Abstract
Bone cancer pain remains one of the most challenging cancer pains to fully control. In order to clarify bone cancer pain mechanisms and examine treatments, animal models mimicking the human condition are required. In our model of Walker 256 tumor cells implantation of the shaft of femur at the third trochanter level, the anatomical structure is relatively simple and the drilled hole is vertical and in the cortical bone only 1–2 mm in depth without injury of the distal femur. Pain behaviors and tumor growth were observed for 21 days. And neurochemical changes were further investigated in this model. The results showed that cancer-bearing rats demonstrated a decreased limb use score from day 14, an increased spontaneous flinching and guarding times from day 7 and a decreased withdrawal threshold from day 6. The tumor infiltration of bone was monitored by MRI and further verified by histological examination. C-fos and the capsaicin receptor (TRPV1) positive neurons were more expressed in cancer-bearing rats and the substance P expression has no difference, suggesting that neurons were activated in the model. Our animal model demonstrated time-dependent tumor growth and pain behaviors and will be a novel animal model of bone cancer pain in the future.
Keywords: bone metastases, cancer pain, rat model, the third trochanter, Walker 256 cell
Introduction
Bone cancer pain may occur in patients from either primary bone tumors (sarcoma or hematological malignancies) or more commonly from skeletal metastases from the most often diagnosed cancers such as breast, prostate and lung carcinomas.1 Most patients with metastatic bone disease experience moderate to severe pain, in whom bone pain is one of the most common types and contribute to a general decrease in quality of life.2 Until now, the treatment of bone cancer pain such as breakthrough bone pain remains one of the most challenging cancer pains to fully control.3 The pathological and molecular mechanisms of bone cancer pain have not been fully elucidated. Thus, in an attempt to examine efficacy of new therapeutic approaches and investigate the pathogenesis of bone cancer pain, animal models which can mimic the human condition are needed.
Previously, several mouse and rats models were used in research on bone cancer pain.4-11 Three most commonly used models are the mouse model produced by injection of osteolytic murine sarcoma cells, the rat model induced by intratibia inoculation of rat metastatic cancer cells and the rat model established by injection of metastatic cancer cells into the distal femur.
In the first model,4-6 osteolytic murine sarcoma cells were extensively used, which can mirror what occurs in patients with primary bone cancers. However, primary bone cancers are extremely rare tumors, which occupy less than 0.2% of all cancers,12,13 and osteosarcoma occurred mainly in children and young adults and most patients can be cured,14 which are different from common metastatic bone cancers. In addition, because of the small limbs of mouse, the femur of mouse is a relative large bone and that can be chosen to make the implantation surgery. The patellar ligament of the femur was cut, which may be susceptible to impaired locomotion and confuse the results of behavior tests of bone pain.
In the light of the relative scarcity of murine metastatic cancer cells that can grow in bone and relative more injuries of the mouse model, the rat models of bone cancer pain may be more appropriate. The rat model induced by intratibia inoculation of rat metastatic cancer cells has the advantage of mirroring metastatic bone pain. In these models, the metastatic cancer cells such as the rat mammary tumor cells (MRMT-1,Walker 256) and prostate cancer cells (AT-3.1) were commonly utilized.7,8,10,11,15-17 The tibia was the most frequent injection site reported in these rat models, possibly because the surgery in the tibia is easier than the femur. The muscle units and tendons passing over or attached to the tibia are much less than to the femur. However, from a clinical point of view, the metastases in the tibia are rare. And the femur on the other hand represents the most frequently affected long bone in breast metastases.18,19
In current two femoral rats models,10,11,20 the implantation sites were both on the distal femur. In one model, the patellar ligament was cut and needed partial alteration of the knee joint, which may also cause more damages mentioned above. In another model, although avoiding arthrotomy, there is also a problem that the injection hole is located on the one side of the distal femoral surface, the drill route must be at the specific acute angle with the long bone and the femur was surrounded with muscles and unexposure, so that the drill angle is difficult to control.
Because of all these shortcomings of current bone cancer pain models, we established a new rat bone cancer pain model of femur at the third trochanter level. In our model, the bony landmark of rat femur is the third trochanter, which can be easily palpated and located for injection surgery in the rat prone position. In addition, the anatomical structure at the third trochanter is relatively simple and the implantation hole can be easily drilled in vertical to the femur shaft. This new model can be easily made and cause minimal injury.
