Abstract
We investigated the effect of dasatinib and sunitinib on tyrosine kinase (TK) signaling, caveolin-1 (Cav-1) expression and secretion and proliferation of PC-3 and DU145 prostate cancer cells in vitro and in vivo. Treatment of both cell lines with either dasatinib or sunitinib reduced phosphorylation of PDGFR, VEGFR2, Akt, FAK, Src (dasatinib only) and Cav-1, and reduced cellular and secreted levels of Cav-1. Both agents dose-dependently inhibited proliferation of these cells. In PC-3 and DU145 subcutaneous xenografts, treatment with dasatinib, sunitinib or anti-Cav-1 antibody (Ab) alone produced significant tumor regression compared with that by vehicle or IgG alone. Combined dasatinib and anti-Cav-1 Ab treatment or sunitinib and anti-Cav-1 Ab produced greater tumor regression than either treatment alone. Serum Cav-1 levels were lower in dasatinib- and sunitinib-treated mice than they were in vehicle-treated mice, and correlated positively with tumor growth in dasatinib- and sunitinib-treated groups (r = 0.48, p = 0.031; r = 0.554, p = 0.0065, respectively), compared with vehicle controls. Cav-1 knockdown, in combination with dasatinib or sunitinib treatment in PC-3 cells, caused a greater reduction in the phosphorylation of PDGFR-β and VEGFR2, and expression and secretion of PDGF-B and VEGF-A than that in PC-3 cells treated with dasatinib or sunitinib alone in control siRNA cells, suggesting that Cav-1 is involved in an autocrine pathway that is affected by these drugs. Overall, our results suggest a role for Cav-1 as a biomarker of response to both dasatinib and sunitinib treatment and as a therapeutic target in prostate cancer.
Keywords: caveolin-1, tyrosine kinase inhibitor, prostate cancer, biomarkers
Introduction
Caveolin-1 (Cav-1) is a major structural component of the caveolae, which are specialized plasma membrane invaginations that are involved in multiple cellular processes, such as molecular transport, cell adhesion and signal transduction.1,2 Cav-1 exerts various biologic functions through protein-protein interactions. Specific proteins, such as receptor tyrosine kinases (RTKs), serine/threonine kinases, phospholipases, G protein-coupled receptors and Src family kinases (SFKs), are localized in lipid rafts and caveolar membranes, where they interact with Cav-1 through the Cav-1 scaffolding domain (CSD). CSD-mediated activities result in the generation of platforms for compartmentalization of discrete signaling events.3
The role of Cav-1 in tumorigenesis is complex and depends on the cell type and biologic context. Under some conditions, Cav-1 may suppress tumorigenesis.4 However, Cav-1 upregulation is also associated with and contributes to malignant progression of multiple malignancies, including prostate cancer (PCa).3-7
Androgen-deprivation therapy is a standard of care treatment for PCa and efficiently controls the growth of androgen-dependent tumors. However, this therapy is limited in duration of response and has numerous side effects.8 When PCa progresses beyond the confines of the prostate gland and metastasizes to distant sites (predominantly bone marrow), the disease is very difficult to control. Results of clinical studies have also indicated that docetaxel chemotherapy provides only modest survival benefits in castrate-resistant PCa.9
Previous studies showed that Cav-1 expression is stimulated by testosterone and by multiple growth factors (GFs) that are known to promote the development and progression of PCa.10,11 Cav-1 overexpression leads to promiscuous binding of Cav-1 to multiple signaling molecules in the cancer tyrosine-kinase (TK) regulatory network, including vascular endothelial GF receptor 2 (VEGFR2), platelet-derived GF receptor α/β (PDGFRα/β), Src, protein phosphatase 1/protein phosphatase 2A (PP1/PP2A, a negative regulator of Akt) and phospholipase Cγ1 (PLCγ1), through CSD-CSD binding-site interactions.12,13 These interactions increase PCa cell survival.12 We have also shown that in PCa cells, a positive-feedback loop is established in which VEGF, transforming GF-β1 (TGF-β1) and fibroblast GF-2 (FGF2) upregulate Cav-1 expression, which, in turn, leads to increased levels of VEGF, TGF-β1 and FGF2 mRNA and protein, resulting in enhanced invasion activities (i.e., migration, motility) of PCa cells.10 In the same study, we found that Akt-mediated Cav-1-enhanced mRNA stability is a major mechanism for the upregulation of these cancer-promoting GFs.
A critically important characteristic of many androgen-insensitive PCa cell lines is secretion of biologically active Cav-1 protein. PCa cell-derived secreted Cav-1 can promote PCa-cell viability through antiapoptotic activities and clonal growth in vitro, similar to those observed following enforced expression of Cav-1 within the cells.12,14-16 We recently showed that recombinant Cav-1 protein is taken up by PCa cells and tumor-associated endothelial cells and promotes angiogenesis by activating Akt- and/or nitric oxide synthase-mediated signaling.17 Additionally, we demonstrated that Cav-1 regulates ligand-stimulated RTK signaling (VEGF/VEGFR2) and downstream biologic activities in PCa and endothelial cells.13
Dasatinib is an orally administrated inhibitor of Bcr-Abl kinase and SFK proteins that is FDA approved as a second-line treatment for chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Recently, dasatinib was shown to inhibit proliferation, cell adhesion, migration and invasion of PCa cells in vitro.18,19
Sunitinib is an oral multi-targeted RTK inhibitor that selectively and potently inhibits VEGFRs 1, 2 and 3; PDGFRs α and β; the stem cell factor receptor KIT; the FMS-like TK-3 receptor (FLT3) and the glial cell line-derived neurotrophic factor receptor “rearranged during transfection.”20-24 Sunitinib has also demonstrated antiangiogenic and antitumor activities in various solid-tumor xenograft models, including colon, renal, breast, lung and PCa.24
In this study, we evaluated the effects of dasatinib and sunitinib on TK signaling, Cav-1 expression and secretion and cell growth in vitro and in vivo. Our results demonstrate that PCa cell-secreted Cav-1 is a potential biomarker for response to dasatinib and sunitinib and a therapeutic target for PCa.
