Abstract
Background
Paraoxonase (PON) is a high-density-lipoprotein (HDL) associated enzyme with antioxidative and anti-atherogenic property. Its function is associated with coronary artery disease and its activity genetically controlled. We evaluated whether genetic variation of PON-1 is associated with clinical outcome in a large cohort of Korean patients with drug-eluting stents implantation.
Methods
A total of 1676 patients with drug-eluting stent implantation were enrolled in the prospective CROSS-VERIFY cohort from June 2006 to June 2010. We genotyped the PON1-Q192R gene, measured clopidogrel on-treatment platelet reactivity (OPR), and analyzed lipid profiles. The primary endpoint was the composite of cardiac death, myocardial infarction, and stent thrombosis at 12 months.
Results
PON-1 genotyping data were available in 1336 patients. Since the Q-allele is associated with decreased PON-activity, we analyzed the outcome between patients with QQ/QR (815 patients, 61%) and those with RR-genotype (521 patients, 39%). After adjustment for common cardiac risk factors, the QQ/QR-genotype was an independent predictor of the primary thrombotic endpoint with an 11-fold increased risk (HR 11.6, 95% CI: 1.55–87.0), but not repeat revascularization (HR 1.12, 95% CI: 0.78–1.61). The QQ/QR-genotype was not associated with OPR (QQ/QR: 231±86 PRU vs. RR 236±82 PRU, p = 0.342) but higher small-dense LDL levels (1.20±0.12 mg/dL vs. 0.76±0.15 mg/dL, p = 0.027). The increased risk of thrombotic outcomes was more profound in acute coronary syndrome (ACS) patients compared with non-ACS patients.
Conclusion
PON1 Q-allele is an independent predictor of worse cardiovascular outcome independent of platelet function and is associated with significantly higher levels of small dense LDL-C.
Introduction
Paraoxonase (PON) is a calcium-dependent, multifunctional antioxidant enzyme that is widely distributed [1]. The activity of PON is genetically controlled, and the Q192R single-nucleotide polymorphism (SNP) in the paraoxonase PON-1 gene is the best known genetic determinant of PON-activity [2]. Recently, this genetic variation was reported to be strongly linked to clopidogrel bioactivation, clopidogrel on-treatment platelet reactivity (OPR), and thrombotic clinical outcome [3]. However, subsequent studies failed to reproduce the association between PON-1 polymorphism and clopidogrel response variability [4]–[7]. Others have suggested that PON-1 genotype may be associated with long term clinical outcome in patients with known CAD possibly due to antioxidant effects on low density lipoprotein-cholesterol (LDL-C) [8].
This study was undertaken to elucidate whether PON-1 genetic variation is associated with clinical outcome after percutaneous coronary intervention (PCI) with drug-eluting stents (DES). We further tried to dissect the mechanism by which PON-1 genetic variation could possibly affect clinical outcome by evaluating the relationship between PON-1 SNP and clopidogrel response variability and lipid parameters including LDL-C particle size.
Methods
Study Population
The CROSS-VERIFY cohort (measuring clopidogrel resistance to assure safety after percutaneous coronary intervention using VerifyNow) is a dynamic cohort with on-going patient recruitment including consecutive patients receiving PCI who agreed to measurement of clopidogrel OPR with the VerifyNow P2Y12 assay after clopidogrel therapy at Seoul National University Hospital since June 1st 2006. The enrollment period for the current study was from June 2006 through June 2010, and only patients with implantation of drug eluting stent (DES) were enrolled. Exclusion criteria were contraindication to aspirin, clopidogrel, or heparin; the use of intravenous glycoprotein IIb/IIIa inhibitor within 5 days before the platelet reactivity test; the concomitant use of cilostazol; uncontrolled malignancy; bleeding tendency; and ethnicity other than Korean heritage. The study complied with Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Seoul National University Hospital. The participants provided their written informed consent to participate in this study. The ethics committee approved the consent procedure.
Platelet Function Test
The inhibitory effect of clopidogrel on platelet reactivity was measured using the VerifyNow P2Y12 assay (Accumetrics Inc., San Diego, CA). Blood sample was obtained 12 to 24 hours after final dose of clopidogrel in patients who had been on 75 mg of clopidogrel for more than 7 days, and 12 to 24 hours after PCI in patients who were loaded with clopidogrel before catheterization. A loading dose of 300 mg clopidogrel was administered in patients who had been taking clopidogrel for less than 7 days; 600 mg was given to clopidogrel-naïve patients. All patients took aspirin at 100 mg per day or 300 mg loading, if not taken previously. The VerifyNow P2Y12 assay reports the results as P2Y12 reaction units (PRU).
Lipid Profile and LDL-C Particle Size Measurement
Fasting blood samples were obtained by venipuncture on the day of the PCI. Serum was separated by centrifugation and biochemical measurements were conducted immediately. Serum total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) were measured enzymatically using the Hitachi 747 chemical analyzer (Hitachi, Tokyo, Japan). LDL particle size was determined using gel electrophoresis (LipoprintTM System; Quantimetrix Corp., Redondo Beach, CA, USA) according to the manufacturer’s instructions [9].
