Abstract
A series of trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 were prepared and evaluated for their antiviral activity against tobacco mosaic virus (TMV) for the first time. The bioassay results showed that most of these compounds exhibited good antiviral activity against TMV, of which compounds 1, 5, 6, 20, 27 and 34 exhibited significantly higher activity against TMV than commercial Ribavirin both in vitro and in vivo. Furthermore, these compounds have more simple structure than commercial Ribavirin, and can be synthesized more efficiently. These new findings demonstrate that trans-3-aryl acrylic acids and their derivatives represent a new template for antiviral studies and could be considered for novel therapy against plant virus infection.
Introduction
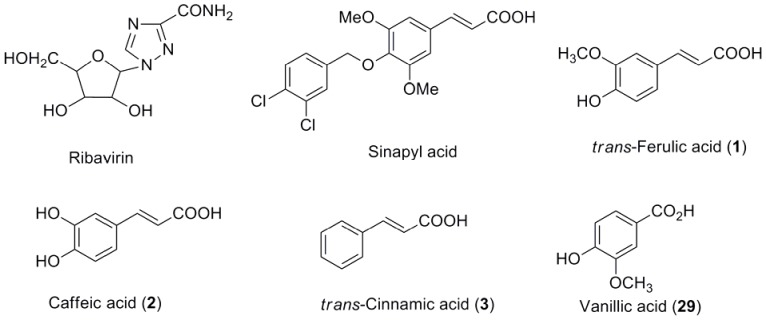
Plant viruses cause dramatic losses in agriculture and horticulture all over the world [1]. Tobacco mosaic virus (TMV), one of the most well-studied plant viruses [2], infects more than 400 plant species belonging to 36 families, such as tobacco, tomato, potato, and cucumber [3], [4]. As a successfully registered plantviral inhibitor, Ribavirin (Figure 1) is widely used to prevent TMV disease [5]. However, the inhibitory effects of Ribavirin are less than 50% at 500 µg/mL, and its effective duration is not long. In fact, there are no super chemical treatments that can absolutely inhibit TMV once it has infected plants. Therefore, the development of highly efficient, novel, environmentally benign antiviral inhibitors has been continuously conducted. During the process for finding an effective way to protect plants from TMV infection, Song et al. have also reported that cyanoacrylate derivatives and amide derivatives containing α-aminophosphonate moiety exhibited moderate to excellent antiviral activity against TMV [6], [7]. Natural phenanthroindolizidine alkaloids have been proved to be efficiency to inhibit TMV by our group [8], [9].
Figure 1. Chemical structure of Ribavirin, acrylic acids 1–3 and acid 29.
Natural product-based agrochemicals offer advantages in that they can sometimes be specific to a target species and often have unique modes of action with little mammalian toxicity. Another benefit is their ability to decompose rapidly, thereby reducing their risk to the environment [10], [11].
trans-3-(Substitutedphenyl)acrylic acids and their derivatives are widely distributed in the plant kingdom and are found to have diverse biological activities. These natural or natural-based compounds have been applied widely in medicinal formulation for their properties of low toxicity and environmental friendly [12]. For example, trans-ferulic acid (1, Figure 1) ubiquitously distributed in primary plant cell walls and crop bran [13] exhibited diverse physiological activities such as reduction of serum cholesterol levels [14], antioxidant properties in several oil models [15], antibacterial [16] and anticancer activity [17], [18]. trans-Cinnamic acid (3, Figure 1) also was found to have allelopathy activity [19], moderate to strong insecticidal activity [20], and a significant inhibitory effect on phenylalanine ammonia-lyase activity in wheat seedlings [21]. Sinapyl acid (Figure 1) possessing the same α, β-unsaturated carbonyl as an important Michael acceptor anticancer pharmacophore displayed potent cytotoxicity against the KB cell line with an IC50 value of 14 µM [22]. Vivanco reported that trans-cinnamic acid, o-coumaric acid and ferulic acid exhibited antimicrobial activity against both soil-borne bacteria and fungi [23]. Antitumor activities of various trans-3-(substitutedphenyl)acrylic acids derivatives were also explored by many research groups [24]–[28]. Hydroxycinnamic acid esters are widely distributed in the plant kingdom and are reported as cellular antioxidants, anti-inflammatory agents, or inhibitors of enzymes involved in cell proliferation [29]–[31]. However, up to now, no one has reported the antiplantviral activity of the trans-3-(substitutedphenyl)acrylic acids or their derivatives.