Materials and Methods
Cell lines, cell culturing, reagents and antibodies
The hormone-refractory PCa cell lines PC-3 and DU145, obtained from the American Type Culture Collection, were cultured in RPMI 1640 medium supplemented with 10% FBS (Atlanta Biological). Dasatinib and sunitinib, purchased from LKT Laboratories, Inc., were dissolved in dimethyl sulfoxide (Sigma-Aldrich) for in vitro use and in citrate buffer (pH 3.0) for in vivo application.
Anti-Cav-1, anti-VEGF-A, anti-PDGF-B, anti-VEGFR2, anti-PDGFRβ, anti–phospho-PDGFRβ (Y857) and anti-VEGFR2 antibodies were purchased from Santa Cruz Biotechnology; anti-phospho-VEGFR2 (Y951) and anti-phospho-FAK (Y861) were purchased from Invitrogen., anti-Src, anti-phospho-Src (Y419) and anti-phospho-Akt (S473) antibodies were purchased from Cell Signaling Technology, and anti-phospho-Cav-1 and anti-Akt antibodies were purchased from BD Biosciences.
RNA interference
Knockdown of Cav-1 in PC-3 cells was achieved by transient transfection of the cells with a pool of Cav-1-specific siRNA (Invitrogen), and a pool of non-targeting siRNA (NCsi) (Invitrogen), as control, by using Lipofectamine RNAiMax transfection reagent (Invitrogen). Cells were treated or used for further analysis after 48 h of transfection.
Proliferation assay
PC-3 and DU145 cells in 0.1 ml of medium were plated in 96-well plates at a concentration of 3–5 × 103 cells/well. Dasatinib and sunitinib were added at concentrations ranging from 0.05–5 μM for dasatinib and 0.2–20 μM for sunitinib, and cell proliferation was determined after 24, 48 and 72 h by using an MTS assay (Promega) according to the manufacturer’s instructions.
Western blot analysis
Cells were treated separately with the two drugs for 2 h for cell extract or 24 h in serum-free medium for condition medium (CM) collection. Cellular extracts were prepared by washing the cells with ice-cold PBS and lysing them in ice-cold RIPA buffer (Cell Signaling) containing protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails I and II (Sigma-Aldrich). The protein lysates or CM were boiled in SDS sample buffer and separated over 4–15% SDS-PAGE gels (BioRad). The resulting separated proteins were transferred onto a nitrocellulose membrane and blotted with specific antibodies; antibody detection was performed by using a chemiluminescence-based detection system (Pierce Biotechnology). Quantification was performed using UN-SCAN-IT gel analysis software and data were expressed as the ratio units of either phosphorylated protein per total protein or total protein per loading control protein β-actin, relative to that in the untreated controls in lane 1.
PC-3 and DU145 xenografts and treatments
Ten-week-old male athymic nu/nu nude mice were injected subcutaneously in the right flank with 5 × 106 cancer cells suspended in Matrigel (BD Biosciences). Tumors were allowed to develop, and tumor growth was measured by caliper twice a week. Tumor volume was calculated according to the formula V = (a2 × b)/2, in which a and b are the minimal and maximal diameters, respectively, in millimeters. Animals were examined daily and body weight and tumor size recorded twice weekly. When tumor volumes reached the range of 150–200 mm3, mice were allocated into five treatment groups of eight to 10 animals each so that all groups had approximately the same mean tumor volume.
For mice bearing PC-3 tumors, treatments consisted of vehicle alone (controls; citrate buffer (100μl q.d., p.o.); immunoglobulin G (IgG; 10 μg q.o.d., i.p.); anti-Cav-1 antibody (10 μg q.o.d., i.p.); dasatinib (15 mg/kg q.d., p.o.) and combined dasatinib (15 mg/kg q.d., p.o.) plus anti-Cav-1 antibody (10 μg q.o.d., i.p.).
For mice with DU145 tumors, treatments consisted of vehicle alone (controls; citrate buffer 100 μl q.d.p.o.); IgG (10 μg q.o.d., i.p.); anti-Cav-1 antibody (10 μg q.o.d., i.p.); sunitinib (10 mg/kg q.d., p.o.) and combined sunitinib (10 mg/kg q.d., p.o.) plus anti-Cav-1 antibody (10 μg q.o.d., i.p.).
Treatments continued for 21 d, and tumor volumes were calculated and recorded as described above. Mice were euthanized, and their tumor tissues and serum were collected for analysis.
Serum Cav-1 assay
The serum concentrations of Cav-1 in the control mice, those treated with dasatinib and those treated with sunitinib were determined according to the previously described sandwich ELISA assay protocol.25 Concentrations are reported as ng/ml.
Statistical analyses
ANOVA (analysis of variance) software (unpaired t-test) was used to compare the tumor weights and volumes as well as serum Cav-1 concentrations between groups. Pearson’s correlation coefficient testing was used to identify any correlation between serum Cav-1 concentrations and tumor weight. All analyses were performed by using Statview 5.0 software (SAS Institute). p < 0.05 was considered statistically significant.
