Abstract
Nitric oxide (NO) has been known to preserve the level of chlorophyll (Chl) during leaf senescence. However, the mechanism by which NO regulates Chl breakdown remains unknown. Here we report that NO negatively regulates the activities of Chl catabolic enzymes during dark-induced leaf senescence. The transcriptional levels of the major enzyme genes involving Chl breakdown pathway except for RED CHL CATABOLITE REDUCTASE (RCCR) were dramatically up-regulated during dark-induced Chl degradation in the leaves of Arabidopsis NO-deficient mutant nos1/noa1 that exhibited an early-senescence phenotype. The activity of pheide a oxygenase (PAO) was higher in the dark-induced senescent leaves of nos1/noa1 compared with wild type. Furthermore, the knockout of PAO in nos1/noa1 background led to pheide a accumulation in the double mutant pao1 nos1/noa1, which retained the level of Chl during dark-induced leaf senescence. The accumulated pheide a in darkened leaves of pao1 nos1/noa1 was likely to inhibit the senescence-activated transcriptional levels of Chl catabolic genes as a feed-back inhibitory effect. We also found that NO deficiency led to decrease in the stability of photosynthetic complexes in thylakoid membranes. Importantly, the accumulation of pheide a caused by PAO mutations in combination with NO deficiency had a synergistic effect on the stability loss of thylakoid membrane complexes in the double mutant pao1 nos1/noa1 during dark-induced leaf senescence. Taken together, our findings have demonstrated that NO is a novel negative regulator of Chl catabolic pathway and positively functions in maintaining the stability of thylakoid membranes during leaf senescence.
Introduction
Chlorophyll (Chl) molecules play a central role in the initial and indispensable processes of photosynthesis, such as harvesting light energy and driving electron transfer. However, like most porphyrins, Chl is also a dangerous molecule and potentially hazardous to plant cells in situations where the photosynthetic apparatus is overexcited and absorbed energy is transferred from chl to oxygen, resulting in the production of reactive oxygen species (ROS) [1], [2]. Indeed, plant cells need a process to inactivate this hazard efficiently through a Chl catabolism pathway. As a dramatically visualized sign of leaf senescence and fruit ripening, loss of green color is resulted from Chl breakdown combined with carotenoid retention or anthocyanin accumulation [2], [3]. Thus, the degradation of Chl is a prerequisite to detoxify the potentially phototoxic pigments in order to remobilize the nitrogen pools of the apoproteins from Chl-binding proteins in chloroplasts during leaf senescence [2]–[5].
In recent years, important progresses have been made in better understanding the pathway of Chl catabolism in higher plants. In brief, the initial reaction during the Chl breakdown pathway is the removal of the phytol residue and the central Mg by chlorophyllase and metal chelating substance, respectively. In 1999, Chlorophyllase genes, termed CLHs, were cloned in Arabidopsis leaves [6]. However, recent studies questioned the involvement of CLHs in Chl breakdown in vivo during leaf senescence [7], [8] even though the two CLHs present in Arabidopsis exhibited chlorophyllase activity in vitro [6]. In 2009, pheophytinase (PPH) was identified to function in porphyrin-phytol hydrolysis involved in senescence-related chlorophyll breakdown in vivo [9]. In the next-step reactions, the resulting pheophorbide (pheide) a is converted into a primary fluorescent chlorophyll catabolite (pFCC), which requires two enzymes including pheide a oxygenase (PAO) and red chl catabolite reductase (RCCR) [10]–[13]. PAO is identical to ACCELERATED CELL DEATH (ACD1) and encodes a chloroplast envelop-bound Rieske-type iron-sulfur oxygenase [12]. Unlike PAO, RCCR is a soluble protein targeted to chloroplasts [11]. PAO catalyzes the cleavage of the porphyrin ring, resulting in the red chlorophyll catabolite, which is further reduced in to pFCC by RCCR. The resulting primary fluorescent catabolite pFCCs are exported from the plastid and after further modification they are imported into their final destination, the vacuole by a primary active transport system [14], [15].
Recently, the genetic lesion identification of stay-green mutants has provided an in-depth understanding of chlorophyll degradation pathway. NON-YELLOW COLORING1 (NYC1) and NYC1-LIKE (NOL) were cloned based on the genetic analysis of the stay-green mutants nyc1 and nol, which selectively retained photosystem II (PSII) light-harvesting complex subunits and exhibited high concentrations of chlorophyll b [16], [17]. NYC1 and NOL, encoding two subunits of chlorophyll b reductase, catalyze the first half of chlorophyll b to chlorophyll a reduction [17]. Besides nyc1/nol, the mutation of the PAO gene caused a stay-green phenotype during dark-induced leaf senescence. It was reported that the pao mutant plants showed a light-dependent lesion mimic phenotype due to the accumulation of phototoxic pheide a [12], [18], [19]. Recent studies on plant senescence research have been focusing on the third type of stay-green mutants, which are defective in a stay-green gene, termed senescence-induced degradation (SID) in the Bf993 mutant of Festuca pratensis [20]–[22]. The orthologous genes of SID, now designated SGR (STAY-GREEN), have been identified in a variety of plant species such as Arabidopsis [23], rice [24]–[26], pea [26], [27], bell pepper [28], [29], and tomato [28]. Interestingly, SGR directly interacts with a subset of the proteins in the light harvesting chlorophyll a/b-protein complex II (LHCPII), implying that SGR may be involved in destabilizing pigment-protein complexes as a prerequisite for chlorophyll degrading enzymes to access their substrate during leaf senescence [2], [25], [30].
Although tremendous progress has been made in the elucidation of the mechanism through which chlorophyll is degraded in the last decade, our current knowledge of the regulatory networks of chlorophyll breakdown during senescence remains limited [2], [3], [30]. Given that plant hormones regulate senescence processes marked as Chl breakdown, it is not unexpected that Chl catabolic gene expression is under hormonal control. The senescence-promoting hormones ethylene and MeJA stimulate CLH activity [31]. The treatment of MeJA activates the expression of AtCLH1 in Arabidopsis [6], and in Citrus, CLH1 is highly up-regulated by ethylene [32]. As a key player of Chl breakdown pathway, PAO is highly expressed in senescent tissues, but at low levels in presenescent leaves [18]. Like AtCLH1 [33], the expression of PAO is also responsive to wounding [34]. Generally, it remains to be shown which hormonal regulators or messenger molecules are critical for the regulation of Chl degradation pathway.
NO has been noted as an antisenescence signal in that NO treatments extend the postharvest life of fruits and vegetables [35]. During storage, NO application delays yellowing and retards the onset of chlorophyll degradation in broccoli (Brassica oleracea) florets [36], [37]. NO preserves the level of Chl in potato leaves infected by Phytophthora infestans [38] and counteracts leaf senescence induced by MeJA [39]. In pea (Pisum sativum), NO levels are significantly reduced during the natural senescence [40] and negatively correlate with ethylene levels [35], [41]. NO treatments can protect against senescence-dependent chlorophyll degradation in soybean (Glycine max) cotyledons [42]. On the other hand, leaf senescence occurs more rapidly in NO-deficient mutants compared with wild type plants [43], [44].
In this study, we have explored the physiological effects of the reduced endogenous NO levels on Chl degradation during dark-induced leaf senescence using the NO-deficient mutant nos1/noa1 [45] in combination with the expression analysis of Chl catabolic genes and the enzymatic activity examination of PAO. Although the role of NOS1/NOA1 in NO biosynthesis remains controversial [45]–[49], it has been demonstrated that the mutation of NOS1/NOA1 causes a significant reduction in NO production in Arabidopsis [45], [50]–[54] and Nicotiana benthamiana [55]. Recent data suggested that NOS1/NOA1 is a functional cGTPase essential for chloroplast function [56], [57] and its role in endogenous NO accumulation needs to be further explored [49], [58], [59]. Interestingly, mutant NOS1/NOA1 lacking the C-terminal domain, although retaining GTPase activity, failed to complement the mutant nos1/noa1, suggesting that this GTPase activity of NOS1/NOA1 is not sufficient to recover the NO deficiency-related phenotypes of the nos1/noa1 plants [49]. In this study, we observed that the rapid loss of Chl occurring in the NO-deficient mutant nos1/noa1in darkness is attributable to the up-regulated expression of the major genes involved in Chl degradation pathway. Here, we also show that the knockout of PAO in the nos1/noa1 mutant retains levels of Chl during dark-induced leaf senescence, suggesting that the PAO mutation-caused accumulation of Chl catabolite pheide a is most likely to inhibit the rapid degradation of Chl resulted from NO deficiency in the nos1/noa1 mutant. Our findings support the hypothesis that NO functions as an anti-senescence messenger molecule through repressing the transcriptional activation of the major Chl catabolic genes during leaf senescence.
