Abstract
An introduction to the accompanying three papers.
Keywords: Sex differences in response to sex steroids, Knee biomechanics and osteoarthritis, Pain perception in knee osteoarthritis, Musculoskeletal tissues, Estrogen, Testosterone, Rapid actions, Ligaments, Tendons, Bones, Animal models of osteoarthritis, Knee as an organ
Review
Introduction
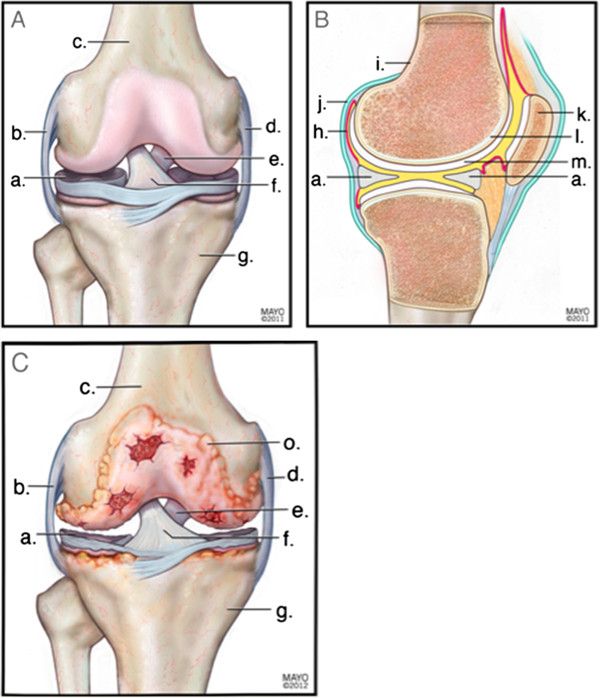
Osteoarthritis (OA) is a leading cause of disability in the United States. It is the most common form of arthritis and afflicts 13.9% of U.S. adults aged 25 and older and 33.6% (12.4 million) of those over 65—an estimated 26.9 million individuals in the United States in 2005 [1]. Although knee OA is typically diagnosed from radiograph images showing narrowing of the joint space and osteophytes, all the components of the knee joint are involved [2,3]. Consequently, OA is a progressive disease involving extensive inflammation and damage to not only the joint cartilage and synovium, but also the joint capsule and the bone, muscle, ligaments and tendons surrounding the joint, with alterations in peripheral innervation and central pain processing [4] (Figure 1A,B). The result is irreversible structural change and consequent joint stiffness, pain, and functional impairment [1-4] (Figure 1C).
Figure 1.
Anterior (A) and lateral (B) views of a healthy knee joint showing the meniscus (a), lateral collateral ligament (b), distal femur (c), medial collateral ligament (d), posterior cruciate ligament (e), anterior cruciate ligament (f), proximal tibia (g), periosteum (i), joint capsule (j), patella (k), subchondral bone (l), and normal articular cartilage (m). Degradation of the articular cartilage due to OA is shown in C.
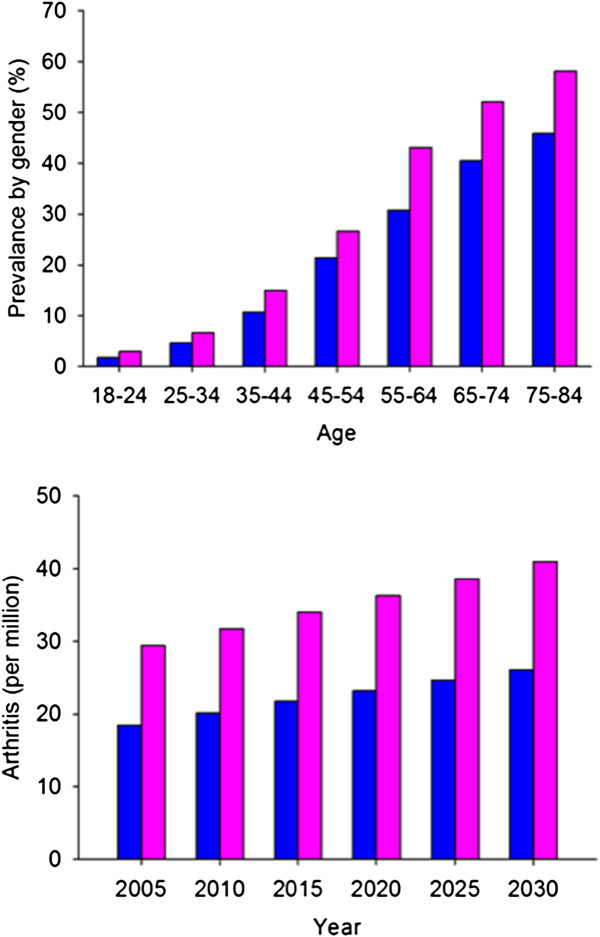
Although our understanding of the underlying causes of OA is increasing, we are only beginning to appreciate differences in the disease that exist with respect to sex or gender. Studies sponsored by the Centers for Disease Control and Prevention and the National Institutes of Health have identified differences in the incidence and severity of OA between men and women as well as between racial and ethnic groups [3,5]. The burden of OA is greater in women (Figure 2), who disproportionately develop knee and hand, but not hip OA [5]. The greater number of women in the aging U.S. population is of clinical concern due to the more severe knee OA experienced by women and its impact on quality of life and independence. Moreover, early onset OA is becoming more common, particularly among women who lead physically active lifestyles, such as athletes and workers in occupations that involve exposure to traumatic injury or mechanical stress. Injuries to the anterior cruciate ligament are of particular concern in young women (16–20 years) as approximately 50% of them will develop knee OA within 10–15 years [6,7]. As described in greater detail in the three accompanying papers, these observations underscore the need for research targeted at understanding how sex differences contribute to the development and progression of OA, and influence prevention and treatment strategies.
Figure 2.