Results
Dasatinib and sunitinib inhibit RTK/TK signaling activities and regulate Cav-1 expression and secretion in PCa cell lines in vitro
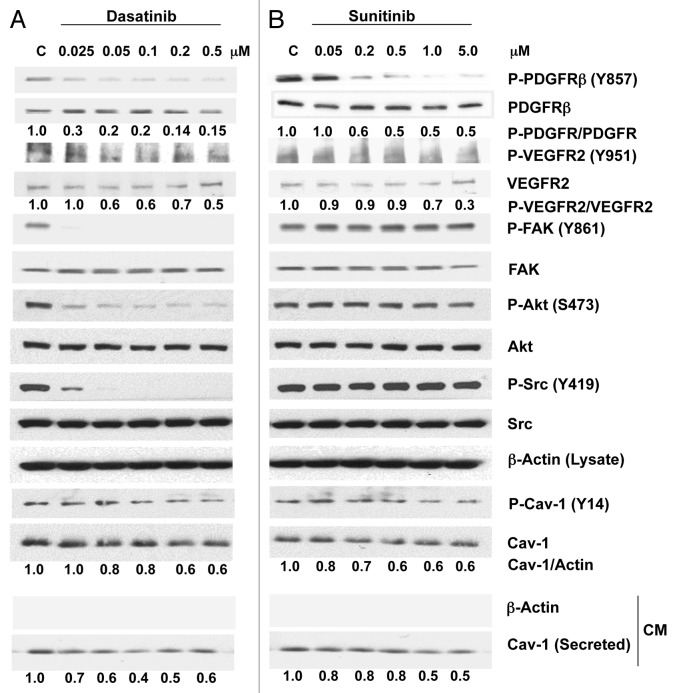
To investigate whether either dasatinib or sunitinib treatment of PCa cells inhibits specific signaling activities and regulates the expression and secretion of Cav-1, we treated PC-3 cells with each drug separately at different concentrations for 2 h. At concentrations ranging from 0.025–0.5 μM, dasatinib caused a marked reduction in the phosphorylation of PDGFRβ (Y857) and moderate reduction in the phosphorylation of VEGFR2 (Y951) (Fig. 1A). As expected, dasatinib also considerably reduced the phosphorylation of Src (Y419) and its downstream target, FAK (Y861). Similarly, dasatinib produced a marked dose-dependent reduction in Akt (S473) phosphorylation. Dasatinib treatment also considerably reduced the phosphorylation of Cav-1 (Y14) in a dose-dependent manner. We further investigated the effect of dasatinib on Cav-1 secretion by analyzing Cav-1 expression in the conditioned medium from PC-3 cells treated with dasatinib for 24 h. It was interesting that Cav-1 secretion was reduced dramatically by dasatinib (60% at 0.1 μM) (Fig. 1A).
Figure 1. Effects of dasatinib and sunitinib on Cav-1 expression and secretion and on TK signaling in PC-3 cells. Dasatinib (A) and sunitinib (B) treatment of PC-3 cells resulted in a dose-dependent decrease in phosphorylation of PDGFRβ, VEGFR2, Akt and Cav-1. Dasatinib but not sunitinib also reduced the phosphorylation of FAK and Src. Both dasatinib and sunitinib dose-dependently reduced the expression and secretion of Cav-1.
Sunitinib treatment of PC-3 cells also caused a reduction in the phosphorylation of PDGFRβ (Y857) and VEGFR2 (Y951) at relatively high sunitinib concentrations. Phosphorylation of Src, Akt and FAK was not reduced by sunitinib treatment, even at the highest concentration, 5.0 μM (Fig. 1B). Sunitinib treatment of PC-3 cells at concentrations ranging from 0.05–5.0 μM reduced the phosphorylation of Cav-1 (Y14) in a dose-dependent manner and slightly reduced total Cav-1 expression (40%). Finally, Cav-1 secretion was reduced significantly (50%) at sunitinib concentrations of 1.0–5.0 μM (Fig. 1B).
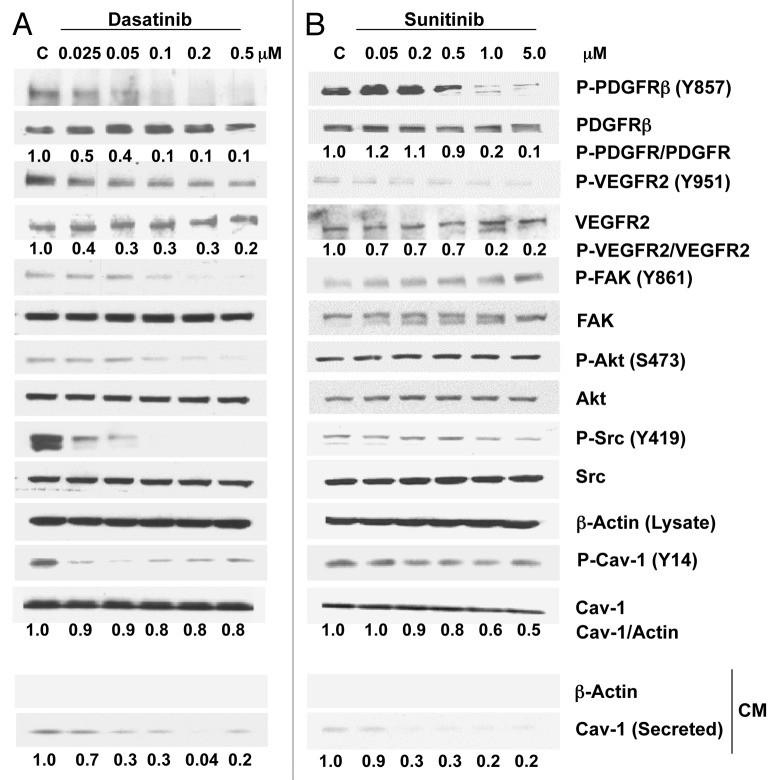
In DU145 cells, treatment with dasatinib (0.025–0.5 μM) caused a dose-dependent marked reduction in the phosphorylation of PDGFRβ (Y857) (90% at 0.1 μM), VEGFR2 (Y951) (70% at 0.1 μM), FAK (Y861), Akt (S473) and Src (Y419) (Fig. 2A). A substantial, although not dose-dependent, reduction was observed at 0.025 μM for Cav-1 (Y14) (Fig. 2A). Dasatinib did not affect Cav-1 cellular expression levels in these cells, but its secretion was reduced dramatically (70%) at doses as low as 0.05 μM.