During our research for potent antiplantviral remedies, we found that trans-ferulic acid showed excellent antiviral activity against TMV. Based on this finding, a series of trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 were designed, synthesized and systematically evaluated for their antiviral activity against TMV.
Results and Discussion
Chemistry
(For experiment details please see Supporting Information: Text S1) trans-Ferulic acid (1), caffeic acid (2), trans-cinnamic acid (3) and vanillic acid (29) are commercially available and other trans-3-aryl acrylic acids were synthesized via Knoevenagel reaction (Figure 2 and Figure 3). The aromatic aldehydes 35a–h, 36a–h, 36p and 36j are commercially available. 1,2,3-Benzothiadiazole-7-carboxaldehyde (36i) [32], 2,3,6,7-tetramethoxy-9-phenanthrenecarboxaldehyde (36k) [33] and 3,6,7-trimethoxy-9-phenanthrenecarboxaldehyde (36l) [33] were synthesized according to reported literatures. (6-Benzyloxy-2,3-dimethoxyphenanthren-9-yl)methanol (37), 6-hydroxy-2,3-dimethoxyphenanthrene-9-carboxylic acid methyl ester (38) and 3-hydroxy-6,7-dimethoxyphenanthrene-9-carboxylic acid methyl ester (39) were prepared according to our reported literature [34]. The synthetic procedure of phenanthrenecarboxaldehyde 36m–o is shown in Figure 4 and Figure 5.
Figure 2. Synthesis of trans-3-(substitutedphenyl)acrylic acids (4–11).
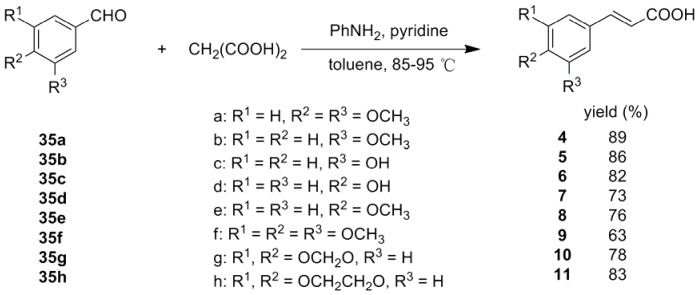
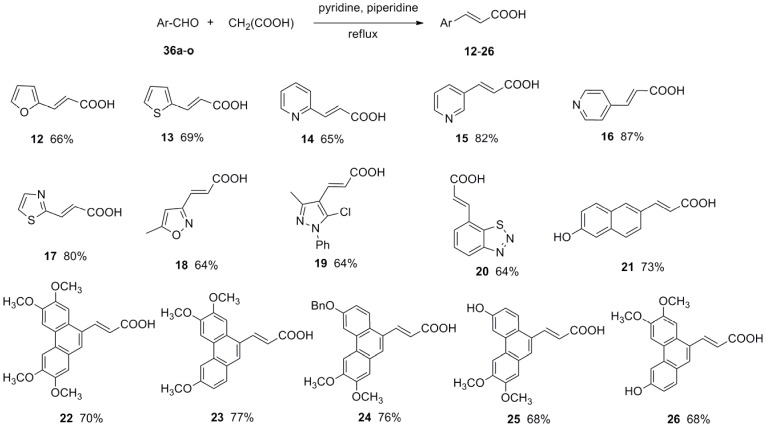
Figure 3. Synthesis of trans-3-aryl acrylic acids (12–26).
Figure 4. Synthesis of 6-benzyloxy-2,3-dimethoxyphenanthren-9-carboxaldehyde (36m).
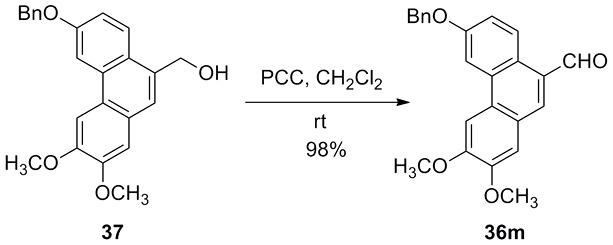
Figure 5. Synthesis of 2,3-dimethoxy6-hydroxyl-phenanthren-9-carboxaldehyde (36n) and 6,7-dimethoxy3-hydroxyl-phenanthren-9-carboxaldehyde (36o).
