Abstract
Recombinant human erythropoietin (rHuEpo) increases haemoglobin mass (Hbmass) and maximal oxygen uptake (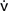 O2 max).
O2 max).
Purpose
This study defined the time course of changes in Hbmass, 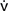 O2 max as well as running time trial performance following 4 weeks of rHuEpo administration to determine whether the laboratory observations would translate into actual improvements in running performance in the field.
O2 max as well as running time trial performance following 4 weeks of rHuEpo administration to determine whether the laboratory observations would translate into actual improvements in running performance in the field.
Methods
19 trained men received rHuEpo injections of 50 IU•kg−1 body mass every two days for 4 weeks. Hbmass was determined weekly using the optimized carbon monoxide rebreathing method until 4 weeks after administration. 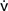 O2 max and 3,000 m time trial performance were measured pre, post administration and at the end of the study.
O2 max and 3,000 m time trial performance were measured pre, post administration and at the end of the study.
Results
Relative to baseline, running performance significantly improved by ∼6% after administration (10∶30±1∶07 min:sec vs. 11∶08±1∶15 min:sec, p<0.001) and remained significantly enhanced by ∼3% 4 weeks after administration (10∶46±1∶13 min:sec, p<0.001), while 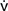 O2 max was also significantly increased post administration (60.7±5.8 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p<0.001) and remained significantly increased 4 weeks after rHuEpo (58.0±5.6 mL•min−1•kg−1, p = 0.021). Hbmass was significantly increased at the end of administration compared to baseline (15.2±1.5 g•kg−1 vs. 12.7±1.2 g•kg−1, p<0.001). The rate of decrease in Hbmass toward baseline values post rHuEpo was similar to that of the increase during administration (−0.53 g•kg−1•wk−1, 95% confidence interval (CI) (−0.68, −0.38) vs. 0.54 g•kg−1•wk−1, CI (0.46, 0.63)) but Hbmass was still significantly elevated 4 weeks after administration compared to baseline (13.7±1.1 g•kg−1, p<0.001).
O2 max was also significantly increased post administration (60.7±5.8 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p<0.001) and remained significantly increased 4 weeks after rHuEpo (58.0±5.6 mL•min−1•kg−1, p = 0.021). Hbmass was significantly increased at the end of administration compared to baseline (15.2±1.5 g•kg−1 vs. 12.7±1.2 g•kg−1, p<0.001). The rate of decrease in Hbmass toward baseline values post rHuEpo was similar to that of the increase during administration (−0.53 g•kg−1•wk−1, 95% confidence interval (CI) (−0.68, −0.38) vs. 0.54 g•kg−1•wk−1, CI (0.46, 0.63)) but Hbmass was still significantly elevated 4 weeks after administration compared to baseline (13.7±1.1 g•kg−1, p<0.001).
Conclusion
Running performance was improved following 4 weeks of rHuEpo and remained elevated 4 weeks after administration compared to baseline. These field performance effects coincided with rHuEpo-induced elevated 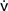 O2 max and Hbmass.
O2 max and Hbmass.
Introduction
Erythropoietin is a glycoprotein hormone produced primarily in the kidney that regulates red blood cell mass by stimulating the survival, proliferation and differentiation of erythrocytic progenitors [1]. In healthy subjects, administration of recombinant human erythropoietin (rHuEpo) increases haemoglobin concentration not only by the well known increase in red blood cell mass but also by a decrease in plasma volume [2], [3], [4]. The decrease in plasma volume, which precedes the increase in red cell mass, appears to be a rapid responding mechanism regulated by the renin-angiotensin-aldosterone system to control haematocrit [2], [3]. Theoretically, normal human red blood cells can persist in the circulation for approximately 17 weeks [5]. However, neocytolysis, the selective hemolysis of young circulating red blood cells which contributes to the regulation of red cell mass, seems to appear during specific conditions that cause a rapid decrease in erythropoietin levels such as spaceflight, high altitude exposure or blood doping [5], [6], [7], [8], [9]. Little is known about the time course of these mechanisms post rHuEpo administrations. In this study, heamoglobin mass (Hbmass) and related blood volumes were repeatedly determined using the optimized carbon monoxide (CO) rebreathing method [10], [11] pre, during and post rHuEpo administration in healthy trained men.
Most studies investigating the effects of rHuEpo on exercise performance have used maximal oxygen uptake (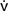 O2 max) tests. However,
O2 max) tests. However, 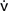 O2 max tests alone may be of little help in predicting exercise performance among athletes of similar ability [12], [13], [14], [15]. One study assessed submaximal performance in healthy male subjects and reported improvements of more than 50% in time to exhaustion on a cycle ergometer at a given 80% of
O2 max tests alone may be of little help in predicting exercise performance among athletes of similar ability [12], [13], [14], [15]. One study assessed submaximal performance in healthy male subjects and reported improvements of more than 50% in time to exhaustion on a cycle ergometer at a given 80% of 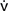 O2 max whereas the increase in
O2 max whereas the increase in 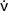 O2 max was approximately 12% [16]. Despite being a very interesting finding which questions the mechanisms by which rHuEpo improves submaximal exercise performance [17], the validity of the time to exhaustion test in assessing human exercise performance has been questioned [18]. In addition to being more variable than time trial tests [19], time to exhaustion tests are less likely to mimic the exercise performance environment [18], [19]. There are indeed only minimal occasions where an athlete is required to maintain a constant intensity until volitional exhaustion compared with self-paced exercise [18], [19].
O2 max was approximately 12% [16]. Despite being a very interesting finding which questions the mechanisms by which rHuEpo improves submaximal exercise performance [17], the validity of the time to exhaustion test in assessing human exercise performance has been questioned [18]. In addition to being more variable than time trial tests [19], time to exhaustion tests are less likely to mimic the exercise performance environment [18], [19]. There are indeed only minimal occasions where an athlete is required to maintain a constant intensity until volitional exhaustion compared with self-paced exercise [18], [19].
