Abstract
Background: The endothelin receptor-A (ETRA) plays an important role in tumor cell migration, metastasis, and proliferation. The endothelin receptor B (ETRB) plays a critical role in angiogenesis and the inhibition of anti-tumor immune cell recruitment. Thus dual blockade of ETRA and ETRB could have significant anti-tumor effects.
Results: Dual ETRA/ETRB blockade with macitentan (or the combination of the ETRA and ETRB antagonists BQ123 and BQ788) did not enhance antitumor immune cell recruitment. In vitro studies demonstrate that ETRA inhibition prevents the induction of ICAM1 necessary for immune cell recruitment. When used as a single agent against human tumor xenografts, macitentan demonstrated non-significant anti-tumor activity. However, when used in combination with chemotherapy, macitentan specifically reduced tumor growth in cell lines with CD133+ cancer stem cells. We found that ETRA is primarily expressed on CD133+ CSC in both cell lines and primary human tumor cells. ETRA inhibition of CSC prevented chemotherapy induced increases in tumor stem cells. Furthermore, ETRA inhibition in combination with chemotherapy reduced the formation of tumor spheres.
Methods: We tested the dual ETRA/ETRB antagonist macitentan in conjunction with (1) an anti-tumor vaccine and (2) chemotherapy, in order to assess the impact of dual ETRA/ETRB blockade on anti-tumor immune cell infiltration and ovarian tumor growth. In vitro murine and human cell line, tumor sphere assays and tumor xenograft models were utilized to evaluate the effect of ETRA/ETRB blockade on cell proliferation, immune cell infiltration and cancer stem cell populations.
Conclusions: These studies indicate a critical role for ETRA in the regulation of immune cell recruitment and in the CSC resistance to chemotherapy.
Keywords: ovarian cancer, ETRA, ETRB, macitentan
Background
The endothelin system, including endothelin 1, 2, 3 and 4 (ET-1, ET-2, ET-3, ET-4) and the endothelin receptors ETRA and ETRB, was first identified as a significant modulator of vascular tone. Endothelins are now also recognized as important factors in the pathophysiology of cancer.1 Endothelins influence tumorigenesis and cancer progression in multiple malignancies including ovarian, breast, colon, lung, stomach, prostate, bladder, renal cell and hepatocellular carcinoma.1-9 The endothelin axis may have particular importance in the pathogenesis of ovarian cancer. Ninety percent of malignant ovarian tumors express endothelin receptors and ET-1, and ET-1 levels are elevated in patients with advanced ovarian cancer.10,11
A significant body of work has linked ETRA and ovarian cancer tumorigenesis. ET1 stimulation of ETRA increases tumor cell proliferation inhibits apoptosis, and increases invasion and tumor migration leading to metastatic spread.1,10-12 The inhibition of ETRA decreases the growth of HEY ovarian cancer xenografts both alone and in combination with paclitaxel.10,13,14-16 ETRA has also been linked to an epithelial-mesenchymal transition (EMT) and chemotherapy resistance in ovarian cancer.17 In addition to their role on cancer cells, ET-1 and ETRA are also overexpressed on tumor-associated cells including fibroblasts, macrophages and endothelial cells and thus endothelins also modulate the tumor microenvironment (inducing angiogenesis, increasing bone metastasis, etc.). Based on the numerous roles of ETRA in tumorigenesis various ETRA specific inhibitors are currently undergoing investigation as anti-cancer therapies in ovarian cancer and castrate-resistant prostate cancer.4,18-20
While less studied, ETRB also appears to play an important role in promoting tumorigenesis. ETRB activation is reported to have overall pro-tumorigenic activity.21 We have demonstrated that inhibition of ETRB expression on tumor vasculature leads to a reduction in anti-tumor immune cell infiltration into the tumor microenvironment thus promoting tumor survival through the evasion of the host immune response.22 Additionally, ETRB plays a role in promoting tumor angiogenesis by inducing endothelial cell survival, proliferation and invasion.14
Given the pro-tumorigenic roles of ETRA and ETRB, dual antagonism of these receptors is an appealing anti-cancer strategy. Macitentan is a high affinity dual ETRA/ETRB antagonist developed for its therapeutic potential in treating pulmonary hypertension.23,24 While macitentan primarily targets ETRA it also inhibits ETRB (~50:1 ratio). In this study, we investigated the effect of combined ETRA/ETRB blockade using the dual ETRA/ETRB antagonist macitentan and combined therapy with the ETRA and ETRB specific antagonists BQ123 and BQ788, respectively, in murine and human tumor xenograft ovarian cancer models. We hypothesize that dual ETRA/ETRB blockade will produce a synergistic effect leading to greater anti-tumor effects compared with blocking either receptor alone. In addition, ETRA/ETRB blockade in conjunction with vaccine therapy could be extremely potent, inhibiting tumor cell growth, angiogenesis and promoting anti-tumor immunity. Our findings indicate that ETRA signaling is necessary for the recruitment of anti-tumor immune cells and that ETRA may play a role in the chemotherapy resistance of cancer stem cells.