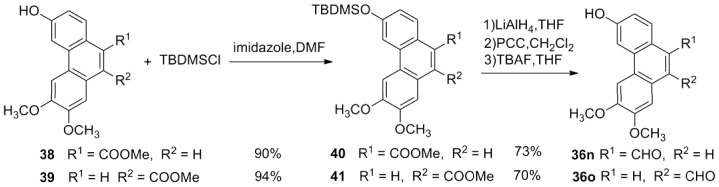
trans-3-Aryl acrylic acids 4–26 were obtained employing aromatic aldehydes 35a–h, 36a–o and malonic acid as starting materials via classical Knoevenagel condensation (Figure 2, Figure 3). However, trans-3-(2-hydroxyl-1-naphthyl)acrylic acid (27) could not be obtained in good yield via Knoevenagel condensation. Then wittig reaction was applied and 27 was prepared from aldehyde 36p in good yield (Figure 6). Most of the aromatic aldehydes were commercially available or prepared according to published procedure, only the preparation of phenanthryl aldehydes 36m–o was described here. 36m was prepared through a PCC oxidation from corresponding alcohol 37 [28] which was an intermediate of phenanthroindolizidine alkaloids (Figure 4). 36n and 36o were prepared from corresponding carboxylates 38 and 39 by the conventional four steps (protection, reduction to alcohol, oxidation to aldehyde and deprotection) in about 65% overall yield (Figure 5).
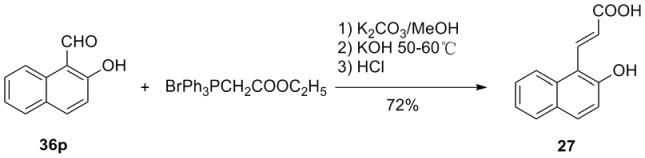
Figure 6. Synthesis of trans-3-(2-hydroxyl-1-naphthyl)acrylic acid (27).
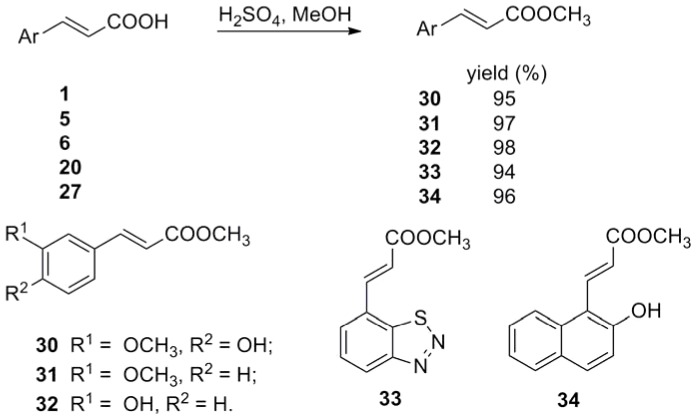
Substituted benzaldehydes 35a–h were used to get trans-3-substituted phenyl acrylic acids 4–11. To further investigate the effect of benzene ring on antiviral activity, a series of simple aromatic rings such as furan ring, thiophene ring, pyridine ring, thiazole ring, isoxazole ring, pyrazole ring, benzothiadiazole ring and naphthalene ring were chose to form trans-3-aryl acrylic acids 12–21. As the phenanthroindolizidine alkaloids and their derivatives were reported to have excellent antiviral activity against TMV by us [8], [9], the phenanthrene rings with different substitutes were also chose to form trans-3-aryl acrylic acids 22–26. In order to investigate the importance of the acrylic acid fragment, the carbon-carbon double bond of trans-ferulic acid (1) was hydrogenated to form 28 (Figure 7) and the trans-3-aryl acrylic acids 1, 5, 6, 20 and 27 was esterified to get the corresponding trans-3-aryl acrylic acid methyl esters 30–34 (Figure 8).
Figure 7. Synthesis of compound 28.
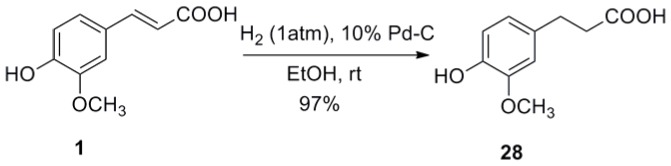
Figure 8. Synthesis of trans-3-aryl methylacrylate 30–34.