Prevalence of arthritis by age group for US men (blue) and women (pink) in 2003–2005 (top panel) and current and projected prevalence of arthritis for US men and women (bottom panel). The graphs are based on data from the Centers for Disease Control website.
Sex differences and models for studying knee OA
Degenerative arthritis has been studied extensively in mice and other laboratory animals such as rats, guinea pigs, rabbits, dogs, and more recently, rhesus monkeys [8]. Such studies have been described as indicative of the human clinical condition because they develop similar histological and morphological abnormalities [8-10] as well as hormonal adaptations and polygenic inheritance in the case of genetic models [11]. Unfortunately, most studies on the mechanisms underlying OA have not considered sex differences, whether the studies used in vitro cell culture or animal models.
That sex differences exist in OA was recognized as far back as 1956 [12]. Nonetheless, even with the many animal models that have been developed and studied since that time, few studies have addressed the issue of sex differences. An extensive review of animal models published in 1994 [13] made no mention of sex differences. In 1996, Carlson et al. observed that the prevalence and severity of OA was similar in a limited population of male and female cynomolgus monkeys, whereas knee OA in humans occurs more commonly in females [14]. As recently as 2001 [15], a review of the literature identified the studies had been conducted using sex-matched animals, but these were limited in number and to a small group of laboratories: Silberberg (8 studies, 1941–1963), Walton (6 studies, 1975–1979) and Sokoloff (5 studies, 1956–1962).
The information provided in Table 1, which summarizes all animal studies of osteoarthritis published between 2002 and 2012, underscores the need for well-designed studies addressing sex differences. Out of a total of 1043 studies, only 32 identified the sex of the animals and only one of these made a comparison of the results between the sexes. Clearly, investigators typically do not consider the potential for sex differences in osteoarthritis. Moreover, the Pubmed database listed 2,968 clinical studies of knee OA in the past 10 years with 2,189 (73.7%) specifying the sex of the clinical population. Within this population, only a small percentage identifies "sex" (198 or 9.0%) or "gender" (142 or 6.5%) as a key parameter of the study (Table 2). These statistics indicate that although sex differences are routinely reported in clinical studies, they are not necessarily the principal factor on which the studies are based.
Table 1.
Summary of osteoarthritis-related journal articles referring to an animal model
| Year | Number of OA animal studies | Studies indicating sex | % indicating sex | Number studying sex |
|---|---|---|---|---|
| 2011 |
102 |
2 |
2 |
0 |
| 2010 |
156 |
5 |
3 |
0 |
| 2009 |
137 |
3 |
2 |
1 |
| 2008 |
118 |
3 |
3 |
0 |
| 2007 |
110 |
3 |
3 |
0 |
| 2006 |
90 |
3 |
3 |
0 |
| 2005 |
88 |
7 |
8 |
0 |
| 2004 |
91 |
3 |
3 |
0 |
| 2003 |
55 |
2 |
4 |
0 |
| 2002 |
57 |
1 |
2 |
0 |
| 2001 | 39 | 0 | 0 | 0 |
Table 2.