Results
NO represses the transcriptional activation of Chl catabolic pathway genes during dark-induced leaf senescence
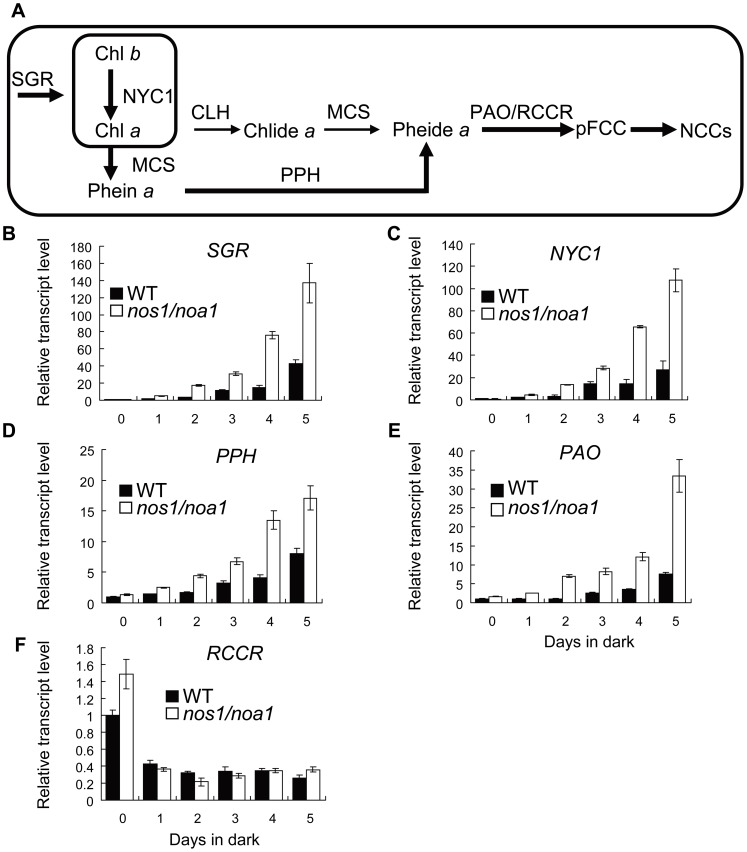
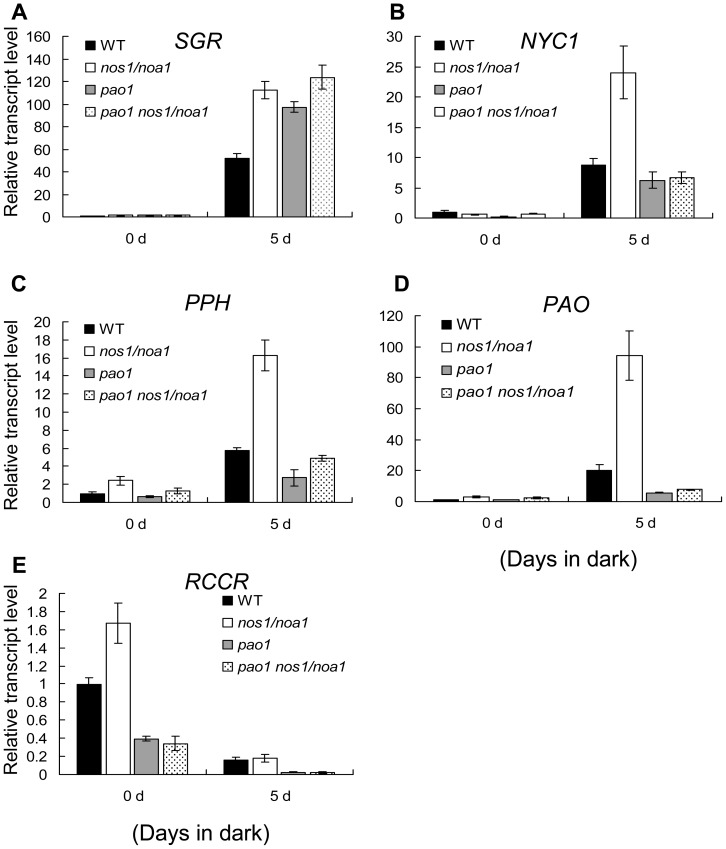
Given that the depletion of endogenous NO leads to an early leaf senescence phenotype in the mutant plants [43], [44], we investigated whether NO plays a role in the regulation of Chl breakdown pathway during leaf senescence using the NO-deficient mutant nos1/noa1. The quantitative reverse transcription PCR technique was used to examine the relative levels of the major genes involving Chl breakdown pathway in the wild type and nos1/noa1 mutant leaves during dark-induced leaf senescence. Upon dark treatment for 5 d, the transcription levels of SGR, NYC1, PPH and PAO were dramatically up-regulated in the nos1/noa1 mutant compared with that in wild type (Fig. 1B–E). The strongest increase (3.4-fold) was detected for PAO, and the increase range for the rest of the genes was from 1.1 to 2.9-fold when leaves were dark-treated to induce senescence. In contrast to the four genes mentioned above, the mRNA level of RCCR was down-regulated at day one and was kept at similar levels in both the wild type and nos1/noa1 mutant leaves through to day 5 in darkness, which is consistent with the previous results that RCCR expression is constitutively active throughout the process of leaf senescence [60], [61]. Interestingly, we found that sodium nitroprusside (SNP) treatment (250 µM) dramatically inhibited the transcriptional activation of Chl breakdown pathway genes, including SGR, NYC1, PPH and PAO in the nos1/noa1 mutant compared with the mutant leaves under control treatment (Fig. S1). On the other hand, we have also performed the NO depletion experiments to further validate our hypothesis using the widely-used NO specific scavenger, cPTIO. We found that upon dark treatment for 4 d, the transcription levels of SGR, NYC1, PPH and PAO were more dramatically up-regulated in the darkened leaves of wild type treated with 500 µM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3 oxide (cPTIO) compared with the leaves of wild type under control condition, which mimics the expression patterns of Chl catabolic genes in the NO-deficient mutant nos1/noa1 during dark-induced leaf senescence (Fig. S2). In addition, it should be mentioned that no significant difference was observed in the transcript levels of SGR, NYC1, PPH and PAO between the wild type and nos1/noa1 mutant at the beginning of dark-treatment. These results indicate that NO represses the transcriptional activation of the major genes involving Chl catabolic pathway during dark-induced leaf senescence.
Figure 1. Transcript levels of chlorophyll breakdown pathway genes in wild type and nos1/noa1 mutant leaves during dark-induced senescence.
(A) A suggestive model for chlorophyll breakdown pathway with integrating the recent findings in Arabidopsis. Thickness of arrows reflects relative activities of respective enzymes (Hörtensteiner, 2009). (B–F) qRT-PCR analysis of mRNA abundance of enzyme genes (SGR, NYC1, PPH, PAO and RCCR) involved in chlorophyll degradation in wild type and nos1/noa1 mutant leaves during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
NO inhibits PAO activity during dark-induced leaf senescence
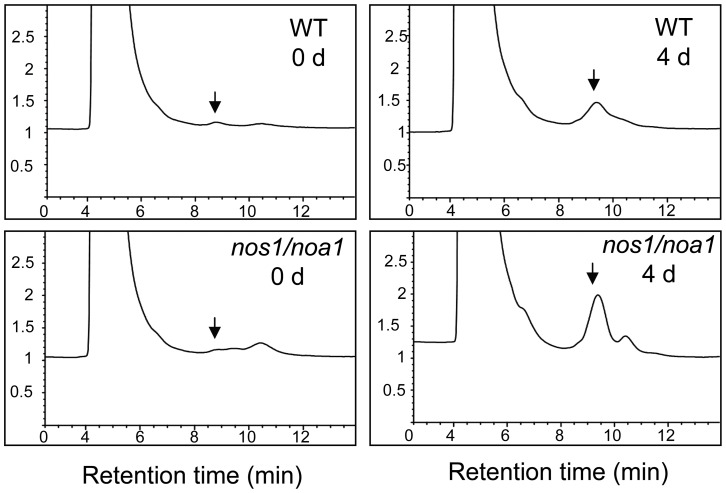
In Arabidopsis, the activity of PAO is enhanced in senescent leaves [12]. To verify the effect of NO on the activity of PAO during dark-induced leaf senescence, we assessed in vivo PAO activity using PAO-RCCR coupled assay with crude extracts of PAO and RCCR from the wild type and nos1/noa1 mutant leaves, respectively, during a 4-d dark treatment. The results showed that the activity of PAO in the nos1/noa1 mutant was significantly higher than that in wild type at day 4 in darkness, although the activities of PAO in both genotypes increased upon dark treatment (Fig. 2 and Table S1). These data further support that NO acts as a negative regulator in modulating chlorophyll breakdown during leaf senescence.