Genetic Analysis
PON-1 (Q192R, rs662) was screened using the TaqMan fluorogenic 5′ nuclease assay (ABI, Foster City, CA). The PCR was performed using 384-well plates by a Dual 384-Well GeneAmp PCR System 9700 (ABI, Foster City, CA) and the endpoint fluorescent readings were performed on an ABI PRISM 7900 HT Sequence Detection System (ABI, Foster City, CA). Duplicate samples and negative controls were included to ensure accuracy of genotyping.
Endpoints
The primary endpoint was the composite of cardiac death, non-fatal myocardial infarction (MI), and stent thrombosis at 12 months. Secondary endpoints included each individual components of the primary end point, target lesion revascularization (TLR) at 12 months, and clopidogrel OPR. All deaths were considered to be cardiac deaths unless an unequivocal non-cardiac cause could be documented. Myocardial infarction was defined according to the universal definition of myocardial infarction [10]. Stent thrombosis was defined according to the Academic Research Consortium definition, including definite and probable stent thrombosis [11].
Follow-up
All patients had regular out-patient visits with a cardiology specialist. Data were prospectively gathered by a dedicated coordinator. When follow-ups were missed or key data were missing, telephone contacts were made by data coordinators. To confirm the completeness of follow-up and minimize underreporting of events, the vital status of all patients were checked with the National Statistical Agency and events of patients with possible follow-up loss with the National Health Insurance Corporation.
Statistical Analysis
The data were presented as numbers and frequencies for categorical variables, and as the mean ± the standard deviation for continuous variables. For comparison among groups, the Chi-square test (or Fisher’s exact test when any expected cell count was <5 for a 2×2 table) was used for categorical variables and the unpaired Student’s t-test or the 1-way analysis of variance were applied for continuous variables. The Chi-square test for goodness of fit was used to verify agreement with Hardy-Weinberg equilibrium using the Fisher’s exact test. The chronological trend of outcomes was expressed as Kaplan–Meier estimates at 12 months and was compared between PON-1 QQ/QR- and RR-genotype carriers. The log-rank test was used to analyze the significant indifferences in clinical outcomes. A multivariable Cox proportional hazards regression model was used to find the independent predictors of thrombotic events after PCI. The analysis of covariance (ANCOVA) was applied to quantify the effect of PON-1 Q192R genotypes on LDL-particle size by entering patients’ genotype status as factor and clinical factors as covariates. In this case, the data were presented as the mean ± the standard error. Two sided p values less than 0.05 were considered statistically significant. Statistical tests were performed using SPSS version 17 (SPSS Inc., Chicago, Illinois, USA).
Results
Study Population
A total of 1676 patients were enrolled in the CROSS-VERIFY Cohort for the current study. We excluded 38 patients without drug eluting stent implantation, 143 patients with concomitant use of cilostazol, 2 Caucasians, and 157 patients who did not agree with genetic analysis or failed PON-1 Q192R genotyping, leaving 1336 patients available for the current analysis.
PON1-genotyping was successful in 98%. The observed genotype distribution was 13.4%, 47.6% and 39% for QQ, QR and RR-genotype, respectively. The genotyping replication rate for PON Q192R was 99%. The minor allele frequency was 0.37 and the genotype distribution was within Hardy-Weinberg-Equilibrium (X 2, P = 0.490). The baseline characteristics of the study population are summarized in Table S1. The mean age was 64 years old, 68% were male, 68% had hypertension, and 32% had diabetes mellitus. Seven hundred and seventy-three patients (57.9%) presented with stable angina, while 563 patients (42.1%) with acute coronary syndrome (ACS). When grouped according to genotype, there was no difference in baseline characteristics among the three groups. There was no relationship between the distribution of the paraoxonase 1 gene polymorphism and clinical presentation (P = 0.418).