Figure 2. Effects of dasatinib and sunitinib on Cav-1 expression and secretion and on TK signaling in DU145 cells. Dasatinib (A) and sunitinib (B) treatment of DU145 cells resulted in a dose-dependent decrease in phosphorylation of PDGFRβ, VEGFR2, Src and Cav-1. Dasatinib, but not sunitinib, also reduced the phosphorylation of FAK and Akt. Both dasatinib and sunitinib dose-dependently reduced significantly the secretion of Cav-1.
Sunitinib treatment of DU145 cells caused notable dose-dependent reductions in the phosphorylation of PDGFRβ (Y857) (80% at 1.0 μM), VEGFR2 (Y951) (80% at 1.0μM) and Cav-1 (Y14) and a modest reduction in the phosphorylation of Src (Y419). Sunitinib had no effect on the phosphorylation of FAK and Akt. Similarly to that observed with dasatinib treatment, Cav-1 secretion was also reduced significantly (80%) by sunitinib treatment (Fig. 2B).
Dasatinib and sunitinib inhibit the growth of hormone-refractory PCa cell lines in vitro
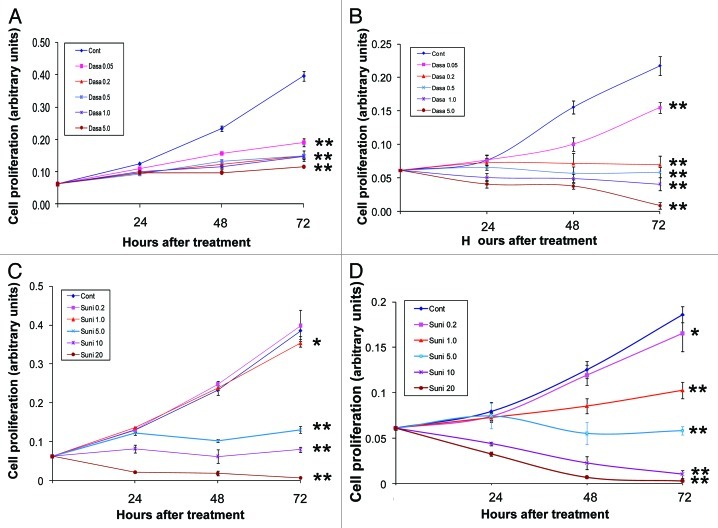
To evaluate the effects of dasatinib and sunitinib on the cellular proliferation of hormone-refractory PCa cell lines, we treated PC-3 and DU145 cells with different concentrations of the inhibitors (ranging from 0.05–5.0 μM for dasatinib and from 0.2–20 μM for sunitinib) for different times (24, 48 and 72 h).
Dasatinib significantly inhibited the proliferation of both PC-3 and DU145 cells in a dose-dependent manner (Fig. 3A and B). Sunitinib significantly inhibited the proliferation of PC-3 cells at concentrations higher than 5.0 mM (Fig. 3C) and inhibited the proliferation of DU145 cells at concentrations higher than 1.0 μM (Fig. 3D). These data show that dasatinib inhibits the cellular proliferation of PC-3 and DU145 to the same extent, whereas the concentration of sunitinib required to obtain significant inhibition of cellular proliferation in PC-3 cells (5.0 μM) is higher than that required in DU145 cells (1.0 μM).
Figure 3. Effects of dasatinib and sunitinib on PC-3 and DU145 cellular proliferation. Dasatinib (Dasa) inhibited proliferation of PC-3 (A) cells and DU145 cells (B) at doses ranging from 0.05–5.0 μM Sunitinib (Suni) inhibited proliferation of PC-3 cells (C) and DU145 cells (D) at doses ranging from 0.2–20 μM. Data are plotted as means ± SD of experiments repeated in triplicate.*, p < 0.05; **, p < 0.0001 by Student’s t-test compared with control group.
These data are generally consistent with the results of previous studies which showed that dasatinib inhibits phosphorylation of Src and FAK in PC-3 cells26 and with those of studies using the SFK-Abl dual kinase inhibitor AZD0530, which showed inhibited proliferation in both PC-3 and DU145 cells.27
Dasatinib and sunitinib, each in combination with anti-Cav-1 antibody, suppress the tumor growth of PC-3 or DU145 xenografts
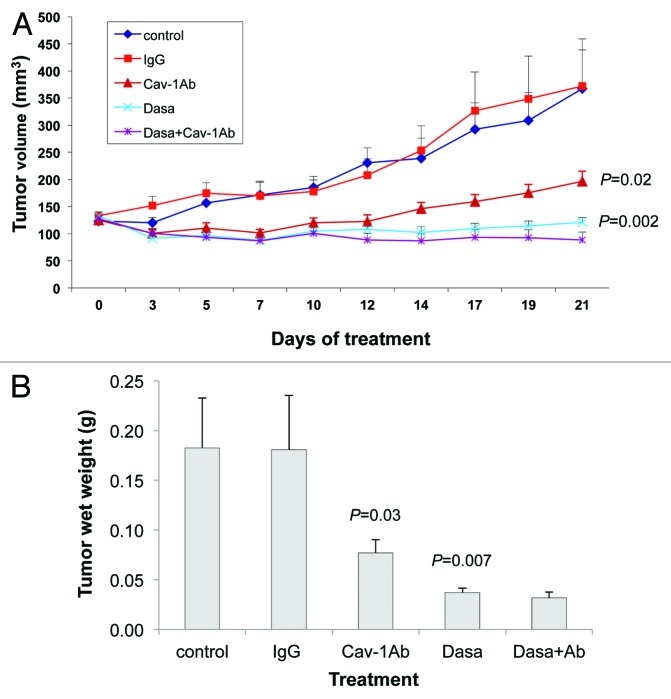
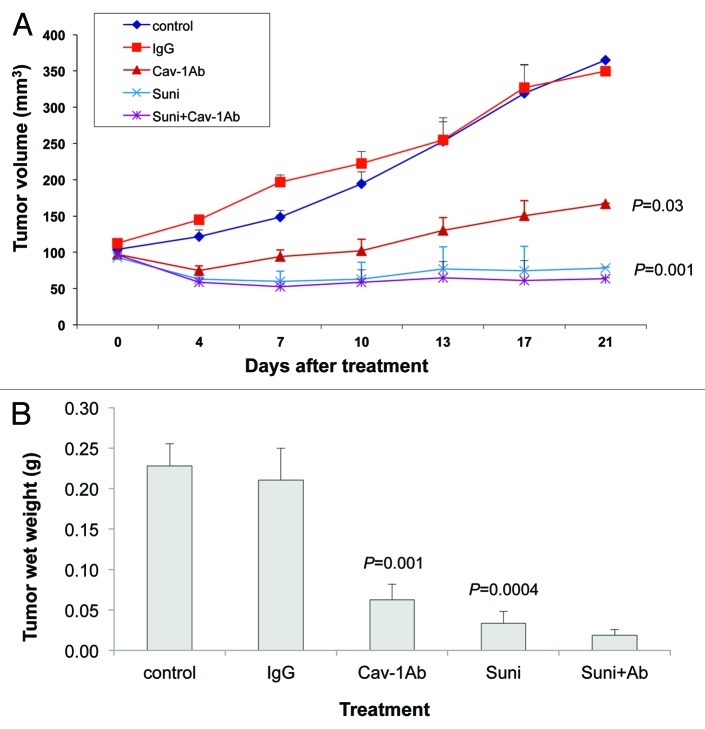
Our observation that dasatinib and sunitinib both reduce the secretion of Cav-1 by PC-3 and DU145 cells, in addition to their inhibitory effects on the RTK/TK signaling modules, led us to investigate the effects of combining anti-Cav-1 antibody with dasatinib and separately with sunitinib on the growth of PC-3 and DU145 xenograft tumors. Treatments were started on day 7 after injection of PC-3 cells into the right flank of nude mice, by which time the tumors were well established (tumor volumes were 150–200 mm3). We treated PC-3 xenografts with dasatinib alone, anti-Cav-1 antibody alone, the combination, control IgG and the vehicle control.
