Abstract
Reconciling traditional classifications, morphology, and the phylogenetic relationships of brown-spored agaric mushrooms has proven difficult in many groups, due to extensive convergence in morphological features. Here, we address the monophyly of the Bolbitiaceae, a family with over 700 described species and examine the higher-level relationships within the family using a newly constructed multilocus dataset (ITS, nrLSU rDNA and EF1-alpha). We tested whether the fast-evolving Internal Transcribed Spacer (ITS) sequences can be accurately aligned across the family, by comparing the outcome of two iterative alignment refining approaches (an automated and a manual) and various indel-treatment strategies. We used PRANK to align sequences in both cases. Our results suggest that – although PRANK successfully evades overmatching of gapped sites, referred previously to as alignment overmatching – it infers an unrealistically high number of indel events with natively generated guide-trees. This 'alignment undermatching' could be avoided by using more rigorous (e.g. ML) guide trees. The trees inferred in this study support the monophyly of the core Bolbitiaceae, with the exclusion of Panaeolus, Agrocybe, and some of the genera formerly placed in the family. Bolbitius and Conocybe were found monophyletic, however, Pholiotina and Galerella require redefinition. The phylogeny revealed that stipe coverage type is a poor predictor of phylogenetic relationships, indicating the need for a revision of the intrageneric relationships within Conocybe.
Introduction
The Family Bolbitiaceae
Brown-spored mushroom genera of the Bolbitiaceae represent members of a large, complex clade in the Agaricales with a considerable history of taxonomic debate. They live as decomposers of leaf-litter and dung and are characterized mostly by tiny fruiting bodies and a cap covering composed of balloon-shaped cells [1], [2]. Some of the species contain hallucinogenic compounds and are toxic [3]. Traditionally the genera Bolbitius, Conocybe, Agrocybe, Galerella, Pholiotina, Descolea, Panaeolus, Panaeolina and a number of smaller genera have been placed in the family [2], [4], [5], [6], [7], [8]. In addition, a number of puffball-like (gasteroid) species have been added, including Gastrocybe, Galeropsis, Agrogaster, Gymnoglossum and Cyttarophyllum, many of which are known only from type materials [2], [9], [10]. Agrocybe has traditionally been classified in the Bolbitiaceae, which has been abandoned by many authors recently [4], [11], [12]. This was in part inspired by hitherto published molecular phylogenies, which included only a handful of species from the Bolbitiaceae, but suggested a polyphyletic origin, with Agrocybe being distantly related to the rest of the genera [3], [13], [14], [15], [16], [17]. This has also been supported by patterns of conidiogenesis [11].
Panaeolus, Panaeolopsis and Panaeolina form a rather isolated group in the Bolbitiaceae. They share several taxonomically important features with the core Bolbitiaceae (Bolbitius, Conocybe and Pholiotina), such as structure of spore wall and cap covering, or ecology, but differ in a number of spectacular features [2]. Among others, the color of the spores is dark reddish-brown to blackish in the panaeoloid species, whereas the bolbitioid genera have yellow- to rusty brown spores. A close affinity between Panaeolus and Panaeolina and Conocybe/Bolbitius has been inferred previously [14], [16] mainly based on nrLSU sequences, however, support for this relationship, and thus the correct phylogenetic classification of the panaeoloid genera remained elusive. The only available multigene datasets, however, suggest that the panaeoloid species are more closely related to species of Tubaria and allied genera than the core Bolbitiaceae [17], [18].
Bolbitius, Conocybe and Pholiotina have emerged as a monophyletic unit in almost all phylogenetic studies involving these species with limited taxon sampling [13], [14], [16], [17]. The generic-level taxonomy of Pholiotina and Conocybe has been a disputed field. Several authors treated Pholiotina as a subgenus (e.g. [7]) within Conocybe. The latter is characterized by special capitate cystidia (lecythiform), which clearly separates it from all but two of the Pholiotina taxa (Ph. brunnea, Ph. intermedia), bearing fusiform - utriform cystidia. Furthermore, many species of Pholiotina possess a protective veil coverage of the young fruiting bodies, while species of Conocybe and some Pholiotina species do not [1], [4], [5]. This puts the monophyly of this genus into question and raises the possibility of a relationship between veil-less Pholiotina and Conocybe taxa. Despite this long-standing debate, no molecular phylogenetic studies have resolved the affinities of Conocybe and Pholiotina or veiled and veil-less species of Pholiotina so far.
The largest genus in the family is Conocybe, with about 500 described species, which, according to a recent critical revision [5], group into ca. 170 morphologically distinguishable taxa (see Fig. 1). Sectional taxonomy of Conocybe predominantly relies on the composition of stipe covering, made up of two types of cystidia, which serve to protect the stipe in early stages of the ontogeny and prevent the fusion of gill edges with the stipe surface when the cap is still closed [2], [4], [5], [6], [7]. These include oil-flask-shaped, lecythiform cystidia, i.e. cells with a broad base and sharply delimited rounded capitulum, and simple hair-like or cylindrical cystidia. The three largest sections of the Conocybe, sect. Conocybe, sect. Pilosellae and sect. Mixtae have lecythiform, hair-like and both types of cystidia on the stipe, respectively [4], [12]. Section Mixtae was erected for species with both lecythiform and hair-like cystidia occurring on the stipe [19]. However, because of the occasional occurrence of distorted or defective cystidia among lecythiform ones, this definition was later emended to comprise only species in which the ratio of lecythiform and hair-like cystidia ranges from 1∶5 to 5∶1 [20], [21]. Based on different types of characters, several morphologically uniform groups have been excepted from this classification, such as section Candidae for species with pseudoparaphyses (spacer cells between basidia), pale colours of the fruiting bodies and (partial) deliquescence, section Ochromarasmius for species with ornamented spores, or section Singerella for volvate taxa [5], [7], [22], [23]. The presence of pseudoparaphyses led some authors to consider species of section Candidae as members of Bolbitius [24]. Despite the mentioned exceptions, whether the traditional view of three major sections can be validated with molecular characters remained untested.
Figure 1. Morphological diversity in the Bolbitiaceae, with examples of Conocybe (A–E, G–J), Bolbitius (F) and Pholiotina (K–M).
A. Conocybe mesospora; B. Conocybe hornana; C. Conocybe digitalina; D. Conocybe anthracophila; E. Conocybe microrhiza; F. Bolbitius titubans; G. Conocybe romagnesii a partially deliquescent species; H and J Conocybe deliquescens upper fresh, lower immature and already collapsed fruiting bodies; I. Conocybe albipes; K. Pholiotina teneroides showing rich veil remnants on stipe; L. Pholiotina brunnea with scanty veil fibrils on cap margin; M. Pholiotina coprophila, a species without veil.
The Evolution of Fruiting Bodies within and Outside of the Bolbitiaceae
The Bolbitiaceae include species with deliquescent fruiting bodies, named after a special phase of the ontogeny, in which the fruiting body is enzymatically digested, resulting in partial autolysis or collapse [2], [25], [26], [27]. Recently, in the Psathyrellaceae, deliquescent fruiting bodies have been identified as a result of convergent, complex evolutionary processes involving correlated gain of several physiological traits. The term coprinoidization was proposed to describe this process, as an analogue of gastromycetation [26], [28]. It has been suggested that deliquescent fruiting bodies represent an adaptation to fast-changing environments, which is achieved by an accelerated ontogeny resulting from quick water uptake of pseudoparaphyses, cystidia and skeletal cells of the cap. Previous phylogenetic results highlighted the polyphyletic nature of deliquescent fruiting bodies at the family level by identifying Coprinus s. str. in the Agaricaceae [29], and the remaining Coprinus sensu lato taxa in the Psathyrellaceae, however, whether deliquescent species were monophyletic within these families remained contentious. Recently the situation has been resolved in the Psathyrellaceae by identifying four lineages with independent acquisitions of deliquescent fruiting bodies [28]. Whether a similar scenario of fruiting body evolution with similar putative adaptations can be discerned in the Bolbitiaceae remained to be tested in order to obtain a more thorough picture on fruiting body evolution in mushrooms.
Alignment Methods for Indel-rich Sequences
Aligning rapidly evolving and indel-rich loci across higher evolutionary distances has been a challenge for evolutionary biologists and considerable effort has been put into developing increasingly sophisticated methods that capture more of the biological realism [30], [31], [32], [33], [34], [35], [36], [37]. The accurate inference of the number of insertions and deletions (indels) and their placement in the alignment has been the task of primary interest, since most of the rapidly evolving phylogenetic markers are also non-coding and thus accumulate indels at a high rate [36], [38]. Recent advances in probabilistic models eliminated the problem of gap costs, i.e. penalizing indels rather than incorporating them into alignment inference [34], [36], [39]. For instance, PRANK achieved theoretical superiority over traditional methods with gap costs, by using an explicit model of indel evolution and distinguishing insertions from deletions [33]. Even with elaborate nucleic acid and indel models, the dependence of progressive algorithms on a guide tree can induce significant bias and should be accounted for in downstream analyses. It has been recognized rather early that rough guide trees built from pairwise or k-mer distances often poorly reflect true relationships and can bias the progressive alignment steps of the algorithms [30]. One approach to mitigate the dependence on a rough guide tree has been the application of few to several iterative refinement steps during alignment estimation, where each step a new alignment is generated, which serves as the input for the estimation of a new guide tree (e.g. [30]). A potential drawback of these algorithms is the poor quality of the guide tree, which is usually built by simple distance-based algorithms. Liu et al. [31], [32] automated the process of alignment estimation and the inference of high quality trees (by Maximum Likelihood) in an algorithm that iteratively improves the alignment. This method, called SATé, uses the likelihood of the resulting ML trees as an optimality criterion for choosing among alignments [32] and has been shown to achieve higher accuracy at a not significantly higher computational cost. SATé uses the whole alignment in the tree inference stage, thus the potential confounding effects of poorly aligned sites may be carried over to the next cycle. Recently, using PRANK, a manual alternative for this has been proposed, which takes advantage only of unambiguously aligned regions [40]. A tree is computed from ungapped (unambiguously aligned) regions of the alignment and used as a guide tree in the next round of alignment estimation. However, the way alignment noise affects the quality of guide trees and the next alignment has not been examined.