Phytotoxic Activity
All the trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 were tested for their phytotoxic activity against Nicotiana tabacum var Xanthi nc, the results indicated that these natural product-based compounds have no phytotoxic activity at 500 µg/mL.
Antiviral Activity In Vitro And In Vivo
The in vitro antiviral results of all the trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 against TMV were listed in Table 1. To make a judgment of the antiviral potency of the synthesized compounds, the commercially available plant virucide Ribavirin was used as the control. Our results indicated that 3-(3-methoxyphenyl)acrylic acid (5) possesses significantly higher in vitro antiviral activity (68.4%) against TMV than Ribavirin (38.5%) at 500 µg/mL. At the same concentration, FA (1) containing hydroxyl, methoxy at the 3, 4-positions of benzene ring, compound 6 containing hydroxyl at the 3-position of benzene ring, compound 20 containing benzothiadiazole ring, compound 27 containing hydroxyl at the 2-position of naphthalene ring, and compound 24 containing benzyloxyl at 3-position of phenanthrene ring also showed higher antiviral activity (47.5%, 40.5%, 52.2%, 45.8% and 42.8%, respectively) than Ribavirin. And the compounds 3, 22, 23, 25, 26 showed in vitro antiviral activity close to Ribavirin. Other trans-3-aryl acrylic acids exhibited lower in vitro antiviral activity against TMV than Ribavirin. All of the methyl acrylates 30–34 exhibited lower in vitro activity than their corresponding trans-3-aryl acrylic acids 1, 5, 6, 20, 27.
Table 1. In Vitro and In Vivo Anti-TMV Activity of Compounds 1–34 at 500 µg/mL.
| Compd. | In vitro inhibition rate (%)a | In vivo | ||
| Inactivation effect (%)a | Curative effect (%)a | Protection effect (%)a | ||
| 1 | 47.5 | 38.5 | 31.2 | 34.3 |
| 2 | 16.5 | 20.8 | 3.9 | 12.0 |
| 3 | 33.4 | 48.4 | 3.3 | 10.1 |
| 4 | 28.7 | 45.6 | 12.6 | 22.4 |
| 5 | 68.4 | 46.6 | 38.9 | 30.8 |
| 6 | 40.5 | 34.7 | 51.7 | 32.9 |
| 7 | 29.1 | 15.1 | 24.3 | 8.9 |
| 8 | 20.6 | 10.4 | 10.3 | 11.6 |
| 9 | 26.9 | 27.0 | 10.9 | 14.7 |
| 10 | 20.4 | 23.8 | 23.8 | 8.3 |
| 11 | 25.3 | 14.8 | 15.4 | 6.0 |
| 12 | 26.8 | 20.7 | 22.1 | 28.4 |
| 13 | 30.0 | 22.9 | 21.4 | 19.3 |
| 14 | 27.5 | 23.4 | 16.8 | 20.2 |
| 15 | 0 | 0 | 0 | 8.3 |
| 16 | 18.5 | 11.1 | 10.3 | 17.2 |
| 17 | 21.3 | 19.2 | 15.2 | 18.6 |
| 18 | 17.5 | 21.9 | 19.3 | 20.4 |
| 19 | 20.0 | 13.7 | 10.2 | 15.2 |
| 20 | 52.2 | 45.0 | 46.2 | 49.7 |
| 21 | 22.3 | 17.1 | 17.5 | 21.4 |
| 22 | 36.7 | 30.1 | 33.3 | 40.2 |
| 23 | 34.4 | 21.0 | 25.3 | 30.7 |
| 24 | 42.8 | 31.3 | 37.9 | 34.4 |
| 25 | 38.3 | 31.6 | 30.0 | 28.8 |
| 26 | 34.4 | 21.0 | 25.3 | 30.7 |
| 27 | 50.4 | 48.8 | 42.5 | 46.6 |
| 28 | 27.2 | 20.8 | 38.7 | 12.5 |
| 29 | 27.1 | 11.4 | 10.8 | 6.1 |
| 30 | 23.8 | 20.0 | 20.6 | 17.3 |
| 31 | 37.5 | 30.0 | 33.3 | 32.8 |
| 32 | 31.7 | 28.9 | 27.2 | 35.4 |
| 33 | 37.6 | 33.3 | 24.6 | 31.8 |
| 34 | 45.8 | 38.9 | 40.5 | 43.2 |
| Ribavirin | 38.5 | 35.9 | 32.3 | 36.4 |
(a: For details please see Supporting Information: Text S1).