The main purpose of the present study was to investigate the time course of Hbmass and related blood parameters as well as changes in running 3,000 m time trial performance following 4 weeks of rHuEpo administration in healthy trained men in order to determine whether the augmented 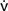 O2 max observed in laboratory based experiments would translate into actual improvements in running performance in the field.
O2 max observed in laboratory based experiments would translate into actual improvements in running performance in the field.
Methods
Ethics Statement
This study was approved by University of Glasgow Ethics Committee and conformed to the Declaration of Helsinki. All subjects underwent a medical assessment and provided written informed consent to participate. This study was part of a larger research project funded by the World Anti-Doping Agency (WADA).
Subjects
Nineteen healthy non-smoking trained men (mean ± SD, age: 26.0±4.5 yr, body mass: 74.8±7.9 kg, height: 179.8±5.4 cm) participated in this study. The subjects were regularly engaged in predominantly endurance-based activities such as running, cycling, swimming, triathlon and team sports. The subjects were divided into two groups for exercise performance analysis. The first group included subjects who had a history of running (n = 10) and the second group included the remaining subjects who were involved in other sporting activities (n = 9). Subjects were requested to maintain their normal training but abstain from official sporting competition for the duration of the research protocol. One of the subjects in the running group suffered from nasal obstruction and coughing during the study and could not perform the time trials post rHuEpo administration. The running performance data of this subject was excluded from the analyses.
Experimental Design
Each subject subcutaneously self-injected 50 IU•kg−1 body mass of rHuEpo (NeoRecormon, Roche, Welwyn Garden City, UK) every second day for 4 weeks. Daily oral iron supplementation (∼ 100 mg of elemental iron, Ferrous Sulphate Tablets, Almus, Barnstaple, UK) was given during the 4 weeks of rHuEpo administration. Venous blood samples from an antecubital vein were obtained in triplicate at baseline (over 2 weeks prior to the first rHuEpo administration), during rHuEpo administration (on days 2, 4, 8, 10, 14, 16, 20, 22, 26, 28) and for 4 weeks after rHuEpo administration (on days 30, 35, 41, 43, 48, 50, 57). All blood samples were taken after 10 min of rest in the supine position [20]. Blood samples were homogenized using a roller mixer and then measured in triplicate using a Sysmex XT-2000i (Sysmex UK, Milton Keynes, UK). The mean value of the triplicate was reported. Resting blood pressure and heart rate were recorded three times on both arms in the supine position before blood sampling using an automated cuff oscillometric device (Boso-Medicus, Bosch & Sohn GmbH, Jungingen, Germany).
Measurements of Haemoglobin Mass and Related Blood Volumes
Hbmass was determined in triplicate prior to the first rHuEpo injection and then weekly up to 4 weeks post rHuEpo administration using the optimized CO-rebreathing method as previously described [10], [11]. Briefly, a bolus of chemically pure CO dose of 1.0 mL•kg−1 body mass was administered with the first breath through a spirometer and rebreathed for 2 min with 4 L of oxygen. Change in percent carboxyhaemoglobin in venous blood samples from baseline to 8 min after CO administration was measured using a blood gas analyzer (ABL 725, Radiometer, Copenhagen, Denmark). CO concentration was measured using the Pac 7000 Draeger CO-analyzer (Draeger Safety, Northumberland, UK) and an estimated alveolar ventilation of 7.5 l•min−1 was used for calculations. Hbmass as well as blood, red cell and plasma volume were calculated as previously described elsewhere [11]. Blood volume was derived by dividing Hbmass by the haemoglobin concentration. Red cell volume was obtained by multiplying blood volume by the haematocrit and plasma volume was then calculated by subtracting red cell volume from blood volume. The typical error of measurement for Hbmass calculated from the two first baseline measurements was 1.5% (95% confidence interval (CI) 1.1 to 2.2%). The typical error of measurement for blood, red cell and plasma volume between the two weeks prior to the first rHuEpo administration were 2.4%, 1.6% and 3.6% (CI 1.8 to 3.5, 1.2 to 2.4 and 2.7 to 5.3), respectively.
Running Performance Assessment
Two 3,000 m time trials separated by at least one day rest were performed on a 200 m indoor athletic track (Kelvin Hall, Glasgow, UK) pre, post rHuEpo administration and at the end of the study. Verbal encouragement was given with feedback provided for the split time and remaining laps. The best performance on each occasion was used for analysis. The typical error of measurement for time trial performance calculated from the two tests on each phase was 1.3% (CI 1.1 to 1.7). Borg’s rating of perceived exertion (RPE) was recorded at the completion of the time trial. Temperature and humidity were recorded using a hygrometer. In addition to the time trial, 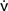 O2 max was determined pre, post rHuEpo administration and at the end of the study using an incremental test to exhaustion on a motorised treadmill. Both continuous or discontinuous protocols were used because of other purposes of the study (the speed was increased by 1 km•h−1•min−1 or by 2 km•h−1 every 3 min with 3 min of active recovery at a walking pace between each bout, respectively). Following a 30 min recovery,
O2 max was determined pre, post rHuEpo administration and at the end of the study using an incremental test to exhaustion on a motorised treadmill. Both continuous or discontinuous protocols were used because of other purposes of the study (the speed was increased by 1 km•h−1•min−1 or by 2 km•h−1 every 3 min with 3 min of active recovery at a walking pace between each bout, respectively). Following a 30 min recovery, 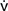 O2 max was verified using a square-wave protocol to exhaustion at a speed equivalent to the end speed attained during the incremental test minus 1 km•h−1
[21], [22]. Gas exchange was measured breath by breath using an automated metabolic gas analysis system (Cosmed Quark b2, Cosmed, Rome, Italy).