Clinical Outcomes
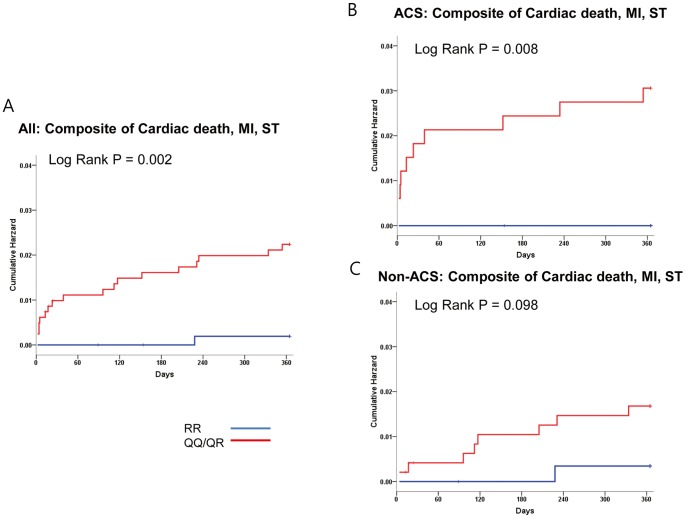
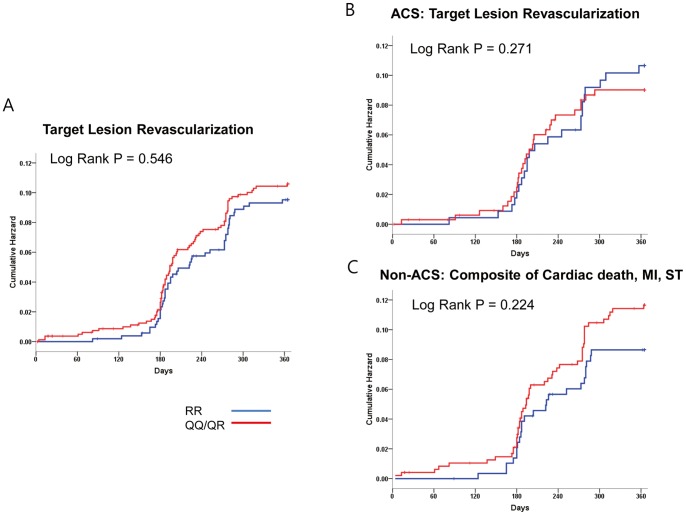
The primary composite endpoint occurred in 1.4% (19 events during the one year follow-up). The baseline characteristics between the both groups with and without clinical events were mostly similar, except that the frequency of QQ/QR genotype was higher in the event group (60.5% vs. 94.7%, P = 0.002) and that of beta-blocking agents higher in the event-free group (51.5% vs. 26.3%, P = 0.029) ( Table 1 ). Cardiac death occurred in 9 patients (0.7%), MI in 11 patients (0.8%), and stent thrombosis in 8 patients (0.6%). Since PON-Q allele is associated with decreased PON activity, and with similar clinical outcome (Figure S1, Table S3), we divided the patients into those with and without the Q-allele. Eight hundred and fifteen patients had QQ or QR genotypes (61%), while 521 patients had RR genotype (39%). The primary composite endpoint (2.2% vs. 0.2%, P = 0.002) as well as the individual endpoints of MI (1.3% vs. 0.3, P = 0.008) and stent thrombosis (1.0% vs. 0%, P = 0.023) occurred more frequently in patients with the QQ/QR genotype compared with those with the RR genotype ( Figure 1A ) ( Table 2 ). This difference in thrombotic outcomes was more profound in the ACS patients compared with the non-ACS patients, although the trend toward higher risk of thrombotic outcome in the QQ/QR group was maintained even in the non-ACS group ( Figure 1B, 1C ). As for repeat revascularization, there was no difference in the risk of TLR between QQ/QR versus the RR genotypes (9.8% vs. 9.0%, P = 0.590) ( Figure 2A ), which was similar in both ACS and non-ACS subgroups ( Figure 2B, 2C ).
Table 1. Baseline characteristics according to primary endpoint.
| Event (−) N = 1317 | Event (+) N = 19 | p-value | |
| Demographic characteristics | |||
| Mean OPR (PRU) | 233±83 | 245±89 | 0.535 |
| PON-1 QQ/QR-genotype (%) | 60.5 | 94.7 | 0.002 |
| Age (years) | 64±9 | 67±10 | 0.111 |
| Men (%) | 67.4 | 78.9 | 0.284 |
| Body mass index (kg/m2) | 25.2±4.9 | 24.1±3.9 | 0.355 |
| Current smoker (%) | 17.7 | 10.5 | 0.555 |
| Hypertension (%) | 67.7 | 63.2 | 0.678 |
| Diabetes mellitus (%) | 32.4 | 15.8 | 0.123 |
| Dyslipidemia (%) | 45.4 | 47.4 | 0.865 |
| Chronic kidney disease (%) | 2.4 | 5.3 | 0.380 |
| - Renal replacement tx. | 1.0 | 0 | >0.999 |
| Presenting symptoms (%) | 0.503 | ||
| - Stable angina | 58 | 47.4 | 0.351 |
| - Unstable angina | 35.2 | 42.1 | 0.529 |
| - NSTEMI | 4.7 | 10.5 | 0.238 |
| - STEMI | 2.1 | 0 | >0.999 |
| Previous PCI (%) | 32.3 | 36.8 | 0.672 |
| Previous CABG (%) | 3.0 | 5.3 | 0.449 |
| Previous MI (%) | 4.6 | 10.5 | 0.219 |
| Congestive heart failure (%) | 0.8 | 0 | >0.999 |
| Cerebrovascular accident (%) | 5.8 | 5.3 | >0.999 |
| Peripheral artery disease (%) | 1.2 | 0 | >0.999 |
| Laboratory finding | |||
| GFR (ml/min/1.73 m2) | 69.3±17.3 | 66.2±20.2 | 0.442 |
| Cholesterol (mg/dL) | 156±40 | 155±35 | 0.902 |
| - Triglyceride (mg/dL) | 143±94 | 128±69 | 0.512 |
| - HDL-C (mg/dL) | 42±12 | 42±13 | 0.765 |
| - LDL-C (mg/dL) | 88 | 87 | 0.910 |
| Concomitant Medication | |||
| ACEi/ARB (%) | 41.9 | 52.6 | 0.345 |
| Beta-blocker (%) | 51.5 | 26.3 | 0.029 |
| Calcium channel blocker (%) | 26.9 | 26.3 | 0.956 |
| -Dihydropyridine CCB (%) | 16.9 | 15.8 | 0.895 |
| -Non-DHP CCB (%) | 10.2 | 15.8 | 0.434 |
| Statin (%) | 62.6 | 52.6 | 0.371 |
| - Lipophilic statin (%) | 41.4 | 42.1 | 0.949 |
| Proton pump inhibitor (%) | 2.2 | 10.5 | 0.070 |
| - Omeprazol (%) | 0.8 | 0 | >0.999 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CCB, calcium channel blocker; HDL, high density lipoprotein; LDL, low density lipoprotein; OPR, on treatment platelet reactivity; PCI, percutaneous coronary intervention; PRU, P2Y12 reaction unit.