Results
Macitentan in vitro activity is dependent on expression of ETRA and ETRB
We first investigated the expression of ETRA and ETRB on human ovarian cancer and endothelial cell lines. Quantitative real time PCR demonstrated that ETRA mRNA is highly expressed in the human ovarian cancer cell lines A2780 and A2008, but is not expressed in Ovcar5 or the murine ID8 ovarian cancer cell line (Fig. 1A). ETRB was primarily expressed on endothelial cells including human dermal microvascular endothelial cells (HDMEC) and the murine brain endothelial cell line bEnd.3 (Fig. 1A). We then treated each cell line with ET-1 with and without macitentan. Cell counts were compared after 72 h of treatment. Consistent with the lack of ETRA or ETRB expression, we found that macitentan had limited activity vs. the OVCAR5 human ovarian cancer cell line and the murine ID8 ovarian cancer cell line (Fig. 1B). Macitentan had greatest activity against A2008 and A2780 ovarian cancer cells (IC50 1 μM) (Fig. 1B, 1). When combined with cisplatin, macitentan demonstrated additive activity against A2780 cells (Fig. 1C). Macitentan also demonstrated growth inhibitory activity against both human and murine endothelial cells (Fig. 1B, 2), which primarily express ETRB, with a similar TD50 of 1 μM. These results demonstrate the specificity of macitentan’s actions on cells which express ETRA or ETRB.
Figure 1. Macitentan in vitro cytotoxicity with and without chemotherapy. (A) qRT-PCR demonstrating the expression of ETRA and ETRB by human (1) and mouse (2) ovarian cancer and endothelial cell lines. Human ovarian cancer cell lines include A2008, A2780 and Ovcar5. Human dermal microvascular endothelial cells (HDMEC) are used as endothelial source. The murine ID8 ovarian cancer cell line and MS1 and bEnd.3 (brain endothelial cells) are used as a source of endothelial cells. (B) Viable cell percentage (relative to DMSO controls) of tumors cells (1) and endothelial cells (2) treated with increasing does of macitentan. Macitentan demonstrates activity (1C50 ~1uM) vs. both cell lines (A2008 and A2780) which demonstrate ETRA expression, but not against OVCAR5 cells which do not express ETRA . Macitentan demonstrates activity against both human and murine endothelial cells. ET-1 and DMSO treatment are shown as controls. (C) Macitentan cytotoxicity in A2008 cells in combination with Cisplatin demonstrates additive activity.
Anti-tumor immune cell recruitment requires ETRA
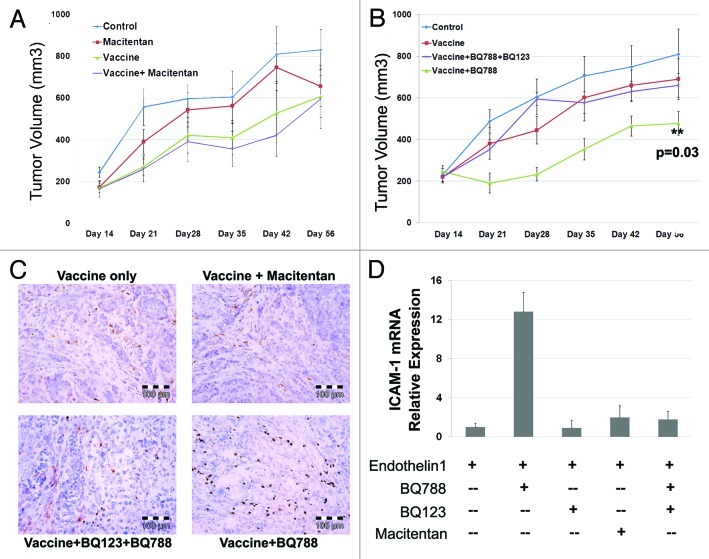
Our previous work demonstrated ETRB blockade leads to an increase in endothelial ICAM-1 expression with a concomitant increase in anti-tumor T cell migration into tumors. Secondary to this amplification in local immune response, the efficacy of vaccine therapy was significantly increased when used in combination with ETRB blockade.22,28 Therefore, we next tested if macitentan could enhance the activity of a tumor vaccine and suppress tumor growth. Animals were vaccinated with apoptotic ID8 cells (or PBS alone controls) and then injected with ID8 tumors as previously described.22 Animals were treated daily via oral gavage with either vehicle or macitentan. Vaccine therapy alone demonstrated modest reductions in tumor volume (Fig. 2A). Consistent with our in vitro data demonstrating limited activity of macitentan on ID8 cells, treatment of ID8 tumors with macitentan alone did not significantly affect tumor volume. Surprisingly, combined treatment with macitentan and vaccine therapy also yielded no enhancement of anti-tumor vaccine activity (Fig. 2A).