O2 max was verified using a square-wave protocol to exhaustion at a speed equivalent to the end speed attained during the incremental test minus 1 km•h−1
[21], [22]. Gas exchange was measured breath by breath using an automated metabolic gas analysis system (Cosmed Quark b2, Cosmed, Rome, Italy).
Statistical Analysis
Individual mean value was calculated when more than one blood sample was collected per week before further analysis. Changes over time in Hbmass, blood, red cell and plasma volume were assessed using t-test to determine whether the slope of these parameters was significantly different from zero. Time trial performance, 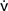 O2 max and blood parameters data for the key stages of the study were analyzed using repeated measures ANOVA. Relationships between time trial performance,
O2 max and blood parameters data for the key stages of the study were analyzed using repeated measures ANOVA. Relationships between time trial performance, 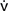 O2 max and Hbmass were assessed using Pearson’s product moment correlation coefficients.
O2 max and Hbmass were assessed using Pearson’s product moment correlation coefficients.
Results
Running Performance (Table 1)
Table 1. Running 3,000 m time trial performance and maximal oxygen uptake.
| Group 1 | Group 2 | |||||
| Baseline | End ofrHuEpo | End of the study | Baseline | End ofrHuEpo | End of the study | |
| 3,000 m total time (min:sec) | 10∶12 (0∶42) | 9∶40* (0∶37) | 9∶53* (0∶43) | 12∶05 (0∶55) | 11∶19* (0∶53) | 11∶39* (0∶58) |
| 1st 1,000 m split (min:sec) | 3∶16 (0∶13) | 3∶10* (0∶12) | 3∶13 (0∶13) | 3∶53 (0∶27) | 3∶36* (0∶14) | 3∶41 (0∶20) |
| 2nd 1,000 m split (min:sec) | 3∶26† (0∶16) | 3∶15*† (0∶14) | 3∶20† (0∶14) | 4∶05 (0∶17) | 3∶54† (0∶26) | 3∶59† (0∶20) |
| 3rd 1,000 m split (min:sec) | 3∶29† (0∶15) | 3∶15* (0∶13) | 3∶20* (0∶16) | 4∶07 (0∶16) | 3∶49*† (0∶15) | 3∶59† (0∶19) |
| RPE scale (6–20) | 18.0 (1.7) | 18.4 (0.9) | 19.0 (1.2) | 17.7 (2.2) | 18.4 (1.6) | 18.6 (1.4) |
| O2 max (mL•min−1•kg−1) | 60.3 (5.0) | 64.4* (3.9) | 61.8 (3.4) | 51.6 (3.5) | 57.0* (5.1) | 54.2* (4.7) |
Group 1 included subjects who had a history of running (n = 9) and group 2 included the other subjects who were involved in other activities (n = 9). Values are means (SD). Significant differences compared to baseline values are indicated by *P<0.05. Significant differences compared to the first 1,000 m split time within the same time trial. † P<0.05. RPE: Borg’s rating of perceived exertion; O2 max: Maximal oxygen uptake.
Running performance data of both groups combined (n = 18) are presented in this paragraph (Table 1 for separate groups). Irrespective of running history, time trial performance significantly improved by ∼6% post administration (10∶30±1∶07 min:sec vs. 11∶08±1∶15 min:sec, p<0.001) and remained significantly enhanced by ∼3% 4 weeks after rHuEpo compared to baseline (10∶46±1∶13 min:sec vs. 11∶08±1∶15 min:sec, p<0.001). RPE did not significantly differ between the time trials (p>0.05). Temperature (19.4±2.8°C) and humidity (46.6±9.0%) remained relatively constant (p>0.05). Relative to baseline, 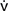 O2 max significantly increased post administration (60.7±5.8 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p<0.001) and remained significantly increased 4 weeks after rHuEpo (58.0±5.6 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p = 0.021).
O2 max significantly increased post administration (60.7±5.8 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p<0.001) and remained significantly increased 4 weeks after rHuEpo (58.0±5.6 mL•min−1•kg−1 vs. 56.0±6.2 mL•min−1•kg−1, p = 0.021). 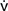 O2 max was at least moderately correlated with Hbmass as well as time trial performance throughout the study (range, r = 0.48 to r = 0.88, p<0.05). However, a significant correlation in individual responses compared to baseline between parameter was only observed for Hbmass and time trial performance at the end of the study (r = −0.68, p = 0.002).
O2 max was at least moderately correlated with Hbmass as well as time trial performance throughout the study (range, r = 0.48 to r = 0.88, p<0.05). However, a significant correlation in individual responses compared to baseline between parameter was only observed for Hbmass and time trial performance at the end of the study (r = −0.68, p = 0.002).
Haematological Parameters and Resting Blood Pressure and Heart Rate (Tables 2 and 3)
Table 2. Haematocrit, haemoglobin concentration, reticulocytes, haemoglobin mass, blood volumes, carboxyhaemoglobin and body mass before, during and 4 weeks post rHuEpo administration.