Figure 1. Kaplan-Meier curves for primary composite end point of cardiac death, myocardial infarction, and stent thrombosis.
Patients with PON-1 QQ/QR genotypes had higher rates for primary endpoint than those with RR-genotype (2.2% vs. 0.2%, Log-rank p = 0.002) (Fig. 1A). This difference in thrombotic outcomes was more profound in the higher risk ACS patients compared with the lower risk non-ACS patients (3% vs. 0%, Log-rank p = 0.008) (Fig. 1B), although the trend toward higher risk of thrombotic outcome in the QQ/QR group was maintained even in the non-ACS group (1.7% vs. 0.3%, Log-rank p = 0.098) (Fig. 1C).
Table 2. Clinical outcome according to PON1-Q192R genotype.
| QQ/QR, n = 815 | RR, n = 521 | p-value | |
| Primary composite end point of cardiac death, MI, and ST | 18 (2.2%) | 1 (0.2%) | 0.002 |
| Secondary end points | |||
| Cardiac Death | 8 (1.0%) | 1 (0.2%) | 0.085 |
| Myocardial infarction | 11 (1.3%) | 0 (0.0%) | 0.008 |
| CVA | 1 (0.1%) | 0 (0%) | 0.422 |
| Stent thrombosis | 8 (1.0%) | 0 (0.0%) | 0.023 |
| Target lesion revascularization | 80 (9.8%) | 47 (9.0%) | 0.590 |
| Composite of Death, MI, CVA | 16 (2.3%) | 4 (0.6%) | 0.002 |
| MACE | 113 (13.9%) | 54 (10.4%) | 0.052 |
MI, myocardial infarction; ST, stent thrombosis; CVA; cerebrovascular accident; MACCE, major adverse cardiovascular event.
P-value by Log-rank test.
Figure 2. Kaplan-Meier curves for target lesion revascularization.
There was no difference in target lesion revascularization rates between PON-1 QQ/QR and RR genotypes either in all patients (9.8% vs. 9.0%, Log-rank P = 0.546) (Fig. 2A), in those with ACS presentation (8.4% vs. 10%, Log rank P = 0.271) (Fig. 2B), or those with non-ACS-presentation (10.8% vs. 8.2%, Log-rank P = 0.224) (Fig. 2C).
To find the independent predictors of clinical outcome, a multivariate Cox-proportional hazard analysis was performed by entering common cardiac risk factors such as age (in decade), diabetes mellitus, dyslipidemia, and chronic kidney disease, along with PON-1 genotype. The QQ/QR genotype was an independent predictor of the primary endpoint with an 11-fold increased risk (HR 11.6, 95% CI: 1.55–87.0) along with age (age in decade HR 1.82, 95% CI: 1.08–3.07) ( Table 3 ). As for repeat revascularization, diabetes mellitus (HR 1.51, 95% CI: 1.06–2.16), and dyslipidemia (HR 1.49, 95% CI: 1.04–2.12) were independent predictors, but not PON-QQ/QR genotype (HR 1.12, 95% CI: 0.78–1.61).
Table 3. Independent predictors of clinical outcomes.
| HR | CI | p-value | |
| Primary end point (composite of cardiac death, MI, Stent thrombosis) | |||
| - PON-QQ/QR0 | 11.6 | 1.55–87.0 | 0.017 |
| - Age (in decade) | 1.82 | 1.08–3.07 | 0.025 |
| - Diabetes mellitus | 0.40 | 0.12–1.34 | 0.146 |
| - Dyslipidemia | 1.23 | 0.50–3.04 | 0.655 |
| - Chronic kidney disease | 0.82 | 0.28–2.39 | 0.720 |
| Target lesion revascularization | |||
| - Diabetes mellitus | 1.51 | 1.06–2.16 | 0.023 |
| - Dyslipidemia | 1.49 | 1.04–2.12 | 0.028 |
| - Chronic kidney disease | 1.37 | 0.92–2.04 | 0.117 |
| - PON-QQ/QR | 1.12 | 0.78–1.61 | 0.542 |
| - Age (in decade) | 0.97 | 0.78–1.18 | 0.772 |
Cox-proportional hazard regression analysis adjusting for PON-192 QQ/QR, age, diabetes mellitus, dyslipidemia, chronic kidney disease as covariates.
PON-1 Genotype, Platelet Function and Lipid-profiles
There was no difference in both the distribution of platelet reactivity and mean OPR among the three genotypes (unadjusted mean OPR: 233±82 PRU, 231±86 PRU, and 236±81 PRU, for GG, GA, and AA genotypes respectively, ANOVA P = 0.596) ( Figure 3 ). In addition, there was no difference in platelet reactivity according to the clopidogrel dosing regimen and the three genotypes (Table S5).
Figure 3. On-treatment platelet reactivity (OPR) according to PON1-Q192R genotypes.