Figure 2. Activities of PAO in wild type and nos1/noa1 leaves during dark-induced leaf senescence.
HPLC analysis of pFCC-1 generated by PAO-RCCR coupled assay with crude extracts of PAO and RCCR from wild type and nos1/noa1 leaves during a 4-d dark treatment. pFCC-1 was eluted after 9 min, as indicated by arrows. The experiment was repeated once with qualitatively identical results.
PAO mutations delay the rapid Chl degradation in the nos1/noa1 mutant
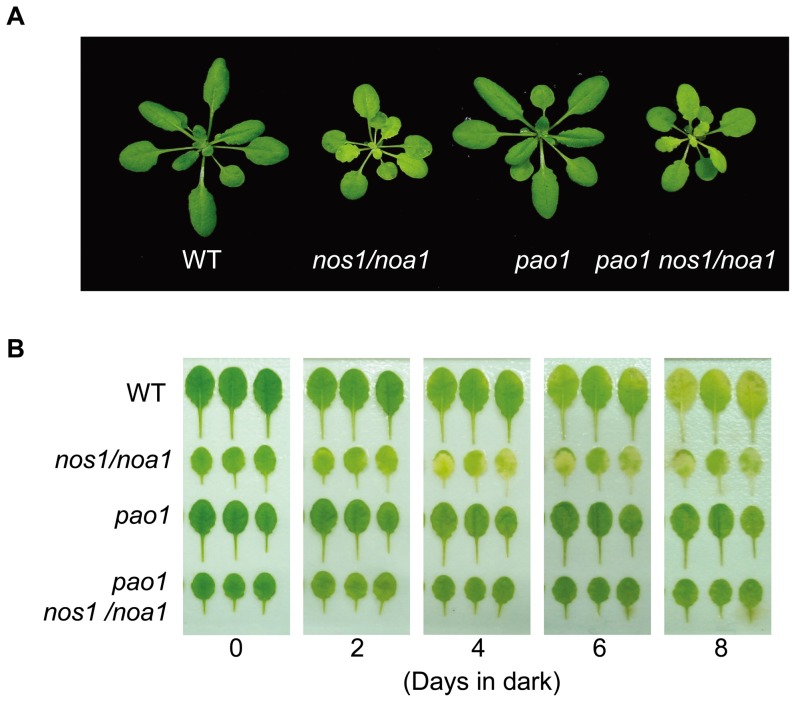
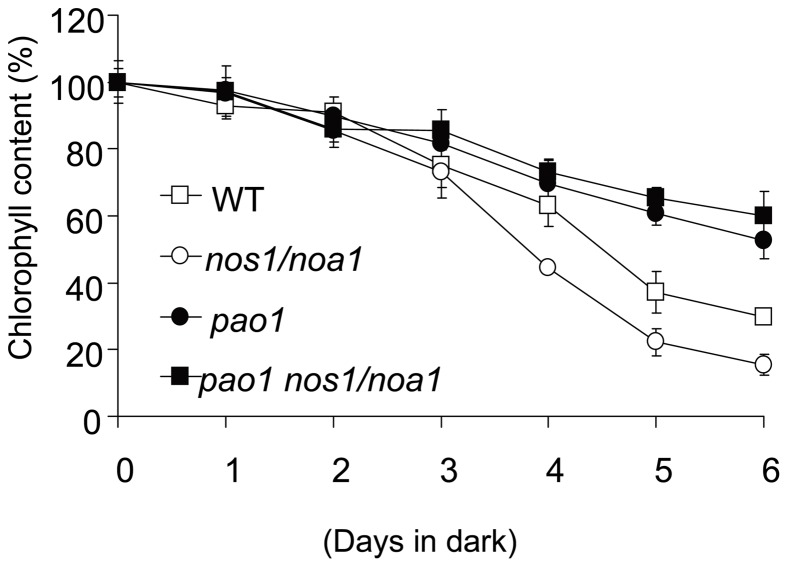
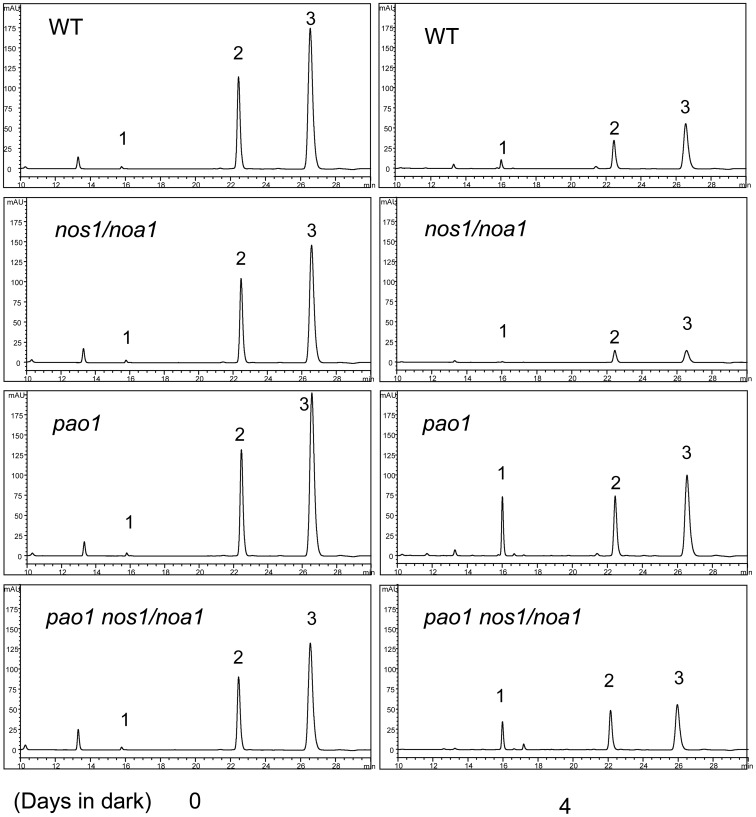
Chl degradation occurs rapidly in the darkened leaves of nos1/noa1 mutant due to endogenous NO deficiency [43]. It is also well known that in pao mutants, Chl is largely retained during senescence [12], [18], which supports the hypothesis that a blockage at the level of PAO inhibits Chl breakdown. To address the question whether the rapid degradation of Chl in the nos1/noa1 mutant during senescence can be inhibited by the accumulation of Pheide a caused by PAO mutations, we generated the double mutant by crossing the nos1/noa1 mutant to a loss-of-function mutant pao1. Interestingly, the double mutant plants showed a smaller size with pale-green leaves just like nos1/noa1 mutant plants (Fig. 3A). When compared with the leaves of the nos1/noa1 mutant, the leaves of the identified double mutant pao1 nos1/noa1 were found to retain the Chl levels as well as the pao1 single mutant throughout to day 8 in darkness (Fig. 3B). For instance, the levels of Chl contents in the double mutant and pao1 were only reduced to around 60% of the initial levels at day 6 upon dark-treatment, whereas the levels of Chl contents in the wild type and nos1/noa1 mutant were severely reduced to around 30% and 20%, respectively (Fig. 4A). Hence, the dark-induced rapid degradation of Chl in the nos1/noa1 mutant was reversed by PAO mutations. These data provided evidence that the accumulation of Chl catabolite Pheide a is most likely to inhibit the rapid Chl breakdown caused by NO-deficiency as exhibited in the nos1/noa1 mutant.
Figure 3. Dark-induced leaf senescence phenotypes of wild type, nos1/noa1, pao1 and the double mutant pao1 nos1/noa1.
(A) 3-week-old seedling phenotypes of wild type, nos1/noa1, pao1 and the double mutant pao1 nos1/noa1. (B) Senescing phenotypes of the detached leaves in dark. The fully-expanded leaves were detached from the plants of the different genotypes shown in (A).
Figure 4. Analysis of chlorophyll contents during dark-induced leaf senescence.
Chlorophyll contents were measured in the detached leaves of the indicated genotypes during a 6-d-dark treatment. Data are means of three replicates. Error bars indicate SD.