| Baseline | Week 2 of rHuEpo | End of rHuEpo | Week 2 postrHuEpo | Week 4 postrHuEpo | |
| Haematocrit (%) | 41.9 (1.8) | 44.7 (2.0)* | 49.2 (2.0)* | 47.7 (2.2)* | 45.1 (1.7)* |
| Haemoglobin (g•dl−1) | 14.4 (0.7) | 15.2 (0.7)* | 16.7 (0.9)* | 16.1 (0.9)* | 15.6 (0.7)* |
| Reticulocytes (%) | 1.07 (0.31) | 2.57 (0.44)* | 1.46 (0.41)* | 0.44 (0.13)* | 0.55 (0.15)* |
| Hbmass (g•kg−1) | 12.7 (1.2) | 13.4 (1.3)* | 15.2 (1.5)* | 15.1 (1.4)* | 13.7 (1.1)* |
| Hbmass (g) | 947 (109) | 1001 (127)* | 1131 (131)* | 1126 (136)* | 1023 (132)* |
| Blood volume (L) | 6.6 (0.9) | 6.6 (1.0) | 6.8 (0.8) | 6.7 (0.9) | 6.6 (0.8) |
| Red cell volume (L) | 2.8 (0.3) | 2.9 (0.3)* | 3.3 (0.4)* | 3.3 (0.4)* | 3.0 (0.4)* |
| Plasma volume (L) | 3.8 (0.6) | 3.7 (0.7) | 3.5 (0.4)* | 3.4 (0.6)* | 3.6 (0.5) |
| Carboxyhaemoglobin (%) | 0.73 (0.15) | 0.77 (0.16) | 1.04 (0.19)* | 1.01 (0.20)* | 0.65 (0.15) |
| Body mass (kg) | 75.1 (8.4) | 74.7 (7.8) | 75.0 (7.9) | 74.6 (7.6) | 74.6 (7.5) |
N = 19. Values are means (SD). Significant differences compared to baseline values are indicated by *P<0.05. Hbmass: Haemoglobin mass.
Table 3. Resting blood pressure and heart rate before, during and 4 weeks post rHuEpo administration.
| Baseline | Week 2 ofrHuEpo | End ofrHuEpo | Week 2 post rHuEpo | Week 4 post rHuEpo | |
| Systolic blood pressure (mmHg) | 128 (9) | 126 (7) | 126 (6) | 126 (7) | 124 (7) |
| Diastolic blood pressure (mmHg) | 70 (8) | 69 (8) | 69 (7) | 70 (7) | 69 (8) |
| Heart rate (beats•min−1) | 62 (8) | 60 (7) | 60 (7) | 59 (7) | 61 (8) |
N = 19. Values are means (SD). No significant differences compared to baseline values were observed.
Table 2 illustrates the changes in the main haematological parameters for five key stages of the study. Relative to baseline values, both haematocrit and haemoglobin concentration gradually increased throughout the rHuEpo administration to reach a maximum approximately one week after the last injection (p<0.001) and remained significantly elevated 4 weeks post rHuEpo administration (p<0.001). Reticulocyte percent increased rapidly after the first two weeks of injections (p<0.001). Nadir with a very low inter-subject variation was reached approximately two weeks after injections ceased, which was significantly lower compared with baseline values (p<0.001). Body mass did not change during the study. rHuEpo administration did not induce any significant changes in resting systolic and diastolic blood pressure as well as in resting heart rate.
Haemoglobin Mass and Blood Volumes (Tables 2, 4 and Fig. 1)
Table 4. Changes in haemoglobin mass, blood, red cell and plasma volume during and for 4 weeks post rHuEpo administration.
| rHuEpo | Post rHuEpo | |
| Haemoglobin mass (g•kg−1•wk−1) | 0.54 (0.46 to 0.63)* | −0.53 (−0.68 to −0.38)* |
| Blood volume (mL•kg−1•wk−1) | 0.34 (−0.36 to 1.05) | −0.60 (−1.20 to 0.00) |
| Red cell volume (mL•kg−1•wk−1) | 1.80 (1.50 to 2.10)* | −1.77 (−2.22 to −1.33)* |
| Plasma volume (mL•kg−1•wk−1) | −1.30 (−1.94 to −0.66)* | 0.50 (0.10 to 0.90)* |
N = 19. Values are means (95% confidence interval). Significant changes are indicated by *P<0.05.
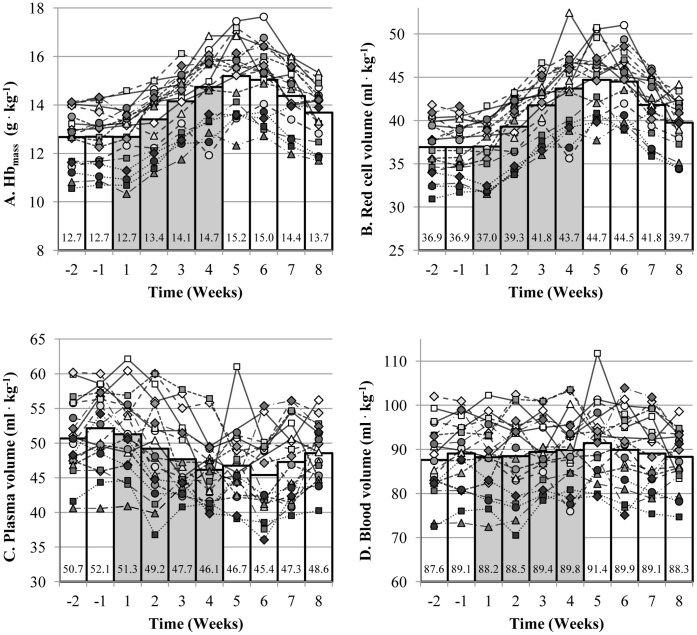
Figure 1. Individual changes in Hbmass (A), red cell (B), plasma (C) and blood (D) volume.
Each line corresponds to one subject and each symbol corresponds to the same subject in all figures. Individual mean value was calculated when more than one blood sample was collected per week. The bar graphs represent the mean values of the 19 subjects. The grey bars represent the 4 weeks of rHuEpo administration.