The in vivo antiviral results of trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 against TMV were listed in Table 1, which gave the protection effect, inactivation effect, and curative effect for the different compounds of this series. Generally, compounds 1, 5, 6, 22 and 24 showed the same activity level as Ribavirin at 500 µg/mL, and compounds 20 and 27 displayed much better activity than Ribavirin in all the three effects. Interestingly, compouds 3, 4 and 5 exhibited higher antiviral activity (48.4%, 45.6% and 46.6%) in the protection effect than Ribavirin (35.9%), while 5 showed lower activitiy in the curative effect, and 3 and 4 showed much lower activity both in the inactivation effect and curative effect. Other trans-3-aryl acrylic acids exhibited lower in vivo antiviral activity against TMV than Ribavirin.
Among compounds 1–11, the more active compounds are compounds 1, 5 and 6 which containing hydroxyl or methoxyl at the 3-position of benzene ring (1 also containing hydroxyl at the 4-position). Removal of hydroxyl or methoxyl at the 3-position of benzene ring (3) caused the decrease of activity. The replacement of hydroxyl or methoxyl by methylenedioxyl or ethylenedioxyl (10 and 11) also caused the decrease of activity. From the structures of 5, 6, 7 and 8, it can be seen that the position difference of hydroxyl or methoxyl caused great changes of activity. From the structures of 2, 6 and 4, 9, it can be concluded that the increase of hydroxyl or methoxyl numbers would cause the decrease of activity. Among compounds 12–27, the more active compounds are compounds 20, 22, 24 and 27. That means besides benzene ring, benzothiadiazole ring, naphthalene ring and phenanthrene ring are also suitable for antiviral against TMV. Comparing the activity of 14–16 and 21, 27, it can be seen that the positions of substituents have an important effect on the antiviral activity both in vitro and in vivo. It could be concluded that the numbers and kinds of substituents also affect the antiviral activity from the structures of 22–26.
To study the structure-activity relationship, different types of structures and the effects of structural changes in different regions of the molecular were considered: elimination of the double bond of the side chain gave the structures of 28 and 29, and change of the carboxy of 1, 5, 6, 20 and 27 by esterifying gave the structures of 30–34. Compound 28 exhibited lower in vitro inhibition rate, inactivation effect and protection effect and higher curative effect than compound 1, which indicates that the different geometry of the alkenyl vs alkyl chain may give different binding properties. Vanillic acid (29) displayed much lower antiviral activity, which indicates that the directly connection of carboxyl group and benzene ring is bad for antiviral activity. Compound 30–33 containing an ester group in the side chain were less active than their corresponding acrylic acids. Though trans-3-(2-hydroxyl-1-naphthyl)methylacrylate (34) showed higher antiviral activity than Ribavirin, it also exhibited slightly lower antiviral activity than trans-3-(2-hydroxyl-1-naphthyl)acrylic acid (27).
Conclusion
In summary, a group of trans-3-aryl acrylic acids 1–27 and their derivatives 28–34 were prepared and evaluated for their antiviral activity against TMV. Most of these compounds exhibited good antiviral activity against TMV and some of them showed activity close to or even higher than Ribavirin at 500 µg/mL. A systematic SAR study on these compounds indicated that the acrylic acid fragment is important for the antiviral activity and the substituents have an important effect on the antiviral activity. Among them, compounds 1, 5, 6, 20, 27, and 34 exhibited remarkable antiviral activity against TMV that indicated benzene ring, benzothiadiazole ring, naphthalene ring and phenanthrene ring are suitable for antiviral activity. Among these compouds, trans-3-(1,2,3-benzothiadiazole-7-yl)acrylic acid (20) and trans-3-(2-hydroxyl-1-naphthyl)acrylic acid (27) showed the highest antiviral activity which is significantly higher than Ribavirin. The remarkable antiviral activity of trans-3-aryl acrylic acids along with their very simple structures give a hope for the future development of new antiviral agents. Further studies on mode of action and toxicity are currently underway in our laboratories.
