Abstract
This study was designed to investigate the role of serum GP73 for diagnosing significant fibrosis in patients with chronic hepatitis B virus (HBV) infections. Two populations were enrollment. All subjects were patients with chronic HBV infections. First population included 761 patients, who received liver stiffness measurement; the second population included 633 patients, who undertaken liver biopsy, in which 472 patients with nearly normal ALT. All patients received serum GP73 test. The effect of GP73 recombinant protein to HepG2 cells and LX2 cells were observed in vitro. Results showed that serum GP73 concentration is correlated with liver stiffness (r = 0.601). The area under ROC curve is 0.76. The sensitivity and specificity of GP73 for significant fibrosis (≥F2) diagnosis were 62.81%, 80.05% respectively (cut off: 76.6 ng/ml). Serum GP73 concentration was significantly correlated with the grading of fibrosis (r = 0.32, and 0.35, in 633 and 472 patients, respectively.) GP73 had a striking performance for diagnosing S2 in patients with chronic HBV infections. In 472 patients with nearly normal ALT, the sensitivity and specificity of GP73 for S2 diagnosis were 62.5% and 80.0% respectively, where the cut-off was set at 82 ng/ml. GP73 recombinant protein may prompt LX2 cells proliferation at the concentration 10–100 ng/ml. The present results indicated that GP73 may be a marker for diagnosing significant fibrosis in patients with chronic HBV infections, and may be a new contributor to fibrogensis.
Introduction
Hepatic fibrosis, the common response associated with almost of all chronic hepatitis B virus (HBV) infection, ultimately leads to cirrhosis [1]. With great advancements in the antiviral therapy used for the treatment of chronic virus hepatitis, the accurate assessment of liver fibrosis is a vital need for successful individualized management. Current guidelines recommend antiviral therapy in chronic hepatitis B patients with significant fibrosis (≥2), whether or not ALT is abnormal [2]. Moreover, the significant fibrosis correlated strongly with poor clinical outcomes, compared with mild fibrosis [3]. Lack of accurate, reproducible and easily applied methods for fibrosis assessment is the major limitation in the clinical management. The current ‘gold standard’ for liver fibrosis detection is liver biopsy [4]. Liver biopsy can provide physicians useful clinical information, such as appropriate time to start antiviral therapy, predicting the response to treatment, assessing the natural course of hepatitis, and estimating prognosis of hepatitis. Although accuracy in detailed fibrosis classification may provide by liver biopsy; however, this method does has innate limits, such as invasive, sampling error and sample size effect, which limits its application. Consequently, the highly specific set of biomarkers for fibrosis grading always pursue by professionals.
In the past two decades, a number of markers in combination with clinical risk factors are used to evaluate fibrosis grade, such as FibroMeter, FibroTest, etc [5], [6], all being used for diagnosing significant fibrosis. In fact, the combination of several biomarker for evaluating fibrosis grading were better prognosis predictors than histological staging [7], [8]. Those methods have one common point, i.e., consisting of several serum marker and mathematical model used to calculate fibrosis index. Since all of those non-invasive models have moderate accuracy for determining significant fibrosis, new biomarkers for the diagnosis of significant fibrosis are still in strong demand by clinicians.
Recently, a novel Golgi protein, GP73, was used to diagnosing hepatocelullar carcinoma [9]. This protein initially reported by Kladney RD, et al [10], and they also found that GP73 expression is increased in cultured cells by viruses infection [10], Subsequently, several investigations demonstrated that GP73 protein is overexpressed in a variety of acute and chronic liver diseases [11], and serum concentration correlated with progression of chronic liver disease [12], [13]. However, the relationship between serum GP73 concentration and staging or grading of chronic liver disease is still a pendent question. The present study was designed to evaluate the serum GP73 for diagnosing significant fibrosis and liver cirrhosis.