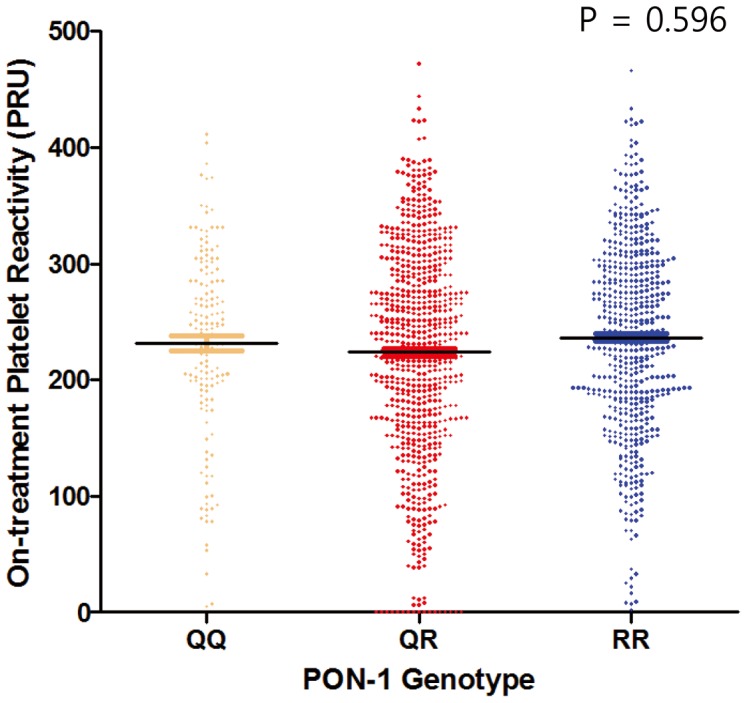
There was no difference in OPR between QQ, QR, and RR genotypes (mean OPR: 233±82 PRU, 231±86 PRU, and 236±81 PRU, for QQ, QR, and RR genotypes respectively, ANOVA P = 0.596).
After adjustment for age (in decade), gender, smoking status, diabetes mellitus, chronic kidney disease, and dyslipidemia, there were no significant differences in OPR within the PON1-genotypes (adjusted mean OPR: 233±6, 231±3, and 234±4 PRU, for QQ, QR, and RR genotypes respectively).
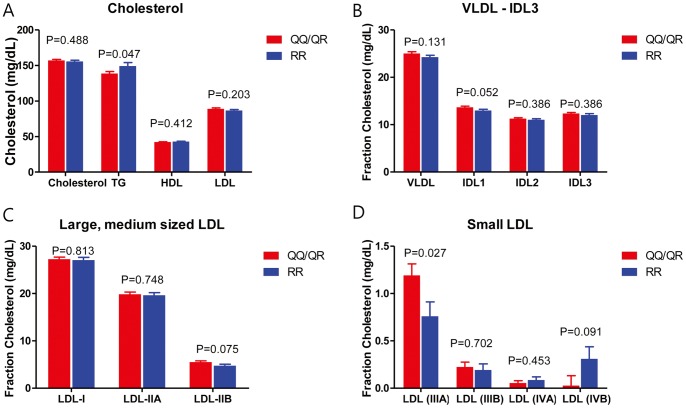
As for the relationship between PON-1 genotype and lipid profile, 63% of patients were on statin therapy, and the serum levels of total cholesterol, TG, HDL-C, and LDL-C in the entire patient cohort were 156±40 mg/dL, 143±94 mg/dL, 43±12 mg/dL, and 88±36 mg/dL, respectively. The baseline lipid parameters were mostly similar between the QQ/QR versus RR genotypes except for TG which was marginally higher in the RR genotype group (139±82 mg/dL vs. 149±110 mg/dL, P = 0.047). A total of 768 patients (57%) underwent LDL-particle size analysis. After adjustment for age, sex, TG, HDL-C, and statin use, patients with QQ/QR genotype had significantly higher small-dense LDL levels than those with RR-genotype (1.20±0.12 mg/dL vs. 0.76±0.15 mg/dL, P = 0.027) ( Figure 4 ) (Table S4). As for the larger particle size LDL-C, i.e. LDL I-IIB, there was no significant difference between the two genotype groups.
Figure 4. Lipid profiles according to genotypes.
Patients with QQ/QR genotypes had lower triglyceride levels (Fig. 4A). As for LDL particle size, there was no difference either in VLDL, IDL between both groups (Fig. 4B), or large and medium sized LDL (Fig. 4C). As for small LDL, patients with QQ/QR genotype had significant higher LDL-IIIA than those RR-genotype (1.20±0.12 mg/dL vs. 0.76±0.15 mg/dL, P = 0.027) (Fig. 4D). Cholesterol level was compared by using one-way ANOVA test. As for LDL-particle size, general linear model analysis was applied to quantify the effect of PON-1 QQ/QR genotype on LDL-particle size after adjustment for age, sex, triglyceride level, HDL-C level, and statin use. Data were presented as the mean ± the SEM.