List of osteoarthritis-related journal articles specifying sex in an animal model
| Date | Journal | Authors | Animal | Sex used | Sex studied |
|---|---|---|---|---|---|
| Oct-11 |
Biochem pharmacol |
Imanishi et al. |
mice |
male and female |
No group comparison |
| Jun-11 |
J Orthop Res |
Watanabe et al. |
mouse |
male and female |
Backcrossing gene |
| Nov-10 |
Osteoarthitis Cartilage |
Schubert et al. |
Wistar rats |
male |
Experimental vs control |
| Sep-10 |
Skeletal Radiol |
Liu et al. |
New Zealand rabbits |
male |
Experimental vs control |
| Aug-10 |
Ann Rheum Dis |
Scott et al. |
Dunkin-Hartley guinea pigs |
male |
Experimental vs control |
| May-10 |
Osteoarthritis Cartilage |
Gurkan et al. |
Hartley guinea pig |
male |
Experimental vs control |
| Jan-10 |
Osteoarthritis Cartilage |
Huang et al. |
Sprague–Dawley rats |
male |
Experimental vs control |
| Apr-09 |
Int J Exp Pathol |
Bowyer et al. |
Dunkin-Hartley guinea pigs |
male |
Experimental vs control |
| Apr-09 |
Osteoarthritis Cartilage |
Chou et al. |
Sprague Dawley rats |
male |
3 groups |
| Feb-09 |
Pain |
McDougall et al. |
Dunkin-Hartley guinea pigs |
male and female |
No differences |
| Sep-08 |
Osteoarthritis Cartilage |
Piscaer et al. |
Wistar rats |
male |
Experimental vs control |
| Jul-08 |
Pharm Res |
Wang et al. |
New Zealand white rabbits |
male |
Experimental vs control |
| Jan-08 |
Am J Physiol Cell |
Kitamura et al. |
mice |
female |
Experimental vs control |
| Jun-07 |
Arthritis Rheum |
Appleton et al. |
Sprague Dawley rats |
male |
Experimental vs control |
| Jun-07 |
J Oral Maxillofac Surg |
Long et al. |
Merino sheep |
male |
3 time points |
| May-07 |
Arthritis Rheum |
Wang et al. |
New Zealand white rabbits |
male |
Experimental vs control |
| Dec-06 |
J Bone Mineral Res |
Kim et al. |
piglets |
male |
Experimental vs control |
| Aug-06 |
Arthritis Rheum |
Cheung et al. |
Hartley guinea pigs, NZW rabbits |
male |
Experimental vs control |
| Jun-06 |
Osteoarthritis Cartilage |
Schueiert & MacDougall |
Wistar rats |
male |
Experimental vs control |
| Nov-05 |
Arthritis Rheum |
Regan et al. |
mice |
male |
2 strains exptl vs control |
| Sep-05 |
Osteoarthritis Cartilage |
Wadhwa et al. |
mice |
male and female |
Genotyped, not compared |
| Jul-05 |
J Orthop Res |
Gushue et al. |
New Zealand white rabbits |
male |
Experimental vs control |
| Apr-05 |
Arthritis Rheum |
Tiraloche et al. |
New Zealand white rabbits |
male |
3 groups |
| Apr-05 |
Pain |
Pomonis et al. |
Sprague Dawley rats |
male |
Experimental vs control |
| Feb-05 |
IEEE Trans Med Imaging |
Patel et al. |
Wistar Hanover rats |
male |
Experimental vs control |
| Feb-05 |
Osteoarthritis Cartilage |
Spriet et al. |
New Zealand rabbits |
male |
Experimental vs control |
| Dec-04 |
Acta Orthop Scand |
Lahm et al. |
Dogs |
male |
Experimental vs control |
| Nov-04 |
Osteoarthritis Cartilage |
Papaioannou et al. |
New Zealand rabbits |
male |
Experimental vs control |
| Sep-04 |
Am J Sports Med |
Murray et al. |
cow, sheep, dog, human |
Men and women |
Animal sex unknown |
| Nov-03 |
Toxicol Pathol |
Guzman et al. |
Wistar rats |
male |
Experimental vs control |
| Jun-03 |
Osteoarthritis Cartilage |
Ciombor et al. |
Hartley guinea pigs |
male |
Experimental vs control |
| Mar-02 | Osteoarthritis Cartilage | Muehleman et al. | New Zealand rabbits | Male | 4 groups |
Cell culture studies are similarly limited with respect to the sex of the cell source. For those studies that use primary cells, whether from animals or humans, most do not directly compare the same set of experimental parameters for both sexes. Moreover, there is often a lack of a statistical design that provides sufficient power to adjust for inter-human variability. When cell lines are used, the sex of the cell is generally not provided. Thus, statements are made concerning underlying mechanisms that may not be accurate for the broader population of subjects.