In the present study, we examine the relationship between alignments and guide trees by applying a manual iterative method to improve the alignments of indel-rich loci and eliminate guide-tree induced errors based on phylogenetic signal from other genes. We do this by using a three-locus dataset of the Bolbitiaceae, a fungal family for which practically no phylogenetic information has been available so far. We used the above alignment strategy to address the alignability of the rapidly evolving nuclear ITS region, which is the most frequently sequenced marker in fungi and plants, as well as two additional genes (nrLSU and EF1-alpha), which generally pose no alignment problems. We demonstrate that the ITS region can be reliably aligned at this scale, by testing for conflict between single-gene trees as well as the ITS alignment with gapped regions removed by an automatic method. The resulting multigene phylogeny is then used to study the evolution of fruiting body types in the Bolbitiaceae and to confirm large-scale patterns of fruiting bodies described recently in other families.
Materials and Methods
Taxon Sampling
To obtain a comprehensive view of the phylogenetic relationships within the Bolbitiaceae, we sampled a morphologically diverse set of species from all major groups of the family. Thus, we gathered specimens of the genera Conocybe, Pholiotina, Bolbitius, Descolea, Galerella and Tubariella, and selected ca. 70% of the species accepted in a recent critical monographic treatment of the genera, covering both temperate and tropical species (Table 1). Psathyrella michiganensis, a species morphologically fitting Conocybe has also been included in the study [41]. Within Conocybe, we selected taxa so as to maximize the morphological diversity in the sample, by sampling species from each of the sections recently established [4], [5]. The final data set contained sequences of 123 specimens of 116 species of Conocybe (91 taxa), Bolbitius (6), Pholiotina (13), Descolea (1), Galerella (2), Panaeolus (2) and Galeropsis (1). All species were represented by at least two genes (except C. volviornata). Altogether 118 ITS, 114 LSU and 87 EF1-alpha sequences have been produced for this study. PCR or sequencing of Galerella plicatella (WU20898), Descolea phlebophora (WU27464), D. recedens (WU27465), D. sp. (WU27469) and Tubariella rhizophora (WU22233) failed, so these species have been omitted from the analyses. The newly generated sequences have been complemented with ITS and LSU sequences of Bolbitius psittacinus (EF648217, EF648218), Panaeolus sphinctrinus (DQ182503, DQ470817) and Panaeolus cambodginiensis (AB158633). Based on the ML phylogeny of species of the agaricoid clade, we chose Panaeolus as the outgroup for the Bolbitiaceae.
Table 1. List of specimens, voucher numbers, countries of origin and GenBank accession numbers of the species used in this study.
| Accession Numbers | |||||
| Taxon name | Voucher No. | Country of Origin | ITS | LSU | ef–1a |
| Bolbitius coprophilus | SZMC-NL-2460 | Hungary | DQ234567 | DQ234567 | DQ234567 |
| Bolbitius elegans | WU23943 | Italy | JX968250 | JX968367 | JX968456 |
| Bolbitius lacteus | WU8327 | Austria | JX968224 | JX968342 | – |
| Bolbitius reticulatus | WU30001 | Hungary | JX968249 | JX968366 | JX968455 |
| Bolbitius subvolvatus | WU28379 | Italy | JX968248 | JX968365 | JX968454 |
| Bolbitius vitellinus | SZMC-NL-1994 | Hungary | JX968252 | JX968369 | – |
| Conocybe aff. ochrostriata | SZMC-NL-0830 | Hungary | JX968236 | JX968354 | JX968447 |
| Conocybe alboradicans | SZMC-NL-3226 | Hungary | JX968219 | JX968336 | JX968435 |
| Conocybe alboradicans | WU14678 | Austria | JX968220 | JX968337 | – |
| Conocybe ammophila | WU23983 | Mongolia | JX968197 | JX968313 | JX968416 |
| Conocybe anthracophila var. ovispora | WU25461 | Italy | JX968237 | JX968355 | – |
| Conocybe anthracophila | WU14367 | Italy | JX968212 | JX968329 | JX968430 |
| Conocybe antipus | WU19791 | Austria | JX968215 | JX968332 | JX968432 |
| Conocybe aurea | WU28161 | Italy | JX968184 | JX968300 | JX968407 |
| Conocybe bispora | SZMC-NL-2573 | Hungary | JX968203 | JX968320 | JX968423 |
| Conocybe brachypodii | SZMC-NL-2105 | Sweden | JX968191 | JX968307 | JX968413 |
| Conocybe brachypodii | SZMC-NL-2189 | Hungary | – | JX968314 | JX968417 |
| Conocybe brachypodii | SZMC-NL-2289 | Sweden | JX968183 | JX968299 | JX968406 |
| Conocybe brachypodii | SZMC-NL-3169 | Hungary | JX968199 | JX968316 | JX968419 |
| Conocybe cettoiana | WU10436 | Italy | JX968218 | JX968335 | – |
| Conocybe crispella | WU27367 | Australia | JX968208 | JX968325 | JX968426 |
| Conocybe cylindracea | WU20796 | Italy | JX968240 | JX968358 | JX968449 |
| Conocybe deliquescens | SZMC-NL-0574 | Hungary | JX968210 | JX968327 | JX968428 |
| Conocybe dumetorum | SZMC-NL-2693 | Sweden | JX968201 | JX968318 | JX968421 |
| Conocybe dunensis | WU27359 | Spain | JX968227 | JX968345 | – |
| Conocybe echinata | SZMC-NL-1007 | Hungary | JX968196 | JX968312 | – |
| Conocybe elegans | SZMC-NL-0908 | Sweden | JX968223 | JX968341 | JX968437 |
| Conocybe enderlei | SZMC-NL-0165 | Sweden | JX968161 | JX968277 | JX968389 |
| Conocybe enderlei | WU21272 | Italy | JX968163 | JX968279 | – |
| Conocybe farinacea | SZMC-NL-2173 | Hungary | JX968167 | JX968283 | – |
| Conocybe fiorii | WU17793 | Italy | JX968217 | JX968334 | JX968434 |
| Conocybe fuscimarginata | SZMC-NL-3668 | Sweden | JX968238 | JX968356 | JX968448 |
| Conocybe gigasperma | SZMC-NL-3972 | Slovakia | JX968179 | JX968295 | JX968403 |
| Conocybe gracilis | WU21277 | Austria | JX968221 | JX968338 | – |
| Conocybe graminis | WU13466 | Austria | JX968195 | JX968311 | – |
| Conocybe herbarum | WU22193 | Austria | JX968193 | JX968309 | – |
| Conocybe hornana | SZMC-NL-3499 | Slovakia | JX968178 | JX968294 | JX968402 |
| Conocybe incarnata | WU21897 | Finland | JX968229 | JX968347 | JX968441 |
| Conocybe ingridiae | WU28158 | Italy | JX968244 | JX968361 | JX968451 |
| Conocybe inocybeoides | SZMC-NL-3589 | Hungary | JX968202 | JX968319 | JX968422 |
| Conocybe inopinata | WU27544 | Italy | JX968165 | JX968281 | JX968392 |
| Conocybe intrusa | WU25546 | Finland | JX968211 | JX968328 | JX968429 |
| Conocybe juniana var. sordescens | SZMC-NL-2304 | Sweden | JX968192 | JX968308 | JX968414 |
| Conocybe karinae | WU28526 | Germany | JX968151 | JX968268 | JX968384 |
| Conocybe lactea | SZMC-NL-1012 | Hungary | JX968209 | JX968326 | JX968427 |
| Conocybe lenticulospora | SZMC-NL-0923 | Sweden | JX968242 | JX968359 | JX968450 |
| Conocybe leporina | SZMC-NL-2380 | Hungary | JX968177 | JX968293 | JX968401 |
| Conocybe lobauensis | WU17826 | Italy | JX968176 | JX968292 | JX968400 |
| Conocybe macrocephala | WU18148 | Austria | JX968182 | JX968298 | – |
| Conocybe macrospora | WU17030 | Germany | JX968175 | JX968291 | – |
| Conocybe merdaria | WU25359 | Austria | JX968174 | JX968290 | – |
| Conocybe microrhiza | SZMC-NL-2180 | Hungary | JX968222 | JX968340 | JX968436 |
| Conocybe microspora | SZMC-NL-1890 | Hungary | JX968160 | JX968276 | – |
| Conocybe monicae | WU22612 | Austria | JX968200 | JX968317 | JX968420 |
| Conocybe moseri var bisporigera | SZMC-NL-1904 | Hungary | JX968235 | JX968353 | JX968446 |
| Conocybe nigrescens | WU27557 | Italy | JX968234 | JX968352 | JX968445 |
| Conocybe ochrostriata var. favrei | WU29786 | Italy | JX968245 | JX968362 | JX968452 |
| Conocybe pallidospora | WU17079 | USA | JX968158 | – | – |
| Conocybe pallidospora | WU7395 | Austria | JX968239 | JX968357 | – |
| Conocybe papillata | SZMC-NL-2370 | Hungary | JX968216 | JX968333 | JX968433 |
| Conocybe pilosella | SZMC-NL-0831 | Hungary | JX968231 | JX968349 | JX968443 |
| Conocybe pseudocrispa | WU18009 | Austria | JX968230 | JX968348 | JX968442 |
| Conocybe pubescens | SZMC-NL-1986 | Romania | JX968173 | JX968289 | JX968399 |
| Conocybe pubescens | WU20759 | Italy | JX968170 | JX968286 | JX968396 |
| Conocybe rickeniana | SZMC-NL-2468 | Hungary | JX968198 | JX968315 | JX968418 |
| Conocybe romagnesii | WU26605 | Italy | JX968206 | JX968323 | JX968424 |
| Conocybe rostellata | SZMC-NL-2499 | Sweden | JX968162 | JX968278 | JX968390 |
| Conocybe sabulicola | WU11185 | Italy | JX968186 | JX968302 | JX968409 |
| Conocybe semiglobata 'type affinis' | WU8794 | Austria | JX968188 | JX968304 | JX968168 |
| Conocybe semiglobata | SZMC-NL-1993 | Hungary | JX968181 | JX968297 | JX968405 |
| Conocybe semiglobata var campanulata | SZMC-NL-3159 | Sweden | JX968284 | – | JX968394 |