Materials and Methods
Study design
This study registered at ChiCTR.org (No.DDT-11001397) Oct, 2010, and included two populations. First population consisted of 761 patients with chronic hepatitis B, who were received liver stiffness measurement; second populations involved 633 patients with chronic HBV infections, in which 472 patients with nearly normal ALT (<80 U/L). Patients in second populations were received liver biopsy and pathological examination. All patients consecutively admitted to two centers (Beijing Ditan Hospital and 302 Military Hospital), between Aug. 2010 and Mar.2012. The study was approved by the Institutional Review Board of the Beijing Ditan Hospital, Capital Medical University. For group enrollment, liver stiffness measurement or liver biopsy were based on clinical requirement. Before initiating drug therapy, the serum samples were collected, and stored at −70°C.
Biochemical analysis
The liver function tests including serum albumin, total bilirubin (TB), and alanine aminotransferase (ALT) were measured using a Roche Hitachi 717 chemistry analyzer at the central laboratory of Beijing Ditan hospital. Quantitative determination of GP73 in serum was performed using commercially available enzyme-linked immunosorbent assay (ELISA) (Hotgen Biotech Inc., Beijing, China), according to the manufacturer's protocol.
Transient elastography measurement
Liver stiffness was measured with a FibroScan® device (FibroScan®, Philips, France), based on manufacturer's protocol. Results were expressed in kilopascals (kPa). Ten successful acquisitions were performed for each patient, and the median value was calculated by the device. The cut-off point of liver stiffness score for significant fibrosis, and liver cirrhosis referenced to the previous report [14], [15] i.e. 8.8 kPa(F2), for significant fibrosis, 16.9 kPa for liver cirrhosis.
Liver biopsy and Immunohistochemistry
Liver biopsies were obtained using 16G disposable needles (Hepafix, Germany). Fibrosis staging was considered reliable when the liver specimen length was ≥1.5 cm or the portal tract number ≥10. Liver specimens were stained with Masson trichrome and interpreted by two highly experienced liver pathologists. Liver fibrosis was scored on a 0–4 scale according to the METAVIR scoring system [16]. For GP73 staining, 3–5 µm formalin-fixed, paraffin-embedded samples were dewaxed and rehydrated. After slides incubating in 3% hydrogen peroxide, sections were incubated with GP73 antibody (HotGen Biotech, Beijing, China) overnight at 4°C; HRP-labeling anti-rabbit (Boster Bio., Wuhan, China) were used as secondary antibodies. 3,3′-Diaminobenzidine (DAB) Substrate Chromogen System (Dako) and was employed in the detection procedure. Images were acquired on an Olympus E520 (Tokyo, Japan) microscope.
Cell culture and proliferation assay
Hepatoma cell line (HepG2) was reserved in our laboratory. Hepatic stellate cell line (LX2) was conferred by Prof. Cheng (Insititute of Infectious Disease, Capital Medical University). LX2 cells line is a widely used hepatic stellate cell in the fibrosis investigation [17]. HepG2 and LX2 cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 in Eagle's minimum essential medium supplemented with10% fetal bovine serum. The ultimate concentration of GP73 recombinant protein added in supernatant was 1.0, 10.0, 20.0, 50.0, and 100.0 ng/ml respectively. After 48 hours coculturing, cell proliferation was evaluated with OD value, which was detected by CCK8 assay kit (Dojindo, Kumamoto, Japan), based on manufacture's protocol.
Western blot
Western blot was performed with standard protocol. Briefly, after cells cocultured with GP73 recombinant protein 48 hours, whole-cell extracts were prepared in assay buffer containing a protease inhibitor cocktail. Protein assays were performed using a BCA Protein assay kit (Pierce/Thermo Scientific, USA) according to the manufacturer's instructions. Total protein was electrophoresed in SDS–PAGE gels, and transferred to nitrocellulose membranes and then blocked with 5% milk in PBS, pH 7.4 with 0.05% Tween-20, incubated with collagen I or collagen III polyclonal antibody (Santa Cruz, USA) and anti-rabbit secondary antibody conjugated to horseradish peroxidase (Santa Cruz., USA). GP73 was detected by chemiluminescence.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0. Student t test was used to compare the difference of serum GP73 concentrations between different patients groups (mild and significant fibrosis group). Correlation between serum GP73 concentration and liver stiffness scores were calculated using Pearson's correlation coefficient (r). Data were expressed as mean ± SEM. P-values <0.05 were considered to be statistically significant. With liver stiffness value (FibroScan) or biopsy as the “gold standard”, the diagnostic performance of GP73 was evaluated by performing the Area under the ROC curve (AUROC) with 95% confidence interval (CI). For adjusting other confounders (Sex, Age, ALT, Total Bilirubin, Albumin, Platelet), we performed multivariate ordinal logistic regression analysis by SPSS 16.0.