Relative to baseline, Hbmass and plasma volume were significantly increased (p<0.001) and decreased (p = 0.004) at the end of the rHuEpo administration, respectively. Hbmass and red cell volume gradually increased by approximately 40 g•wk−1 (p<0.001) and 135 mL•wk−1 (p<0.001), respectively (Fig. 1A and 1B). Plasma volume decreased significantly by approximately 100 mL•wk−1 (p<0.001) (Fig. 1C) while blood volume remained relatively unchanged (p = 0.32) (Fig. 1D). From week 1 to week 4 post rHuEpo administration, the rate of decrease in Hbmass and red cell volume toward baseline values was similar to that of the increase during administration (Fig. 1A and 1B) but both Hbmass and red cell volume were still significantly elevated 4 weeks post administration compared to baseline (p<0.001). Plasma volume was restored to pre-injection levels 4 weeks post administration (p = 0.108) (Fig. 1C).
Carboxyhaemoglobin (Table 2 and Fig. 2)
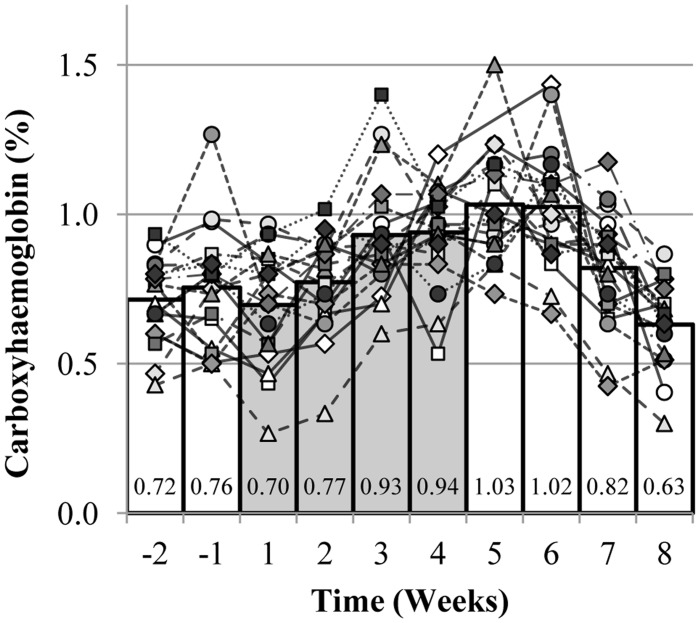
Figure 2. Individual changes in carboxyhaemoglobin.
Each line corresponds to one subject. Individual mean value was calculated when more than one blood sample was collected per week. The bar graphs represent the mean values of the 19 subjects. The grey bars represent the 4 weeks of rHuEpo administration.
Relative to baseline values, basal percentage of carboxyhaemoglobin prior to the CO-rebreathing procedure was significantly elevated at the end of the rHuEpo administration (p<0.001). Percentage of carboxyhaemoglobin returned to pre-injection values 4 weeks post rHuEpo administration.
Discussion
The aim of this study was to investigate the time course of Hbmass and related blood parameters as well as changes in running 3,000 m time trial performance following 4 weeks of rHuEpo administration in order to determine whether the augmented 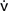 O2 max would translate into actual improvements in running performance in the field. The main findings of this study are that, relative to baseline, running performance was significantly improved following 4 weeks of rHuEpo administration and remained significantly elevated 4 weeks post administration and that these performance effects coincided with significantly rHuEpo-induced elevated
O2 max would translate into actual improvements in running performance in the field. The main findings of this study are that, relative to baseline, running performance was significantly improved following 4 weeks of rHuEpo administration and remained significantly elevated 4 weeks post administration and that these performance effects coincided with significantly rHuEpo-induced elevated 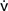 O2 max and Hbmass.
O2 max and Hbmass.
Running Performance
The effects of rHuEpo on exercise performance have principally been investigated using 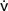 O2 max tests. Despite slight differences in the frequency of injections and in the dosage used, rHuEpo administration for 4 to 6 weeks in healthy subjects has been shown to increase
O2 max tests. Despite slight differences in the frequency of injections and in the dosage used, rHuEpo administration for 4 to 6 weeks in healthy subjects has been shown to increase 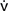 O2 max by approximately 8%, which was confirmed by our observations [23], [24], [25], [26], [27], [28], [29]. However,
O2 max by approximately 8%, which was confirmed by our observations [23], [24], [25], [26], [27], [28], [29]. However, 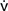 O2 max considerably varies among professional athletes [30], [31] and
O2 max considerably varies among professional athletes [30], [31] and 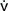 O2 max tests alone may be of little help in determining and ranking exercise performance when athletes of similar endurance ability are compared [12], [13], [14], [15]. Time trial performance more closely reproduces competition conditions and more importantly allows the subjects to choose their own pace. It has previously been observed that blood doping via one unit of autologous blood transfusion, which increased the haematocrit by 5%, improved 10,000 m running performance by approximately one minute in 6 highly trained male distance runners, which corresponded to a ∼3% improvement [32]. However, to our knowledge, no study has measured running time trial performance in order to determine whether the augmented
O2 max tests alone may be of little help in determining and ranking exercise performance when athletes of similar endurance ability are compared [12], [13], [14], [15]. Time trial performance more closely reproduces competition conditions and more importantly allows the subjects to choose their own pace. It has previously been observed that blood doping via one unit of autologous blood transfusion, which increased the haematocrit by 5%, improved 10,000 m running performance by approximately one minute in 6 highly trained male distance runners, which corresponded to a ∼3% improvement [32]. However, to our knowledge, no study has measured running time trial performance in order to determine whether the augmented 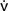 O2 max after rHuEpo administration would translate into actual improvements in performance in the field.
O2 max after rHuEpo administration would translate into actual improvements in performance in the field.