Figure 2. Macitentan treatment of ID8 tumors with and without vaccine therapy. (A) ID8 tumors injected in mice treated with macitentan alone, ID8 cell vaccination alone or combination of macitentan and vaccine. Tumor volume over 56 d demonstrates modest activity of the tumor vaccine but no additional activity with the addition of macitentan. (B) ID8 tumors in mice treated with ID8 cell vaccination alone, vaccine + BQ788 (ETRB antagonist) or vaccine + BQ788 (ETRB antagonist) + BQ123 (ETRA antagonist). Tumor volume over 56 d was measured demonstrating additive activity of vaccine+BQ788 which is inhibited in the presence of ETRA antagonism with BQ123. (C) Immunohistochemical CD8 labeling of ID8 tumors treated in B demonstrating increased CD8 T cell infiltration in tumors treated with vaccine and BQ788. D. Levels of ICAM-1 mRNA in HDMEC cells after 24 h of treatment with ET-1 (necessary for increased ICAM-1 expression) and BQ123, BQ788, BQ123 + BQ788 or macitentan.
In order to determine if the lack of macitentan enhancement of vaccine activity was due to insufficient ETRB blocking activity of macitentan or due to the presence of ETRA blockade, we repeated this tumor vaccine experiment using the ETRA and ETRB specific antagonists BQ123 and BQ788. Confirming our previously reported results, ETRB antagonism with BQ788 enhanced the activity of anti-tumor immune responses and restricted tumor growth (Fig. 2B). However, like that observed with macitentan, combined ETRA/ETRB blockade with BQ123 and BQ788 did not demonstrate any enhancement of vaccine efficacy (Fig. 2B). Immunohistochemical analysis of tumors confirmed increased infiltration by CD8+ cells only in BQ788 treated tumors (Fig. 2C). Thus in the presence of ETRA antagonism, the enhancement of tumor vaccine activity induced by ETRB blockade is lost. This suggests an essential role for ETRA in the recruitment of anti-tumor T cells.
ICAM-1 expression with ETRA and ETRB blockade
Since previous reports demonstrated ETRB antagonism leads to increased ICAM-1 expression on endothelial cells thus promoting immune cell recruitment, we next investigated the effect of specific ETRA blockade, ETRB blockade and combined ETRA/ETRB blockade on ICAM-1 expression. HDMEC cells were treated with ET-1 alone or in combination with the ETRA antagonist BQ123, the ETRB antagonist BQ788, macitentan, or a combination of BQ123 and BQ788. As observed previously, treatment with the ETRB specific antagonist BQ788 led to increased expression of ICAM mRNA (Fig. 2D). Treatment with the ETRA specific antagonist BQ123 alone had no effect on ICAM expression. Consistent with the essential role observed for ETRA in immune cell recruitment in vivo, treatment with macitentan or dual blockade of ETRA and ETRB did not result in increased ICAM expression. These results demonstrate that in the presence of ETRA blockade, ETRB blockade does not increase ICAM-1 expression. This implies that ETRA stimulation is necessary for the increase in ICAM-1 expression produced by ETRB antagonism.
Macitentan treatment of ovarian cancer mouse xenografts
Next we tested the in vivo efficacy of macitentan in a human ovarian cancer cell line xenograft tumor model. We used both the A2008 and the A2780 cell lines which demonstrated response to macitentan in vitro, with and without cisplatin. One week after subcutaneous tumor implantation, mice were treated daily via gavage with vehicle control or macitentan. Animal survival was recorded over an 80 d period. In both A2008 and A2780 tumor cell lines, the Kaplan-Meier survival curve demonstrated no significant (p = 0.14 and p = 0.24, respectively) increase in survival of mice treated with macitentan alone (Fig. 3A, 1 and 2). Given the increased activity of macitentan in combination with cisplatin in vitro, we repeated this experiment comparing cisplatin treatment alone and cisplatin + macitentan therapy. The addition of macitentan to cisplatin had no impact on A2008 cell growth. However, the addition of macitentan to cisplatin demonstrated a significant improvement in response of the A2780 cells to cisplatin therapy.
Figure 3. Macitentan treatment of ovarian cancer human tumor xenografts. (A) (1) A2008 xenografts and (2) A2780 xenografts treated with macitentan daily vs. placebo demonstrating increased survival for A2780 tumors treated with macitentan. (B) (1) A2008 and (2) A2780 xenografts treated with cisplatin with and without macitentan. Once again the addition of macitentan improved survival in the A2780 tumors only.