Results
Patient's characteristics
From Aug. 2010 to Mar 2012, 761 patients received liver stiffness measurements; 633 patients received liver biopsy, in which 472 patients with nearly normal ALT. Those patients consecutively admitted into Beijing Ditan Hospital, Capital Medical University and 302 Military Hospital. The demological materials of two populations were showed in table 1.
Table 1. Patient's clinical characteristic.
| Parameter | Description | |
| Group | FibroScan (761) | liver biopsy (633) |
| Sex/age | ||
| Male | 492 (39.48±12.32 yrs) | 440(36.11±10.72 yrs) |
| Female | 269 (40.62±13.76 yrs) | 193(34.07±10.37 yrs)* |
| Clinical diagnosis | ||
| Chronic hepatitis | 649 (85.28%) | 582(91.94%) |
| Liver cirrhsis | 112 (14.72%) | 51(8.06%) |
| Comorbidity | ||
| Diabetes | 48(6.31%) | 17(2.69%) |
| Hypertension | 11(1.45%) | 7(1.11%) |
| Coronary heart disease | 4(0.53%) | 2(0.32%) |
| HBeAg | ||
| Positive | 436(57.29%) | 397(62.72%) |
| Negative | 325(42.71%) | 236(37.28%) |
| HBV DNA | ||
| < 2 log | 68(8.94%) | 36(5.69%) |
| ≥ 2 log | 693(91.06%) | 597(94.31%) |
| BMI (Kg/m2) | ||
| Male(mean ± S.D.) | 22.60±5.12 | 24.26±6.67 |
| Female(mean ± S.D.) | 20.73±4.93* | 21.33±3.91* |
| Total bilirubin (μmol/L) | 22.79±38.0 | 21.30±37.54 |
| Albumin (g/L) | 43.36±6.11 | 42.86±6.06 |
| Prothrombin time (s) | 12.88±2.20 | 12.71±9.35 |
Compared with male group, p<0.05.
Since without any patients with ascites, no related information was showed.
Serum GP73 concentration significantly correlated with hepatic stiffness
Based on more recently report, the diagnostic thresholds of liver stiffness in discriminating fibrosis stages ≥ F1, ≥ F2, ≥ F3 and = F4 were 7.1 kPa, 8.8 kPa, 10.7 kPa, and 16.9 kPa, respectively. Based on data of liver stiffness, 57.95% (441/761) patients had mild fibrosis (324 patients with very mild fibrosis; 117 patients with F1 grade). 42.05% (320/761) patients were significant or severe fibrosis (F2:79, F3:105, F4:136). Obviously, more cirrhotic patients were confirmed with liver stiffness than initially clinician's diagnosis (Fig. 1A, Table 2).
Figure 1. Serum GP73 concentration was correlated with liver stiffness (761 patients).
A: Different GP73 levels were observed in patients with different groups of liver stiffness. B: serum GP73 concentration was correlated with liver stiffness. C and D: the ROC analysis of GP73 was performed on diagnosis of significant fibrosis and liver cirrhosis. The numbers after symbols “<”or “ = ” are p value.
Table 2. Serum GP73 concentration related with liver stiffness ( FibroScan), ALT, Fibrosis grading, and serum HBV DNA.