To further investigate whether the endogenous Pheide a accumulation in the double mutant pao1 nos1/noa1 affects the rapid up-regulation of Chl catabolic pathway genes, we verified the expression patterns of SGR, NYC1, PPH, PAO and RCCR in the mutants and wild type leaves after dark-treatment by qRT-PCR. Interestingly, we found that the senescence-induced up-regulation of NYC1, PPH and PAO in the double mutant pao1 nos1/noa1 leaves, in which the Pheide a accumulation was validated using HPLC (Fig. 5), was dramatically inhibited in comparison with that in the nos1/noa1 mutant and wild type leaves (Fig. 6B–D). It should be noted that the up-regulated expression of SGR was not significantly affected in the double mutant in reference to the expression level of SGR in the nos1/noa1 mutant (Fig. 6A), indicating that the blockage of Chl degradation pathway at PAO has little feed-back inhibitory effect on SGR expression. These results suggest that the accumulation of Pheide a caused by PAO mutations might act as feed-back signals to repress the senescence-activated expression of the major genes involving Chl catabolic pathway.
Figure 5. Green pigment changes in the leaves of wild type, nos1/noa1, pao1 and pao1 nos1/noa1 during dark-induced leaf senescence by HPLC analysis.
Representative data of green pigment changes in the leaves of the indicated genotypes after a 4-d-dark incubation. Numerals 1 to 3 indicate Pheide a, Chl b, and Chl a, respectively. The experiment was repeated twice.
Figure 6. The effects of accumulated Pheide a on the transcript levels of chlorophyll breakdown pathway genes during dark-induced senescence.
(A–E) qRT-PCR analysis of mRNA abundance of chlorophyll catabolic genes (SGR, NYC1, PPH, PAO and RCCR) in wild type and the indicated mutant leaves during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
NO deficiency impairs the stability of photosynthetic complexes during dark-induced leaf senescence
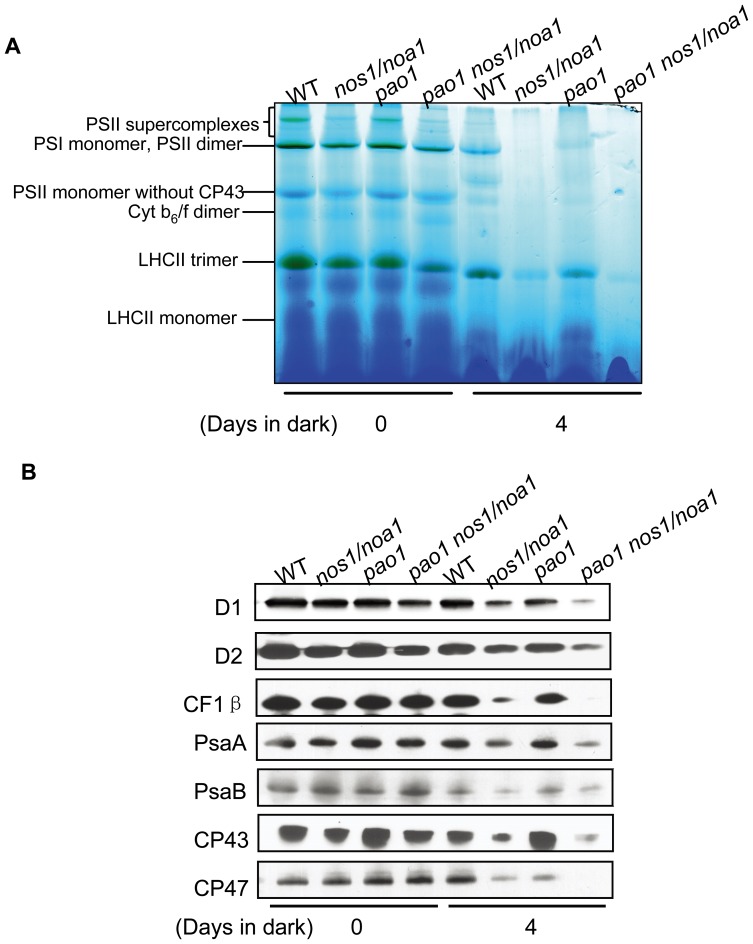
Given that NO deficiency accelerates Chl breakdown during senescence, it is attempted to test whether NO modulates degradation of photosynthetic complexes upon dark-treatment. Thylakoid membranes were isolated from the detached leaves incubated in darkness for 4 d and solubilized mildly with detergent (dodecyl β-D-maltoside). The resulting thylakoid membrane protein complexes were separated by Blue-Native gel electrophoresis (BN-PAGE) under native conditions in the presence of Serva Blue G. As shown in Fig. 7A, the typical thylakoid membrane protein complexes of the wild type include the higher molecular weight complexes corresponding to PSII supercomplexes, the PSI monomer and PSII dimer, PSII core monomer, PSII monomer without the CP43 subunit, Cyt b6/f dimer, LHCII trimer and LHCII monomer. Notably, all of the thylakoid membrane protein complexes were extremely less abundant in the nos1/noa1 mutant as well as in the double mutant pao1 nos1/noa1 after a 4-d dark treatment in comparison with that in wild type (Fig. 7A), indicating that NO deficiency destabilizes all of the thylakoid membrane protein complexes during dark-induced leaf senescence, and the Chl retention caused by the mutations of PAO in the double mutant does not improve the stability of the indicated complexes. Moreover, all of the thylakoid membrane protein complexes were less abundant in the wild type leaves treated with cPTIO after a 4-d dark treatment in comparison with that in wild type under control conditions (Fig. S3), indicating that NO deficiency induced by cPTIO treatments destabilizes all of the thylakoid membrane protein complexes during dark-induced leaf senescence. Interestingly, the abundance of the thylakoid membrane protein complexes in the pao mutant was significantly higher than that in the double mutant pao1 nos1/noa1 after a 4-d dark treatment, implying that NO deficiency and the accumulation of Pheide a may have synergistic effects on the stability loss of the indicated complexes (Fig. 7A). In addition, we observed that under normal growth conditions, the abundance of the indicated complexes in both the nos1/noa1 mutant and the pao1 nos1/noa1 double mutant was slightly reduced compared with the wild type, whereas the pao1 mutant exhibited a similar abundance level to the wild type (Fig. 7A), supporting that NO is a positive regulator in stabilizing thylakoid membranes under both normal and stress conditions.
Figure 7. Abundance analysis of thylakoid membrane protein complexes from leaves incubated in dark.
(A) Blue native-PAGE analysis of thylakoid membrane protein complexes from the detached leaves of wild type and the indicated mutants after a 4-d-dark treatment. (B) Western blotting analysis of thylakoid membrane protein degradation from the detached leaves of wild type and the indicated mutants after a 4-d-dark treatment.
To further explore the effects of NO on the stability of thylakoid membrane proteins, western blotting was performed to examine the abundance of photosynthetic proteins in wild type and the indicated mutants upon dark treatment as shown in Fig. 7B. As revealed by western blotting data, substantial amounts of photosynthetic proteins were lost in both the nos1/noa1 mutant and the pao1 nos1/noa1 double mutant 4 d after dark treatment with respect to the wild type, but the abundance levels of the indicated photosynthetic proteins were quite similar between the wild type and the pao1 mutant (Fig. 7B).
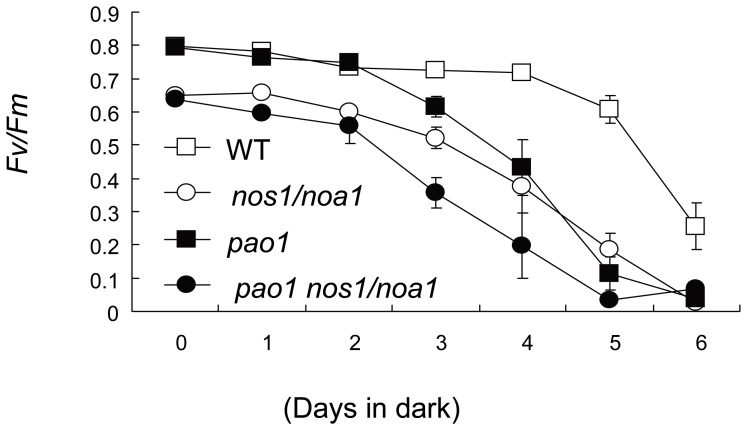
Given that the significant changes in protein abundance of PSII subunits as observed in the indicated mutant leaves upon dark treatment, the photosynthetic Chl fluorescence parameter Fv/Fm was measured in order to test the maximal quantum yield of PSII. Interestingly, a typical Fv/Fm value (0.8) for healthy plants was measured in wild-type leaves, whereas the Fv/Fm ratio in both the nos1/noa1 mutant and the pao1 nos1/noa1 double mutant leaves was around 0.65, indicating a rather severe defect in PSII (Fig. 8). During the dark treatment, the Fv/Fm values of both the nos1/noa1 mutant and the pao1 nos1/noa1 double mutant leaves were found to be 48% and 73% less compared with the wild type respectively, when leaves were darkened for 4 d (Fig. 8). Consistent with the results obtained in BN-PAGE (Fig. 7A) and western blotting (Fig. 7B), it is noteworthy that the Fv/Fm ratio of the pao1 mutant leaves was less than 40% reduced in comparison with the wild type leaves after 4-d dark treatment. The Fv/Fm values of all the mutant leaves decreased progressively during the following days. By contrast, the Fv/Fm in the wild type leaves only slightly decreased from 0.8 to 0.69 through 5 d in darkness and dropped at day 6 due to a large decrease in PSII quantum efficiency (Fig. 8). In the darkened leaves of the indicated mutants as shown in Fig. 8, dark-induced PSII damages were more pronounced, and PSII damaging proceeded at a faster rate relative to wild-type leaves.