ETRA blocks chemotherapy induced tumor stemness
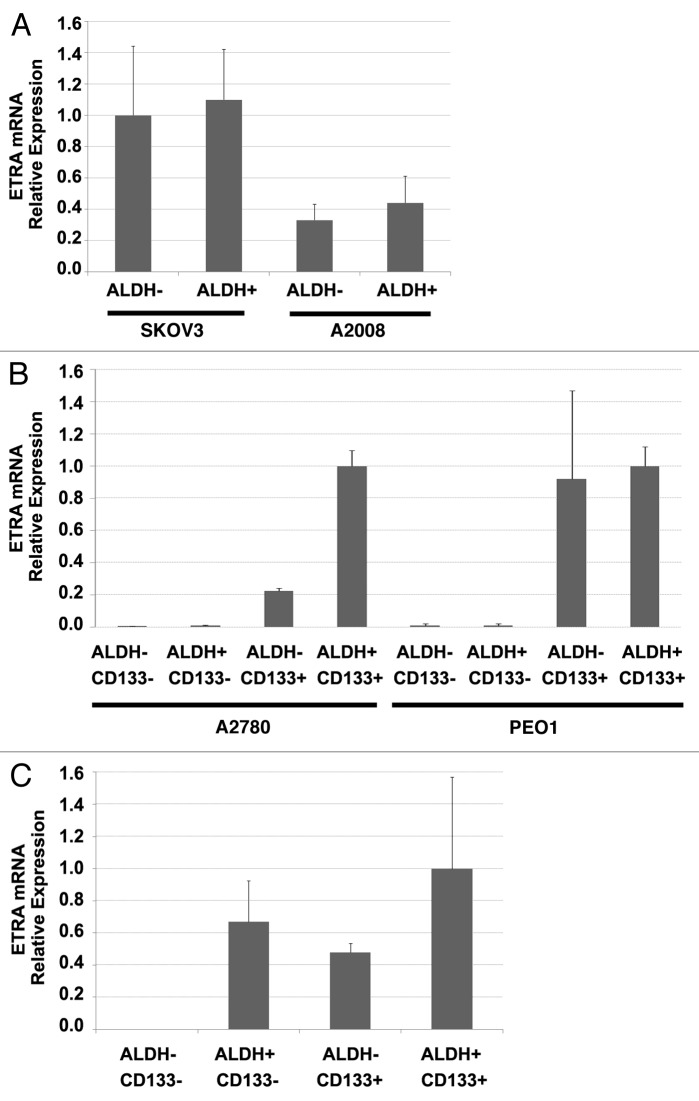
ETRA activation has been associated with an epithelial-mesenchymal transition (EMT) and chemoresistance in ovarian cancer cells.17 Both EMT and chemoresistance are hallmarks of cancer stem cells (CSC).29,30 Our group and others have demonstrated that ovarian cancer stem cells in ~40% of patients can be defined by the expression of CD133.25,31 In CD133(-) tumors, ALDH enzymatic activity can be used to define CSC.25,32 Given the increased efficacy of cisplatin therapy with macitentan and the link of ETRA with phenotypic characteristics of CSC, we analyzed ETRA expression in the different populations of ovarian CSC. We analyzed ETRA mRNA expression in FACS isolated ALDH+ and ALDH- cells from ovarian cancer cell lines which do not express CD133 (A2008 and SKOV3) and in ALDH+/−CD133+/− cells from cell lines which do express CD133(A2780 and PEO1). We found that in CD133(-) cell lines, ETRA was equally expressed in ALDH+ and ALDH- cells (Fig. 4A). In contrast, among the CD133+ cell lines (A2780 and PEO1) we found that ETRA expression was primarily expressed in the ALDH+/CD133+ and ALDH-/CD133+ cells (Fig. 4B). Interestingly, in cells isolated from primary human ovarian tumors, we observed ETRA expression in ALDH+CD133-, ALDH-CD133+, and ALDH+CD133+ CSC but no expression in the ALDH-CD133- cells (Fig. 4C).
Figure 4. ETRA is preferentially expressed in CD133+ ovarian cancer stem cells. (A) qRT-PCR demonstrating a lack of differential expression of ETRA in ADLH (+) and ALDH (-) cell populations of SKOV3 and A2008 cell lines (which do not express CD133). (B) qRT-PCR demonstrating preferential expression of ETRA in CD133+ cells from A2780 and PEO1 cell lines. (C) qRT-PCR demonstrating preferential expression of ETRA in ALDH+CD133- ALDH-CD133+ and ADLH+CD133+ primary human ovarian cancer stem cells vs. ALDH-CD133- progenitor cells.