Discussion
To the best of our knowledge, this is the first study, which investigated the relationship between PON-1 genetic variants and clinical outcome in an exclusive cohort of patients who received DES. We report that PON-1 Q-allele is associated with increased risk for composite thrombotic outcome of cardiac death, MI and stent thrombosis, but not with increased risk for TLR. Patients with QQ/QR genotype have 11-fold increased risk for thrombotic complications than those with RR-genotype. However, QQ/QR genotype was not associated with clopidogrel response variability but with higher small dense (sd)-LDL level.
PON-1 Q192R Genotype and Cardiovascular Outcome
The association between PON-1 Q192R genotypes and clinical outcome in coronary artery disease (CAD)-patients has been investigated in a couple of aspects. A cross-sectional genetic-association meta-analysis by Wheeler et al [12] involving 23,998 cases revealed no or minimal association between Q192R polymorphism and CAD. Reny et al also reported in a meta-analysis with 5,302 patients that he PON1-Q192R polymorphism has no major impact on the risk of MACE in clopidogrel-treated patients. [13].
In contrast, Mackness et al [14] showed that lower PON activity was associated with an increased risk for cardiovascular events in males, which was more pronounced in those with pre-existing CAD. The positive association between PON-1 genetic variation and clinical outcome in CAD-patients has been reported for long-term cardiovascular mortality and morbidity in 793 statin treated CAD patients in the REGRESS study [8]. In addition, Bhattacharyya et al [15] showed that those with the QQ-genotype were at increased risk for major adverse cardiovascular events. In line with these previous studies, our study comprised of patients receiving DES showed that the Q-allele carriers (PON-1 192QQ/QR genotypes) had an 11-fold risk increase for composite endpoint of cardiac death, MI and stent thrombosis. The association between PON-1 genotype and cardiovascular outcome was more profound in patients with ACS.
In contrast, we could not find any association between TLR and PON-1 genotypes. Chen et al [16] and Turban et al [17] also reported that Q192R genotype were not associated with severity, progression or regression of coronary atherosclerosis.
Potential Mechanisms
Previously, Bouman et al reported that PON-1 Q192R was strongly linked to clopidogrel metabolism, and with clinical outcome including stent thrombosis, identifying the PON enzyme activity as a key step for clopidogrel bioactivation. This could be an important mechanism by which Q allele carriers are at increased risk for cardiovascular events. However, this cannot explain the association between PON-1 genotype and clinical outcome in many patients who were not taking clopidogrel in previous studies. Furthermore, subsequent studies by Sibbing et al, Fontana et al, Trenk et al, and Lewis et al could not reproduce the association between PON-1 polymorphism and clopidogrel response variability [4]–[6], [18]. Likewise, our results did not show any association between PON-1 Q192R polymorphism and mean OPR, either. Even the distribution of platelet function was very similar for the three genotype groups, suggesting mechanisms other than the effect on clopidogrel metabolism may be responsible for the different clinical outcome.
The first report on the genetic regulation of PON activity suggested that PON activity was controlled by two alleles at a single autosomal locus [19]. Later, Adkins et al identified that the serum paraoxonase-phenotype is associated with the ARG/Gln structural polymorphism at amino acid position 192 [2]. The 192R-alloenzyme was shown to bind to the HDL particle with 3-fold higher affinity than 192Q-alloenzyme, consequently exhibiting increased stability [20], and individuals with PON-1 192R-allele have increased PON activity. [3], [21] HDL-associated PON-1 (i) increases the reverse cholesterol transport, (ii) endothelial nitric oxide (NO) production, and (iii) promotes pronounced systemic antioxidant effects.
In this study, we found that patients with PON-1 QQ/QR genotypes had significantly higher sd-LDL-C level compared to those with RR-genotype, in particular LDL-IIIA. There are seven major LDL-subfractions (I (large), IIA and IIB (medium), IIIA and IIIB (small), and IVA and IVB (very small)). Predominance of LDL with small particle size has been associated with increased cardiovascular risk [22], and has been accepted as an emerging cardiovascular risk factor by the National Cholesterol Education Program Adult Treatment Panel III [23]. Small dense-LDL resides longer in plasma, has enhanced oxidizability, arterial proteoglycan binding and permeability through the endothelial barrier [24]. We hypothesize that impaired PON activity may lead to increased level of more atherogenic and rupture-prone sd-LDL-C resulting in thromboembolic events.
Another possible mechanism may be the endothelial dysfunction caused by decreased PON-1 activity. HDL-associated PON-1 activity plays a crucial role in maintaining the endothelial-atheroprotective effects of HDL. It stimulates the endothelial NO-production. NO has important antithrombotic properties, i.e. inhibiting leukocyte adhesion, limiting platelet adhesion and aggravation, and the expression of plasminogen activator inhibitor-1 [25]. Impaired HDL-associated PON-1 activity leads to decreased endothelial nitric oxide synthase (eNOS)-activity. Reduced endothelial NO bioavailability contributes to impaired endothelial repair, to the development and progression of atherosclerosis. Besler et al demonstrated that HDL from PON-1-deficient mice failed to stimulate NO production [26]. Patients with decreased paraoxonase activity, i.e. those with 192Q-allele, may have decreased HLD-associated anti-inflammatory and anti-thrombotic effect. Consequently, they would be at increased risk for thromboembolic complication. Besler et al also demonstrated that HDL from healthy subjects stimulated endothelial NO production, whereas that from stable CAD patients did not. Interestingly, HDL from ACS patients even inhibited the endothelial NO production. We showed that (i) 192Q-allele carriers had higher risk for thrombotic complication, and that (ii) this risk was more pronounced in ACS patients. Taken together, ACS patients are more susceptible to thrombotic complication due to HDL-PON mediated inhibition of endothelial NO synthesis, and this effect is exaggerated by the low PON activity due to PON-1 192Q-alleles.