Figure 8. Analysis of PSII photochemical efficiency (Fv/Fm) of detached leaves during dark-induced senescence.
The fifth leaves were excised from wild type and the indicated mutant plants (21-d-old). Error bars indicate SE (n = 5).
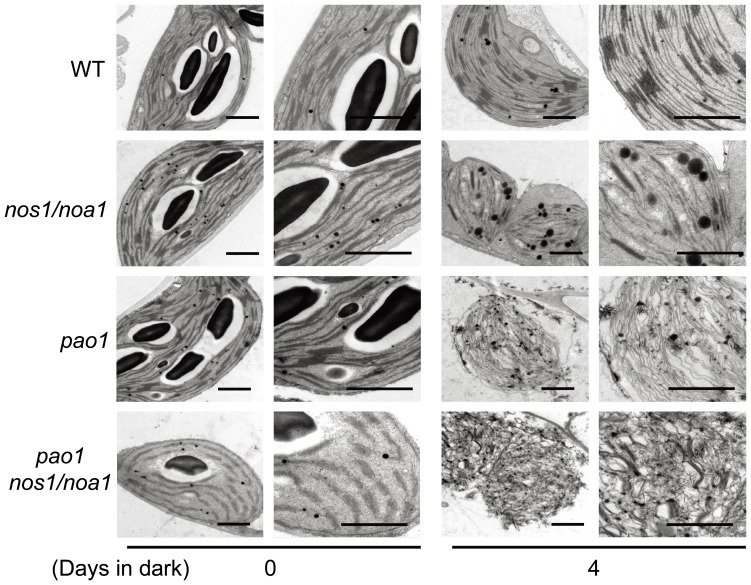
To further explore the effect of NO deficiency on the stability maintenance of thylakoid membranes, we conducted examinations of chloroplast ultrastructural alterations by transmission electron microscopy (TEM). The thylakoid membranes in the chloroplasts of the mutant nos1/noa1 appeared distorted and began to decompose after 4 d dark-treatment, whereas wild type thylakoid systems, including grana and stroma membranes, retained their initial configurations (Fig. 9). In agreement with the findings by BN-PAGE and western blotting, detailed examinations by TEM revealed that the ultrastructures of chloroplasts in the double mutant pao1 nos1/noa1 were most severely disrupted and thylakoid membranes were almost totally decomposed with respect to that in wild type, pao1 and nos1/noa1 mutants upon a 4-d dark treatment (Fig. 9). Thus, the findings by TEM examinations further support that NO deficiency and the accumulation of Pheide a have synergistic effects on the stability loss of thylakoid membranes upon dark-treatment. Taken together, these data support the notion that NO deficiency affects the maximal quantum yield of PSII through modulating the stability of thylakoid membranes during dark-induced leaf senescence.
Figure 9. Chloroplast ultrastructures of detached leaves during dark-induced senescence.
Cross-sectional analysis of thylakoid membranes in chloroplasts from the fully-expanded leaves detached from wild type, nos1/noa1, pao1 and the double mutant pao1 nos1/noa1 plants after 0 d and 4 d of dark-treatment by transmission electron microscopy (TEM). Low magnification overview (left) and close-up images (right) of the chloroplast ultrastructure were shown respectively at day 0 and day 4. Bars = 1 µm.
Discussion
Accumulated studies pointed to the critical roles of plant hormones in triggering, accelerating or even reversing the overall process of senescence by activating specific genes [62]–[66]. It appears that plants may integrate these different hormonal signaling factors through endogenous signaling networks in order to decide whether the senescence process should be executed or not. Two classical hormones ethylene and cytokinin have been recognized to influence leaf senescence through acting as a senescence-promoting factor and as a senescence-delaying factor, respectively in higher plants [67], [68]. Although it has been noted that NO plays a role in senescence by retarding the onset of chlorophyll degradation in different plant species [36]–[39], [42], the mechanisms through which NO modulates Chl degradation during senescence remain elusive.
In this study, the NO-deficient mutant nos1/noa1 was employed to address the effect of NO on Chl breakdown during leaf senescence since the dark-induced leaf senescence occurs more rapidly in the mutant compared with wild type plants [43]. Considering that leaf senescence occurs sequentially in individual leaves within the rosette rather than in the whole plant in Arabidopsis [69], dark-induced senescence of detached leaves was employed in this study. Our present results demonstrate that the expression of the genes encoding enzymes involved in Chl catabolism is severely repressed, except for RCCR during dark-induced leaf senescence. In our senescence induction system, RCCR is not an inducible gene upon dark treatment, whereas its transcript level is slightly reduced during senescence. Our data further confirm that RCCR seems to be constitutively active and its activity is rather constant during all phases of leaf development [5], [18].
The hypothesis underlying the findings presented in this report is that endogenous NO deficiency caused by NOS1/NOA1 mutations leads to loss of signal that represses the transcriptional activation of Chl catabolic genes during dark-induced leaf senescence. To our knowledge, endogenous NO, acting as a negative messenger molecule in regulating Chl breakdown at transcriptional level during leaf senescence, has not been previously reported. Previous reports suggest that endogenous NO levels negatively correlate with senescence progression in many different plant species. Nitric oxide (NO) has been characterized as an anti-senescence agent. It was showed that NO content in peroxisomes isolated from senescent pea leaves is less than that in young leaves [70]. NO emission is negatively correlated with senescence processes [35], [40]. Moreover, the NO-deficient mutant nos1/noa1 exhibits an early-senescence phenotype and dark-induced senescence and chlorophyll loss can be rescued in nos1/noa1 leaves by treatments with NO donors [43]. Interestingly, expressing a NO degrading enzyme in Arabidopsis led to early leaf senescence phenotypes with a massive up-regulation of SENESCENCE-ASSOCIATED GENEs (SAGs) [44]. In agreement with the above findings described for the role of NO in regulating Chl degradation and leaf senescence, our data support the model that decreases in NO levels occurring during leaf senescence under normal conditions relieve the repression of the enzymatic activities involved in Chl catabolic pathway at the transcriptional level, which is likely to be an adaptation for plants to accelerate Chl degradation in accordance with leaf senescence programming.
In addition to negatively regulating the expression levels of Chl catabolic pathway genes, our findings presented in this study have demonstrated that NO plays a positive role in maintaining stability of thylakoid membranes during dark-induced leaf senescence. This conclusion has been drawn based on several lines of evidence described in this study. Firstly, NO deficiency caused by NOS1/NOA1 mutations accelerates the stability loss of thylakoid membrane complexes during dark-induced leaf senescence by BN-PAGE. Secondly, western blotting data indicate that the abundance of thylakoid membrane proteins rapidly declined in the nos1/noa1 mutant upon dark-treatment. Thirdly, in accordance with BN-PAGE and western blotting findings, NO deficiency leads to the collapsed ultrastructures of thylakoid membranes in chloroplasts of the darkened leaves. As the final stage of leaf development, leaf senescence undergoes a programmed cell death process, referred to as the senescence syndrome [65], [66]. Chl breakdown is a dramatically visualized sign of the leaf senescence syndrome [3], [71]. During senescence, the degeneration of chloroplasts and disruption in photosynthesis occurs first with deterioration and leakiness of the thylakoid membranes [63], [64], [66]. Importantly, our data have provided evidence to support that NO plays a dual role in regulating the process of leaf senescence as a negative regulator for Chl degradation pathway and as a positive regulator in maintaining the stability of thylakoid membranes. On the other hand, the analysis of the pao1 nos1/noa1 double mutant has led us to reveal that the retained Chl levels caused by PAO mutations in the double mutant dose not improve the stability of thylakoid membranes with respect to the nos1/noa1 mutant during dark-induced leaf senescence, indicating that NO is a determinant factor in regulation of the degradation of thylakoid membrane complexes during leaf senescence.