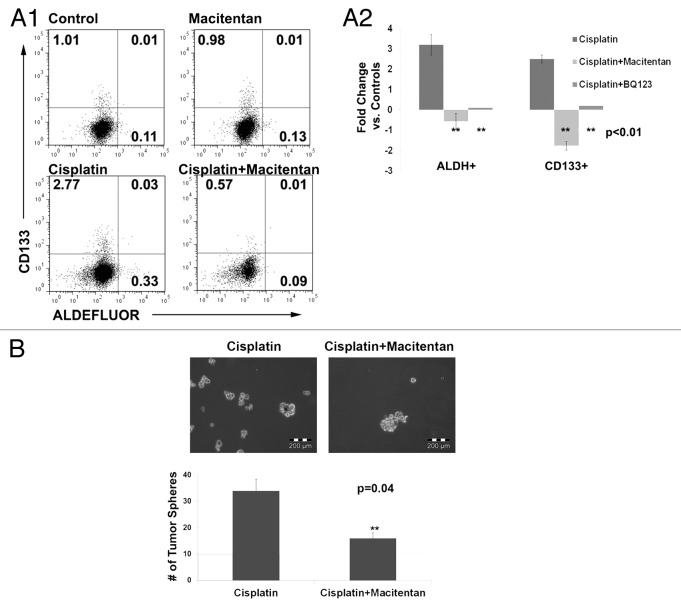
We next tested the impact of macitentan on the percentages of CSC in the above cell lines. As single agent therapy, macitentan had no impact on the percent of CSCs. Consistent with previous reports, cisplatin therapy was associated with a concomitant increase in the percentage of both ALDH+ and ALDH+/CD133+ CSCs.25 We then tested the impact of concurrent cisplatin and macitentan therapy on CSC percentages. Interestingly, treatment with macitentan or the ETRA specific antagonist BQ123 prevented this chemotherapy induced increase in stemness (Fig. 5A). The ETRB antagonist BQ788 had no impact on stem cell percentages (data not shown). Finally, we tested the impact of cisplatin vs. cisplatin + macitentan on the formation of tumor spheres from CD133+ ascites cells of a patient with platinum refractory ovarian cancer. The addition of macitentan to cisplatin lead to a 2 fold reduction in the number of tumor spheres (Fig. 5B). This data indicates that ETRA may play a role in CD133+ tumor stem cell resistance to chemotherapy.
Figure 5. (A) FACS analysis (1) and average fold change (2) of A2780 cells treated with cisplatin, macitentan, or cisplatin + macitentan. Cell percentages are indicated in the respective quadrants. (B) Tumor spheres formed from 5,000 ascites cells collected from a platinum refractory ovarian cancer patient treated with cisplatin or cisplatin + macitentan.
Discussion
We demonstrate here that, consistent with previous studies, the endothelin axis represents a potential therapeutic target in ovarian cancer. The dual ETRA/ETRB antagonist macitentan demonstrated anti-ovarian cancer activity primarily in combination with cisplatin. We found that ETRA is preferentially expressed in CD133+ ovarian CSC and may play a role in CSC resistance to chemotherapy. However, our study also elucidates some of the difficulties of targeting the endothelin axis and may explain the disappointing results of endothelin antagonist in the clinic. Specifically, we have found that ETRA activation appears to be necessary for the recruitment of anti-tumor T cells. Thus, while ETRA may effectively inhibit the growth of cancer cells, the benefit of these actions on cancer cells may be counter-acted by the inhibition of the anti-tumor immune response.
Endothelin receptors and anti-tumor immunity
Both the dual endothelin receptor antagonist macitentan and combined therapy with the specific ETRA/ETRB antagonists BQ123 and BQ788 prevented the increase in vaccine therapeutic efficacy associated with isolated ETRB inhibition. Consistent with this, our in vitro studies indicated that ETRA stimulation is necessary for the upregulation of ICAM-1 in the presence of ETRB blockade. Our studies are also in line with reports demonstrating an essential role for ETRA in lymphocyte recruitment in models of pleurisy33 and the role of Endothelin in regulating the cellular migration of several cell types.34-37 Our finding implies opposing roles of ETRA and ETRB in the regulation of immune cell recruitment. Similarly opposing roles for ETRA and ETRB have been reported in the regulation of dendritic cells, cardiovascular disease, and pulmonary function.38 Our data continues to support a role for targeting the endothelin axis as a potential means to enhance anti-tumor immune response based therapies. However, ETRB specific antagonists need to be developed for clinical use.
ET receptors and cancer stem cells
Consistent with reports for endothelin receptors as active in both hematopoietic stem cells and brain cancer stem cells,39,40 we observed that ETRA was specifically expressed on CD133+ ovarian CSC in cell lines and in patient samples. In patient samples we also observed some expression in ALDH+CD133- cells, however, given the mixed populations of cells derived from whole tumor cell suspensions, it is possible that the ETRA expression in these ALDH+CD133- cells is derived from non-tumor cells.
While ETRA antagonism had minimal impact on CSC as a single agent, it significantly abrogated the chemotherapy induced increase in cancer stem cells. Given CSC have been linked with epithelial mesenchymal transition (EMT), our observations linking ETRA and the induction of ‘cancer stemness’ is in line with a link between the endothelin axis and EMT.17,29 Recent reports suggest that cancer stem cell markers are induced in patient samples following treatment with chemotherapy.41 Of particular interest, CD133 was the only stem cell marker which correlated with platinum resistance. Our findings for a role of ETRA in ovarian cancer stemness have significant clinical implications. If these antagonists are capable of potentiating chemotherapy by preventing an increase in “cancer stemness” they could significantly reduce the risk of ovarian cancer recurrences. Our studies also indicate that these agents will work primarily in conjunction with chemotherapy and not as a single agent. This may explain some of the disappointing clinical trial results observed with ETRA inhibitors as a single agent,42,43 and supports the use of ETRA targeting agents in conjunction with chemotherapeutics.44
Targeting endothelin receptors in cancer patients
Our results add to a growing literature regarding the role of the endothelin axis in tumorigenesis. In particular our studies support targeting ETRA to enhance chemotherapeutic efficacy.45,46 However, the results are tempered by our findings that ETRA antagonism also blocks the recruitment of anti-tumor immune cells to the tumor microenvironment. Taken together, we would propose that the best means to target the endothelin axis in ovarian cancer patients would be to use ETRA specific antagonists in combination with chemotherapy as primary treatment. After completion of chemotherapy, ETRB specific antagonists could be used as consolidative/maintenance therapy to both inhibit angiogenesis and promote anti-tumor immunity.