With regard to HDL-C level, there are conflicting results on the association between PON Q192R genotype and serum HDL-C levels. In our study we could not demonstrate any association. In a study by Srinivasan et al [27] with 1,786 black and white young adults, the frequency of the PON-1 R-allele was higher in blacks than in whites (0.668 versus 0.297, P<0.001), and the R-allele was associated with increased HDL-C in whites (P = 0.041), whereas the opposite was true in blacks (P = 0.008). Ethnicity might play a role in differences in phenotypic expression of PON1 genotype on lipid levels.
The Frequency of PON-1 Q192R Genotypes in Koreans
It is essential to determine first whether the differences in genotype frequencies observed in our study are attributed to the differences due to ethnicity or due to selection of patients with PCI-phenotype. Two studies reported the frequencies of PON Q192R-genotypes in healthy Koreans. In a study by Eom et al [28] the frequency of QQ, QR and RR genotypes was 13%, 42% and 45%, respectively, and in another study by Lee et al [29], it was 6.2%, 45.2%, and 41.2%, respectively. These study results suggest that the distribution of Q and R alleles is inverted in Koreans compared with Caucasians, and the low Q-allele frequency in our study population is not due to the selection of a cohort with PCI-phenotypes.
If the PON 192Q-allele is associated with increased risk for atherosclerotic burden, it is likely that the frequency of the 192 Q–allele is higher in the PCI cohort than the general Korean population. Unfortunately, we did not perform genotyping of PON Q192R patient in healthy Koreans, consequently we cannot prove this hypothesis.
Limitation
There are several limitations in this study. First, we did not measure the PON activity in each patient. But several previous studies already reported that the PON activity is genetically controlled and 192Q-allele is associated with low activity [3], [15]. Second, there is a discrepancy in PON-1 Q192R allele frequency. The distribution of QQ, QR and RR genotypes were 35%, 47% and 18% in Bouman’s, 53%, 39%, 8% in Sibbing’s, 52%, 38%, 10% in Fontana’s study population. But in our study population, it was 13%, 48%, 39%, for QQ, QR and RR, respectively. The distribution of Q and R alleles differs in many populations. For example, the Q- and R-allele frequencies in Caucasian populations are 0.7 and 0.3, respectively, whereas this is inverted in African and Asian populations [30]. Therefore, our results cannot be directly extrapolated to Western populations, and vice versa. Third, all participants had significant CAD and received DES implantation. Therefore our findings are limited to the population analyzed in this study and cannot be generalized to others with mild CAD or those that do not receive PCI with DES. Most importantly, the low number of events in the study may raise the concern that the results may be by chance. Our results are hypothesis generating at best and further studies are needed to confirm our finding. Finally, since the LDL particle size analysis was performed at physicians’ discretion, patients at increased risk would more likely undergo LDL-particle analysis which is reflected by the differences in the baseline characteristics (Table S2).
Conclusion
In patients receiving DES, PON1 Q-allele is an independent predictor of worse cardiovascular outcome independent of platelet function and is associated with significantly higher levels of small dense LDL-C. PON-1 genotype may serve as a novel genetic risk factor for adverse events after PCI.
Supporting Information
Kaplan Meier Survival analysis of composite of cardiac death, MI, ST according to genotypes.
(TIF)
(TIF)
Baseline characteristics of the study population.
(DOC)
Baseline characteristics of the study population according to LDL-particle size analysis.
(DOC)
Clinical outcomes according to PON1-Q192R genotype.
(DOC)
Lipid profile according to PON-1 Q192R genotype.
(DOC)
Clopidogrel on-treatment platelet reactivity according to different loading regimen.