| Conocybe semiglobata var campanulata | WU26395 | Germany | JX968169 | JX968285 | JX968395 |
| Conocybe siennophylla | SZMC-NL-1210 | Hungary | JX968246 | JX968363 | JX968453 |
| Conocybe siennophylla | WU17988 | Germany | JX968243 | JX968360 | – |
| Conocybe siliginea | SZMC-NL-1211 | Hungary | JX968159 | JX968275 | – |
| Conocybe siliginea | SZMC-NL-2313 | Sweden | JX968225 | JX968343 | JX968438 |
| Conocybe singeriana | WU22129 | Austria | JX968166 | JX968282 | JX968393 |
| Conocybe solitaria | WU20903 | India | JX968214 | JX968331 | JX968431 |
| Conocybe sp. | SZMC-NL-1455 | Hungary | JX968194 | JX968310 | JX968415 |
| Conocybe subovalis | SZMC-NL-1415 | Hungary | JX968190 | JX968306 | JX968412 |
| Conocybe subpubescens | SZMC-NL-0162 | Sweden | JX968189 | JX968305 | JX968411 |
| Conocybe subpubescens | SZMC-NL-2181 | Hungary | JX968171 | JX968287 | JX968397 |
| Conocybe subxerophytica | SZMC-NL-0164 | Sweden | JX968187 | JX968303 | JX968410 |
| Conocybe tenera | SZMC-NL- | Hungary | JX968185 | JX968301 | JX968408 |
| Conocybe tenera | SZMC-NL-1615 | Hungary | JX968180 | JX968296 | JX968404 |
| Conocybe tetrasporoides | WU17385 | New Zealand | JX968232 | JX968350 | – |
| Conocybe tuxlaensis | SZMC-NL-1897 | Hungary | JX968164 | JX968280 | JX968391 |
| Conocybe vaginata | WU25703 | Sri Lanka | JX968204 | JX968321 | – |
| Conocybe velutinomarginata | WU28695 | Germany | JX968226 | JX968344 | JX968439 |
| Conocybe velutipes | SZMC-NL-2187 | Hungary | JX968228 | JX968346 | JX968440 |
| Conocybe velutipes var. nitrophila | WU20916 | India | JX968233 | JX968351 | JX968444 |
| Conocybe volvata | WU20900 | India | JX968205 | JX968322 | – |
| Conocybe volviornata | WU22218 | Indonesia | – | JX968339 | – |
| Conocybe watlingii | WU22744 | Finland | JX968172 | JX968288 | JX968398 |
| Conocybe zeylandica | WU20185 | La Réunion | JX968207 | JX968324 | JX968425 |
| Conocybe zuccherellii | WU12421 | Italy | JX968213 | JX968330 | – |
| Descolea maculata var. occidentalis | WU21819 | Portugal | JX968155 | JX968272 | – |
| Galerella floriformis | WU22833 | Vanuatu | JX968254 | JX968371 | JX968458 |
| Galerella nigeriensis | CNF1/5859 | Nigeria | JX968251 | JX968368 | JX968457 |
| Galeropsis desertorum | SZMC-NL-1863 | Hungary | JX968154 | JX968271 | JX968387 |
| Pholiotina aberrans | SZMC-NL-3161 | Sweden | JX968256 | JX968373 | JX968459 |
| Pholiotina aeruginosa | WU27104 | Germany | JX968247 | JX968364 | – |
| Pholiotina aporos | SZMC-NL-1241 | Hungary | JX968260 | JX968376 | JX968462 |
| Pholiotina arrheni | SZMC-NL-2509 | Sweden | JX968261 | JX968377 | – |
| Pholiotina brunneola | SZMC-NL-1216 | Hungary | JX968259 | JX968375 | JX968461 |
| Pholiotina coprophila | SZMC-NL-2176 | Hungary | JX968156 | JX968273 | – |
| Pholiotina cyanopus | WU2134 | Austria | JX968157 | JX968274 | JX968388 |
| Pholiotina dasypus | SZMC-NL-2279 | Hungary | JX968152 | JX968269 | JX968385 |
| Pholiotina indica | WU20891 | India | JX968263 | JX968378 | JX968464 |
| Pholiotina nemoralis var. dentatomarginata | SZMC-NL-2921 | Hungary | JX968258 | JX968374 | JX968460 |
| Pholiotina nemoralis var. dentatomarginata | SZMC-NL-2962 | Hungary | JX968257 | – | – |
| Conocybe pygmaeoaffinis | WU16600 | Austria | JX968149 | JX968382 | – |
| Pholiotina striipes | WU26997 | Austria | JX968150 | JX968267 | JX968383 |
| Pholiotina sulcata | SZMC-NL-1975 | Hungary | JX968153 | JX968270 | JX968386 |
| Pholiotina teneroides | SZMC-NL-3501 | Slovakia | JX968264 | JX968379 | JX968465 |
| Pholiotina utricystidiata | WU20164 | Germany | JX968262 | JX968463 | – |
| Pholiotina vestita | SZMC-NL-2191 | Hungary | JX968266 | JX968381 | JX968467 |
| Pholiotina vexans | SZMC-NL-3967 | Slovakia | JX968265 | JX968380 | JX968466 |
| Psathyrella michiganensis | SMITH 10920 TYPE | USA | JX968241 | – | – |
Laboratory Protocols
Genomic DNA was extracted from 2–10 mg of dried herbarium specimens, using the DNeasy Plant Mini Kit (QIAGEN) according to the manufacturer’s instructions. We amplified the ITS1-5.8S-ITS2 (ca. 700 bp), nrLSU (ca. 1500 bp) and the EF1-alpha (ca. 1200 bp) regions using the primer combinations ITS1/4, LROR/LR7 and 983F/2218R, respectively [28]. Amplification protocols and PCR conditions were as described previously [28]: 95 C° for 5 min, 95 C° for 0.5 min, 48–52 C° for 0.5 min, 72 C° for 0.3 min, repeated for 30 cycles, and a final extension at 72° for 4 min. Cleaning and sequencing of PCR products was performed commercially by LGC Genomics (Berlin). Single reads were assembled to contigs by the PreGap and Gap4 programs of the Staden package [42].
Alignment Strategy
Previous studies revealed that alignment errors can have profound effects on phylogeny reconstruction [58], [59], especially in the case of indel-rich alignments [40]. Of the markers used in this study, the ITS is the most prone to such bias, due to the high number of insertion-deletion events in the ITS1 and ITS2 loci (but not in the 5.8S gene), whereas alignment of the two other loci is straightforward at this taxonomic scale. These length mutations cause most of the problems when aligning distantly related ITS sequences. In order to examine the effects of alignment assumptions on the resulting phylogeny, we applied two different alignment strategies for the ITS locus. The two approaches were common in iteratively improving the alignment based on new guide trees estimated from the results of the previous alignment. However, the first strategy, performed in SATé 1.4 ([32], settings: 100 replicates, aligner: PRANK, tree estimator: RAxML, alignment merger:OPAL, model: GTR+G, other options left at default) used only the ITS alignment as an input for estimating a new guide tree, whereas the second one employed information from all three genes to infer a tree that serves as the guide tree in the next alignment inference stage. This latter was performed manually by first inferring an alignment for the ITS sequences in PRANK ([34], using the +F option to fix already inferred indels, otherwise as default), then merging the resulting ITS alignment with the nrLSU and EF1-alpha alignments, and running a Bayesian MCMC analysis on the combined matrix (see below). The 50% majority rule consensus tree computed on the basis of the Bayesian run (excluding burn-in) was then used as input for the next alignment of ITS sequences. Polytomies of the consensus tree were randomly resolved to zero-length branches in Mesquite [43]. This procedure was repeated until no change to the resulting Bayesian consensus tree topology was observed (see below).
Combinability Tests
We used the congruence of single-gene ML trees as a criterion for detecting incongruence between the single-gene trees. We performed a Maximum Likelihood bootstrap analysis for each single-locus alignment (as described below). Incongruent, strongly supported (70% or greater) nodes were regarded as a signature of significant conflict.
Phylogenetic Analyses
We estimated phylogenetic relationships and support values using the final concatenated alignment (ITS, LSU and EF1-alpha) using Bayesian MCMC and ML bootstrapping. Best-fit models of evolution were selected for each locus using the AICc criterion in jModelTest [44]. The proportion of invariant sites (“I”) was omitted from all models, since this accounts for the same phenomenon as the gamma distribution, and convergence problems in Bayesian analyses have been identified when the two were applied simultaneously.
For the final Bayesian analyses, we coded all indels in the ITS alignment as a separate partition of binary presence/absence characters following Simmons and Ochoterena [45]. The simple indel coding algorithm [45] considers all contiguous sets of gap characters as one single evolutionary event, as opposed to e.g. fifth state coding (used in parsimony) where each gap character is considered a separate event. We checked the congruence of the phylogenetic signal in the gap characters by running a Bayesian MCMC analysis using the indel data only and manually comparing the clade structure to that obtained from nucleic acid data. The model implemented for restriction sites in MrBayes was used for the indel partition with the command "coding = variable" to adjust for constant characters not included. As an alternative to indel-coding, we excluded gapped sites from the ITS alignment by using GBlocks 0.91 [46]. For GBlocks, we used the “less stringent” set of parameters, allowing at most half of the sequences to contain a gap in a single column of the alignment.