Supporting Information
Experimental data of the synthesized compounds.
(DOC)
Funding Statement
The authors gratefully acknowledge the National Key Project for Basic Research (2010CB126106) and the National Natural Science Foundation of China (21132003, 21121002) and China Postdoctoral Science Foundation (20090460057, 2011M500519, 2012T50207) for financial support. The authors thank China Agricultural University to supply some of chemical reagents and the National Key Technology Research and Development Program (2012BAK25B03-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bos L (1982) Crop losses caused by viruses. Crop Prot 1: 263–282. [Google Scholar]
- 2. Ren Y, Wong SM, Lim LY (2010) Application of plant viruses as nano drug delivery systems. Pharm Res 27: 2509–2513. [DOI] [PubMed] [Google Scholar]
- 3. Craeger AN, Scholthof KB, Citovsky V, Scholthof HB (1999) Tobacco mosaic virus: pioneering research for a century. Plant Cell 11: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritzenthaler C (2005) Resistance to plant viruses: old issue, new answer. Curr Opin Biotechnol 16: 118–122. [DOI] [PubMed] [Google Scholar]
- 5. Wei LZ, Meng FS (2010) Antiviral composition containing fucoidan and ribavirin for preventing and treating plant viral diseases. CN 101869111A, 20101027, CAN 153: 573294. [Google Scholar]
- 6. Long N, Cai XJ, Song BA, Yang S, Chen Z, et al. (2008) Synthesis and antiviral activities of cyanoacrylate derivatives containing an α-aminophosphonate moiety. J Agric Food Chem 56: 5242–5246. [DOI] [PubMed] [Google Scholar]
- 7. Hu DY, Wang QQ, Yang S, Song BA, Bhadury PS, et al. (2008) Synthesis and antiviral activities of amide derivatives containing the α-aminophosphonate moiety. J Agric Food Chem 56: 998–1001. [DOI] [PubMed] [Google Scholar]
- 8. An TY, Huang RQ, Yang Z, Zhang DK, Li GR, et al. (2001) Alkaloids from Cyanachum komarovii with inhibitory activity against the tobacco mosaic virus. Phytochemistry 58: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 9. Wang KL, Su B, Wang ZW, Wu M, Li Z, et al. (2010) Synthesis and antiviral activities of phenanthroindolizidine alkaloids and their derivatives. J Agric Food Chem 58: 2703–2709. [DOI] [PubMed] [Google Scholar]
- 10. Qian XH, Lee PW, Cao S (2010) China: Forward to the green pesticides via a basic research program. J Agric Food Chem 58: 2613–2623. [DOI] [PubMed] [Google Scholar]
- 11. Seiber JN (2011) Sustainability and agricultural and food chemistry. J Agric Food Chem 59: 1–21. [DOI] [PubMed] [Google Scholar]
- 12. Hu YY, Xu XY (2006) Study on the chemical and Pharmacological properties of ferulic acid. Chin Trad Patent Med 28: 253–255. [Google Scholar]
- 13. Tanaka A (1971) Separation and quantitative analysis of ferulates. Yukagaku 20: 792–797. [Google Scholar]
- 14. Guardiola F, Codony R, Addis PB, Rafecas M, Oatella J (1996) Biological effects of oxysterols: Current status. Food Chem Toxicol 34: 193–211. [DOI] [PubMed] [Google Scholar]
- 15. Nystrom L, Makinen M, Lampi A, Piiromen V (2005) Antioxidant activity of steryl ferulate extract from rye and wheat bran. J Agric Food Chem 53: 2503–2510. [DOI] [PubMed] [Google Scholar]
- 16. Van der Watt E, Pretorius JC (2001) Purification and identification of active antibacterial components in Carpobrotus edulis L. J Ethnopharmacol 76: 87–91. [DOI] [PubMed] [Google Scholar]
- 17. Hudson EA, Dinh PA, Kokubun T, Simmonds MSJ, Gescher A (2000) Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers PreVent 9: 1163–1170. [PubMed] [Google Scholar]