Treatment of the PC-3 tumor-bearing mice showed that tumor volume was reduced significantly by both dasatinib alone and anti-Cav-1 antibody alone, compared with that in the IgG-treated control mice (p = 0.0022 and 0.02, respectively). Further, the combination of dasatinib and anti-Cav-1 antibody produced greater tumor regression than did either treatment alone (Fig. 4A). Similarly, treatment with dasatinib alone and anti-Cav-1 antibody alone significantly reduced the tumor wet weight compared with that in the mice treated with vehicle only and IgG only (p = 0.0072 and 0.0307, respectively). The combination of dasatinib and anti-Cav-1 antibody also produced a greater reduction in tumor wet weight than did either treatment alone (Fig. 4B).
Figure 4. Antitumor activity of dasatinib (Dasa) in combination with anti-Cav-1 antibody (Cav-1 Ab) on PC-3 xenografts in nude mice. (A) Dasatinib alone and anti-Cav-1 antibody alone significantly reduced the tumor volume of PC-3 cells growing as xenografts compared with those of vehicle- and IgG-treated controls (p = 0.002 and p = 0.02, respectively). Anti-Cav-1 antibody treatment also enhanced the efficacy of dasatinib. Each data point represents the mean tumor volume in each group containing 9–16 mice (B), dasatinib alone and anti–Cav-1 antibody alone significantly reduced the tumor wet weight in nude mice bearing PC-3 tumor xenografts compared with the weights in vehicle- and IgG-treated controls (p = 0.0072 and p = 0.0307, respectively). Data represents the mean tumor weight ± SEM in each group containing 9–16 mice.
In the DU145 xenografts, tumor volume was significantly reduced by treatment with sunitinib alone and with anti-Cav-1 antibody alone, compared with that in the vehicle-only and the IgG-treated controls (p = 0.0015 and 0.0377, respectively; Fig. 5A). In addition, both treatments alone significantly reduced the tumor wet weights, compared with those in the vehicle-only and IgG-treated controls (p = 0.0004 and 0.0016, respectively; Fig. 5B). Finally, the combination of sunitinib and anti-Cav-1 antibody produced greater tumor regression (tumor volume and wet weight) than did either treatment alone (Fig. 5A and B).
Figure 5. Antitumor activity of sunitinib (Suni) alone and in combination with anti–Cav-1 antibody (Cav-1Ab) on DU145 xenografts in nude mice. (A) Sunitinib alone and anti-Cav-1 antibody alone significantly reduced the tumor volume of DU145 cells growing as xenografts compared with those of vehicle- and IgG-treated controls (p = 0.0015 and p = 0.0377, respectively). Anti-Cav-1 antibody treatment also enhanced the efficacy of sunitinib. Each data point represents the mean tumor volume in each group containing 7–10 mice. (B) Sunitinib alone and anti-Cav-1 antibody alone significantly reduced the tumor wet weight in nude mice bearing DU145 tumor xenografts compared with those in vehicle- and IgG-treated control mice (p = 0.0004 and p = 0.0016, respectively). Data represents the mean tumor weight ± SEM in each group containing 7–10 mice.