Bayesian MCMC analyses were performed in MrBayes 3.1.2 [47] and BEAST 1.6.1. [48]. We ran two replicates of four chains with default priors in MrBayes and three replicates of one chain in BEAST were run for 20.000.000 generations, sampling every 100th tree. The data were divided into ITS1, 5.8S ITS2, nrLSU and EF1-alpha partitions and the parameters of the model were unlinked across partitions. For each partition, we used the GTR+G model. To avoid potential over-partitioning of the dataset, we monitored the posterior distributions and associated parameter variances in Tracer [49] for each partition. High variance and low effective sample sizes were used as signatures of over-partitioning. The burn-in was designated at sufficient topological convergence, as judged by the average standard deviation of split frequencies (<0.01) and the Cumulative and Compare functions of AWTY (ceb.csit.fsu.edu/ awty /) [Wilgenbusch 2004]. The resulting tree samples were used to compute 50% majority rule consensus trees in MrBayes and Sumtrees [50].
Maximum Likelihood inference and bootstrapping was performed in 1000 replicates in RAxML 7.0.4 [51], using the same partitioning scheme as above and the GTRGAMMA model. Bootstrap trees were summarized by the SumTrees script of the Dendropy package.
Constraint Analyses
The monophyly of volvate species, the genus Pholiotina, as well as that of veil-less Pholiotina species were tested. For this we inferred ten unconstrained and ten constraint trees in RAxML based on the final concatenated alignment, using the above mentioned settings, and calculated single-site likelihoods for all trees. Constraint trees were constructed manually in Mesquite. Because Galerella nigeriensis nested within Pholiotina, we constructed the constraint trees allowing this taxon to be resolved outside Pholiotina. This resulted in a polytomy, which was resolved according to the ML solution around that node. The CONSEL package was used with default settings to calculate approximately unbiased (AU) test p-values [52].
Maximum Likelihood Phylogeny of the Agaricoid Clade
We assembled an LSU alignment of the agaricoid clade with the aims to select an outgroup for the Bolbitiaceae and identify the phylogenetic position of deliquescent lineages. For this we downloaded nrLSU sequences (>500 bp) of all species of the agaricoid clade represented in GenBank. Due to inconsistencies in species limits, we did not attempt to reduce this dataset to one sequence per species; however, completely unidentified and environmental sequences were excluded. This dataset included Cyttarophyllum, a gasteroid representative of the Bolbitiaceae. An alignment was computed by using MUSCLE [30], followed by minor manual refinement. A ML tree inferred in PhyML 3.0 [53], using the GTR+G model of evolution with 4 rate categories, Subtree Pruning and Regrafting (SPR) as the branch swapping algorithm. As a mean of branch support, we performed approximate Likelihood Ratio Tests, which is a fast alternative of bootstrapping [54]. The aLRT support corresponds to the probability that the branches exist, as compared to the null hypothesis of it having zero-length [54]. A list of sequences included in this alignment is available in the Supplementary Information. The alignment of the entire agaricoid clade contained 1608 nrLSU sequences and 1358 characters.
Ancestral State Reconstruction
To examine whether coprinoid lineages in the Bolbitiaceae emerged via parallel gains or multiple losses of the coprinoid fruiting body type, we performed ancestral state reconstructions on the most recent common ancestor of the Bolbitiaceae, using the tree obtained from the concatenated data matrix including indels. Fruiting body types were coded as either coprinoid or non-coprinoid. Taxa were coded as coprinoid when their fruiting bodies collapse or deliquesce upon maturing, possess pseudoparaphyses and a plicate cap surface. We coded species with partial deliquescence as coprinoid if they show the above syndrome. This way, we could exclude ambiguities arising from the determination of the level of deliquescence in species with tiny fruiting bodies, which desiccate before autolysis. An Maximum Likelihood approach was chosen and performed with the program BayesTraits 1.0 [55]. We used 1000 phylograms, subsampled by Mesquite from the output of the final Bayesian analysis and reconstructed ancestral states on each tree, by using the 'addmrca' command, which allows topological uncertainty to be taken into account by reconstructing the ancestral state for the least inclusive node which contains all the specified taxa. 25 attempts were made to maximize the likelihood on each tree (mltries = 25). Based on the results of preliminary parsimony mapping, we selected five nodes around which switches in fruiting body type might have occurred (Table 2). A Markov model with two states and no restrictions was applied. Statistical significance was measured by fixing the node of interest in one or the alternative state and comparing the mean of the resulting likelihoods. A difference of two logL unit was taken as evidence for significant support [56]. A fully Bayesian method was also considered, but given the uncertainties in prior optimization, we chose Maximum Likelihood reconstruction over a set of trees.
Table 2. Summary of ancestral states of fruiting body types inferred by ML reconstructions in BayesTraits.
| Node | Probability of state (0) | –lnL(0) | –lnL(1) | –(lnL(1)+lnL(0)) |
| Node 1 (Bolbitiaceae) | 0.937 | 31.52 | 33.72 | 2.205 |
| Node 2 (Bolbitius+Pholiotina 1) | 0.763 | 31.53 | 33.43 | 1.9 |
| Node 3 (Bolbitius) | 0.311 | 31.71 | 32.29 | 0.59 |
| Node 4 (Conocybe 6) | 0.673 | 31.53 | 33.54 | 2.0047 |
| Node 5 (section Candidae) | 0.014 | 34.59 | 31.54 | 3.0419 |
See Fig. 3 for the position of nodes on the phylogeny. State 0 and 1 re present the non-coprinoid and coprinoid fruiting body morphologies, respectively.
Stipe covering was also scored for all taxa in the tree, either as consisting entirely of lecythiform (oil-flask-shaped), fusiform-utriform or both types of cystidia. Since there are three major types of stipe covering, we tested both additive binary coding and multistate coding. Additive binary coding comprised two characters, the presence or absence of hair-like, and that of lecythiform cystidia. Under multistate coding we distinguished 'hair-like only' (state 0), 'hairs and lecythiform' (state 1) and 'lecythiform only' (state 2) conditions. We reconstructed ancestral states for the most recent common ancestor of Conocybe. Information on the mean number and types of state changes was obtained by the ‘Summarize state changes on trees’ command in Mesquite 3.0 [43]. 50 mappings were performed on each tree.
Results
Alignment
Alignment of the nrLSU and EF1-alpha genes was straightforward, neither contained indels. After trimming non-overlapping leading and trailing gaps, the nrLSU and EF1-alpha alignments were and 1303 and 1179 bp long, respectively. The EF1-alpha alignment contained three introns (positions 1–90, 756–813 and 956–1016), which could not be aligned unambiguously and were excluded from the analyses. For the ITS region, we used two different alignment strategies. Using SATé, we generated 100 alignments, of which the one yielding the tree with the best likelihood score (–18953.91) contained 1542 sites (ITS1∶1–800, 5.8S: 801–969, ITS2∶970–1542) and was retained, combined with nrLSU and EF1-alpha alignments and used in a Bayesian MCMC analysis. In the manual refinement strategy, we found that five iterations were sufficient to flat out changes in the alignments, which were reflected by alignment lengths, the topology of single-gene ML phylograms and Bayesian consensus trees. The guide tree had a profound effect on the inferred alignment length. In the first alignment step by PRANK, by using the first guide tree estimated from the data, the length of the resulting alignment contained 4478 sites, the second, computed with a guide tree estimated from the first alignment contained 3102 sites, whereas subsequent alignments, computed with guide trees estimated from all three genes were around 1500 bp long (the final being: ITS1∶1–730, 5.8S: 731–902, ITS2∶903–1614). To estimate the phylogenetic relationships in the Bolbitiaceae, this final ITS alignment has been concatenated with the two other loci. Alignments and phylogenetic trees have been uploaded to TreeBase (No: 13626).
We did not encounter significant conflict between the single-gene trees. To address whether the ITS region contains phylogenetic signal, or the alignment is merely a result of forcing unalignable sequences into an alignment, we inferred single-gene ITS trees with and without applying GBlocks as well as inferred trees from the recoded gap data only (Figures S1–S4). We found that these trees are congruent with each other (as judged by mutually exclusive clades with strong ML bootstrap support) as well as LSU and EF1-alpha trees, differing only in the number of strongly supported clades. This suggests that the ITS alignment contains phylogenetic signal, as opposed to the scenario where forced alignment would contain random noise. The final concatenated alignment thus contained all three genes, plus the recoded binary indel characters, totaling to 4075 nucleic acid sites and 864 presence/absence characters. Of the indel characters, 450 were parsimony informative. The nrLSU alignment of the agaricoid clade contained 1367 characters.