Conclusions
We evaluated the impact of dual ETRA/ETRB antagonism in ovarian cancer cell lines. We observed that dual ETRA/ETRB antagonism has mixed activity on the tumor. Our previous results demonstrated that ETRB blockade can enhance anti-tumor immune cell recruitment. Here we show that the enhancement of immune cell recruitment is lost in the presence of ETRA antagonism. However, we also observed that ETRA is specifically expressed on a subset of CD133+ ovarian cancer stem cells. ETRA blockade appears to prevent chemotherapeutic induction of cancer stem cells and works best in combination with chemotherapy.
Materials and Methods
Cell culture and cytotoxicity assays
ID8 and A2008 cells were obtained from Dr. Coukos (University of Pennsylvania). A2780 and PEO1 cells were obtained from Dr. Murphy (Duke University). OVCAR5 cells were obtained from Dr. Liu (University of Michigan). All cells were cultured in RPMI10 with 10% FBS and penicillin-streptomycin. For cytotoxicity studies, ~2,000 cells were plated at ~30% confluence overnight in 96 well plates and then treatment with macitentan (macitentan given daily for 3 d) with or without cisplatin (250 ng/ml given × 1 d). After 72 h cell viability was assessed using MTT assay (Invitrogen) per manufacturer’s instructions. For tumor sphere assays, A2780 cells were plated at 40% confluence overnight and then treated with cisplatin (250 ng/ml) with or without macitentan (1 uM, given daily for 3 d). On day 5, 2,000 live cells (based on trypan blue staining) from each treatment group were plated in low adhesion plates in tumor sphere media.25 Total sphere number was counted 7 d after plating in sphere media.
Quantitative real time PCR (qRT-PCR)
RNA was isolated using traditional TRIZol or Nano-RNA isolation protocols (nano protocol was used for RNA isolated from FACS isolated CSC) per manufacturer’s protocol (Invitrogen). mRNA was then reverse transcribed Superscript III first-stand synthesis system for RT-PCR (Invitrogen) according to the manufacturer’s recommendations. 10 ng cDNA was then used for each subsequent PCR reaction. All samples were analyzed in duplicate with SYBR-green based using the StepOne system (Applied Biosystems) as previously described.22
Flow cytometry
APC-Anti-CD133 (Miltenyi Biotec) and ALDEFLOUR (StemCell Technologies) enzymatic activity based cell isolation was performed as previously described.25 Flow cytometric analysis was performed on a BD Biosciences FACS Calibur through the University of Michigan Flow Cytometry Core, and data analysis performed using FlowJo (Tree Star, Inc.).
Tumor xenograft studies
C57BL6 mice or NOD-SCID mice were obtained from Charles River Laboratories. Animals were maintained in accordance with institutional policies and all studies were performed with approval of the University Committee on Use and Care of Animals. All tumor vaccine studies were performed as previously described.22 Briefly, C57BL6 mice were vaccinated with 20 × 106 UV irradiated/apoptotic ID8 cells. Animals were vaccinated weekly ×3 weeks and then after one week 20 × 106 live ID8 cells were injected in 200 μl of Matrigel (BD Biosciences) subcutaneously into the axilla of C57BL6 mice. Treatment with macitentan (20 μg/Kg given daily via gavage dissolved in 0.5% (w/w) methylcellulose/water), or BQ788 or BQ123 (300 μg/dose/mouse given IP 3 ×/week for 2 weeks) was initiated one week after tumor injection. Tumor growth was measured using calipers and volumes were calculated based on the modified ellipsoid formula (L × W × W/2). At the time of sacrifice, tumors were resected and snap frozen for IHC with anti-CD8 as previously described.
Human tumor xenografts were generated as previously described.26 Briefly, 1 × 106 A2008 or A2780 cells were injected in 200 μl of growth factor reduced Matrigel into the axillae of immunodeficient mice. Tumors were allowed to engraft for 3 d and then were treated with macitentan daily as above with our without Cisplatin (250 μg/kg) given IP daily for 3 d.
Human tumor ascites processing and tumor sphere assays
Tumor ascites was processed as previously described.27 Briefly, ascites associated cells were concentrated by centrifugation and then red blood cells were lysed using ACK buffer (Lonza). Cells were then passed through a 40 um filter followed by 4 passes through a round tip 28G needle to isolate single cells. 5000 cells were then plated in tumor sphere media in non-adherent plates. Spheres were allowed to form for 14 d and were then mechanically separated by pipetting and replated together with cisplatin (100 ng/ml) or cisplatin + macitentan. After 4 d, media was replaced and total sphere number was assessed after 14 d of growth.