The knee as an organ
Diagnosis of knee OA is based on evidence of joint pain or reduced space between articulating bone surfaces due to thinning of the opposing articular cartilages. However, multiple tissues that comprise the knee joint appear to be compromised by the disease, including subchondral bone, articular cartilage, meniscus, anterior cruciate ligament, synovium and synovial fluid, and the innervation of these tissues. A change in any of these tissues can influence the distribution of load across the joint, with corresponding adaptations in the other tissues and ultimately the cartilages [2]. Such pathophysiologic changes may exacerbate age-related physiologic changes in joint function attributable to genetic characteristics, age, sex, and health status, leading to greater cartilage damage. Thus, to better understand how knee OA is differentially expressed in males and females, it is critical to view the knee as an organ, rather than focusing only on the articular cartilage [2,3]. Moreover, the development of OA can involve multiple mechanisms, including mechanical loading, fluctuations in hormonal levels, and modulation of nervous system pathways.
Biomechanics and etiology of knee OA
Experimental and computational data suggest that contact stress in joint cartilage is a significant predictor of the risk for developing knee OA. Limb alignment, a major determinant of mechanical stresses within the knee, can predict the development of radiographic signs of knee OA, but these data do not indicate how limb alignment could contribute to sex differences. Similarly, little is known about how sex-related changes in muscle function might contribute to the worsening of knee OA. Significant gaps in knowledge remain as to how changes in musculoskeletal traits, such as obesity, disturb the normal mechanical environment of the knee joint and contribute to sex differences in the initiation and progression of knee OA [16].
Hormonal modulation of the knee
Knee tissues are modulated by sex hormones during tissue development and throughout the life cycle in both males and females. Whereas menopause in women is associated with an increase in OA severity, systemic estrogen alone cannot explain the observed sex differences [17]. Recent data, for example, show that sex-specific variations in the responses of chondrocytes to sex steroids are due to differences in receptor number as well as mechanisms of hormone action [18]. Moreover, the reduction in systemic estrogens is accompanied by changes in the relative levels of other steroid hormones, but how this impacts knee physiology is not known.
Neurologic contributions to knee OA
In addition to a greater prevalence of knee OA, women also often report greater pain and more substantial reductions in function and quality of life than men [19]. OA pain can be related to the innervation of the knee joint, but the pain does not always match the degree of injury and can continue even after total joint replacement. The mechanisms underlying these differences in pain between men and women with knee OA are unknown [20]. By improving our understanding of the mechanisms responsible for sex differences in the perception of pain in OA, more effective, and possibly sex-specific, treatment strategies will emerge.
Conclusion
Epidemiologic studies have established that there are sex differences in the incidence and severity of knee OA. Therapeutic approaches to the treatment of OA, particularly regenerative medicine strategies, have not yet taken these sex differences into consideration [21,22]. Effective interventions, however, will require a better understanding of the mechanisms involved in the disease and its differential expression in men and women. Although little is known about the mechanisms that underlie disparities between men and women in disease incidence and severity, it is likely that mechanical, hormonal, and neural events in the joint are involved. The papers that follow in this series review the literature related to sex differences and OA and identify gaps in our understanding with the goal of motivating research on this important problem.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
This paper was written by the authors to provide an overview of the gaps of knowledge that exist in the study of sex differences related to osteoarthritis. BDB wrote the initial draft, which was then edited by the other authors. All authors read and approved the final manuscript.
Contributor Information
Barbara D Boyan, Email: barbara.boyan@bme.gatech.edu.
Laura L Tosi, Email: ltosi@childrensnational.org.
Richard D Coutts, Email: rdcoutts1@gmail.com.
Roger M Enoka, Email: enoka@colorado.edu.
David A Hart, Email: hartd@ucalgary.ca.
Daniel P Nicolella, Email: dnicolella@gmail.com.
Karen J Berkley, Email: kberkley@psy.fsu.edu.
Kathleen A Sluka, Email: kathleen-sluka@uiowa.edu.