Phylogenetic Analyses
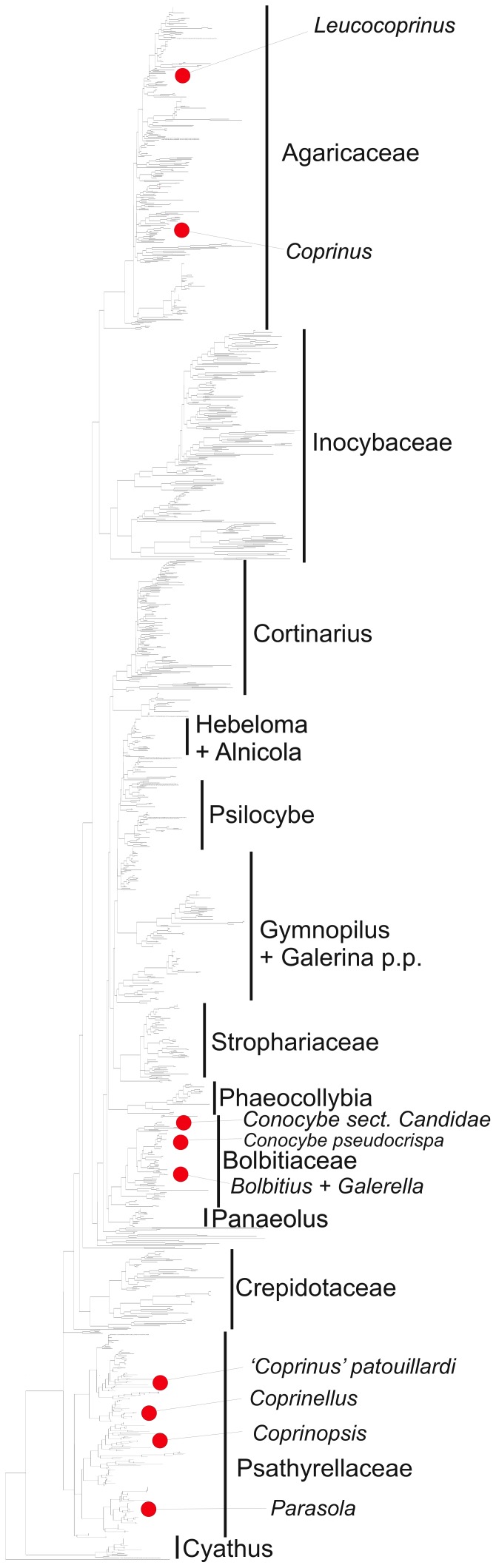
For examining the phylogenetic distribution of coprinoid species, we downloaded all nrLSU sequences of the agaricoid clade from GenBank, excluding sequences shorter than 500 bp and those of environmental origin. The ML phylogeny completed within 24 hours, 425 and 252 branches receiving probabilities >0.90 and >0.95 from approximate likelihood ratio tests (aLRT). The phylogeny revealed many of the major agaricoid clades recovered by Matheny et al., [17], including the Inocybaceae, Psathyrellaceae, Cortinariaceae (paraphyletic), Bolbitiaceae, Crepidotaceae, Agaricaceae, Strophariaceae, Hebeloma+Alnicola, etc. Coprinoid lineages were found in the Agaricaceae, Bolbitiaceae and Psathyrellaceae.
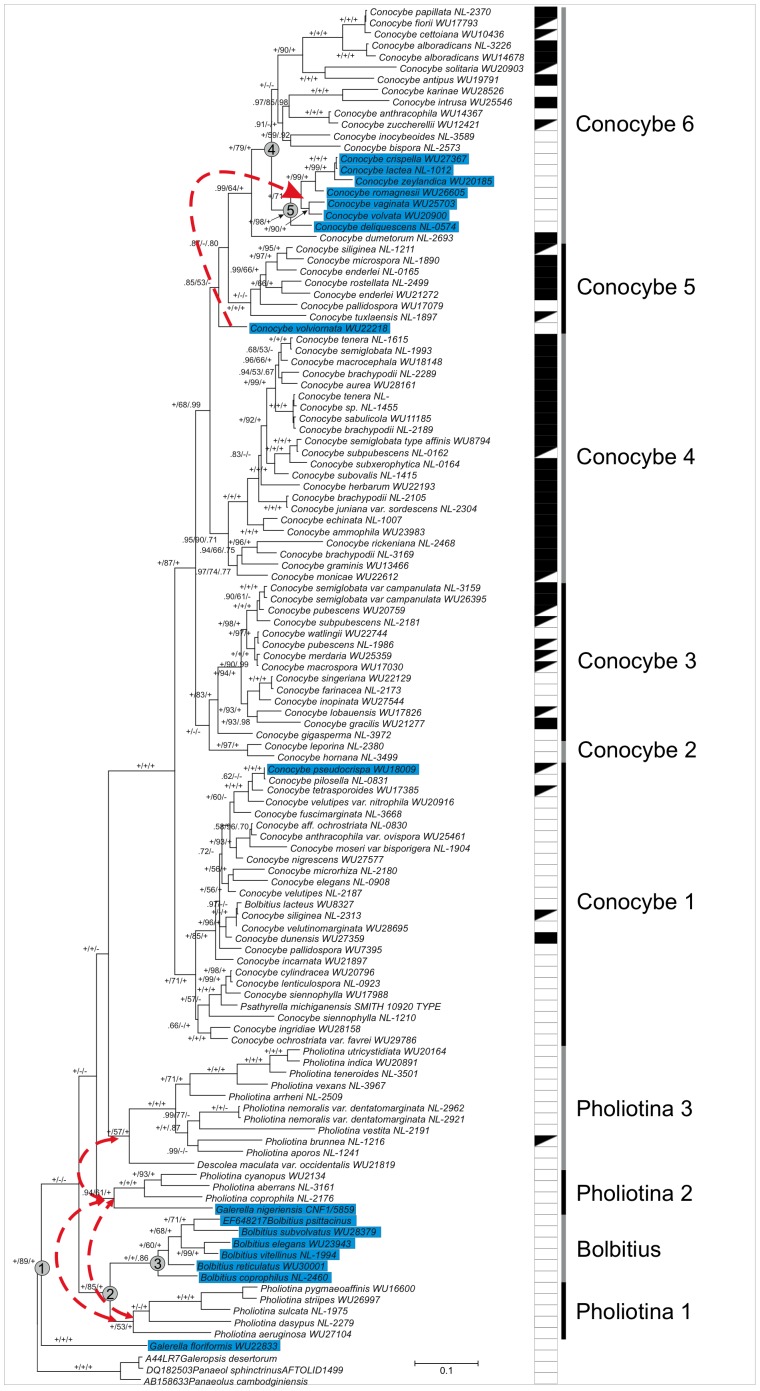
In total, we performed the following Bayesian runs for this study: five for each iteration of the manual alignment refinement, one for the SATé alignment, one for the gap data only, one for GBlocks-curated alignments as well as a BEAST analysis of the final nucleic acid dataset. All analyses converged sufficiently to the stationary distributions, so we established the burn-in as 15.000.000 generations. The consensus tree obtained in the final Bayesian analysis using MrBayes is presented in Fig. 2. All analyses provided strong support for both early and more recent nodes with a few exceptions. Conocybe volviornata had an ambiguous position, probably due to the amount of missing data for this species. In the following, BPP, BBPP and MLBS stand for Bayesian posterior probabilities inferred using MrBayes, Bayesian posterior probabilities inferred using BEAST and Maximum Likelihood bootstrap support inferred using RAxML for the final concatenated dataset. The analyses recovered eight major clades within the family, Pholiotina 1 (BPP:1.0, BBPP:1.0, MLBS:53%), Pholiotina 2 (BPP:1.0, BBPP:1.0, MLBS:100%), Pholiotina 3 (BPP: 0.94, BBPP:1.0, MLBS:61%), Bolbitius (BPP:1.0, BBPP:0.86, MLBS:100%), Conocybe 1 (BPP:1.0, BBPP:1.0, MLBS:71%), Conocybe 2 (BPP: 1.0, BBPP:1.0, MLBS:97%), Conocybe 3 (BPP:1.0, BBPP:1.0, MLBS: 83%), Conocybe 4 (BPP:0.95, BBPP:0.71, MLBS:90%), Conocybe 5 (BPP:1.0, BBPP:1.0, MLBS:100%) and Conocybe 6 (BPP:0.99, BBPP:1.0, MLBS: 64%). Of the three secotioid species (i.e. species with closed fruiting bodies, but well-developed lamellae) sequenced, Conocybe deliquescens ( = Gastrocybe lateritia) was nested in Conocybe clade 6, C. cylindracea in Conocybe clade 1, whereas Galeropsis desertorum clustered in the outgroup (Panaeoloidae). The two sequenced species of Galerella did not form a monophyletic clade of their own, G. floriformis appears basal within the family, whereas G. nigeriensis is close to the Pholiotina 2 clade.
Figure 2. Phylogenetic distribution of the coprinoid fruiting body type in the agaricoid clade (sensu [17]) shown on a Maximum Likelihood tree inferred from LSU sequences of all taxa of the agaricoid clade present in Genbank, supplemented with LSU sequences generated in this and a previous study ([26]).
Tree topologies of various analyses were largely congruent. The branching order around the Pholiotina 3 and Bolbitius plus Pholiotina 1 clades are, however somewhat uncertain, appearing in contradicting positions on the MrBayes and BEAST consensus trees, although the BPP-s were 1.0 for both alternatives. The placement of these clades on the Maximum Likelihood tree was congruent with the BEAST tree, but ML bootstrap support was lower than 50%.
The tree inferred from the recoded characters of indel presence/absence recovered the same major clades (Fig. S1), although the resolution of the 50% majority rule consensus tree was much lower (Fig. S2, which is well explained by the small number of characters (864, of which 450 was parsimony informative). This tree contained one large polytomy comprising the Conocybe 1–6 as well as the Pholiotina 3 clades. Galerella floriformis and the Bolbitius+Pholiotina 1 clades were supported as monophyletic, and appeared basal to the rest of the family. Since support was weak at these nodes, they do not imply significant conflict with the nucleotide-based analysis in statistical terms. Two specimens, lacking the ITS region were also placed in polytomy. Thus, we conclude that indel data are congruent with nucleic acids and provide a reliable signal with regard to the phylogeny of the Bolbitiaceae.
The tree inferred from the three-locus dataset with gapped sites deleted from the ITS region (by GBlocks) was congruent with trees inferred from the whole alignment (Figs. S3–S4).
Constraint Analyses
We tested two morphologically informed hypotheses by constraint analyses. The first was the monophyly of the three volvate species C. volvata, C. vaginata and C. volviornata, which were inferred in different positions on the tree. Based on p-values of the approximately unbiased test, trees where these species appear as monophyletic cannot be rejected (p = 0.138–0.139). Since C. volviornata is the only species represented only by one locus, which is the most conserved of the three loci (nrLSU) we are inclined to attribute its ambiguous position to an effects of missing data.
The monophyly of Pholiotina could be rejected (p-values: 0.004–4*10−5). The constraint trees were designed to allow Galerella nigeriensis to be resolved outside Pholiotina, however, it was resolved within the latter genus. Similarly, forcing Pholiotina species without veil to be monophyletic results in significantly worse likelihoods (au test p-values: 0.001 for all 10 trees).