Results
Implantation of breast cancer cells Walker 256 in the femur induce pain behaviors in rats
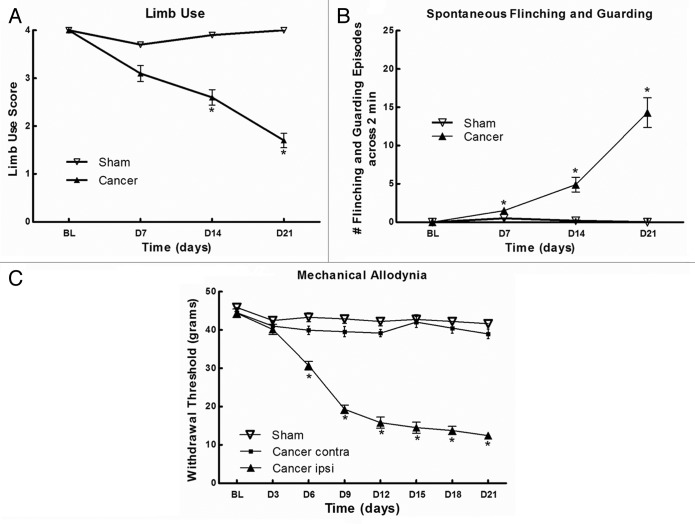
According to the data (Fig. 1), sham rats showed no signs of movement-evoked pain, spontaneous pain or mechanical allodynia throughout the behavioral test period, which can indicate that the impact of surgery at the third trochanter level was minimal, so that it did not affect the pain behaviors. Cancer cells did not induce mechanical allodynia on the contralateral side of rats (Fig. 1C). Cancer-bearing rats demonstrated a decreased limb use score from day 14 (2.60 ± 0.16; p < 0.05; Fig. 1A), a increased spontaneous flinching and guarding times across 2 min from day 7 (1.50 ± 0.27; p < 0.05; Figure 1B) and a decreased withdrawal threshold in the dynamic von Frey test from day 6 (30.7 ± 4.7 g; p < 0.05; Figure 1C). These results show that Walker 256 cells can elicit movement-evoked pain (limb use), spontaneous pain behaviors (flinching and guarding) and mechanical allodynia in femur, as previously reported in the tibia.16,21
Figure 1. Evaluation of pain behaviors following the implantation of Walker 256 cells in rat left femur. (A) Movement-evoked pain was determined using limb use score. Significant impaired limb use was observed at days 14 and 21. (B) Spontaneous flinching and guarding times across 2 min indicate spontaneous pain behaviors and the significant differences were found at days 7, 14 and 21. (C) Mechanical allodynia was determined using the von Frey test. The withdrawal threshold diminishes progressively from day 6. All graphs show means ± SEM, n = 10 rats/group, *p < 0.05.
Radiological and histochemical analysis of tumor development in the femur
To determine cancer development, the femurs of rats were monitored by MRI at days 7, 14 and 21 following the implantation (Fig. 2). Sagittal MR images showed homogeneous signal of the bone marrow in the untreated rats (naive) and Hank's solution injected rats (sham). And tumor-bearing rats demonstrated the time-dependent nature of tumor progression. At day 7, diffuse hyperintense medullary infiltration was observed and a regular cortical line except implantation site was presented. At day 14, tumor proliferation affects both the bone marrow and the regular cortical line. And at day 21, the whole medullary cavity was invaded. Then the femora were embedded and stained with Harris’ hematoxylin and eosin to verify cancer cell infiltration (Fig. 3).
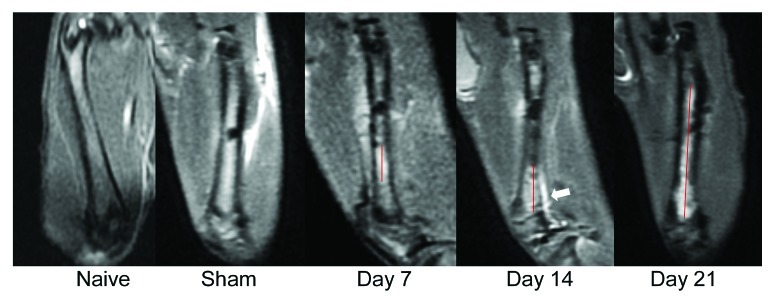
Figure 2. MRI images of bone cancer-bearing rat femurs. Sagittal planes of the femur at different stages of tumor progression. T2 magnetic resonance images (MRI) of a rat coping with femoral bone show tumor-affected part of the femur (red line). Tumor proliferation affects the regular cortical line at day 14 (white arrowhead).