| Parameter | N | GP73(ng/mL) | |
| Mean ± SD | 95%CI | ||
| Liver stiffnessa | |||
| < F1 | 324 | 53.70±28.84 | 50.55–56.85 |
| F1 | 117 | 62.54±34.31 | 56.27–68.60 |
| F2 | 79 | 78.46±45.35 | 68.30–88.61 |
| F3 | 105 | 91.90±44.51 | 73.28–90.51 |
| F4 | 136 | 166.0±87.39 | 151.2–180.8 |
| Fibrosis gradingb | |||
| 0.5 | 48 | 56.75±38.33 | 45.62–67.88 |
| 1.0 | 232 | 60.37±40.07 | 55.19–65.55 |
| 1.5 | 35 | 72.03±37.75 | 59.06–84.99 |
| 2.0 | 57 | 80.03±59.48 | 64.25–95.81 |
| 2.5 | 26 | 93.80±49.04 | 73.99–113.6 |
| 3.0 | 40 | 92.28±69.69 | 68.10–120.7 |
| 3.5 | 18 | 94.40±52.88 | 68.10–120.7 |
| 4.0 | 16 | 458.1±119.1 | 94.67–221.6 |
| ALT(U/L)c | |||
| ≤ 40 | 274 | 71.90±55.28 | 65.33–78.48 |
| < 80 | 197 | 72.52±53.07 | 65.08–79.98 |
| 80–200 | 93 | 88.35±77.53 | 72.38–104.3 |
| > 200 | 69 | 137.0±89.25 | 115.5–158.4 |
| HBV DNA(log10 IU/ml)b | |||
| negative | 25 | 49.13±17.39 | 41.95–56.31 |
| < 4 | 99 | 65.00±41.52 | 56.72–73.29 |
| ≥ 4 | 106 | 91.88±88.7* | 74.79–109.0 |
| ≥ 6 | 194 | 81.93±63.81 | 72.89–90.96 |
| ≥ 8 | 48 | 83.08±58.15 | 66.20–99.97 |
. 761 patients received liver stiffness measurements; b. 472 patients with nearly normal ALT; c. 633 patients with chronic hepatitis B infections; * P<0.05 Compared with patients with HBV DNA less than 4 Log.
To ascertain the correlation between serum GP73 concentration and liver stiffness, we firstly performed correlated analysis. Results showed that serum GP73 concentration is correlated with the value of liver stiffness (Correlation coefficient r = 0.601, p<0.0001). (Fig. 1B) Serum GP73 concentrations were significantly different between patients with different fibrotic group, except for F3 group (Fig. 1A). The important is that GP73 levels in patients of F2 group (78.46±45.35 ng/ml) is significantly higher than those of in patients of F1 group (62.54±34.31 ng/ml, p<0.01). This result suggested that GP73 concentration may be a marker for differentiating significant fibrosis (≥ F2) with mild fibrosis (<F2). The other interesting result is, for most cirrhotic patients, serum GP73 concentrations were especially higher than patients in F2 or F3 group. Despite serum GP73 concentration in patients of F3 group (81.90±44.51 ng/ml) were higher than those of patients in F2 group (78.46±45.35 ng/ml), this difference is no significant.
To clarify the optimal cut-off value of GP73 in diagnosing significant fibrosis (≥F2) in patients with chronic hepatitis B, we performed receiver operator characteristic (ROC) curve analysis. ROC curve analyses showed that the sensitivity and specificity of GP73 for significant fibrosis diagnosis were 62.81% (95% CI: 57.26%–68.12%), 80.05% (95% CI: 80.05%–83.68%) respectively, where the cut-off value was set at 76.6 ng/ml. The area under ROC curve is 0.76 (95% CI: 0.73–0.80). The positive predictive value (PPV), the negative predictive value (NPV), and acuuracy were 74.73%, 67.69, and 72.01%, respectively. If the diagnostic cut-off value was set at 135.4 ng/ml, the sensitivity and specificity of GP73 for diagnosing liver cirrhosis (F4) were 60.29% (95% CI: 51.55%–68.58%), 94.01% (95% CI: 91.84%–95.75%) respectively. The area under ROC curve is 0.88 (95%CI: 0.85–0.92). (Fig. 1.C, D). The PPV, the NPV, and acuuracy were 91.68%, 60.29%, and 86.07%, respectively.