Serum Cav-1 concentration correlates with PC-3 and DU145 tumor growth and suppression induced by drug treatment in vivo
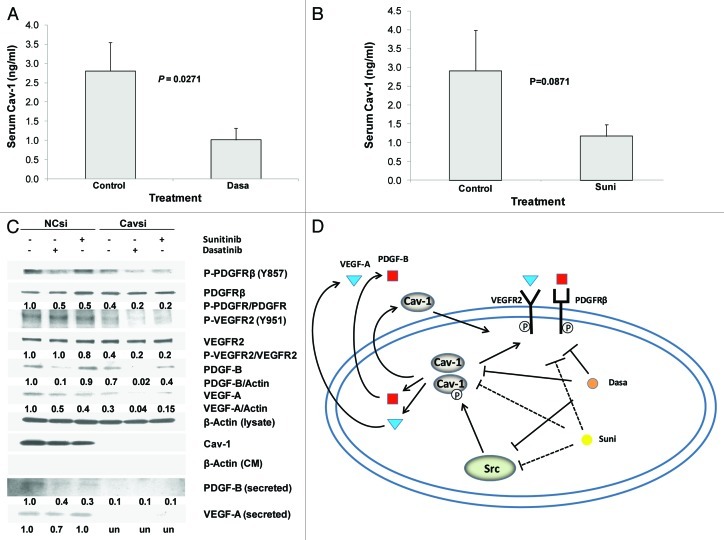
We further investigated the effects of dasatinib and sunitinib treatments on the secretion of Cav-1 in vivo by using our established Cav-1 ELISA procedure25,28 to measure the serum Cav-1 concentrations in the treated PC-3 and DU145 tumor-bearing mice. Serum Cav-1 concentrations in the dasatinib-treated PC-3 tumor-bearing mice were significantly lower than they were in the vehicle only-treated control mice (p = 0.0271; Fig. 6A). In addition, sunitinib treatment of the DU145 tumor-bearing mice yielded lower serum Cav-1 concentrations than those we found in the vehicle-only control mice, although those differences were not statistically significant (p = 0.0871; Fig. 6B).
Figure 6. Dasatinib and sunitinib suppress RTK signaling by downregulation of Cav-1. (A) Serum Cav-1 concentrations in mice treated with dasatinib were significantly reduced compared with those in the control group (p = 0.027). (B) Serum Cav-1 concentration in mice treated with sunitinib were reduced compared with that in the control group, although the differences did not achieve statistical significance (p = 0.0871). (C) Cav-1 knockdown in PC-3 cells treated with dasatinib or sunitinib caused greater reduction in the phosphorylation of PDGFRβ and VEGFR2 than control siRNA, and suppressed cellular and secreted PDGF-B and VEGF-A [secreted VEGF-A were reduced to undetectable levels (un) in response to Cavsi alone and Cavsi dasatinib or sunitinib treatment]. (D) Dasatinib and sunitinib inhibit PDGFRβ and VEGFR2 signaling by downregulation of Cav-1 expression and secretion, in turn, leads to suppression of expression and secretion of PDGF-B and VEGF-A. Data in (A and B) represent mean serum Cav-1 + SD in each group.
Moreover, when we compared the serum Cav-1 concentrations in the vehicle-treated with those in the dasatinib-treated mice and the concentrations in the vehicle-treated with those in the sunitinib-treated mice, we found that the serum Cav-1 concentration correlated positively with tumor growth (wet weight) (r = 0.48, p = 0.031 for dasatinib and r = 0.554, p = 0.0065 for sunitinib). Together, these results provide further evidence that dasatinib and sunitinib each reduce secretion of Cav-1 in vivo.
Dasatinib and sunitinib inhibit RTK/TK signaling through the downregulation of Cav-1 expression and secretion
To investigate the potential role of Cav-1 in dasatinib or sunitinib regulation of the RTK/TK signaling, we analyzed the effect of Cav-1 knockdown in PC-3 cells treated with dasatinib (0.05 μM) or sunitinib (0.2 μM) on the RTK signaling. Cav-1 knockdown by Cav-1-specific small interfering RNA (Cavsi) caused a marked reduction (60%) in the phosphorylation of PDGFRβ (Y857), VEGFR2 (Y951), cellular expression of PDGF-B (30%), VEGF-A (70%) and, interestingly, the secretion of PDGF-B (90%) and VEGF-A (reduced to undetectable levels) compared with that of NCsi. Treatment of the cells with dasatinib caused a marked reduction in P-PDGFRβ (50%) in NCsi cells compared with that of NCsi-untreated cells (Fig. 6C). Dasatinib did not cause a notable reduction of P-VEGFR2, but did cause a marked reduction in cellular and secreted PDGF-B and VEGF-A, in NCsi compared with NCsi-untreated cells. Sunitinib treatment caused a marked reduction in P-PDGFRβ (50%); a moderate reduction in P-VEGFR2 and cellular PDGF-B and a marked reduction in cellular VEGF-A (60%) and secreted PDGF-B (70%) in NCsi compared with NCsi-untreated cells (Fig. 6C). Sunitinib treatment did not cause a notable reduction in secreted VEGF-A in NCsi compared with NCsi-untreated cells. Importantly, for both dasatinib and sunitinib the reductions in P-PDGFRβ, P-VEGFR2 and cellular and secreted PDGF-B and VEGF-A were markedly enhanced (45–80% in each case) in Cavsi-treated cells compared with NCsi-treated cells. These results strongly suggest that dasatinib and sunitinib inhibits RTK/TK signaling, in part, through inhibition of Cav-1 which, in turn, leads to inhibition of cellular and secreted PDGF-B, and VEGF-A (Fig. 6D).