C Kent Kwoh, Email: kentkwoh2@gmail.com.
Mary I O’Connor, Email: oconnor.mary@mayo.edu.
Wendy M Kohrt, Email: wendy.kohrt@ucdenver.edu.
Eileen Resnick, Email: emresnick@gmail.com.
References
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new Insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- Frank CB, Shrive NG, Boorman RS, Lo IKY, Hart DA. New perspectives on bioengineering of joint tissues: joint adaptation creates a moving target for engineering replacement tissues. Ann Biomed Engin. 2004;32:458–465. doi: 10.1023/b:abme.0000017548.85451.b7. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis. A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ. Sex differences in osteoarthritis of the hip and knee. J Am Acad Orthop Surg. 2007;15:522–525. [PubMed] [Google Scholar]
- Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Bien DP. Rationale and implementation of anterior cruciate ligament injury prevention warm-up programs for female athletes. J Strength Cond Res. 2011;25:271–285. doi: 10.1519/JSC.0b013e3181fb4a5a. [DOI] [PubMed] [Google Scholar]
- Brophy RH, Silvers HJ, Mandelbaum BR. Anterior cruciate ligament injuries; etiology and prevention. Sports Med Arthrosc. 2010;18:2–11. doi: 10.1097/JSA.0b013e3181cdd195. [DOI] [PubMed] [Google Scholar]
- Van Sickle DC. In: Comparative pathobiology of major age-related diseases: current status and research frontiers. Scarpelli DG, Migaki G, editor. New York: Alan R Liss; 1984. Experimental models of Osteoarthritis; pp. 175–188. [Google Scholar]
- Silberberg M, Silberberg R. Age changes of bones and joints in various strains of mice. Am J Anat. 1941;68:69–95. doi: 10.1002/aja.1000680104. [DOI] [Google Scholar]
- Walton M. Studies of degenerative joint disease in the mouse knee joint; scanning electron microscopy. J Pathol. 1977;123:211–217. doi: 10.1002/path.1711230403. [DOI] [PubMed] [Google Scholar]
- Cohen BJ. Mammalian models for research on aging. Washington, DC: National Academy Press; 1981. pp. 23–57. [Google Scholar]
- Sokoloff L. Natural history of degenerative joint disease in small laboratory animals. 1. Pathological anatomy of degenerative joint disease in mice. Arch Pathol. 1956;62:118–128. [PubMed] [Google Scholar]
- Pritzker KP. Animal models for osteoarthritis: processes, problems, and prospects. Ann Rheum Dis. 1994;53:406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA. Epidemiology of osteoarthritis. Clin Geriatr Med. 1988;4:241–255. [PubMed] [Google Scholar]
- Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- Nicollela DP, O’Connor MJ, Enoka RM, Boyan BD, Hart DA, Resnick I, Berkley KJ, Sluka KA, Kwoh CK, Tosi LL, Coutts RD, Kohrt WM. Mechanical contributors to sex differences in knee osteoarthritis. Biol Sex Differences. accompany paper. [DOI] [PMC free article] [PubMed]
- Boyan BD, Hart DA, Enoka RM, Nicolella DP, Resnick E, Berkley KJ, Sluka KA, Kwoh CK, Tosi LL, O’Connor MJ, Coutts RD, Kohrt WM. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol Sex Differences. accompany paper. [DOI] [PMC free article] [PubMed]
- Kinney RC, Schwartz Z, Week K, Lotz MK, Boyan BD. Human articular chondrocytes exhibit sexual dimorphism in their responses to 1tbeta-estradiol. Osteoarthritis Cartilage. 2005;13:330–337. doi: 10.1016/j.joca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systemic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Berkley KJ, O’Connor MJ, Nicollela DP, Enoka RM, Boyan BD, Hart DA, Resnick E, Kwoh CK, Tosi LL, Coutts RD, Kohrt WM. Neural and psychological contributions to sex differences in knee osteoarthritic pain. Biol Sex Differences. accompany paper. [DOI] [PMC free article] [PubMed]
- Pelletier JP, Martel-Pelletier J, Raynauld JP. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther. 2006;8:206. doi: 10.1186/ar1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach CA, Carr AJ, Loughlin J. Recent advance in the genetic investigation of osteoarthritis. Trends Mol Med. 2005;11:186–191. doi: 10.1016/j.molmed.2005.02.005. [DOI] [PubMed] [Google Scholar]