In conclusion, this study sheds light on the critical role of NO in regulating the stability of thylakoid membrane complexes in coordination with the degradation of the pigment apparatus during leaf senescence in the dark-induction course of yellowing of leaves. Considering that temporal progress of maturation and senescence goes hand in hand with a significant decrease of NO emission [35], [40], NO may play important roles in reprogramming the overall organization of senescing events such as Chl breakdown and the degeneration of chloroplasts with deterioration and leakiness of the thylakoid membranes. It is known that NO acts as a signaling molecule involving in a variety of biotic and abiotic stress responses in higher plants. Our studies also provide insight into better understanding the entire process of leaf senescence in response to environmental stresses.
Materials and Methods
Plant material and growth conditions
The Columbia (Col-0) ecotype of Arabidopsis (Arabidopsis thaliana) was used as the wild type. The T-DNA insertion mutant nos1/noa1 was kindly provided by Dr. Nigel Crawford (University of California at San Diego, USA) and the mutant pao1 (stock number: CS3733) was obtained from the Arabidopsis Biological Resource Centre (ABRC, Ohio State University, USA). The pao1 nos1/noa1 double mutant was obtained by crossing and the putative double mutants were confirmed by performing PCR and sequence analyses. Plants were grown on peat soil under a long-day condition, 16 h of white light (80 µmol m−2 s−1) and 8 h in dark, with 60% relative air humidity at 21°C.
Senescence induction
Fully extended rosette leaves were detached from 3-week-old plants and used for dark-induced leaf senescence. The detached leaves were incubated on Petri dishes containing three layers of filter papers soaked in 15 ml distilled water. Petri dishes were wrapped with double-layer aluminum foil, and kept in dark at 22°C.
RT-PCR and real time RT-PCR
Total RNA was isolated using the Plant RNeasy kit (Qiagen). After DNA digestion with RQ1 DNase (Promega), first-strand cDNA was synthesized using the high efficient reverse transcription kit ReverTra Ace-α-® (TOYOBO). Quantitative real-time PCR was performed with SYBR Premix Ex TaqII (Takara) using a MyiQ5 single color Real-Time PCR Detection System (Bio-Rad). The comparative threshold cycle (Ct) method was used for determining relative transcript levels (iQ5 admin, Bio-Rad) using ACTIN2 as an internal control. Specific primers to respective genes were as follows:
ACT2 (forward, 5′- GCCATCCAAGCTGTTCTCTC-3′ and reverse, 5′- GCTCGTAGTCAACAGCAACAA-3′);
SGR (forward, 5′-GCAAGGATGGGCAAATAGG-3′ and reverse, 5′-CACCGCTTATGTGACAATGAAC-3′);
PAO (forward, 5′- ACGGCATGGTAAGAGTCAGC -3′ and reverse, 5′- AAACCAGCAAGAACCAGTCG -3′);
RCCR (forward, 5′- ATCGCCTCCAATCACAACTC-3′ and reverse, 5′- TTAGCACAAGCGACTTGGAA-3′);
PPH (forward, 5′- CAATCATGCTTGCTCCTGGTG-3′ and reverse, 5′- CTACCAATCCTGGACTCCTCC-3′);
NYC1 (forward, 5′- ATGTGATGAGCAGACAGCACAGTG-3′ and reverse, 5′- CCTTAGCCAGGAAGTGACAATAGC-3′).
PAO enzyme assays
The activities of PAO were measured using a coupled assay with RCCR according to previous reports, with minor modifications [11], [23], [72]. Briefly, leaf samples were processed (5 ml/g fresh weight) in a homogenization buffer containing 400 mM sorbitol, 25 mM Tricine-KOH, pH 8.0, 2 mM EDTA, 1 mM MgCl2, 0.1% bovine serum albumin (W/V), 5 mM polyethylene glycol 4000, and 0.5 mM dithiothreitol with chilled mortars and pestles. Next, the homogenized samples were filtrated through two layers of nylon cloth, and the resulting homogenate was centrifuged at 7,000 g for 4 min. The resulting green sediment was washed once with the washing medium (the homogenization medium with EDTA, MgCl2, and polyethylene glycol 4,000 omitted). After centrifugation at 8,000 g for 5 min, the resulting pellets were re-suspended in the washing medium (2 ml/g fresh weight leaf tissue). The re-suspended samples were further centrifuged at 14,000 g for 5 min. The resulting sediments were frozen in liquid nitrogen and stored at −80°C. According to the previous procedures, soluble stroma protein (S1 fraction) was used as the source of RCCR and solubilized membrane protein in osmotic buffer (Tris-MES, pH 8.0, 0.1% bovine serum albumin) with 1% Triton X-100 was used as the source of PAO, respectively.
To test the activities of PAO, Pheide a (30 µg), used as substrate, was mixed with the ferredoxin-reduced system containing 10 µg ferredoxin (Sigma), 1 mM NADPH (Sigma), 1 mM Glc-6-P (G6P), and 10 milliunits of G6P dehydrogenase. The final reaction mixture volume was 50 µl. After incubation in the dark at 25°C for 1 h, the reaction was terminated with methanol at a final concentration of 70%. The reaction mixture was centrifuged at 14,000 g for 10 min, and the supernatant was analyzed for pFCC-1 by reverse-phase HPLC with 0.1 M potassium phosphate (pH 7.0)/methanol (32.5%∶67.5%, V/V).
pFCC-1 was analyzed by HPLC with an Agilent 1100 HPLC ChemStation coupled to a fluorescence detector equipped with a ZorbaxSB C-18 column (4.6 mm×25 cm, 5-µm particle diameter). Samples (40 µl) were injected at a flow rate of 0.8 ml/min. Under these conditions, pFCC-1was eluted after 9 min. Fluorescence was recorded at 320 nm (excitation)/450 nm (emission). The amount of pFCC-1 was calculated according to integrated peak areas.
Chlorophyll measurements
The fully extended rosette leaves detached from 3-week-old plants were used for dark-induced leaf senescence. Chlorophyll was extracted from the detached leaves after dark-induction. The chlorophyll in leaf samples was extracted with 80% (V/V) acetone. The chlorophyll contents were determined spectrophotometrically at 645 and 663 nm as described previously by [73].
Chlorophyll fluorescence measurements
The maximum photochemical efficiency of PSII was determined from the ratio of variable (Fv) to maximum (Fm) fluorescence (Fv/Fm) with an LI-6400XT Portable Photosynthesis System (LI-COR Biosciences, Lincoln, Nebraska USA).
Separation of thylakoid membranes and BN-PAGE
For the preparation of thylakoid membranes, leaves (0.5 g) were homogenized in 5 ml isolation buffer (400 mM sucrose, 2 mM MgCl2, 10 mM NaCl, and 50 mM HEPES, Ph 7.8) with chilled mortars and pestles. After filtrating through two layers of nylon cloth, samples were washed twice with the isolation buffer, and centrifuged at 5,000 g for 10 min. The resulting sediments were suspended in 100 µl re-suspension buffer (20% [w/v] glycerol and 25 mM BisTris-HCl, pH 7.0) and stored in −80°C for use. BN-PAGE was performed as described [74], [75].
Western blot analysis
Immunodetection of thylakoid membrane proteins was performed using the indicated primary antibodies against thylakoid membrane proteins (Agrisera). Alkaline-phosphatase-conjugated goat anti-rabbit IgG (Chemicon) was used as a secondary antibody and reaction was revealed using an ECL kit (Amersham).
Transmission electron microscopy
For transmission electron microscopy processing, the 5th leaves of the 21-d-old wild-type and mutant plants were collected. The leaf samples were fixed in 2.5% glutaraldehyde (v/v) for 48 h at 4°C. Thin sections were examined by a transmission electron microscope (H-7650, Hitachi) using a voltage of 80 kV.
HPLC Analysis of Chlorophyll and Chlorophyll Catabolites
For pigment extraction, leave samples (about 0.1 g) were ground in a mortar with liquid nitrogen. Pigments were extracted with 10% (v/v) 0.2 M Tris-HCl, pH 8.0, in acetone. The extracts were incubated at −20°C for 2 h in the dark. After centrifugation at 14,000 g, 4°C for 20 min, 20 µl supernatants were analyzed on Agilent 1100 HPLC ChemStation as described [18], [76].