Sensitivity and specificity of GP73 for diagnosis significant fibrosis
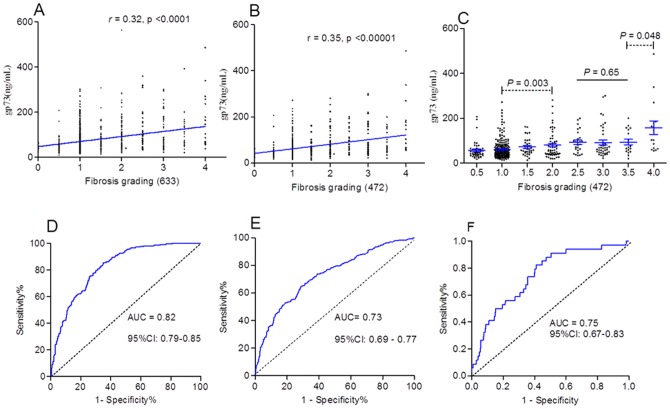
Serum GP73 concentration was significantly correlated with the grading of fibrosis (correlation coefficient r = 0.32, and 0.35, in 633 patients with chronic hepatitis B, and in which 472 patients with nearly normal ALT, respectively.) (Fig. 2.A, B). The mean GP73 concentration increased with liver grading aggravation, but significantly statistical differences only observed in several groups (Table 2; Fig. 2C).
Figure 2. Serum GP73 was correlated with grading of patients.
A: serum GP73 was correlated with grading of 633 patients. B and C: serum GP73 was correlated with grading of 472 patients with nearly normal ALT. D, E, F: ROC analysis of GP73 was performed on diagnosing S2(D), G2(E), and cirrhosis (F) respectively.
To characterize GP73 as a new diagnostic tissue marker of significant fibrosis (≥S2), or moderate/severe inflammation (≥G2), we conducted a ROC analysis. The results showed that GP73 had a striking performance for diagnosing S2 or G2 in patients with chronic HBV infections. In patients with nearly normal ALT, the sensitivity and specificity of GP73 for S2 diagnosis were 62.5% (95%CI: 56.26–68.45%) and 80.0% (75.9–83.68%) respectively, where the cut-off was set at 82 ng/ml (Fig. 2D); The PPV, NPV, and diagnosing accuracy were 87.76%, 67.98%, and 80.29%, respectively. For G2 diagnosis, the sensitivity and specificity were 53.2% (95% CI: 47.35–58.95%) and 80.21(75.89–84.05%) respectively, the cut-off was 85 ng/ml (Fig. 2, E). The PPV, NPV, and diagnosing accuracy were 73.11%, 54.78%, and 68.64%, respectively. If the cut-off was set at 138.4 ng/ml for cirrhosis (S 3.5–4) diagnosis, the sensitivity and specificity were 38.24% (22.17–56.44%) and 90.18% (87.00–92.80%) respectively (Fig. 2.F). The PPV, NPV, and diagnosing accuracy were 94.95%, 23.21%, and 86.44%, respectively.
Which factors related with serum GP73 levels?
Multivariate ordinal logistic regression analysis was performed to adjust other potential confounders (Sex, Age, ALT, total bilirubin, albumin, Platelet). The results showed that serum GP73 was an independent risk factor of liver fibrogenesis in CHB patients with nearly normal ALT. The relative risk was increased 1.106 with the serum GP73 increasing 10 ng/mL. The results were showed in table 3. To explore the causes of GP73 increasing in serum, we further performed a Pearson's correlation analysis on several biomarkers, which reflected virus replication, hepatocytes injury, and other liver functions. As Fig. 3A and 3B showed, that ALT, total bilirubin (Tbil) were positively correlated with serum GP73 concentration. The correlation coefficient r were 0.25, 0.18, respectively (Fig. 3, B, F). The interesting data was that ALT seemed not significantly correlated with GP73 concentration in 472 patients with nearly normal ALT (r = 0.014, p = 0.76), by contraries, the total of 633 patients was (r = 0.25, p = 0.0003) (Fig. 3B, Table 2). Similarly, although HBV DNA was not correlated with serum GP73 concentration (r = 0.01, p = 0.89), the serum GP73 concentration of patients with HBV DNA above 4log was significantly higher than those of patients with HBV DNA below 4 log (p = 0.007) (Fig. 3C and 3D; Table 2). The other interesting result was that patient's GP73 levels were negatively correlated with their ALB levels (Fig. 3E).