- 18. Yasukawa K, Akihisa T, Kimura Y, Tamura T, Takido M (1998) Inhibitory effect of cycloartenyl ferulate, a component of rice bran, on tumor promotion in two stage carcinogenesis in mouse skin. Biol Pharm Bull 21: 1072–1076. [DOI] [PubMed] [Google Scholar]
- 19. Chaves N, Sosa T, Alias JC, Escudero JC (2001) Identification and effects of interaction phytotoxic compounds from exudates of Cistus ladanifer leaves. J Chem Ecol 27: 611–621. [DOI] [PubMed] [Google Scholar]
- 20. Park IIK, Lee HS, Lee SG, Park JD, Ahn YJ (2000) Insecticidal and fumigant activities of cinnamomum cassia Bark-derived materials against mechoris ursulus (Coleoptera: Attelabidae). J Agric Food Chem 48: 2528–2531. [DOI] [PubMed] [Google Scholar]
- 21. Lam M, Scaman CH, Clemens S, Kermode A (2008) Retention of phenylalanine ammonia-lyase activity in wheat seedlings during storage and in vitro digestion. J Agric Food Chem 56: 11407–11412. [DOI] [PubMed] [Google Scholar]
- 22. Zou HB, Dong SY, Zhou CX, Hu LH, Wu YH, et al. (2006) Design, synthesis, and SAR analysis of cytotoxic sinapyl alcohol derivatives. Bioorg Med Chem 14: 2060–2071. [DOI] [PubMed] [Google Scholar]
- 23. Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003) Metabolic profiling of root exudates of arabidopsis thaliana . J Agric Food Chem 51: 2548–2554. [DOI] [PubMed] [Google Scholar]
- 24. Luo Y, Qiu KM, Lu X, Liu K, Fu J, et al. (2011) Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Bioorg Med Chem 19: 4730–4738. [DOI] [PubMed] [Google Scholar]
- 25. Li DD, Lv PC, Zhang H, Zhang HJ, Hou YP, et al. (2011) The combination of 4-anilinoquinazoline and cinnamic acid: A novel mode of binding to the epidermal growth factor receptor tyrosine kinase. Bioorg Med Chem 19: 5012–5022. [DOI] [PubMed] [Google Scholar]
- 26. Mariano C, Marder M, Blank VC, Roguin LP (2006) Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem 14: 2966–2971. [DOI] [PubMed] [Google Scholar]
- 27. Han CK, Ahn SK, Choi NS, Hong RK, Moon SK, et al. (2000) Design and synthesis of highly potent fumagillin analogues from homology modeling for a human MetAP-2. Bioorg Med Chem Lett 10: 39–43. [DOI] [PubMed] [Google Scholar]
- 28. Zhou HB, Wu H, Zhang XN, Zhao Y, Stöckigt J, et al. (2010) Synthesis, biological evaluation, and structure–activity relationship study of novel cytotoxic aza-caffeic acid derivatives. Bioorg Med Chem 18: 6351–6359. [DOI] [PubMed] [Google Scholar]
- 29. Chan RI, San RH, Stich HF (1986) Mechanism of inhibition of N-methyl-N′-nitro-N-nitrosoguanidine-induced mutagenesis by phenolic compounds. Cancer Lett 31: 27–34. [DOI] [PubMed] [Google Scholar]
- 30. Chen H, Muramato K, Yamauchi F, Nokihara K (1996) Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agric Food Chem 44: 2619–2623. [Google Scholar]
- 31. Laranjinha J, Vierira O, Almeida L, Madeira V (1996) Inhibition of metmyoglobin/H2O2-dependent low density lipoprotein lipid peroxidation by naturally occurring phenolic acids. Biochem Pharmacol 51: 395–402. [DOI] [PubMed] [Google Scholar]
- 32. Zhong JC, Bian QH, Wang M (2005) Synthesis of benzo[d][1,2,3]thiadiazole-7-carbaldehyde. Chemical Reagents 27: 241–242. [Google Scholar]
- 33. Yamashita S, Kurono N, Senboku H, Tokuda M, Orito K (2009) Synthesis of phenanthro[9,10-b]indolizidin-9-ones, phenanthro[9,10-b]quinolizidin-9-one, and related benzolactams by Pd(OAc)2-catalyzed direct aromatic carbonylation. Eur J Org Chem 8: 1173–1180. [Google Scholar]
- 34. Wu M, Li L, Su B, Liu ZH, Wang QM (2011) First total synthesis of (−)- and (+)-6-O-desmethylantofine. Org Biomol Chem 9: 141–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental data of the synthesized compounds.
(DOC)