Figure 3. Hematoxylin and eosin staining in the rat femur at day 14 post-surgery. Panels (A) and (B) demonstrate the sham surgery group showing healthy bone structures. And panels (C) and (D) demonstrate that breast cancer cells filled the intramedullary space of the femur. (A and C: original magnification 40× ; B and D: original magnification 200× ).
Neuronal plasticity, the capsaicin receptor TRPV1 and substance P (SP) in cancer-treated rats
The immunohistochemical analysis for c-fos, TRPV1 and SP expression were performed through the L4–L6 spinal cord segments at day 14 following cancer cells injection (Fig. 4). C-fos and TRPV1 positive neurons were more expressed in cancer-bearing rats (Figs. 4A, 4B, 4C, 4D). In selected area of ipsilateral dorsal horn localized in laminae V-VI, random 3 fields under 200× magnification were selected and the c-fos and TRPV1 positive neurons were counted, which were significantly more in cancer-bearing rats than in sham animals (Fig. 4G; p < 0.01). And the SP expression has no difference between the two groups.
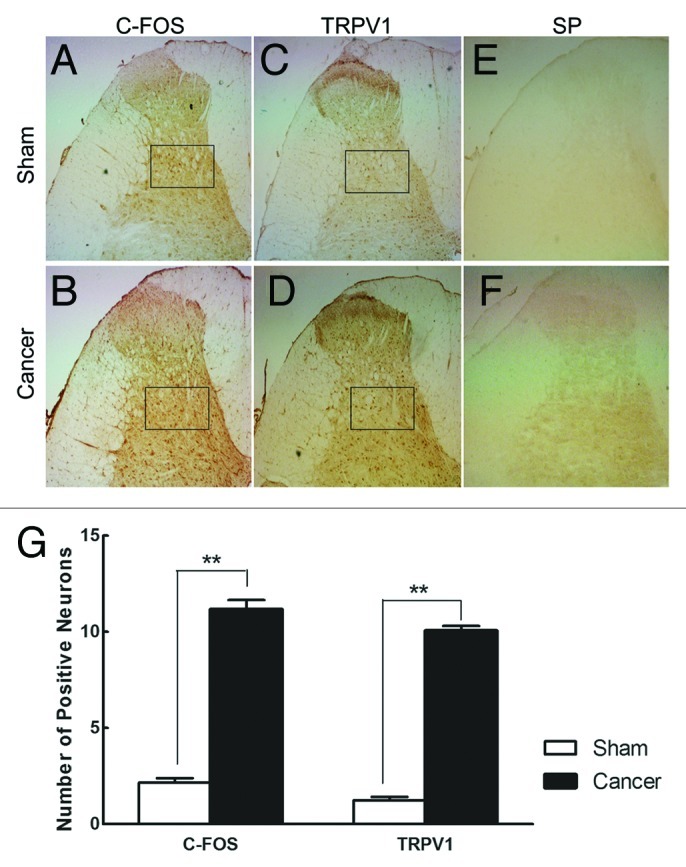
Figure 4. Immunohistochemical staining of spinal c-Fos, TRPV1 and SP. (A–F) Photographs of c-fos, TRPV1 and SP proteins’ ipsilateral dorsal horn profiles in both cancer-bearing and sham rats, respectively. (G) Number of c-fos and TRPV1 positive neurons in selected areas (black box, randomly selected 3 fields under 200 × magnification) for sham and cancer rats. Data are mean ± SEM, **p < 0.01.
Figure 5 demonstrated the western blot analysis of rat L4–L6 ipsilateral spinal cord tissue with c-fos antibody. To quantitate the protein samples that were loaded, the parallel membrane was probed with GAPDH antibody as the internal control. C-fos expression was increased with time. From day 7, c-fos/GAPDH ratio has been significantly higher than sham (p < 0.05).