Relative to baseline, running performance improved post rHuEpo administration and remained enhanced 4 weeks after administration by approximately 6% and 3%, respectively. Following rHuEpo administration, subjects were able to maintain a faster pace throughout the 3,000 m time trial compared to baseline. While the augmented oxygen transport capacity illustrated by the increase in Hbmass can explain the increase in 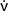 O2 max post rHuEpo administration, it cannot really explain the significant improvement in running performance. Indeed, as oxygen supply and demand are adequately matched during submaximal exercise in healthy men, the rHuEpo-induced augmented oxygen transport capacity cannot be the reason of the improvement in time trial running performance observed in our study [12], [17]. In addition, failure to observe a strong correlation between individual changes in
O2 max post rHuEpo administration, it cannot really explain the significant improvement in running performance. Indeed, as oxygen supply and demand are adequately matched during submaximal exercise in healthy men, the rHuEpo-induced augmented oxygen transport capacity cannot be the reason of the improvement in time trial running performance observed in our study [12], [17]. In addition, failure to observe a strong correlation between individual changes in 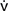 O2 max, Hbmass and running performance may imply that rHuEpo administration improves submaximal exercise performance by other mechanisms, although it could also be partly explained by the limited sensitivity of the methods of measurement and by the limitations of the study (see next paragraph). Other mechanisms than the increase in oxygen transport capacity have been proposed to explain the effects of rHuEpo on exercise performance [33], [34]. For instance, it has been demonstrated that self-reported mood, cognitive function and perceived physical condition were improved following rHuEpo administration [35], [36]. rHuEpo may indeed exert effects in nonhematopoietic tissues including the brain [37], [38], [39]. Admittedly, two different studies conducted by the same research group did not reveal any measurable nonhematopoietic ergogenic effect of rHuEpo on exercise performance and the authors therefore concluded that the increased oxygen carrying capacity is the main if not the only reason why rHuEpo enhances exercise performance in healthy men [40], [41]. On the other hand, the central governor model can give an alternative view [12]. As the perception of effort of the subjects did not change and because the subjects were not limited by an inadequate oxygen supply neither before nor after administration as they ran at submaximal levels, the central governor model would argue that the subjects ran faster after rHuEpo administration because of greater recruitment of motor units allowed by a control mechanism of feed-forward and feedback to the brain [12; especially Fig. 2 of that paper].
O2 max, Hbmass and running performance may imply that rHuEpo administration improves submaximal exercise performance by other mechanisms, although it could also be partly explained by the limited sensitivity of the methods of measurement and by the limitations of the study (see next paragraph). Other mechanisms than the increase in oxygen transport capacity have been proposed to explain the effects of rHuEpo on exercise performance [33], [34]. For instance, it has been demonstrated that self-reported mood, cognitive function and perceived physical condition were improved following rHuEpo administration [35], [36]. rHuEpo may indeed exert effects in nonhematopoietic tissues including the brain [37], [38], [39]. Admittedly, two different studies conducted by the same research group did not reveal any measurable nonhematopoietic ergogenic effect of rHuEpo on exercise performance and the authors therefore concluded that the increased oxygen carrying capacity is the main if not the only reason why rHuEpo enhances exercise performance in healthy men [40], [41]. On the other hand, the central governor model can give an alternative view [12]. As the perception of effort of the subjects did not change and because the subjects were not limited by an inadequate oxygen supply neither before nor after administration as they ran at submaximal levels, the central governor model would argue that the subjects ran faster after rHuEpo administration because of greater recruitment of motor units allowed by a control mechanism of feed-forward and feedback to the brain [12; especially Fig. 2 of that paper].
This study was part of a larger research project funded by WADA and was designed primarily for other purposes. The present study was not blinded, did not include a control group and the subjects, yet involved in endurance activities, were not all runners. As such, we were unable to adequately assess changes in the training load during the study due to the absence of a placebo group and the heterogeneity of sporting/training activity of the subjects. It cannot totally be excluded that the performance enhancements observed may, therefore, be partly due to placebo effect, to an increase in the training load and to an improvement in pacing strategy especially for the non-runners. It was however reassuring that the typical error of measurement for the two time trials conducted on each phase was low and that the amplitude of the improvement observed was well above the confidence interval.
Haemoglobin Mass and Haematological Parameters
The level of Hbmass prior to the intervention reflected the regular participation in endurance activities of the subjects [42]. Following the first week of rHuEpo administration, Hbmass gradually increased and reached its maximum one week after the last injection. The weekly increase of approximately 40 g is equivalent to the haemoglobin contained in one bag of 450 mL stored blood [43]. The delay of approximately one week between rHuEpo injections and the observed responses in Hbmass and red cell volume is explained by the reticulocyte maturation time of 1–4 days [44]. While normal human red blood cells can theoretically persist in the circulation for approximately 17 weeks [5], Hbmass and red cell volume started to decrease toward baseline values from approximately the second week post rHuEpo administration with a similar rate to that of the increase during administration. This decrease in Hbmass and red cell volume post rHuEpo administration entailed the haemolysis of circulating red blood cells and therefore the release of heme from heamoglobin [45]. The heme degradation is catalyzed by heme oxygenase and generates one molecule of CO per molecule of heme oxidized [46]. The CO produced by heme metabolism represents more than 85% of the endogenous CO production in healthy non-smoking subjects [46]. Although measurement of blood carboxyhaemoglobin is influenced by environmental factors such as air pollution [5], carboxyhaemoglobin can be used as an index of haemolysis [47]. Indeed, basal carboxyhaemoglobin levels increased at the end of the rHuEpo administration and remained elevated for 2 weeks post administration reflecting accelerated haemolysis. Despite being only an indirect marker, these results are in agreement with the negative regulation of red cell mass by neocytolysis when supraphysiologic red cell mass and endogenous erythropoietin suppression occur (induced by rHuEpo administration, for instance) [7], [8]. In addition, this study confirmed that 4 weeks of rHuEpo administration sufficient to induce a significant increase in haematocrit did not increase resting blood pressure in healthy subjects when blood pressure was assessed by standard techniques compared to intra-arterial pressure transducers [40]. This study also demonstrates that the optimized CO-rebreathing method performed weekly for ten weeks is safe and well tolerated by healthy subjects.