Table 3. The Multivariate ordinal logistic regression analysis for the factors assocaited with Fibrogenesis.
| Parameter | b | stb | Wald χ2 | P | OR | 95%CI for OR | |
| Lower | Upper | ||||||
| Fibrosis grading | |||||||
| 4 | 0.25 | 0.88 | 0.08 | 0.771 | - | - | - |
| 3.5 | 1.22 | 0.86 | 2.02 | 0.155 | - | - | - |
| 3 | 2.21 | 0.86 | 6.62 | 0.010 | - | - | - |
| 2.5 | 2.64 | 0.86 | 9.39 | 0.002 | - | - | - |
| 2 | 3.37 | 0.87 | 15.17 | <.0001 | - | - | - |
| 1.5 | 3.73 | 0.87 | 18.41 | <.0001 | - | - | - |
| 1 | 6.59 | 0.91 | 52.65 | <.0001 | - | - | - |
| 0.5 | 8.00 | 0.95 | 70.86 | <.0001 | - | - | - |
| GP73 (per 10 ng/mL) | 0.01 | 0.00 | 30.62 | <.0001 | 1.010 | 1.007 | 1.014 |
| ALB (per 10 g/L) | −0.08 | 0.02 | 18.03 | <.0001 | 0.927 | 0.895 | 0.960 |
| PLT (per 10 ×109/L) | −0.01 | 0.00 | 30.87 | <.0001 | 0.992 | 0.990 | 0.995 |
Note: Adjusted the factors including Sex, Age, ALT, total bilirubin, albumin, and platelet.
Figure 3. Serum GP73 concentration was related with levels of different biochemical marker.
A and B: serum GP73 concentration was correlated with ALT in patients with ALT ≥ 80 U/L, but nearly normal ALT was not. Although different HBV DNA levels had their different GP73 concentration (C), the correlation was not significant (D). Sample number may be one of most important causes. GP73 were also correlated with total bilirubin (F), especially, significantly correlated with serum ALB negatively (E).
To further validate GP73 expression in liver tissue with different fibrotic gradings, we observed character of GP73 staining in different biopsy sample. Immunohistochemical analysis showed that GP73 positive cells mainly scattered in parenchymal cells, but several non parenchymal cells also positive staining (Fig. 4). This result was consistent with Iftikhar R, et al. [12], report. Compared with mild fibrotic tissue, GP73 was strongly expressed in significant or severe fibrotic liver tissue.
Figure 4. GP73 were stained in different liver tissue.

GP73 was stained in brown. Arrow indicated positive cells. A: mild fibrosis (S1); B: significant fibrosis (S2); C: severe fibrosis (S3–4); D: cirrhosis (S4).
Serum GP73 may be a contributor to liver fibrosis
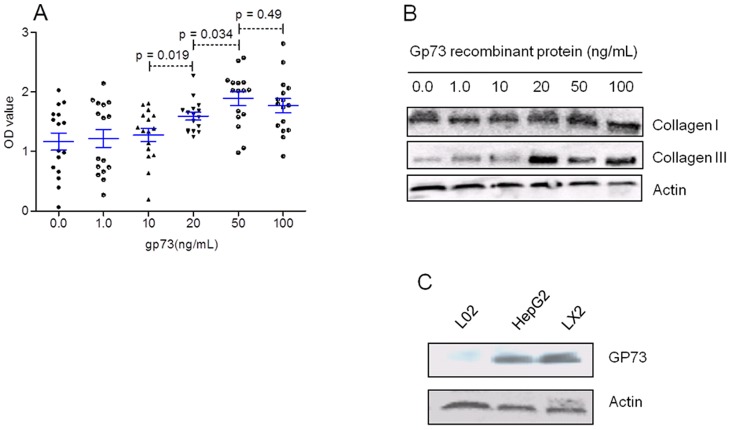
To investigate the effect of GP73 to hepatocytes or hepatic stellate cells, we used different concentration of GP73 recombinant protein (1.0, 10.0, 20.0, 50.0, and 100.0 ng/ml) coculturing with HepG2 cells, or LX2 cells. The result showed that GP73 may obviously prompt proliferation of LX2 cells (Table 4; Fig. 5A), but without any effect on HepG2 cells in vitro (data not show). With concentration of GP73 recombinant protein increasing (from 10 ng/mL to 80 ng/mL), the OD values of cultured LX2 cells also increased (Fig. 5A). The results suggested that GP73 recombinant protein may prompt LX2 cells proliferation in vitro. After cocultured 48 hours, the collagen III expression in LX2 cells was increased, but the collagen I was not (Fig. 5B). We speculated that GP73 might regulate hepatic stellated cells by autocrine, since LX2 also expressed GP73 in vitro (Fig. 5C).