Discussion
Because the activities of the RTKs and SFKs are central to the development and progression of PCa, inhibition of those activities is a valid therapeutic strategy.29,30 Nearly all of the small-molecule TK inhibitors (TKIs) commonly used for treating cancer are known to inhibit ATP’s binding to the ATP-binding site (with the exception of temsirolimus, which targets mTOR), but none of those inhibitors are specific for a single kinase. For example, dasatinib and sunitinib, the 2 TKIs we tested in this study, inhibit a broad spectrum of kinases, including Bcr-Abl, SFK, VEGFR2, ROCK, KIT, mTOR, EGFR and PDGFR.31
An important note about the clinical use of TKIs, however, is that it has met with limited success owing to the selective therapeutic responses of various malignancies. This is due to both the insufficient expression of the appropriate therapeutic target and the complexity of the response to these available agents, resulting partly from their lack of target specificity.32 Additional studies that address the signaling responses to specific TKIs such as dasatinib and sunitinib may reveal unique molecular activities that would be useful as candidate therapeutic targets for combination therapeutic strategies and/or biomarkers to better stratify and monitor patients undergoing TKI therapy. This study revealed that both dasatinib and sunitinib reduce VEGFR2 and PDGFRβ phosphorylation, but only dasatinib reduced the phosphorylation of Src and FAK in PC-3 and DU145 cells. We further found dose-dependent growth suppression caused by dasatinib and sunitinib in those cells. These data highlight the importance of dasatinib and sunitinib in inhibiting cellular proliferation and add support to the results of previous studies.26,27,33,34
Our results revealed that both dasatinib and sunitinib suppress the expression and secretion of Cav-1 in PC-3 and DU145 cells in a dose-dependent fashion. Dasatinib treatment was reported previously to cause a significant reduction in Cav-1 mRNA in breast cancer cell lines,35 which suggests possible modulation of Cav-1 expression by dasatinib at the transcriptional level. An interesting note is that both of these drugs also caused a dose-dependent reduction in Cav-1 phosphorylation. To date, the role of the phosphorylated or activated form of Cav-1 has not been clarified. Cav-1 is phosphorylated at the tyrosine residue (Y14) in response to a number of stimuli, including oxidative stress.36 This phosphorylation process may serve as a mechanism through which Cav-1 signals to the intracellular machinery36 and may indicate a change in its function.37 Indeed, a number of studies have shown that P-Cav-1 plays a role in tumor progression as a growth-stimulatory or antiapoptotic signal. For example, P-Cav-1 was shown to promote tumor progression by stimulating proliferation, migration, invasion or survival in melanoma cells,38 mesangial cells,39 kidney cells40 and metastatic human breast and PCa cells.41
We further demonstrated in this study that both dasatinib and sunitinib treatment significantly reduced Cav-1 secretion. We previously demonstrated that many androgen-insensitive PCa cell lines secrete biologically active Cav-1 protein, which promotes PCa cell viability through antiapoptotic activities and clonal growth in vitro, similar to those activities observed after enforced expression of Cav-1.12,14,16 Further, we previously found that recombinant Cav-1 protein is taken up by PCa cells and endothelial cells in vitro and that recombinant Cav-1 increases angiogenic activities both in vitro and in vivo by activating Akt- and/or nitric oxide synthase–mediated signaling.17 Cav-1-stimulated autocrine and paracrine engagement of the local tumor microenvironment involve, but are not likely, limited to its proangiogenic activities. Besides the local effects produced by tumor cell-derived secreted Cav-1, serum Cav-1 can promote metastasis at distant sites.7 To date, various mechanisms have been proposed as underlying Cav-1 secretion. The results of an early study demonstrated that Cav-1 phosphorylation at Ser 80 is required for its retention by the endoplasmic reticulum and its entry into the regulated secretory pathway.42 Other investigators reported that Cav-1 is secreted through exosomes by melanoma cells43 and through oncosomes or prostasomes by PCa cells.44,45
Our new results demonstrate that anti-Cav-1 antibody significantly suppressed the tumor growth in PC-3 and DU145 xenografts, which confirms the role of secreted Cav-1 in the stimulation of PCa tumor progression. The findings that both dasatinib and sunitinib suppress tumor growth and that each, when combined with anti-Cav-1 antibody, results in greater tumor regression than did either drug alone suggest that dasatinib and sunitinib exert their therapeutic effects in part through suppressing the expression and secretion of Cav-1.
It is important to note that our results also support the concept that PCa cell-derived secreted and/or soluble Cav-1 is a therapeutic target in PCa. Both dasatinib and sunitinib significantly reduced the serum concentration of Cav-1, and that concentration correlated positively with tumor growth in both treated groups. The limitation of prostate-specific antigen (PSA) as a biomarker is increasingly recognized in the assessment of response to treatment in men with metastatic castration-resistant PCa. For example, the 2008 recommendations of the Prostate Cancer Clinical Trials Working Group46 say that early changes in PSA, in the absence of other objective evidence of disease progression, should not indicate the need for prompt discontinuation of drug therapy. That recommendation is particularly applicable because PSA changes may not be predictive of radiographic or clinical response and/or benefit.
Other previous reports have shown that the use of gene profiling may be a useful approach in the identification of biomarkers associated with sensitivity of PCa cells to dasatinib.47 Additional studies have shown that a six-gene set, including Cav-1, was useful in determining the response to dasatinib in a panel of breast cancer cell lines in vitro.35 The results of our study substantially extend these previous results and raise the possibility of using serum Cav-1 as a biomarker for dasatinib and sunitinib response in PCa patients.
The results of Cav-1 knockdown experiments clearly indicate that reduction in the expression of Cav-1 by Cavsi treatment caused a marked reduction in the phosphorylation of PDGFRβ and VEGFR2 similar to that observed by dasatinib or sunitinib treatment (Fig. 6C). Cav-1 knockdown, in combination with dasatinib or sunitinib treatment in PC-3 cells, caused a greater reduction in the phosphorylation of PDGFR-β and VEGFR2, and expression and secretion of PDGF-B and VEGF-A than that observed in PC-3 cells treated with dasatinib or sunitinib alone in control siRNA cells. These data strongly suggest that these drugs exert their inhibitory function via the reduction of Cav-1 expression and secretion which leads to the reduction of expression and secretion of GFs such PDGF-B and VEGF-A, thereby causing a reduction in the phosphorylation of both PDGFRβ and VEGFR2 through ligand depletion. Our results of a marked reduction in the phosphorylation of PDGFRβ and VEGFR2 in response to dasatinib or sunitinib in Cavsi-treated cells suggest a possible role for Cav-1 in protecting the cells from the inhibitory action of these drugs (Fig. 6D).
