor necrosis factor-α
- TNFR1
TNF type-1 receptor
- TUNEL
terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling
- V-ATPase
vacuolar ATPase
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-09-0662) on December 21, 2012.
REFERENCES
- Adam-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Kronke M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J Leukoc Biol. 1998;63:678–682. doi: 10.1002/jlb.63.6.678. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- Boatright KM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bowman A, Nusse R. Location, location, location: FoxM1 mediates β-catenin nuclear translocation and promotes glioma tumorigenesis. Cancer Cell. 2011;20:415–416. doi: 10.1016/j.ccr.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- Gagliardi S, Rees M, Farina C. Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase. Curr Med Chem. 1999;6:1197–1212. [PubMed] [Google Scholar]
- Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS. RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Huang X, Masselli A, Frisch SM, Hunton IC, Jiang Y, Wang JY. Blockade of tumor necrosis factor-induced Bid cleavage by caspase-resistant Rb. J Biol Chem. 2007;282:29401–29413. doi: 10.1074/jbc.M702261200. [DOI] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- Inoue T, Wang Y, Jefferies K, Qi J, Hinton A, Forgac M. Structure and regulation of the V-ATPases. J Bioenerg Biomembr. 2005;37:393–398. doi: 10.1007/s10863-005-9478-8. [DOI] [PubMed] [Google Scholar]
- Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Morikawa T, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452–1462. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ozturk S, Schleich K, Lavrik IN. Cellular FLICE-like inhibitory proteins (c-FLIPs): fine-tuners of life and death decisions. Exp Cell Res. 2012;318:1324–1331. doi: 10.1016/j.yexcr.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, et al. Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J Clin Invest. 2006;116:2901–2913. doi: 10.1172/JCI23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S, Shibata T, Sakamoto M, Hirohashi S. Target disruption of the mutant β-catenin gene in colon cancer cell line HCT116: preservation of its malignant phenotype. Oncogene. 2002;21:5906–5911. doi: 10.1038/sj.onc.1205756. [DOI] [PubMed] [Google Scholar]
- Senthivinayagam S, Mishra P, Paramasivam SK, Yallapragada S, Chatterjee M, Wong L, Rana A, Rana B. Caspase-mediated cleavage of β-catenin precedes drug-induced apoptosis in resistant cancer cells. J Biol Chem. 2009;284:13577–13588. doi: 10.1074/jbc.M900248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry. 2010;49:4715–4723. doi: 10.1021/bi100397s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol. 2006;8:72–77. doi: 10.1038/ncb1340. [DOI] [PubMed] [Google Scholar]
- van Raam BJ, Salvesen GS. Proliferative versus apoptotic functions of caspase-8 hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta. 2012;1824:113–122. doi: 10.1016/j.bbapap.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zeng Q, Yu G, Li S, Wang CY. Wnt/β-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 2006;18:679–687. doi: 10.1016/j.cellsig.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Walker F, Kiflemariam S, Whitehead RH, Williams D, Phillips WA, Mikeska T, Dobrovic A, Burgess AW. Selective inhibition of proliferation in colorectal carcinoma cell lines expressing mutant APC or activated B-Raf. Int J Cancer. 2009;125:297–307. doi: 10.1002/ijc.24289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.