Table 4. Effects of gp73 recombinant protein on LX2 cells.
| GP73 recombinant Protein (ng/ml) | N | OD value | |
| Mean ± SD | 95%CI | ||
| 0.0 | 16 | 1.17±0.58 | 0.86–1.48 |
| 1.0 | 16 | 1.22±0.61 | 0.90–1.54 |
| 10.0 | 16 | 1.27±0.44 | 1.04–1.51 |
| 20.0 | 16 | 1.59±0.27 | 1.45–1.73 |
| 50.0 | 16 | 1.89±0.46 | 1.64–2.13 |
| 100.0 | 16 | 1.77±0.48 | 1.52–2.03 |
Figure 5. Gp73 recombinant protein prompted LX2 cells proliferation.
A: when the concentration of GP73 recombinant protein was above 20 ng/ml, the LX2 proliferation was prompted. B: GP73 recombinant protein up-regulated collagen III expression, but collagen I was not. C: GP73 expression evaluated in different cells in vitro.
Discussion
The ultimate aim of fibrosis grading is provided clinicians with accurate information for treatment decision and prognosis judgment. Identifying significant fibrosis is also one of critical factors for treatment decision, especially for patients with mild abnormal ALT [18]. Avoided or reduced times of liver biopsy, but obtained pathological information from liver tissue, is always pursued by clinicians. Multi-marker combination can provide more accurate information about fibrosis [19], but result in increasing the patient's expenditure and clinician's working load. Based on our present data, GP73 might be a useful single marker for diagnosing significant fibrosis and cirrhosis in patients with chronic HBV infections.
The first question is why serum GP73 concentration correlated with liver stiffness? Based on recently reports, serum GP73 concentration related with progression of chronic liver diseases [13], [20]. Different with other HCC marker, increased serum GP73 is related to hepatic impairment and chronic fibrosis [21], [20]. In patients with Wilson disease, serum GP73 levels were associated with liver inflammation, fibrosis, and dysplasia, rather than copper overload [22]. More importantly, other experimental research showed that hepatic stellate cells are also expressed GP73 [23]. This result consistent with our data, and indicated that more hepatic stellate cells activation, more significant fibrosis, and resulting in serum GP73 more increasing.
Strict adherence to practice guidelines of chronic hepatitis B, will make a number of patients with nearly normal ALT lost opportunities of receiving antiviral therapy. In fact, recommended ALT thresholds may not absolutely reflect disease activity or degree of fibrosis [24]. More importantly, significant fibrosis (≥F2, or S2), or moderate hepatocytes injury (G2) are markers for beginning antiviral therapy in patients with chronic hepatitis B, based on present guideline [25]. Compared with other multi- parameter prediction models for grading fibrosis, GP73 is a single marker, which can be analysis with general enzyme-linked immunosorbent method. This new marker may be conveniently used in clinical practice, especially in developing countries for differentiating significant fibrosis with mild fibrosis in patients with chronic hepatitis B.
Liver stiffness is believed one of best non-invasive methods for evaluation liver fibrosis stage and disease progression. However, one question is what optimal cut-off value being chosen for fibrosis grading. Because numerous investigations provided different cut-off value for liver fibrosis classification, it was difficult to select optimal grading standard [26]. Based on recently reports, different research team presented different cut-off value for diagnosing significant fibrosis. Guha IN, et al [27], Stabinski L. et al [28], and Fung J, et al [29], presented 8.8 kPa, 9.3 kPa, 8.1 kPa respectively as optimal cut-off value for diagnosing significant fibrosis (≥F2). Since too higher cut-off value may be to lower the diagnostic sensitivity, we selected the relatively higher cut-off value, 8.8 kPa, for diagnosing significant fibrosis, in order to increase diagnostic specificity and accuracy. Difference of body constitution between east and west countries is other factor in our consideration, because liver stiffness variation in different populations [30]. Based on our present results, significant statistical differences only observed in several groups, although serum GP73 concentrations increasing with fibrosis progression. We speculated that these phenomena may be, at least in part, result in numbers of sample.