Plasma Volume
Apart from confirming previous observations that rHuEpo administration increases haemoglobin concentration by increasing Hbmass and by decreasing plasma volume [2], [3], the present study showed that plasma volume returned to pre-injection values only approximately 4 weeks post rHuEpo administration. Previous studies have concluded that plasma volume returned rather rapidly toward baseline value thus restoring pre-administration haemoglobin concentration values without affecting red cell volume [3], [4]. Olsen et al. [3] used injections of 5000 IU rHuEpo administered every second day for 2 weeks and thereafter only once a week for the 2 consecutive weeks to demonstrate that rHuEpo down-regulates the renin-angiotensin-aldosterone system as well as the rate of proximal renal tubular reabsorption and glomerular filtration. The longer and more contrasted rHuEpo administration regimen used in the present study with a more abrupt erythropoiesis stimulation termination may have induced a more profound down-regulation of the renin-angiotensin-aldosterone system and therefore, may explain why plasma volume had not been restored until 4 weeks after rHuEpo administration. In agreement with previous findings which used a similar rHuEpo regimen than the present study, pre-injection haemoglobin concentration values were not restored 4 weeks post administration [28].
In conclusion, relative to baseline, running performance was significantly improved following 4 weeks of rHuEpo administration in trained men and remained significantly elevated 4 weeks after administration by approximately 6% and 3%, respectively. These performance effects coincided with significantly rHuEpo-induced elevated 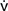 O2 max and Hbmass.
O2 max and Hbmass.
Acknowledgments
The authors wish to specially thank the subjects for their participation and cooperation. We would like also to thank students who helped with data collection as well as the members of the WADA Project (08C19YP) Steering Committee and especially Professor T.D. Noakes, Dr. S. Padmanabhan and Dr. G. Gmeiner.
Funding Statement
This study was part of a larger research project supported and funded by World Anti-Doping Agency (08C19YP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jelkmann W (2011) Regulation of erythropoietin production. J Physiol 589: 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, et al. (2007) Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol 578: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen NV, Aachmann-Andersen NJ, Oturai P, Munch-Andersen T, Borno A, et al. (2011) Erythropoietin down-regulates proximal renal tubular reabsorption and causes a fall in glomerular filtration rate in humans. J Physiol 589: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lundby C, Olsen NV (2011) Effects of recombinant human erythropoietin in normal humans. J Physiol 589: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franco RS (2009) The measurement and importance of red cell survival. Am J Hematol 84: 109–114. [DOI] [PubMed] [Google Scholar]
- 6. Rice L, Ruiz W, Driscoll T, Whitley CE, Tapia R, et al. (2001) Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med 134: 652–656. [DOI] [PubMed] [Google Scholar]
- 7. Rice L, Alfrey CP (2005) The negative regulation of red cell mass by neocytolysis: physiologic and pathophysiologic manifestations. Cell Physiol Biochem 15: 245–250. [DOI] [PubMed] [Google Scholar]
- 8. Chang CC, Chen Y, Modi K, Awar O, Alfrey C, et al. (2009) Changes of red blood cell surface markers in a blood doping model of neocytolysis. J Investig Med 57: 650–654. [DOI] [PubMed] [Google Scholar]
- 9. Alfrey CP, Rice L, Udden MM, Driscoll TB (1997) Neocytolysis: physiological down-regulator of red-cell mass. Lancet 349: 1389–1390. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt W, Prommer N (2005) The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95: 486–495. [DOI] [PubMed] [Google Scholar]
- 11.Durussel J, Ross R, Raj Kodi P, Daskalaki E, Pantazis T, et al.. (2011) Precision of the optimized carbon monoxide rebreathing method to determine total haemoglobin mass and blood volume. Eur J Sport Sci.