Disclosure of Potential Conflcits of Interest
This work was supported in part by a $25,000 research grant from Actelion.
Acknowledgments
This work was supported in part by the ovarian cancer research fund and a $25,000 research grant from Actelion. R.J.B. is supported by the NIH New Investigator Innovator Directors Award grant #00440377.
Author contributions: L.C. prepared the manuscript and analyzed data; C.M. performed in vitro cell line studies, in vivo tumor studies and assisted in manuscript preparation; J.L. performed in vitro tumor cell studies and FACS analysis; S.B. assisted with in vivo studies; I.S. performed initial in vitro and vaccine studies; Y.G. performed chemo-synergy studies; K.Y. assisted in in vitro studies; R.J.B. was responsible for study conception, design and coordination as well as manuscript preparation. All authors have read and approved the manuscript.
Glossary
Abbreviations:
- ETRA
endothelin receptor-A
- ETRB
endothelin receptor-B
- ET-1
endothelin-1
- BQ123
endothelin receptor-A specific inhibitor
- BQ788
endothelin receptor-B specific inhibitor
- ICAM-1
intercellular adhesion molecule-1
- CSC
cancer stem cell
- HDMEC
human dermal microvascular endothelial cells
- bEnd.3
brain endothelial cells
- EMT
epithelial mesenchymal transition
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22959
References
- 1.Bhalla A, Haque S, Taylor I, Winslet M, Loizidou M. Endothelin receptor antagonism and cancer. Eur J Clin Invest. 2009;39(Suppl 2):74–7. doi: 10.1111/j.1365-2362.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 2.Grimshaw MJ. Endothelins in breast tumour cell invasion. Cancer Lett. 2005;222:129–38. doi: 10.1016/j.canlet.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Hagemann T, Binder C, Binder L, Pukrop T, Trümper L, Grimshaw MJ. Expression of endothelins and their receptors promotes an invasive phenotype of breast tumor cells but is insufficient to induce invasion in benign cells. DNA Cell Biol. 2005;24:766–76. doi: 10.1089/dna.2005.24.766. [DOI] [PubMed] [Google Scholar]
- 4.Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. J Transl Med. 2004;2:16. doi: 10.1186/1479-5876-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–6. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 6.Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–7. [PubMed] [Google Scholar]
- 7.Kojima K, Nihei Z. Expression of endothelin-1 immunoreactivity in breast cancer. Surg Oncol. 1995;4:309–15. doi: 10.1016/S0960-7404(10)80043-X. [DOI] [PubMed] [Google Scholar]
- 8.Nakamuta M, Ohashi M, Tabata S, Tanabe Y, Goto K, Naruse M, et al. High plasma concentrations of endothelin-like immunoreactivities in patients with hepatocellular carcinoma. Am J Gastroenterol. 1993;88:248–52. [PubMed] [Google Scholar]
- 9.Shankar A, Loizidou M, Aliev G, Fredericks S, Holt D, Boulos PB, et al. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg. 1998;85:502–6. doi: 10.1046/j.1365-2168.1998.00660.x. [DOI] [PubMed] [Google Scholar]
- 10.Bagnato A, Spinella F, Rosanò L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–72. doi: 10.1677/erc.1.01077. [DOI] [PubMed] [Google Scholar]
- 11.Salani D, Di Castro V, Nicotra MR, Rosanò L, Tecce R, Venuti A, et al. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157:1537–47. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morbidelli L, Orlando C, Maggi CA, Ledda F, Ziche M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am J Physiol. 1995;269:H686–95. doi: 10.1152/ajpheart.1995.269.2.H686. [DOI] [PubMed] [Google Scholar]
- 13.Rosanò L, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A. Endothelin-1 promotes proteolytic activity of ovarian carcinoma. Clin Sci (Lond) 2002;103(Suppl 48):306S–9S. doi: 10.1042/CS103S306S. [DOI] [PubMed] [Google Scholar]
- 14.Salani D, Taraboletti G, Rosanò L, Di Castro V, Borsotti P, Giavazzi R, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–11. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoosein MM, Dashwood MR, Dawas K, Ali HM, Grant K, Savage F, et al. Altered endothelin receptor subtypes in colorectal cancer. Eur J Gastroenterol Hepatol. 2007;19:775–82. doi: 10.1097/MEG.0b013e3282c563de. [DOI] [PubMed] [Google Scholar]
- 16.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res. 2006;12:6296s–300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 17.Rosanò L, Cianfrocca R, Spinella F, Di Castro V, Nicotra MR, Lucidi A, et al. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17:2350–60. doi: 10.1158/1078-0432.CCR-10-2325. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Kim JS, Kim SW, Brantley E, Yun SJ, He J, et al. Macitentan (ACT-064992), a tissue-targeting endothelin receptor antagonist, enhances therapeutic efficacy of paclitaxel by modulating survival pathways in orthotopic models of metastatic human ovarian cancer. Neoplasia. 2011;13:167–79. doi: 10.1593/neo.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James ND, Caty A, Payne H, Borre M, Zonnenberg BA, Beuzeboc P, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106:966–73. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 20.Witteveen PO, van der Mijn KJ, Los M, Kronemeijer RH, Groenewegen G, Voest EE. Phase 1/2 study of atrasentan combined with pegylated liposomal doxorubicin in platinum-resistant recurrent ovarian cancer. Neoplasia. 2010;12:941–5. doi: 10.1593/neo.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziche M, Morbidelli L, Donnini S, Ledda F. ETB receptors promote proliferation and migration of endothelial cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S284–6. [PubMed] [Google Scholar]