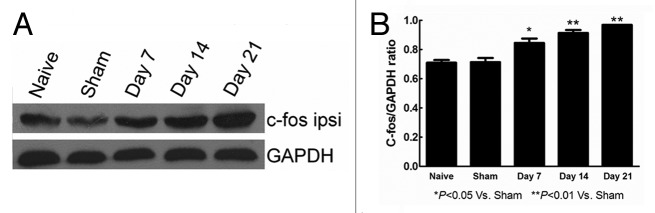
Figure 5. The expression of c-fos protein determined by western blot in naive, sham, day 7, 14, 21 rats. (A) The western blot results of c-fos and GAPDH were showed. (B) C-fos/GAPDH ratio histogram reveals that the c-fos expression were significantly higher in day 7, day 14, day 21 than in sham group. *p < 0.05, **p < 0.01.
Discussion
The model of bone cancer pain includes two factors, tumor infiltration of bone and pain behaviors induced by the tumor. The present study demonstrated that Walker 256 rat mammary gland carcinoma cells in the femur can induce pain behaviors, including movement-evoked pain, spontaneous pain and mechanical allodynia, which were previously presented only in the tibia models.16,21,22
It may be found that all of bone cancer pain models involve the direct injection of cancer cells into the intramedullary space of the mouse or rats’ tibia or femur. The injection method can be considered the most general method because, first, the tumor site is definite, avoiding the animal-to-animal variability in the sites, size and extent of the blood-borne metastasis; second, tibia or femur is not the vital organ, thus, the animal general health is better than that in the blood-borne metastasis, of which the tumors frequently metastasize to vital organs such as the lung or liver and the behavioral assessment of bone pain in former is relatively stable and can last more days; and third, the pain of tibia or femur can be easily detected by animal behavioral changes including flinch, guarding and limb.
In this study, the femora of rats were first perforated in the shaft at the third trochanter level instead of both ends of the femur. The incision can be located clearly by touching the third trochanter in the rat prone position. And the anatomical structure at this site is relatively simple. After incision of skin and subcutaneous fascia, the third trochanter and attached muscles were exposed. The rectus femoris muscle could be peeled along the medical edge of femur in order to reduce the muscle damage. And the exposed bone surface was on the shaft of femur and relatively flat, so that a vertical hole was drilled in the cortical bone only 1–2 mm in depth and the syringe could reach bone marrow. In previous femur models,10,11 the implantation hole was located in the distal femur, either in the intercondylar notch or between the medial epicondyle and the adductor tubercle and both the perforation holes were through cortical bone, epiphysis and metaphysis. The former was straightly 3–5 mm in depth and the latter was at the specific acute angle and 5–6 mm in depth, which may cause more bone injury. The injection surgery causes the minimal injury the better, which can avoid bias of the followed evaluation of pain-related behaviors.
In this model, the impact of implantation surgery was minimal. According to the data, sham operation group did not show pain behaviors and there was no difference between sham group and cancer-bearing group at day 3 following surgery.
In bone cancer pain animal models, it is needed to confirm that the pain behaviors are indeed induced by cancer, or there is tumor infiltration in bone,23 so that a suitable imaging method is important for diagnosis of tumor growth in rat model of bone cancer pain. According to previous clinical data and meta-analysis results,24,25 the MRI is more sensitive and specific than (18) FDG-PET and bone scintigraphy for detecting bone metastases in breast cancer patients. Therefore, it may be more appropriate to choose MRI technique to monitor tumor progression in bone for rat models. In this study, the tumor infiltration of bone can be monitored by MR images, even at early stage (7 d following surgery). And the bone destruction was time-dependent. These cancer-induced bone changes were further verified by bone histological examination.
In addition, the pain of this bone cancer model can be further investigated by neurochemical changes after rats were sacrificed. From more than 20 y, the proto-oncogene c-fos has been considered to be a marker for neuronal activation in the spinal cord and topographically correlated with that of primary afferent projection from the hindpaw.26 In our pain model, the c-fos expression of L4–L6 ipsilateral spinal cord tissue in cancer group was significantly higher than that in sham group, suggesting that neurons were activated in our bone cancer pain model. And the c-fos expression was also time-dependent. Since c-fos can be rapidly induced within minutes by stimulation of a neurotransmitter,27 the early pain can be detected by comparison of c-fos expression (day 7, p < 0.05). And the bone pain induced by cancer might be lasting and accumulated. In previous studies, it has been shown that the capsaicin receptor, transient receptor potential vanilloid (TRPV1) is involved in the induction of bone cancer pain and is activated by local tissue acidosis.28-30 Our study confirmed this observation. Acid environment may contribute to bone cancer pain. And the substance P did not change in our bone cancer model, which is also similar with the results of reported articles.31-33 Nevertheless, the SP expression was increased in neuropathic pain and inflammatory pain models.34-37
Conclusion
The present study demonstrates that inoculation of Walker 256 cells in the shaft of femur at the third trochanter level produces progressive tumor growth and pain behaviors. Neuronal activation and local tissue acidosis may be involved in our bone cancer pain model. And this model will be a new model used to investigate mechanisms and clinical approaches for bone cancer pain.