Accession numbers for the sequence data and Arabidopsis seed stocks
Sequence data from this article can be found in the Arabidopsis Genome Initiative and GenBank/EMBL data libraries under accession numbers: NOS1/NOA1 (At3g47450); PAO (At3g44880); SGR (At4g22920); NYC1 (At4g13250); PPH (At5g13800); RCCR (At4g37000); ACTIN2 (At3g18780). The ABRC seed stock numbers for the nos1/noa1 and pao1 mutants are CS6511 and CS3733, respectively.
Supporting Information
Effects of SNP, an NO donor, on the transcript levels of chlorophyll breakdown pathway genes in the leaves of wild type and nos1/noa1 mutant during dark-induced senescence. (A–E) qRT-PCR analysis of mRNA abundance of enzyme genes (SGR, NYC1, PPH, PAO and RCCR) involved in chlorophyll degradation in the leaves of wild type and nos1/noa1 mutant treated with or without 250 µM SNP during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
(TIF)
Effects of cPTIO, an NO scavenger, on the transcript levels of chlorophyll breakdown pathway genes in wild type leaves during dark-induced senescence. (A–E) qRT-PCR analysis of mRNA abundance of enzyme genes (SGR, NYC1, PPH, PAO and RCCR) involved in chlorophyll degradation in wild type leaves treated with or without 500 µM cPTIO during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
(TIF)
Effects of cPTIO on abundance of thylakoid membrane protein complexes from wild type leaves incubated in dark. Blue native-PAGE analysis of thylakoid membrane protein complexes from the detached leaves of wild type after a 4-d-dark treatment in combination with or without 500 µM cPTIO.
(TIF)
Analysis of PaO-RCCR activity in wild type and nos1/noa1. Detached leaves incubated in darkness for 4 days from both genotypes were used for crude extraction of PaO and RCCR. With all necessary cofactors, reaction mixtures were incubated at 25°C for 1 h and stopped by cold methanol. LU, Fluorescence unit.
(TIF)
Acknowledgments
We are grateful to X.-Y. Gao, W.-L. Hu and G.-Q. Yan (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for assistance with electron microscopy and HPLC analysis, respectively. We thank The Arabidopsis Biological Resource Center for providing Arabidopsis mutant seeds.
Funding Statement
This research was supported in parts by the Ministry of Science and Technology of China (2012CB944802 and 2012AA101103), the Ministry of Agriculture of China (2011ZX08009-003-002), the National Natural Science Foundation of China (31170244 and 30770198) and the CAS/SAFEA International Partnership Program for Creative Research Teams. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399. [DOI] [PubMed] [Google Scholar]
- 2. Hörtensteiner S (2009) Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends in Plant Sci 14: 155–162. [DOI] [PubMed] [Google Scholar]
- 3. Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev of Plant Biol 57: 55–77. [DOI] [PubMed] [Google Scholar]
- 4. Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53: 927–937. [DOI] [PubMed] [Google Scholar]
- 5. Matile P, Hortensteiner S, Thomas H (1999) Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol 50: 67–95. [DOI] [PubMed] [Google Scholar]
- 6. Tsuchiya T, Ohta H, Okawa K, Iwamatsu A, Shimada H, et al. (1999) Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proc Natl Acad Sci USA 96: 15362–15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 55–77. [DOI] [PubMed] [Google Scholar]
- 8. Schenk N, Schelbert S, Kanwischer M, Goldschmidt EE, Doermann P, et al. (2007) The chlorophyllases AtCLH1 and AtCLH2 are not essential for senescence-related chlorophyll breakdown in Arabidopsis thaliana . Febs Letters 581: 5517–5525. [DOI] [PubMed] [Google Scholar]
- 9. Schelbert S, Aubry S, Burla B, Agne B, Kessler F, et al. (2009) Pheophytin Pheophorbide Hydrolase (Pheophytinase) Is Involved in Chlorophyll Breakdown during Leaf Senescence in Arabidopsis . Plant Cell 21: 767–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodoni S, Muhlecker W, Anderl M, Krautler B, Moser D, et al. (1997) Chlorophyll breakdown in senescent chloroplasts - Cleavage of pheophorbide a in two enzymic steps. Plant Physiol 115: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wuthrich KL, Bovet L, Hunziker PE, Donnison IS, Hörtensteiner S (2000) Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J 21: 189–198. [DOI] [PubMed] [Google Scholar]
- 12. Pruzinska A, Tanner G, Anders I, Roca M, Hörtensteiner S (2003) Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc Natl Acad Sci USA 100: 15259–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pruzinska A, Anders I, Aubry S, Schenk N, Tapernoux-Luthi E, et al. (2007) In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 19: 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinder B, Schellenberg M, Rodon S, Ginsburg S, Vogt E, et al. (1996) How plants dispose of chlorophyll catabolites - Directly energized uptake of tetrapyrrolic breakdown products into isolated vacuoles. J Biol Chem 271: 27233–27236. [DOI] [PubMed] [Google Scholar]
- 15. Tommasini R, Vogt E, Fromenteau M, Hortensteiner S, Matile P, et al. (1998) An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J 13: 773–780. [DOI] [PubMed] [Google Scholar]
- 16. Kusaba M, Ito H, Morita R, Iida S, Sato Y, et al. (2007) Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19: 1362–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato Y, Morita R, Katsuma S, Nishimura M, Tanaka A, et al. (2009) Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J 57: 120–131. [DOI] [PubMed] [Google Scholar]
- 18. Pruzinska A, Tanner G, Aubry S, Anders I, Moser S, et al. (2005) Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol 139: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka R, Hirashima M, Satoh S, Tanaka A (2003) The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: Inhibition of the pheophorbide a oxygenase activity does not lead to the “Stay-Green” phenotype in Arabidopsis . Plant Cell Physiol 44: 1266–1274. [DOI] [PubMed] [Google Scholar]
- 20. Thomas H (1987) Sid - a Mendelian Locus Controlling Thylakoid Membrane Disassembly in Senescing Leaves of Festuca-Pratensis. Theor Appl Genet 73: 551–555. [DOI] [PubMed] [Google Scholar]
- 21. Armstead I, Donnison I, Aubry S, Harper J, Hörtensteiner S, et al. (2006) From crop to model to crop: identifying the genetic basis of the staygreen mutation in the Lolium/Festuca forage and amenity grasses. New Phytol 172: 592–597. [DOI] [PubMed] [Google Scholar]
- 22. Armstead I, Donnison I, Aubry S, Harper J, Hörtensteiner S, et al. (2007) Cross-species identification of Mendel's/locus. Science 315: 73–73. [DOI] [PubMed] [Google Scholar]
- 23. Ren G, An K, Liao Y, Zhou X, Cao Y, et al. (2007) Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis . Plant Physiol 144: 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang HW, Li MR, Liang NB, Yan HB, Wei YL, et al. (2007) Molecular cloning and function analysis of the stay green gene in rice. Plant J 52: 197–209. [DOI] [PubMed] [Google Scholar]
- 25. Park S-Y, Yu J-W, Park J-S, Li J, Yoo S-C, et al. (2007) The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 19: 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato Y, Morita R, Nishimura M, Yamaguchi H, Kusaba M (2007) Mendel's green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc Natl Acad Sci USA 104: 14169–14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aubry S, Mani J, Hörtensteiner S (2008) Stay-green protein, defective in Mendel's green cotyledon mutant, acts independent and upstream of pheophorbide a oxygenase in the chlorophyll catabolic pathway. Plant Mol Biol 67: 243–256. [DOI] [PubMed] [Google Scholar]
- 28. Barry CS, McQuinn RP, Chung M-Y, Besuden A, Giovannoni JJ (2008) Amino acid substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol 147: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borovsky Y, Paran I (2008) Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor Appl Genet 117: 235–240. [DOI] [PubMed] [Google Scholar]
- 30. Barry CS (2009) The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci 176: 325–333. [Google Scholar]
- 31. Drazkiewicz M (1994) Chlorophyll - Occurrence, Functions, Mechanism of Action, Effects of External and Internal Factors. Photosynthetica 30: 321–331. [Google Scholar]
- 32. Jacob-Wilk D, Holland D, Goldschmidt EE, Riov J, Eyal Y (1999) Chlorophyll breakdown by chlorophyllase: isolation and functional expression of the Chlase1 gene from ethylene-treated Citrus fruit and its regulation during development. Plant J 20: 653–661. [DOI] [PubMed] [Google Scholar]