Ancestral State Reconstruction
Ancestral states were reconstructed for five nodes on a sample of 1000 post-burn-in trees using ML, including the root node of the Bolbitiaceae (Node 1–5. Table 2, Fig. 3). Ancestral states and corresponding hypothesis tests are summarized in Table 2. The ancestral fruiting body type of the Bolbitiaceae was inferred as non-deliquescent with significant support (d(L1–L2) = 2.205), followed by independent emergence of the deliquescent physiology in the genus Bolbitius, section Candidae of Conocybe, Bolbitius lacteus, etc. Taken together, these reconstructions revealed six independent acquisitions of deliquescence in the Bolbitiaceae (Fig. 3). Given the uncertainties of the phylogeny, first of all, the ambiguous position of C. volviornata (see above) and C. pseudocrispa, however, this number may be biased upward. Consequently, the number of independent acquisitions of deliquescence drops to five when C. volviornata is monophyletic with C. volvata and C. vaginata and four when the placement of C. pseudocrispa (belonging to section Candidae based on morphology) is also regarded ambiguous.
Figure 3. 50% Majority rule consensus phylogram showing the phylogenetic relationships in the Bolbitiaceae.
Branch support values are MrBayes posterior probabilities, ML bootstrap values, BEAST posterior probabilities, in order. Nodes for which ancestral fruiting body type has been reconstructed are labeled nodes 1–5, corresponding to the Bolbitiaceae, Bolbitius+Pholiotina 1, Bolbitius, Conocybe 6 and section Candidae. Taxa with coprinoid combination of characters (pseudoparaphyses, ephemeral fruiting bodies which collapse upon maturity, plicate cap surface) are highlighted in blue. Relationships tested by constraint analyses are marked by dashed lines. The composition of stipe covering either as hairs only (open squares), lecythiform cystidia only (filled squares) and both types (fountain fill) is shown to the right of the tree. Note that ML bootstrap values and Bayesian posterior probabilities from the BEAST runs were obtained without the indel data.
The type of cheilocystidia was inferred as fusiform-utriform in all early nodes of the Bolbitiaceae, with one gain on the branch leading to Conocybe and one in the terminal branch of Ph. brunnea. Stipe covering was inferred to have undergone more changes with a mean of 11.3 and 14.2 state changes per tree under multistate and binary coding, respectively. Under binary coding, the number of gains/losses of lecythiform and hair-like cystidia was inferred as 5.29/2.18 and 0.40/5.76, respectively. Simultaneous loss of hairs and gain of lecythiform cystidia were inferred at a relatively low rate (mean 2.45 per tree) under multistate coding, which may explain the differences observed between the two coding regimes. The ancestral type of stipe coverage was estimated to having hairs only.
Discussion
Alignment Undermatching?
Progressive alignment methods are well-known to be sensitive to the order in which sequences are input to the calculation, most often determined by a guide tree which is estimated from the sequences to be aligned [35], [38], [57], [58]. Consistent with the expectations, our results show that the guide tree had a profound effect on the inferred alignment. However, hitherto documented instances of guide tree bias [40], [59] differ from what we have observed for PRANK. Unlike most other algorithms, PRANK do not overcondense the alignments by erroneously inferring spans of non-homologous sequences as homologous [34]. However, we observed that it infers false negative homologies, resulting in unrealistically long alignments. We term this phenomenon alignment undermatching, referring to alignment overmatching, a pattern observed for ClustalW and MAFFT [33]. The length of the alignment decreased when the accuracy of the guide tree increased (although we cannot assess accuracy objectively with the empirical data at hand, the trees inferred from a multigene alignment more likely to be correct than one based on genetic distances). The excessive length of the first alignments, including the one which PRANK produced after its built-in second iteration was caused by several, noticeably homologous blocks of sequences inferred as non-homologous, separated by gapped sites. These evidently biased alignment lengths upward and, although the proportion of false positive homologies is not expected to increase as a result of "alignment undermatching", the proportion of accurately aligned residues decrease, and thus the phylogenetic signal may be biased. Although PRANK is successful in avoiding nearby indels to collapse, which would result in overcondensed alignments (as is often encountered with ClustalW, MAFFT, etc.), the phenomenon called alignment overmatching by Löytynoja and Goldman [33] our results suggest that in the absence of a reasonably accurate guide tree, on the other hand, its strategy for distinguishing and fixing insertions and deletions can cause undermatching of the sequences, resulting in multiple homologous blocks inferred as separate insertion/deletion events. Because in PRANK, the distinction between insertions and deletions is made on the basis of an outgroup-rooted tree [33], [34], the accuracy of the guide tree becomes the primary determinant of how accurately indels are placed in the alignment. As our analyses predict, the guide trees calculated by PRANK may not always allow reasonably accurate gap placement.
Unambiguous parts of the alignment have recently been used to overcome this issue [40]. This is related to the approach proposed in this study, however, we used all data (including two easily alignable genes) to estimate new guide trees in an iterative framework. With regard to the results, our approach and another iterative alignment refinement strategy, SATé [31] performed similarly, converging to the same answer for the problem of ITS alignment. We conclude that improved guide trees are needed for alignment algorithms, and that even alignments produced by highly sophisticated algorithms, such as PRANK could be further improved by supplying a reasonably accurate guide tree or iterative refinement of a starting tree - alignment pair. This strategy allows the inference of improved alignments for datasets not tractable by using simultaneous phylogeny and alignment inference algorithms (such as Bali-Phy), which can more naturally handle this uncertainty in a Bayesian framework.
Phylogenetic Relationships in the Bolbitiaceae
Our analyses included all but one genera (the exception being Tubariella) of the Bolbitiaceae. The analysis of >1500 nrLSU sequences of the agaricoid clade confirms the monophyly of the Bolbitiaceae in a restricted sense [11], that is, excluding Agrocybe, Leratiomyces and Cyttarophyllum [11], [12], [14], [16], [60]. These three genera show an affinity to Hebeloma and Psilocybe on the basis of the Maximum Likelihood tree (the sequence of Agrocybe pediades within Stropharia probably represents a misidentification). Agrocybe was not monophyletic on the ML tree. This is consistent with findings of Moncalvo et al. [14], Gulden et al. [13], Walther et al. [16] and provides clear evidence for the exclusion of Agrocybe from the Bolbitiaceae, as proposed by Walther and Weiss [11]. Panaeolus, Panaeolina and Galeropsis formed a sister group of the Bolbitiaceae.
Three analyzed species of Descolea (D. gunnii, D. maculata, D. antarctica), a genus classified either in the Cortinariaceae or the Bolbitiaceae, show an affinity to annulate Pholiotina species. This relationship is consistent with the macromorphology of the two clades, both are characterized by small and relatively slender fruiting bodies, with a bell-shaped pileus and a well-developed, grooved ring half-way on the stipe. On the other hand, the limoniform, ornamented spores and a putative mycorrhizal lifestyle [61], [62] represent shared features with the Cortinariaceae. Phylogenies seem to consistently support the placement of Descolea in the Bolbitiaceae [13], [14], [17], where it would represent the only ectomycorrhizal (ECM) lineage of the family.
The core Bolbitiaceae include the genera Conocybe, Pholiotina, Bolbitius, Galerella, Descolea as well as the sequestrate genus Gastrocybe. The latter has recently been recombined in Conocybe (as C. deliquescens, [5]), which was suggested by phylogenetic analyses of ITS sequences [3]. Its phylogenetic position is also verified by this study using three markers (ITS, 5′ LSU, EF1 alpha).
Out of the two species of Galerella included in this study, one was inferred as the basalmost clade in the Bolbitiaceae, while the other as a sister group of Pholiotina 2, implying polyphyly of its genus. The most important taxonomic character of Galerella is the brittle, ephemeral fruiting bodies with strikingly plicate - sulcate cap surface [4], [20], [63]. This suite of traits match that of coprinoid fruiting bodies completely, which has been shown to emerge convergently in several clades of the Agaricales [26], [28], [29]. In light of the convergent nature of coprinoid morphology, a straightforward interpretation of the phylogenetic relationships is that Galerella, as circumscribed now is an artificial grouping of species which convergently evolved the coprinoid morphology within various lineages of the Bolbitiaceae. This interpretation of the phylogenetic results is analogous to the case of Coprinus sensu lato [64], and should lead to the splitting of Galerella. However, more species and collections should be examined to confirm this result.
At genus level, Pholiotina seems to be a phylogenetically heterogeneous group, split into three clades on the Bayesian trees. Of these, Pholiotina 3 contains species with a rich veil, usually forming a spectacular ring on the stipe (sections Vestitae, Pholiotina and Intermediae, [4]). Pholiotina 1 and 2 contain species without veil, but instead with pileocystidia or a slimy cap surface (section Cyanopodae, Piliferae, Vestitae p.p., Verrucisporae). Representatives of these two clades are characterized by a poorly developed veil, which never forms a distinct annulus on the stipe. Constraint analyses forcing Pholiotina to be monophyletic returned low p-values (<<0.01), which underpins the need for splitting Pholiotina into smaller genera. In that case, the use of the generic name, originally given to an annulate taxon [65] should be restricted to species of Pholiotina 3 clade. The paraphyly of Pholiotina conclusively rejects classifications, which treat Pholiotina as a subgenus of Conocybe [7].
Species of Bolbitius form a well-supported clade (BPP: 1.0, BBPP: 0.86, MLBS: 100%) sister to Pholiotina 1. This finding is supported by the morphological uniformity of Bolbitius, i.e. viscid to glutinous pileus, which gets sulcate-plicate, the presence of pseudoparaphyses and a partial deliquescence or collapse of the mature fruiting bodies.