Materials and Methods
Cell culture
Walker 256 rat mammary gland carcinoma cells were kindly provided by the laboratory of Department of Anesthesiology in Tongji Hospital (Huazhong University of Science and Technology, Wuhan, China) and maintained in RPMI 1640 medium (Gibco, Canada) containing 10% fetal bovine serum and 2% penicillin/streptavidin and cultured in a water-saturated incubator in 5% CO2: 95% air. Cells were collected by centrifuging medium for 3 min at 1,200 rpm, rinsed with calcium-and magnesium-free Hank's solution, counted with a hemocytometer and re-centrifuged in the same conditions. The final pellet was diluted to a final concentration for injection of 105 cells in 10 μl Hank's solution and kept on ice prior to surgery.
Animals
Adult female Wistar rats weighing 220–250 g (Experimental Animal Research Center, Hubei, China; SCXK E 2008- 0005) were housed in a temperature-controlled (22 ± 0.5°C) room with a 12-hlight/dark cycle and given food and water ad libitum. All experiments were conducted in accordance with the ethical guidelines of the National Institutes of Health.
Implantation surgery
Rats were completely anesthetized with 6% chloral hydrate i.p. and laid in the prone position. The left leg was shaved and the skin was disinfected with 7% tincture of iodine. A 1-cm-long skin incision was made over the third trochanter. The rectus femoris muscle was gently incised along the medical edge (5–8mm in length) to expose the shaft of femur. (Fig. S1) A 23-gauge needle was drilled vertically in the shaft of femur at the third trochanter level, allowing it to reach the intramedullary canal of the femur. The needle was then replaced with a 20 μl microinjection syringe containing 10 μl volume of Walker 256 cells (105 cells) or Hank's solution (sham group). After slow injection and a 2 min delay to allow cells to fill the bone cavity, the syringe was removed and the drill hole was sealed using bone wax. The site was thoroughly washed with sterile deionized water. The muscle and skin were finally stitched and disinfected.
Behavioral tests
Each rat was tested for movement-evoked pain (limb use), spontaneous pain behaviors (flinching and guarding) and mechanical allodynia before cancer cells or sham injections and then on days 7, 14 and 21, or days 3, 6, 9, 12, 15, 18 and 21 post-surgery. Rats were allowed to habituate for a period of 30 min before following tests: (1) Limb use was evaluated by limping and guarding behavior score of the rats, which were placed in an open field and observed while normal ambulation. The left (cancer treated) hind-limbs of rats were rated on the following scale: 0, complete lack of use, 1, partial non-use, 2, limping and guarding; 3, limping; 4, normal walking. (2) The numbers of spontaneous guarding and flinching, representative of behaviors indicative of spontaneous pain, were recorded during a 2 min observation period. (3) Mechanical allodynia was measured as previously reported,11 using a dynamic plantar esthesiometer (Ugo Basile S. R. L 21025 Comerio VA). Briefly, rats were placed in boxes on an elevated wire mesh. And a straight metal filament was orientated upwards until it touched the plantar surface of the hind paw and began to exert an upward force. When the paw was withdrawn or the preset cut-off was reached (50 g), the force was automatically registered in grams and the filament was automatically pulled down.
Magnetic resonance imaging
MRI studies were performed with 3.0T scanner (Signal, HDxt, GE Healthcare, USA) before and on days 7, 14 and 21 post-surgery. Rats were anaesthetized with 6% chloral hydrate and placed in the supine position. The MRI used a gradient echo sequence with TR: 2160 ms, TE: 40 ms, matrix: 192 × 160, FOV: 60 × 60 mm2, Slice thickness: 1 mm and ETL: 16.