In conclusion, our results strongly suggest that dasatinib and sunitinib inhibit specific signaling activities and suppress the growth of the PCa cell lines PC-3 and DU145 in vitro and in vivo, in part, through downregulation of Cav-1 expression and secretion of Cav-1, PDGF-B and VEGF-A. In addition, through the use of combined treatment with anti–Cav-1 antibody plus dasatinib or sunitinib, our results demonstrate the potential of PCa cell-derived secreted and/or soluble Cav-1 as a biomarker and therapeutic target for PCa.
Acknowledgments
The authors thank Karen F. Phillips, ELS, for her expert editorial assistance and Dr. Jianxiang Wang for technical assistance.
Disclosure of Potential Conflicts of Interest
This work was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant (CA016672) and grant R01CA68814 (to T.C.T.) and by the United States Department of Defense grant DAMD 17–98–1-8575 (to T.C.T.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22633
References
- 1.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–51. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg PW, Schmid SL. Caveolin, cholesterol and Ras signalling. Nat Cell Biol. 1999;1:E35–7. doi: 10.1038/10028. [DOI] [PubMed] [Google Scholar]
- 3.Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84:177–89. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- 4.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 5.Thompson TC, Tahir SA, Li L, Watanabe M, Naruishi K, Yang G, et al. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2010;13:6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280:25134–45. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, Yang G, Cao G, Tahir SA, Naruishi K, Tabata K, et al. Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Mol Cancer Res. 2009;7:1446–55. doi: 10.1158/1541-7786.MCR-09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. American Society of Clinical Oncology Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Ren C, Yang G, Goltsov AA, Tabata K, Thompson TC. Caveolin-1 promotes autoregulatory, Akt-mediated induction of cancer-promoting growth factors in prostate cancer cells. Mol Cancer Res. 2009;7:1781–91. doi: 10.1158/1541-7786.MCR-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61:4386–92. [PubMed] [Google Scholar]
- 12.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahir SA, Park S, Thompson TC. Caveolin-1 regulates VEGF-stimulated angiogenic activities in prostate cancer and endothelial cells. Cancer Biol Ther. 2009;8:2286–96. doi: 10.4161/cbt.8.23.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–5. [PubMed] [Google Scholar]
- 15.Bartz R, Zhou J, Hsieh JT, Ying Y, Li W, Liu P. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2008;122:520–5. doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Foreman TL, Gregory CW, McJilton MA, Wescott GG, Ford OH, et al. Protein kinase cepsilon has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423–9. [PubMed] [Google Scholar]
- 17.Tahir SA, Yang G, Goltsov AA, Watanabe M, Tabata K, Addai J, et al. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68:731–9. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 18.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 19.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 20.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–8. [PubMed] [Google Scholar]
- 21.O’Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–76. [PubMed] [Google Scholar]
- 22.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 23.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 24.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 25.Tahir SA, Ren C, Timme TL, Gdor Y, Hoogeveen R, Morrisett JD, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9:3653–9. [PubMed] [Google Scholar]
- 26.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 27.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27:6365–75. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahir SA, Frolov A, Hayes TG, Mims MP, Miles BJ, Lerner SP, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res. 2006;12:4872–5. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 29.Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I. Tyrosine kinase blockers: new hope for successful cancer therapy. Anticancer Agents Med Chem. 2009;9:66–76. doi: 10.2174/187152009787047752. [DOI] [PubMed] [Google Scholar]
- 30.Duckett DR, Cameron MD. Metabolism considerations for kinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol. 2010;6:1175–93. doi: 10.1517/17425255.2010.506873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demetri GD. Differential properties of current tyrosine kinase inhibitors in gastrointestinal stromal tumors. Semin Oncol. 2011;38(Suppl 1):S10–9. doi: 10.1053/j.seminoncol.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Tolmachev V, Stone-Elander S, Orlova A. Radiolabelled receptor-tyrosine-kinase targeting drugs for patient stratification and monitoring of therapy response: prospects and pitfalls. Lancet Oncol. 2010;11:992–1000. doi: 10.1016/S1470-2045(10)70088-7. [DOI] [PubMed] [Google Scholar]
- 33.Huynh H, Ngo VC, Choo SP, Poon D, Koong HN, Thng CH, et al. Sunitinib (SUTENT, SU11248) suppresses tumor growth and induces apoptosis in xenograft models of human hepatocellular carcinoma. Curr Cancer Drug Targets. 2009;9:738–47. doi: 10.2174/156800909789271530. [DOI] [PubMed] [Google Scholar]
- 34.Abouantoun TJ, Castellino RC, MacDonald TJ. Sunitinib induces PTEN expression and inhibits PDGFR signaling and migration of medulloblastoma cells. J Neurooncol. 2011;101:215–26. doi: 10.1007/s11060-010-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 36.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253:629–36. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 37.Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol. 2000;20:1507–14. doi: 10.1128/MCB.20.5.1507-1514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felicetti F, Parolini I, Bottero L, Fecchi K, Errico MC, Raggi C, et al. Caveolin-1 tumor-promoting role in human melanoma. Int J Cancer. 2009;125:1514–22. doi: 10.1002/ijc.24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Percy CJ, Pat BK, Healy H, Johnson DW, Gobe GC. Phosphorylation of caveolin-1 is anti-apoptotic and promotes cell attachment during oxidative stress of kidney cells. Pathology. 2008;40:694–701. doi: 10.1080/00313020802436402. [DOI] [PubMed] [Google Scholar]
- 41.Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–20. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 42.Schlegel A, Arvan P, Lisanti MP. Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation. Protein sorting at the level of the endoplasmic reticulum. J Biol Chem. 2001;276:4398–408. doi: 10.1074/jbc.M005448200. [DOI] [PubMed] [Google Scholar]
- 43.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–9. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llorente A, de Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117:5343–51. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- 46.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XD, Reeves K, Luo FR, Xu LA, Lee F, Clark E, et al. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 2007;8:R255. doi: 10.1186/gb-2007-8-11-r255. [DOI] [PMC free article] [PubMed] [Google Scholar]