- 33. Benedetti CE, Costa CL, Turcinelli SR, Arruda P (1998) Differential expression of a novel gene in response to coronatine, methyl jasmonate, and wounding in the coi1 mutant of Arabidopsis . Plant Physiol 116: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang ML, Wardzala E, Johal GS, Gray J (2004) The wound-inducible Lls1 gene from maize is an orthologue of the Arabidopsis Acd1 gene, and the LLS1 protein is present in non-photosynthetic tissues. Plant Mol Biol 54: 175–191. [DOI] [PubMed] [Google Scholar]
- 35. Leshem YY, Wills RBH, Ku VVV (1998) Evidence for the function of the free radical gas - nitric oxide (NO) - as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36: 825–833. [Google Scholar]
- 36. Eum HL, Hwang DK, Lee SK (2009) Nitric Oxide Reduced Chlorophyll Degradation in Broccoli (Brassica oleracea L. var. italica) Florets During Senescence. Food Sci Technol Int 15: 223–228. [Google Scholar]
- 37. Eum HL, Lee SK (2007) Nitric oxide treatment reduced chlorophyll degradation of broccoli florets during senescence. Hortsci 42: 927–927. [Google Scholar]
- 38. Laxalt AM, Beligni MV, Lamattina L (1997) Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur J Plant Pathol 103: 643–651. [Google Scholar]
- 39. Hung KT, Kao CH (2004) Nitric oxide acts as an antioxidant and delays methyl jasmonate-induced senescence of rice leaves. J Plant Physiol 161: 43–52. [DOI] [PubMed] [Google Scholar]
- 40. Corpas FJ, Barroso JB, Carreras A, Quiros M, Leon AM, et al. (2004) Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol 136: 2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leshem YY, Pinchasov Y (2000) Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Duch.) and avocados Persea americana (Mill.). J Exp Bot 51: 1471–1473. [PubMed] [Google Scholar]
- 42. Jasid S, Galatro A, Javier Villordo J, Puntarulo S, Simontacchi M (2009) Role of nitric oxide in soybean cotyledon senescence. Plant Sci 176: 662–668. [Google Scholar]
- 43. Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17: 3436–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mishina TE, Lamb C, Zeier J (2007) Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ 30: 39–52. [DOI] [PubMed] [Google Scholar]
- 45. Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302: 100–103. [DOI] [PubMed] [Google Scholar]
- 46. Zemojtel T, Froehlich A, Palmieri MC, Kolanczyk M, Mikula I, et al. (2006) Plant nitric oxide synthase: a never-ending story? Trends in Plant Sci 11: 524–525. [DOI] [PubMed] [Google Scholar]
- 47. Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, et al. (2006) Plant nitric oxide synthase: back to square one - Response. Trends in Plant Sci 11: 526–527. [Google Scholar]
- 48. Guo F-G (2006) Plant nitric oxide synthase: AtNOS1 is just the beginning - Response. Trends in Plant Sci 11: 527–528. [Google Scholar]
- 49. Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/AtNOA1 Is a Functional Arabidopsis thaliana cGTPase and Not a Nitric-oxide Synthase. J Biol Chem 283: 32957–32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He YK, Tang RH, Hao Y, Stevens RD, Cook CW, et al. (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305: 1968–1971. [DOI] [PubMed] [Google Scholar]
- 51. Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122. [DOI] [PubMed] [Google Scholar]
- 52. Zhao M-G, Tian Q-T, Zhang W-H (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis . Plant Physiol 144: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zottini M, Costa A, De Michele R, Ruzzene M, Carimi F, et al. (2007) Salicylic acid activates nitric oxide synthesis in Arabidopsis . J Exp Bot 58: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 54. Zeidler D, Zahringer U, Gerber I, Dubery I, Hartung T, et al. (2004) Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA 101: 15811–15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana . Plant Cell 20: 1390–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gas E, Flores-Perez U, Sauret-Gueto S, Rodriguez-Concepcion M (2009) Hunting for plant nitric oxide synthase provides new evidence of a central role for plastids in nitric oxide metabolism. Plant Cell 21: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu H, Lau E, Lam MPY, Chu H, Li S, et al. (2010) OsNOA1/RIF1 is a functional homolog of AtNOA1/RIF1: implication for a highly conserved plant cGTPase essential for chloroplast function. New Phytol 187: 83–105. [DOI] [PubMed] [Google Scholar]
- 58. Sudhamsu J, Lee GI, Klessig DF, Crane BR (2008) The Structure of YqeH an AtNOS1/AtNOA1 ortholog that couples GTP hydrolysis to molecular recognition. J Biol Chem 283: 32968–32976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Ree K, Gehl B, Chehab EW, Tsai Y-C, Braam J (2011) Nitric oxide accumulation in Arabidopsis is independent of NOA1 in the presence of sucrose. Plant J 68: 225–233. [DOI] [PubMed] [Google Scholar]
- 60. Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98: 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yao N, Greenberg JT (2006) Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 18: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, et al. (2003) The molecular analysis of leaf senescence–a genomics approach. Plant Biotechnol J 1: 3–22. [DOI] [PubMed] [Google Scholar]
- 63. Jing HC, Hille J, Dijkwel RR (2003) Ageing in plants: Conserved strategies and novel pathways. Plant Biol 5: 455–464. [Google Scholar]
- 64. Guo YF, Gan SS (2005) Leaf senescence: Signals, execution, and regulation. Curr Top Dev Biol Vol 71: 83–112. [DOI] [PubMed] [Google Scholar]
- 65. Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5: 278–282. [DOI] [PubMed] [Google Scholar]
- 66. Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136. [DOI] [PubMed] [Google Scholar]
- 67. Gan SS, Amasino RM (1995) Inhibition of Leaf Senescence by Autoregulated Production of Cytokinin. Science 270: 1986–1988. [DOI] [PubMed] [Google Scholar]
- 68. Grbic V, Bleecker AB (1995) Ethylene Regulates the Timing of Leaf Senescence in Arabidopsis . Plant J 8: 595–602. [Google Scholar]
- 69. Hensel LL, Grbic V, Baumgarten DA, Bleecker AB (1993) Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidoposis . Plant Cell 5: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Del Rio LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochem 65: 783–792. [DOI] [PubMed] [Google Scholar]
- 71. Hörtensteiner S (2009) Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci 14: 155–162. [DOI] [PubMed] [Google Scholar]
- 72. Hörtensteiner S, Vicentini F, Matile P (1995) Chlorophyll breakdown in senescent cotyledons of rape, Brassica-Napus L - enzymatic cleavage of phaeophorbide-a in-vitro . New Phytol 129: 237–246. [DOI] [PubMed] [Google Scholar]
- 73. Arnon DI (1949) Copper enzymes in isolated chloroplasts - polyphenoloxidase in beta-vulgaris . Plant Physiol 24: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schagger H, Cramer WA, Vonjagow G (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane-protein complexes by 2-dimensional native electrophoresis. Anal Biochem 217: 220–230. [DOI] [PubMed] [Google Scholar]
- 75. Cline K, Mori H (2001) Thylakoid delta pH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J Cell Biol 154: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Langmeier M, Ginsburg S, Matile P (1993) Chlorophyll breakdown in senescent leaves - demonstration of Mg-dechelatase activity. Physiol Plant 89: 347–353. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of SNP, an NO donor, on the transcript levels of chlorophyll breakdown pathway genes in the leaves of wild type and nos1/noa1 mutant during dark-induced senescence. (A–E) qRT-PCR analysis of mRNA abundance of enzyme genes (SGR, NYC1, PPH, PAO and RCCR) involved in chlorophyll degradation in the leaves of wild type and nos1/noa1 mutant treated with or without 250 µM SNP during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
(TIF)
Effects of cPTIO, an NO scavenger, on the transcript levels of chlorophyll breakdown pathway genes in wild type leaves during dark-induced senescence. (A–E) qRT-PCR analysis of mRNA abundance of enzyme genes (SGR, NYC1, PPH, PAO and RCCR) involved in chlorophyll degradation in wild type leaves treated with or without 500 µM cPTIO during dark-induced senescence. ACTIN2 was used as the internal standard. Error bars indicate standard deviations of three technical replicates, and the results were consistent in three biological replicates.
(TIF)
Effects of cPTIO on abundance of thylakoid membrane protein complexes from wild type leaves incubated in dark. Blue native-PAGE analysis of thylakoid membrane protein complexes from the detached leaves of wild type after a 4-d-dark treatment in combination with or without 500 µM cPTIO.
(TIF)
Analysis of PaO-RCCR activity in wild type and nos1/noa1. Detached leaves incubated in darkness for 4 days from both genotypes were used for crude extraction of PaO and RCCR. With all necessary cofactors, reaction mixtures were incubated at 25°C for 1 h and stopped by cold methanol. LU, Fluorescence unit.
(TIF)