(DOC)
(DOCX)
Funding Statement
This study was supported by a grant from the Clinical Research Center for Ischemic Heart Disease, Seoul, Republic of Korea (A102065) and a grant from the Innovative Research Institute for Cell Therapy, Seoul National University Hospital (A062260), sponsored by the Ministry of Health, Welfare & Family, Republic of Korea. This study was funded in part by a grant from the Korean Liver Research Foundation, Seoul, Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. La Du BN, Adkins S, Kuo CL, Lipsig D (1993) Studies on human serum paraoxonase/arylesterase. Chem Biol Interact 87: 25–34. [DOI] [PubMed] [Google Scholar]
- 2. Adkins S, Gan KN, Mody M, La Du BN (1993) Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet 52: 598–608. [PMC free article] [PubMed] [Google Scholar]
- 3. Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, et al. (2011) Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17: 110–116. [DOI] [PubMed] [Google Scholar]
- 4. Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, et al. (2011) No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J 32: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 5. Fontana P, James R, Barazer I, Berdague P, Schved JF, et al. (2011) Relationship between paraoxonase-1 activity, its Q192R genetic variant and clopidogrel responsiveness in the ADRIE study. J Thromb Haemost 9: 1664–1666. [DOI] [PubMed] [Google Scholar]
- 6. Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, et al. (2011) Paraoxonase-1 Q192R Polymorphism and Antiplatelet Effects of Clopidogrel in Patients Undergoing Elective Coronary Stent Placement. Circ Cardiovasc Genet 4: 429–436. [DOI] [PubMed] [Google Scholar]
- 7. Campo G, Ferraresi P, Marchesini J, Bernardi F, Valgimigli M (2011) Relationship between paraoxonase Q192R gene polymorphism and on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention. J Thromb Haemost 9: 2106–2108. [DOI] [PubMed] [Google Scholar]
- 8. Regieli JJ, Jukema JW, Doevendans PA, Zwinderman AH, Kastelein JJ, et al. (2009) Paraoxonase variants relate to 10-year risk in coronary artery disease: impact of a high-density lipoprotein-bound antioxidant in secondary prevention. J Am Coll Cardiol 54: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 9. Hoefner DM, Hodel SD, O’Brien JF, Branum EL, Sun D, et al. (2001) Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem 47: 266–274. [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, White HD (2007) Universal definition of myocardial infarction. J Am Coll Cardiol 50: 2173–2195. [DOI] [PubMed] [Google Scholar]
- 11. Pinto Slottow TL, Waksman R (2007) Overview of the 2006 Food and Drug Administration Circulatory System Devices Panel meeting on drug-eluting stent thrombosis. Catheter Cardiovasc Interv 69: 1064–1074. [DOI] [PubMed] [Google Scholar]
- 12. Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J (2004) Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet 363: 689–695. [DOI] [PubMed] [Google Scholar]
- 13. Reny JL, Combescure C, Daali Y, Fontana P (2012) Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost 10: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, et al. (2003) Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 107: 2775–2779. [DOI] [PubMed] [Google Scholar]
- 15. Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, et al. (2008) Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299: 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Q, Reis SE, Kammerer CM, McNamara DM, Holubkov R, et al. (2003) Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Am J Hum Genet 72: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turban S, Fuentes F, Ferlic L, Brugada R, Gotto AM, et al. (2001) A prospective study of paraoxonase gene Q/R192 polymorphism and severity, progression and regression of coronary atherosclerosis, plasma lipid levels, clinical events and response to fluvastatin. Atherosclerosis 154: 633–640. [DOI] [PubMed] [Google Scholar]
- 18. Lewis JP, Fisch AS, Ryan K, O’Connell JR, Gibson Q, et al. (2011) Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther 90: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Playfer JR, Eze LC, Bullen MF, Evans DA (1976) Genetic polymorphism and interethnic variability of plasma paroxonase activity. J Med Genet 13: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaidukov L, Rosenblat M, Aviram M, Tawfik DS (2006) The 192R/Q polymorphs of serum paraoxonase PON1 differ in HDL binding, lipolactonase stimulation, and cholesterol efflux. J Lipid Res 47: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 21. Mackness MI, Mackness B, Durrington PN, Fogelman AM, Berliner J, et al. (1998) Paraoxonase and coronary heart disease. Curr Opin Lipidol 9: 319–324. [DOI] [PubMed] [Google Scholar]
- 22. Gardner CD, Fortmann SP, Krauss RM (1996) Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA 276: 875–881. [PubMed] [Google Scholar]
- 23. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 24. Krauss RM (1994) Heterogeneity of plasma low-density lipoproteins and atherosclerosis risk. Curr Opin Lipidol 5: 339–349. [DOI] [PubMed] [Google Scholar]
- 25. Loscalzo J (2001) Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res 88: 756–762. [DOI] [PubMed] [Google Scholar]
- 26. Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, et al. (2011) Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srinivasan SR, Li S, Chen W, Tang R, Bond MG, et al. (2004) Q192R polymorphism of the paraoxanase 1 gene and its association with serum lipoprotein variables and carotid artery intima-media thickness in young adults from a biracial community. The Bogalusa Heart Study. Atherosclerosis 177: 167–174. [DOI] [PubMed] [Google Scholar]
- 28. Eom SY, Kim YS, Lee CJ, Lee CH, Kim YD, et al. (2011) Effects of intronic and exonic polymorphisms of paraoxonase 1 (PON1) gene on serum PON1 activity in a Korean population. J Korean Med Sci 26: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee CH, Lee KY, Choe KH, Hong YC, Kim YD, et al. (2005) [Effects of oxidative DNA damage induced by polycyclic aromatic hydrocarbons and genetic polymorphism of the paraoxonase-1 (PON1) gene on lung cancer]. J Prev Med Public Health 38: 345–350. [PubMed] [Google Scholar]
- 30. Huang Q, Liu YH, Yang QD, Xiao B, Ge L, et al. (2006) Human serum paraoxonase gene polymorphisms, Q192R and L55M, are not associated with the risk of cerebral infarction in Chinese Han population. Neurol Res 28: 549–554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan Meier Survival analysis of composite of cardiac death, MI, ST according to genotypes.
(TIF)
(TIF)
Baseline characteristics of the study population.
(DOC)
Baseline characteristics of the study population according to LDL-particle size analysis.
(DOC)
Clinical outcomes according to PON1-Q192R genotype.
(DOC)
Lipid profile according to PON-1 Q192R genotype.
(DOC)
Clopidogrel on-treatment platelet reactivity according to different loading regimen.
(DOC)
(DOCX)