- 22.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 23.Sidharta PN, van Giersbergen PL, Halabi A, Dingemanse J. Macitentan: entry-into-humans study with a new endothelin receptor antagonist. Eur J Clin Pharmacol. 2011;67:977–84. doi: 10.1007/s00228-011-1043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raja SG. Macitentan, a tissue-targeting endothelin receptor antagonist for the potential oral treatment of pulmonary arterial hypertension and idiopathic pulmonary fibrosis. Curr Opin Investig Drugs. 2010;11:1066–73. [PubMed] [Google Scholar]
- 25.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winer I, Wang S, Lee YE, Fan W, Gong Y, Burgos-Ojeda D, et al. F3-targeted cisplatin-hydrogel nanoparticles as an effective therapeutic that targets both murine and human ovarian tumor endothelial cells in vivo. Cancer Res. 2010;70:8674–83. doi: 10.1158/0008-5472.CAN-10-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulaski HL, Spahlinger G, Silva IA, McLean K, Kueck AS, Reynolds RK, et al. Identifying alemtuzumab as an anti-myeloid cell antiangiogenic therapy for the treatment of ovarian cancer. J Transl Med. 2009;7:49. doi: 10.1186/1479-5876-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, et al. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161:2295–309. doi: 10.1016/S0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgos-Ojeda D, Rueda BR, Buckanovich RJ. Ovarian cancer stem cell markers: prognostic and therapeutic implications. Cancer Lett. 2012;322:1–7. doi: 10.1016/j.canlet.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–18. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 32.Landen CN, Jr., Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–99. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampaio AL, Rae GA, Henriques MM. Role of endothelins on lymphocyte accumulation in allergic pleurisy. J Leukoc Biol. 2000;67:189–95. doi: 10.1002/jlb.67.2.189. [DOI] [PubMed] [Google Scholar]
- 34.Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol. 2010 doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- 35.Ma HJ, Zhu WY, Wang DG, Yue XZ, Li CR. Endothelin-1 combined with extracellular matrix proteins promotes the adhesion and chemotaxis of amelanotic melanocytes from human hair follicles in vitro. Cell Biol Int. 2006;30:999–1006. doi: 10.1016/j.cellbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11:6144–51. doi: 10.3748/wjg.v11.i39.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura A, Kagami S, Urushihara M, Kondo S, Yoshizumi M, Tamaki T, et al. Endothelin-1 is a potent stimulator of alpha1beta1 integrin-mediated collagen matrix remodeling by rat mesangial cells. Biochem Biophys Res Commun. 2002;299:555–61. doi: 10.1016/S0006-291X(02)02693-1. [DOI] [PubMed] [Google Scholar]
- 38.Guruli G, Pflug BR, Pecher S, Makarenkova V, Shurin MR, Nelson JB. Function and survival of dendritic cells depend on endothelin-1 and endothelin receptor autocrine loops. Blood. 2004;104:2107–15. doi: 10.1182/blood-2003-10-3559. [DOI] [PubMed] [Google Scholar]
- 39.Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22:817–23. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Angeli S, Del Pup L, Febas E, Conconi MT, Tommasini M, Di Liddo R, et al. Adrenomedullin and endothelin-1 stimulate in vitro expansion of cord blood hematopoietic stem cells. Int J Mol Med. 2004;14:1083–6. [PubMed] [Google Scholar]
- 41.Steg AD, Bevis KS, Katre AA, Ziebarth A, Alvarez RD, Zhang K, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. Atrasentan Phase III Study Group Institutions A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 43.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Atrasentan Phase 3 Study Group Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witteveen PO, van der Mijn KJ, Los M, Kronemeijer RH, Groenewegen G, Voest EE. Phase 1/2 study of atrasentan combined with pegylated liposomal doxorubicin in platinum-resistant recurrent ovarian cancer. Neoplasia. 2010;12:941–5. doi: 10.1593/neo.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S-J, Kim JS, Kim SW, Yun SJ, He J, Brantley E, et al. Antivascular therapy for multidrug-resistant ovarian tumors by macitentan, a dual endothelin receptor antagonist. Transl Oncol. 2012;5:39–47. doi: 10.1593/tlo.11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosanò L, Cianfrocca R, Spinella F, Di Castro V, Nicotra MR, Lucidi A, et al. Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17:2350–60. doi: 10.1158/1078-0432.CCR-10-2325. [DOI] [PubMed] [Google Scholar]