The genus Conocybe formed a large, well-supported clade (BPP: 1.0, BBPP: 1.0, MLBS: 100%). Based on the clade structure within Conocybe, we distinguish five subclades, however, the subclades do not correspond to the current infrageneric classification of the genus. The three major sections (Conocybe, Pilosellae, Mixtae) can be recognized in our trees, although none of them appears as monophyletic, suggesting that other morphological traits may serve better for circumscribing sections in Conocybe. Smaller sections defined on the basis of fragile, pale fruiting bodies (section Candidae) or the presence of volva (section Singerella) were inferred as monophyletic. The pattern seen in Conocybe raises the question how the composition of stipe covering evolved in the Bolbitiaceae. In addition to the stipe covering, the cystidia at the gill edge (cheilocystidia) are also of interest in this respect, since the same oil-flask-shaped cystidia characterize the genus Conocybe. Ancestral state reconstructions show that the hairs represent the ancestral cystidial morphology in the Bolbitiaceae, and lecythiform cystidia evolved multiple times. Lecythiform cheilocystidia show two gains, one in Pholiotina brunnea, the other in the most recent common ancestor of Conocybe species. Stipe covering shows a more complex evolutionary history. Both with additive binary and multistate coding, the lecythiform type of cystidia shows on average 5.2 gains and 2.1 losses per tree, whereas the hair-like morphology is lost more often than gained (mean gain/loss: 0.4/5.8). This, combined with evidence for the most recent common ancestor of Conocybe having had a stipe covering of hairs only, depicts an inverse relationship in the evolution of hair-like and lecythiform cystidia. That is, hair-like cystidia, the ancestral condition in Conocybe is gradually replaced by lecythiform cystidia. Although the difference between the adaptive value of these two character states is poorly understood, this evolutionary scenario suggests that the composition of stipe covering may have limited taxonomic value. Thus, we raise the possibility that for the infrageneric taxonomy of Conocybe other morphological traits should be sought, such as those used to delimit sections Candidae and Singerella, for which the phylogenetic analyses support monophyly. The presence of pseudoparaphyses in some species of section Singerella supports its monophyly with section Candidae. It is noteworthy that species of section Singerella have veil remnants at the stipe base (volva) or rarely on the margin of the cap. Interestingly, spurious veil remnants have been examined in C. deliquescens (section Candidae) also, which further supports the relatedness of the two groups.
It is noteworthy, that C. dumetorum, the single representative of section Ochromarasmius harbours a sister clade of Conocybe 6. This species is a representative of Conocybe species with rough to verrucose spores, often observable only under SEM [1], [4], [66].
Deliquescent Lineages in the Agaricales
Deliquescence, i.e. the enzymatic autodigestion of mature fruiting bodies, is one of the several ways of departure from the usual pileate-stipitate fruiting body form in the Agaricales [26], [29], [67], [68]. Like gasteroid species [14], deliquescent lineages do not form a monophyletic group. Recently, a complex series of phenotypic changes have been identified which correlate with changes from non-deliquescent to deliquescent fruiting bodies, which include the evolution of pseudoparaphyses to replace basidioles, bimorphic basidia, accordion-like folded, plicate pileus surface and an increase in the size of hymenial cystidia [26]. The word coprinoidization have been proposed for the series of these changes following fruiting body evolution. All these changes are concordant in that they contribute to the acceleration of the ontogeny of the fungus by opening the way for rapid turgor manipulation in the inflating cells [25], [26]. Also, because of the apparent lack of a direct adaptive advantage imposed by autolysis itself, we hypothesized that it might be the rapid ontogeny that is an important aspect in the altered adaptation capabilities of deliquescent lineages as compared to non-deliquescent ones. Thus, we think coprinoid lineages should not only comprise those with a pronounced autodigestive phase, resulting in the well-known inky fluid, but also those in which the above mentioned syndrome of increase in hymenial cell-sizes and consequently a rapid cap expansion, can be observed. In support of this, partial deliquescence, or a sudden collapse of the fruiting body is often observable in species with no actual enzymatic decay but pseudoparaphyses, large hymenial cystidia, plicate cap surface and bimorphic basidia. Within the Psathyrellaceae, such taxa can be found in the genera Parasola and Coprinellus (e.g. C. disseminatus). According to this logic, species of the genera Bolbitius, Galerella as well as section Candidae of Conocybe should be regarded as coprinoid. All of these taxa possess pseudoparaphyses, more or less enlarged hymenial cystidia and the surface of their cap is plicate, however bimorphic basidia are absent. In this study, the above mentioned species formed 4 clades, with strong support from both ML and Bayesian MCMC analyses. Thus, the present phylogeny extends the number of known coprinoid lineages with four in the Bolbitiaceae. Within the Psathyrellaceae, at least four deliquescent lineages have been identified, although uncertainty in the phylogeny raised the possibility of additional two lineages in Coprinopsis and Coprinellus. Within the Agaricaceae, Coprinus s. str. represents an additional deliquescent lineage, with 3–4 species, including the well-known C. comatus. An additional group of taxa with plicate cap surface, bi(tri-)morphic basidia and pseudoparaphyses is found in Leucocoprinus (Agaricaceae) [2], [69]. Molecular phylogenies suggested Leucocoprinus to have emerged from within Leucoagaricus ([14], [70], Fig. 2), from which it only differs in the presence of pseudoparaphyses, bimorphic basidia and the plicate cap surface. Both published phylogenies [14], [70] and the ML tree inferred in this study leave uncertainties about the monophyly of coprinoid Leucocoprinus species (i.e. L. birnbaumii, L. brebissonii, L. fragilissimus, not counting Leucocoprinus spp in the wide sense, for the nomenclatural controversies over this genus see [70]), and thus the number of coprinoid lineages they add to our understanding of the phylogenetic distribution of coprinoidization. Nevertheless, taken together, at least 10 independent lineages with coprinoid fruiting bodies have been identified in the Psathyrellaceae (∼4), Bolbitiaceae (∼4) and in the Agaricaceae (∼2). Interestingly, all these lineages appear among brown-spored mushrooms in the agaricoid clade (sensu [17]). The occurrence of multiple distantly related lineages that convergently acquired the same mechanism of adaptation suggests the presence of easily accessible pathway(s) of developmental specialization. Whether coprinoidization and the accelerated ontogeny are achieved by the same cellular or subcellular mechanisms in the various lineages is a question with a potential to reveal some of the general mechanisms of fruiting body evolution, but requires more research on the ontogeny and subcellular mechanisms of the species involved.
Supporting Information
50% Majority Rule phylogram inferred with gapped sites of the ITS alignment AND recoded gap characters excluded from the analysis (in MrBayes).
(DOCX)
50% Majority Rule phylogram inferred from recoded binary gap characters of the ITS alignment. A total of 864 characters (450 parsimony informative) of gap presence/absence (0/1) were used for the analysis (in MrBayes).
(DOCX)
Maximum Likelihood Phylogram inferred from the concatenated three-locus dataset, without the indel characters (from RAxML).
(DOCX)
Maximum Clade Credibility tree from the BEAST analysis of the concatenated three-locus dataset. Note that branch lengths were omitted from the figure.
(DOCX)
List of Accession Numbers of GenBank sequences used for assessing the phylogenetic distribution of coprinoid fruiting bodies.
(DOCX)
Acknowledgments
The authors are thankful for the curators of the herbaria WU and MICH for arranging loans. Zdenko Tkalcec is thanked for providing material for G. nigeriensis. Else Vellinga contributed valuable suggestions about coprinoid lineages in Leucocoprinus.
Funding Statement
This research was supported by the Hungarian Research Fund (OTKA NN106394). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hausknecht A (2009) Fungi Europaei, Volume 11: Conocybe and Pholiotina. Edizioni Candusso 968 pp.
- 2.Singer R (1986) The Agaricales in modern taxonomy, 4th edition. Koeltz Scientific Books, Koenigstein, Germany.
- 3. Hallen HE, Watling R, Adams GC (2004) Taxonomy and toxicity of Conocybe lactea and related species. Mycol Res 107: 969–979. [DOI] [PubMed] [Google Scholar]
- 4. Hausknecht A, Kalamees K, Knudsen H, Mukhin V (2009) The genera Conocybe and Pholiotina (Agaricomycotina, Bolbitiaceae) in temperate Asia. Folia Cryptogamica Estonica 45: 23–47. [Google Scholar]
- 5. Hausknecht A, Krisai-Greilhuber I (2006) Infrageneric division of the genus Conocybe - a classical approach. Öst Z Mykol 15: 187–212. [Google Scholar]
- 6.Moser M (1983) Die Röhrlinge und Blätterpilze. in GAMS H: Kleine Kryptogamenflora IIb/2, Gustav-Fischer-Verlag, Jena.
- 7.Watling R (1982) British Fungus Flora, Agarics and Boleti, 3/Bolbitiaceae: Agrocybe, Bolbitius & Conocybe. Edinburgh: HMSO. 139 pp.
- 8.Watling R, Gregory N (1981) Census catalogue of world members of the Bolbitiaceae. Bibliotheca Mycologica 82.
- 9. Smith AH (1966) Notes on Dendrogaster, Gymnoglossum, Protoglossum and species of Hymenogaster. Mycologia 58: 100–124. [Google Scholar]
- 10. Watling R (1968) Observations on the Bolbitiaceae. IV. A new genus of gastromycetoid fungi. The Michigan Botanist 7: 19–24. [Google Scholar]
- 11. Walther G, Weiss M (2006) Anamorphs of the Bolbitiaceae (Basidiomycota, Agaricales). Mycologia 98: 792–800. [DOI] [PubMed] [Google Scholar]
- 12.Arnolds E (2005) Conocybe. In Noordeloos, ME Kuyper, TW, Vellinga, EC (Eds): Flora Agaricina Neerlandica 6 Taylor and Francis.