Immunohistochemistry
Rats were deeply anaesthetized with 10% chloral hydrate. The spinal cord segments (L4-L6) were removed, immediately O.C.T. embedded and snap frozen in ice-cold isopentane for 1 min, minimizing ice crystal formation.38 Serial frozen spinal cord sections were cut with a freezing microtome (Leica CM 1900 UV, Leica) at a thickness of 15 μm, collected and processed on slides. These sections were stained using a standard avidin-biotin-peroxidase complex (ABC) technique. Briefly, the sections were pre-incubated in a solution containing 0.3% TritonX-100 in PBS after blocking endogenous peroxidase. After incubated for 40 min at room temperature in a blocking solution of 5% normal goat serum in PBS, the sections were incubated overnight at 4°C with primary antiserum (c-fos, 1: 400, sc-52; TRPV1, 1: 400, sc-28759; SP, 1: 100, sc-9758, Santa Cruz Biotechnology). And then, the sections were incubated with biotinylated goat antirabbit IgG solution for 2 h (1: 200, Zhongshan Goldenbrige Biotechnology). After reacted with avidin-biotinreagents (1:100) for 1 h at room temperature, the sections then were incubated for 3 min with 0.02% diaminobenzidine with 0.01% hydrogen peroxide and finally dehydrated with ethanol, cleared in xylene and coverslipped.
The femora of rats were also removed to determine cancer cell infiltration. Femurs were fixed in 4% paraformaldehyde for 2 d and decalcified in 10% EDTA for 3 weeks. After embedded in paraffin, the femurs were cut in 4 μm and stained with Harris’ hematoxylin and eosin.
Western blot analysis
L4–6 spinal cords were collected from sham and cancer animals. The total protein of ipsilateral and contralateral spinal cords was extracted using radio immune precipitation buffer (RIPA) protein lysis buffer. The protein concentrations were measured using a BCA assay (Beyotime Biotechnology, China). Samples (200 μg protein in each lane) were separated using SDS-PAGEs (10%) and transferred onto PVDF membranes. The membranes were blocked in 10% non-fat dry milk for 3 h at room temperature and then incubated overnight in 4°C with primary antibody (c-fos, 1: 1000, sc-52, Santa Cruz Biotechnology; GAPDH, 1: 10000, Epitomics, USA). And then, the membranes were probed for 2 h with an anti-rabbit horseradish peroxidase conjugated with a secondary antibody (1: 5000, Santa Cruz Biotechnology). Finally, the bands were visualized in ECL solution (Pierce) and exposed onto X-films.
Statistical analyses
The SPSS 12.0 software package was used to perform statistical tests. Pain behaviors data were analyzed using one-way ANOVA followed by Bonferroni’s post-hoc test. Data from the immunohistochemical and Western blot studies were analyzed using student’s t-test. The significance level was set at p < 0.05.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Shijiang Yang for his surgical suggestions. We thank Dr. Changbin Ke for his provided the Walker 256 cells.
Glossary
Abbreviations:
- C-fos
a proto-oncogene
- TRPV1
transient receptor potential vanilloid 1
- SP
substance P
- MRI
magnetic resonance image
- RIPA
radio immune precipitation buffer
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- FDG
an analogue of glucose
- PET
Positron emission tomography
- TR
repetition time
- TE
echo time
- FOV
field-of-view
- ETL
echo train length
- OCT
optimum cutting temperature
- ABC
avidin-biotin-peroxidase complex
- EDTA
ethylene diamine tetraacetic acid
- PVDF
polyvinylidene difluoride
Supplemental Materials
Supplemental materials may be found here:
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/23291
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–94. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1588::AID-CNCR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–81. doi: 10.1111/j.1749-6632.2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeppetella G. Impact and management of breakthrough pain in cancer. Curr Opin Support Palliat Care. 2009;3:1–6. doi: 10.1097/SPC.0b013e3283260658. [DOI] [PubMed] [Google Scholar]
- 4.Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med. 2000;1:303–9. doi: 10.1046/j.1526-4637.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 5.Sevcik MA, Luger NM, Mach DB, Sabino MA, Peters CM, Ghilardi JR, et al. Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis. Pain. 2004;111:169–80. doi: 10.1016/j.pain.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010;6:87. doi: 10.1186/1744-8069-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, et al. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008;154:1533–8. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colvin L, Fallon M. Challenges in cancer pain management--bone pain. Eur J Cancer. 2008;44:1083–90. doi: 10.1016/j.ejca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, et al. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–36. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 10.De Ciantis PD, Yashpal K, Henry J, Singh G. Characterization of a rat model of metastatic prostate cancer bone pain. J Pain Res. 2010;3:213–21. doi: 10.2147/JPR.S14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doré-Savard L, Otis V, Belleville K, Lemire M, Archambault M, Tremblay L, et al. Behavioral, medical imaging and histopathological features of a new rat model of bone cancer pain. PLoS One. 2010;5:e13774. doi: 10.1371/journal.pone.0013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(Suppl):203–10. doi: 10.1002/1097-0142(19950101)75:1+<203::AID-CNCR2820751308>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 14.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 15.Roudier MP, Bain SD, Dougall WC. Effects of the RANKL inhibitor, osteoprotegerin, on the pain and histopathology of bone cancer in rats. Clin Exp Metastasis. 2006;23:167–75. doi: 10.1007/s10585-006-9026-x. [DOI] [PubMed] [Google Scholar]
- 16.Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, et al. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. 2006;345:1292–8. doi: 10.1016/j.bbrc.2006.04.186. [DOI] [PubMed] [Google Scholar]
- 17.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, et al. A rat model of bone cancer pain. Pain. 2002;96:129–40. doi: 10.1016/S0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 18.Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36:476–82. doi: 10.1016/S0959-8049(99)00331-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–80. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 20.Proschek D, Mack MG, Kurth AA, Proschek P, Martin B, Hansmann ML, et al. Radiofrequency ablation of experimental bone metastases in nude rats. Anticancer Res. 2008;28(2A):879–85. [PubMed] [Google Scholar]
- 21.Yu S, Peng HD, Ju DW, Wei PK, Xu L, Lao LX, et al. Mechanisms of treatment of cancer pain with a topical Chinese herbal formula in rats. Chin Med J (Engl) 2009;122:2027–31. [PubMed] [Google Scholar]
- 22.Cao F, Gao F, Xu AJ, Chen ZJ, Chen SS, Yang H, et al. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res. 2010;1326:162–73. doi: 10.1016/j.brainres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Ohlmann-Knafo S, Kirschbaum M, Fenzl G, Pickuth D. [Diagnostic value of whole-body MRI and bone scintigraphy in the detection of osseous metastases in patients with breast cancer--A Prospective Double-Blinded Study at two Hospital Centers] Rofo. 2009;181:255–63. doi: 10.1055/s-0028-1109104. [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Cheng T, Xu W, Yan WL, Liu J, Yang HL. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–31. doi: 10.1007/s00256-010-0963-8. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–4. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–3. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- 28.Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–31. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, et al. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142:27–35. doi: 10.1016/j.pain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148:560–72. doi: 10.1016/j.neuroscience.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 31.Park HC, Seong J, An JH, Kim J, Kim UJ, Lee BW. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys. 2005;61:1523–34. doi: 10.1016/j.ijrobp.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 32.Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(Suppl):866–73. doi: 10.1002/cncr.11144. [DOI] [PubMed] [Google Scholar]
- 33.Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain. 2009;13:138–45. doi: 10.1016/j.ejpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Nitzan-Luques A, Devor M, Tal M. Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. Pain. 2011;152:2413–26. doi: 10.1016/j.pain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Peng X, Hagshenas J, Insolera R, Fink DJ, Mata M. A novel cell-cell signaling by microglial transmembrane TNFα with implications for neuropathic pain. Pain. 2010;151:296–306. doi: 10.1016/j.pain.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parenti C, Aricò G, Ronsisvalle G, Scoto GM. Supraspinal injection of Substance P attenuates allodynia and hyperalgesia in a rat model of inflammatory pain. Peptides. 2012;34:412–8. doi: 10.1016/j.peptides.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Geng X, Zhang L, Zeng Y, Dong H, Yu H. Substance P expression in the distal cerebrospinal fluid-contacting neurons and spinal trigeminal nucleus in formalin-induced the orofacial inflammatory pain in rats. Brain Res Bull. 2009;78:139–44. doi: 10.1016/j.brainresbull.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Erickson QL, Clark T, Larson K, Minsue Chen T. Flash freezing of Mohs micrographic surgery tissue can minimize freeze artifact and speed slide preparation. Dermatol Surg. 2011;37:503–9. doi: 10.1111/j.1524-4725.2011.01926.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.