- 12. Noakes TD (2008) Testing for maximum oxygen consumption has produced a brainless model of human exercise performance. Br J Sports Med 42: 551–555. [DOI] [PubMed] [Google Scholar]
- 13. Shephard RJ (2009) Is the measurement of maximal oxygen intake passe? Br J Sports Med 43: 83–85. [DOI] [PubMed] [Google Scholar]
- 14. Costill DL, Thomason H, Roberts E (1973) Fractional utilization of the aerobic capacity during distance running. Med Sci Sports 5: 248–252. [PubMed] [Google Scholar]
- 15. Levine BD, Stray-Gundersen J (1992) A practical approach to altitude training: where to live and train for optimal performance enhancement. International journal of sports medicine 13 Suppl 1S209–212. [DOI] [PubMed] [Google Scholar]
- 16. Thomsen JJ, Rentsch RL, Robach P, Calbet JA, Boushel R, et al. (2007) Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol 101: 481–486. [DOI] [PubMed] [Google Scholar]
- 17. Noakes TD (2008) Mechanism by which rHuEPO improves submaximal exercise performance. Eur J Appl Physiol 103: 485. [DOI] [PubMed] [Google Scholar]
- 18. Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports medicine 40: 1–25. [DOI] [PubMed] [Google Scholar]
- 19. Laursen PB, Francis GT, Abbiss CR, Newton MJ, Nosaka K (2007) Reliability of time-to-exhaustion versus time-trial running tests in runners. Med Sci Sports Exerc 39: 1374–1379. [DOI] [PubMed] [Google Scholar]
- 20. Ahlgrim C, Pottgiesser T, Robinson N, Sottas PE, Ruecker G, et al. (2010) Are 10 min of seating enough to guarantee stable haemoglobin and haematocrit readings for the athlete’s biological passport? Int J Lab Hematol 32: 1–6. [DOI] [PubMed] [Google Scholar]
- 21. Kirkeberg JM, Dalleck LC, Kamphoff CS, Pettitt RW (2011) Validity of 3 protocols for verifying VO2 max. Int J Sports Med 32: 266–270. [DOI] [PubMed] [Google Scholar]
- 22.Pettitt RW, Clark IE, Ebner SM, Sedgeman DT, Murray SR (2012) Gas Exchange Threshold and VO2max Testing for Athletes: An Update. J Strength Cond Res. [DOI] [PubMed]
- 23. Russell G, Gore CJ, Ashenden MJ, Parisotto R, Hahn AG (2002) Effects of prolonged low doses of recombinant human erythropoietin during submaximal and maximal exercise. Eur J Appl Physiol 86: 442–449. [DOI] [PubMed] [Google Scholar]
- 24. Audran M, Gareau R, Matecki S, Durand F, Chenard C, et al. (1999) Effects of erythropoietin administration in training athletes and possible indirect detection in doping control. Med Sci Sports Exerc 31: 639–645. [DOI] [PubMed] [Google Scholar]
- 25. Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, et al. (2000) Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc 32: 1238–1243. [DOI] [PubMed] [Google Scholar]
- 26. Berglund B, Ekblom B (1991) Effect of recombinant human erythropoietin treatment on blood pressure and some haematological parameters in healthy men. J Intern Med 229: 125–130. [DOI] [PubMed] [Google Scholar]
- 27. Wilkerson DP, Rittweger J, Berger NJ, Naish PF, Jones AM (2005) Influence of recombinant human erythropoietin treatment on pulmonary O2 uptake kinetics during exercise in humans. J Physiol 568: 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parisotto R, Gore CJ, Emslie KR, Ashenden MJ, Brugnara C, et al. (2000) A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica 85: 564–572. [PubMed] [Google Scholar]
- 29. Connes P, Perrey S, Varray A, Prefaut C, Caillaud C (2003) Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch 447: 231–238. [DOI] [PubMed] [Google Scholar]
- 30. Mujika I, Padilla S (2001) Physiological and performance characteristics of male professional road cyclists. Sports Med 31: 479–487. [DOI] [PubMed] [Google Scholar]
- 31. Lucia A, Hoyos J, Perez M, Santalla A, Chicharro JL (2002) Inverse relationship between VO2max and economy/efficiency in world-class cyclists. Med Sci Sports Exerc 34: 2079–2084. [DOI] [PubMed] [Google Scholar]
- 32. Brien AJ, Simon TL (1987) The effects of red blood cell infusion on 10-km race time. JAMA 257: 2761–2765. [PubMed] [Google Scholar]
- 33.Boning D, Maassen N, Pries A (2008) No proof for augmented arterial oxygen content as only factor influencing exercise capacity after Epo doping. J Appl Physiol 105: 1988; author reply 1989. [DOI] [PubMed]
- 34. Boning D, Maassen N, Pries A (2011) The hematocrit paradox–how does blood doping really work? Int J Sports Med 32: 242–246. [DOI] [PubMed] [Google Scholar]
- 35. Ninot G, Connes P, Caillaud C (2006) Effects of recombinant human erythropoietin injections on physical self in endurance athletes. J Sports Sci 24: 383–391. [DOI] [PubMed] [Google Scholar]
- 36. Miskowiak K, Inkster B, Selvaraj S, Wise R, Goodwin GM, et al. (2008) Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology 33: 611–618. [DOI] [PubMed] [Google Scholar]
- 37.Jelkmann W, Depping R, Metzen E (2009) Nonhematopoietic effects of erythropoiesis-stimulating agents. In: Elliott SG, Foote M, Molineux G, editors. Erythropoietin, Erythropoietic Factores, and Erythropoiesis. Basel: Birkhäuser. 299–317.
- 38. Brines M, Cerami A (2005) Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci 6: 484–494. [DOI] [PubMed] [Google Scholar]
- 39. Ehrenreich H, Bartels C, Sargin D, Stawicki S, Krampe H (2008) Recombinant human erythropoietin in the treatment of human brain disease: focus on cognition. J Ren Nutr 18: 146–153. [DOI] [PubMed] [Google Scholar]
- 40. Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, et al. (2008) Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol 105: 581–587. [DOI] [PubMed] [Google Scholar]
- 41. Rasmussen P, Foged EM, Krogh-Madsen R, Nielsen J, Nielsen TR, et al. (2010) Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol 109: 476–483. [DOI] [PubMed] [Google Scholar]
- 42. Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmid A, et al. (2001) Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med 22: 504–512. [DOI] [PubMed] [Google Scholar]
- 43.Morkeberg J, Sharpe K, Belhage B, Damsgaard R, Schmidt W, et al.. (2009) Detecting autologous blood transfusions: a comparison of three passport approaches and four blood markers. Scand J Med Sci Sports: 1–9. [DOI] [PubMed]
- 44. Banfi G (2008) Reticulocytes in sports medicine. Sports Med 38: 187–211. [DOI] [PubMed] [Google Scholar]
- 45. Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G (2010) Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryter SW, Otterbein LE (2004) Carbon monoxide in biology and medicine. Bioessays 26: 270–280. [DOI] [PubMed] [Google Scholar]
- 47. Engel RR, Rodkey FL, Krill CE Jr (1971) Carboxyhemoglobin levels as an index of hemolysis. Pediatrics 47: 723–730. [PubMed] [Google Scholar]