- 13. Gulden G, Stensrud O, Shalchian-Tabrizi K, Kauserud H (2005) Galerina Earle: A polyphyletic genus in the consortium of dark-spored agarics. Mycologia 97: 823–837. [DOI] [PubMed] [Google Scholar]
- 14. Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, et al. (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23: 357–400. [DOI] [PubMed] [Google Scholar]
- 15.Rees BJ, Marchant AD, Zuccarello GC, Heslewood MM, Bartlett J (2003) A southern hemisphere contribution to the phylogenetic study of agarics. Australasian Mycologist 21.
- 16. Walther G, Garnica S, Weiss M (2005) The systematic relevance of conidiogenesis modes in the gilled Agaricales. Mycol Res 109: 525–544. [DOI] [PubMed] [Google Scholar]
- 17. Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, et al. (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995. [DOI] [PubMed] [Google Scholar]
- 18. Bodensteiner P, Binder M, Moncalvo JM, Agerer R, D SH (2004) Phylogenetic relationships of cyphelloid homobasidiomycetes. Mol Phylogenet Evol 33: 501–515. [DOI] [PubMed] [Google Scholar]
- 19.Singer R (1969) Mycoflora australis. Nova Hedwigia Beiheft 29.
- 20.Hausknecht A (2003) Beitrage zur kenntnis der Bolbitiaceae 9. Conocybe sekt. Mixtae. Öst Z Mykol 12.
- 21. Watling R (1986) Observations on the Bolbitiaceae. 28. The Conocybe pubescens (C. GlLLET) KÜHNER complex. Galera neoantipus and its various interpretations. Bol Soc Micol Madrid 11: 91–96. [Google Scholar]
- 22.Singer R (1962) Diagnoses Fungorum novorum Agaricalium II. Sydowia 15.
- 23.Watling R (1979) Observations on the Bolbitiaceae XVII. Volvate species of Conocybe. Sydowia, Beih 8.
- 24. Bon MC (1990) Combinaisons nouvelles et validations. Doc Mycol 20/78: 37–40. [Google Scholar]
- 25. Kües U (2000) Life history and developmental processes in the basidiomycete Coprinus cinereus. Microb Mol Biol Rev 64: 316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy GL, Walther G, Házi J, Vágvölgyi C, Papp T (2011) Understanding the Evolutionary Processes of Fungal Fruiting Bodies: Correlated Evolution and Divergence Times in the Psathyrellaceae. Syst Biol in press. [DOI] [PubMed]
- 27.Nagy LG, Házi J, Szappanos B, Kocsubé S, Bálint B, et al.. (2012) The Evolution of Defense Mechanisms Correlate with the Explosive Diversification of Autodigesting Coprinellus Mushrooms (Agaricales, Fungi. Syst Biol in press. [DOI] [PubMed]
- 28. Nagy LG, Urban A, Orstadius L, Papp T, Larsson E, et al. (2010) The evolution of autodigestion in the mushroom family Psathyrellaceae (Agaricales) inferred from Maximum Likelihood and Bayesian methods. Mol Phylogenet Evol 57: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 29. Hopple JS Jr, Vilgalys R (1999) Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: divergent domains, outgroups, and monophyly. Mol Phylogenet Evol 13: 1–19. [DOI] [PubMed] [Google Scholar]
- 30. Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu K, Raghavan S, Nelesen S, Linder CR, Warnow T (2009) Rapid and accurate large-scale coestimation of sequence alignments and phylogenetic trees. Science 324: 1561–1564. [DOI] [PubMed] [Google Scholar]
- 32. Liu K, Warnow T, Holder MT, Nelesen S, Yu J, et al. (2012) SATé-II: Very Fast and Accurate Simultaneous Estimation of Multiple Sequence Alignments and Phylogenetic Trees. Syst Biol 60: 90–106. [DOI] [PubMed] [Google Scholar]
- 33. Loytynoja A, Goldman N (2005) An algorithm for progressive multiple alignment of sequences with insertions. Proc Natl Acad Sci U S A 102: 10557–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loytynoja A, Goldman N (2008) Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science 320: 1632–1635. [DOI] [PubMed] [Google Scholar]
- 35. Loytynoja A, Goldman N (2009) Evolution. Uniting alignments and trees. Science 324: 1528–1529. [DOI] [PubMed] [Google Scholar]
- 36. Redelings BD, Suchard MA (2007) Incorporating indel information into phylogeny estimation for rapidly emerging pathogens. BMC Evol Biol 7: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roshan U, Livesay DR (2006) Probalign: Multiple sequence alignment using partition function posterior probabilities. Bioinformatics 22: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 38. Dwivedi B, Gadagkar SR (2009) Phylogenetic inference under varying proportions of indel-induced alignment gaps. BMC Evol Biol 9: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J, Keightley PD, Johnson T (2006) MCALIGN2: faster, accurate global pairwise alignment of non-coding DNA sequences based on explicit models of indel evolution. BMC Bioinformatics 7: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith AH (1972) The North American species of Psathyrella. Mem NY Bot Gard 24: 1–633. [Google Scholar]
- 42. Staden R, Beal KF, Bonfield JK (2000) The Staden Package. Methods Mol Biol 132: 115–130. [DOI] [PubMed] [Google Scholar]
- 43.Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 2.6. http://mesquiteprojectorg.
- 44. Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 45. Simmons MP, H O (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49: 369–381. [PubMed] [Google Scholar]
- 46. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552. [DOI] [PubMed] [Google Scholar]
- 47. Altekar G, Dwarkadas S, Huelsenbeck JP, Ronquist F (2004) Parallel Metropolis coupled Markov chain Monte Carlo for Bayesian phylogenetic inference. Bioinformatics 20: 407–415. [DOI] [PubMed] [Google Scholar]
- 48.Rambaut A, Drummond A (2008) BEAST. Software distributed by the author at http://beast.bio.ed.ac.uk/.
- 49.Rambaut A, Drummond A (2008) Tracer v 1.4.1. Software distributed by the authors at http://beast.bio.ed.ac.uk/.
- 50. Sukumaran J, Holder MT (2010) Dendropy: A phython library for phylogenetic computing. Bioinformatics 26: 1569–1571. [DOI] [PubMed] [Google Scholar]
- 51. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 52. Shimodaira H, Hasewaga M (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17: 1246–1247. [DOI] [PubMed] [Google Scholar]
- 53. Guindon S, Gascuel O (2003) PhyML – A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 54. Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol 55: 539–552. [DOI] [PubMed] [Google Scholar]
- 55.Pagel M, Meade A (2007) Bayestraits 1.0. Software distributed by the author at http://www.evolution.rdg.ac.uk.
- 56.Pagel M, Meade A (2007) BayesPhylogenies v1.0. Software distributed by the authors.
- 57. Morrison DA (2009) Why would phylogeneticists ignore computerized sequence alignment? Syst Biol 58: 150–158. [DOI] [PubMed] [Google Scholar]
- 58. Simmons MP, Müller K, Webb CT (2008) The relative sensitivity of different alignment methods and character codings in sensitivity analysis. Cladistics 24: 1039–1050. [DOI] [PubMed] [Google Scholar]
- 59. Penn O, Privman E, Landan G, Graur D, Pupko T (2012) An alignment confidence score capturing robustness to guide tree uncertainty. Mol Biol Evol 27: 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nauta MM (2004) Notulae ad Floram agaricinam neerlandicam - XLIII. Notes on Agrocybe. Persoonia 18: 429–433. [Google Scholar]
- 61. Bougher NL, Malajczuk N (1985) A new species of Descolea (Agaricales) from Western Australia, and aspects of its ectomycorrhizal status. Australian J Bot 33: 619–627. [Google Scholar]
- 62. Horak E, Desjardin DE, Hemmes DE (1996) Agaricales of the Hawaiian Islands. 3: The genus Galerina and selected other brown-spored agarics. Mycologia 88: 278–294. [Google Scholar]
- 63. Tkalcec Z, Mesic A, Cerkez M (2010) Galerella nigeriensis (Agaricales), a new species from tropical Africa. Mycotaxon 114: 263–270. [Google Scholar]
- 64. Redhead SA, Vilgalys R, Moncalvo JM, Johnson J, Hopple JS (2001) Coprinus Persoon and the disposition of Coprinus species sensu lato. Taxon 50: 203–241. [Google Scholar]
- 65. Fayod V (1889) Prodrome d'une histoire naturelle des Agaricinees. Ann Sci Nat Bot 9: 181–411. [Google Scholar]
- 66. Watling R (1976) Observations on the Bolbitiaceae XV. The taxonomic position of those species of Conocybe possessing ornamented basidiospores. Rev Mycologie 40: 31–37. [Google Scholar]
- 67. Hibbett D (2004) Trends in morphological evolution in homobasidiomycetes inferred using maximum likelihood: a comparison of binary and multistate approaches. Syst Biol 53: 889–903. [DOI] [PubMed] [Google Scholar]
- 68. Hibbett DS, Binder M (2002) Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proc Biol Sci 269: 1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vellinga EC, Davis RJ (2006) Lepiotaceous fungi in California, U.S.A. –1. Leucoagaricus amanitoides sp. nov. Mycotaxon 98: 197–204. [Google Scholar]
- 70. Vellinga EC, Sysouphanthog P, Hyde KD (2011) The family Agaricaceae: phylogenies and two new white-spored genera. Mycologia 103: 494–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
50% Majority Rule phylogram inferred with gapped sites of the ITS alignment AND recoded gap characters excluded from the analysis (in MrBayes).
(DOCX)
50% Majority Rule phylogram inferred from recoded binary gap characters of the ITS alignment. A total of 864 characters (450 parsimony informative) of gap presence/absence (0/1) were used for the analysis (in MrBayes).
(DOCX)
Maximum Likelihood Phylogram inferred from the concatenated three-locus dataset, without the indel characters (from RAxML).
(DOCX)
Maximum Clade Credibility tree from the BEAST analysis of the concatenated three-locus dataset. Note that branch lengths were omitted from the figure.
(DOCX)
List of Accession Numbers of GenBank sequences used for assessing the phylogenetic distribution of coprinoid fruiting bodies.
(DOCX)