Based on data of stiffness measurement, setting 76.6 ng/ml as cut-off value may be appropriate for significant fibrosis diagnosis in chronic hepatitis B population. The impressive finding of this study was a obvious difference in GP73 concentration in patients with different fibrotic grading, especially in patients with nearly normal ALT (Table 2). According to results of liver biopsy, 80.21 ng/ml and 85 ng/ml, may effectively differentiate significant fibrosis (S2) or moderate injury (G2) from mild fibrosis or injury respectively. Integrating all abovementioned results, we proposed that 85 ng/ml may be an appropriate cut-off value for diagnosing significant fibrosis of moderate/severe hepatocytes injury from patients with chronic HBV infections. If the cut-off value was set at 135 ng/ml, GP73 was also a potent marker for diagnosing liver cirrhosis.
Although GP73 (tr/tr) mice (with a severe truncation of the GP73 C-terminus) developed marked abnormity in liver, the role of GP73 in liver disease is still unknown [31]. The other interesting result is that GP73 may be not only a fibrosis marker, but also a contributor to fibrogenesis in patients with chronic HBV infections. Since unexplained high GP73 serum concentration was observed in patients with chronic HBV infection, this suggested that soluble GP73 may be playing a role in disease progression. This histological information indicated that non parenchymal cells may be another source of serum GP73. The present interpretation to serum GP73 levels is that HBV replication might increase GP73 secretion, and inflammation might result in GP73 releasing from hepatocytes. The molecular mechanism of GP73 mediating hepatic stellate cells proliferation needed to further elucidated. The main defects of our study is that patients received liver biopsy did not perform liver stiffness measurement, or vice versa, since most patients was willing to undertake FinroScan test, rather than liver biopsy. In fact, only thirteen patients received liver biopsy and liver stiffness measurements. We did not perform analysis to those patients separately.
In summary, GP73 may be a useful marker for liver fibrosis grading, especially for diagnosing significant fibrosis and cirrhosis in patients with chronic HBV infections.
Acknowledgments
We thank Dr. Gang Wan f or some statistical help.
Funding Statement
Supported by grant from the National Natural Science Foundation of China (No.30872243; No.81071411) to Prof. HW; and grant from the Fund of Capital Clinical Investigation (No.111107058811068) to Prof. BL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, et al. (2006) CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12: 671–696. [DOI] [PubMed] [Google Scholar]
- 2. Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM (2008) Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology 48: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 3. Hoefs JC, Shiffman ML, Goodman ZD, Kleiner DE, Dienstag JL, et al. (2011) Rate of progression of hepatic fibrosis in patients with chronic hepatitis C: results from the HALT-C Trial. Gastroenterology 141: 900–908.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, et al. (2004) Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med 10: 429–434. [DOI] [PubMed] [Google Scholar]
- 5. Munteanu M, Imbert-Bismut F, Messous D, Morra R, Thabut D, et al. (2008) Reproducibility of non-invasive fibrosis biomarkers, FibroMeter and FibroTest, could be improved by respecting the analytical standardizations. Clin Biochem 41: 1113–1114. [DOI] [PubMed] [Google Scholar]
- 6. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, et al. (2001) Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 7. Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, et al. (2008) Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 48: 1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, et al. (2009) Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology 49: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 10. Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, et al. (2000) GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 249: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Wan X, Li Z, Lin C, Zhan Y, et al. (2011) Golgi protein 73(GP73), a useful serum marker in liver diseases. Clin Chem Lab Med 49: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 12. Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Gräff A, Gusmirovic I, et al. (2004) Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol 99: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 13. Sun Y, Yang H, Mao Y, Xu H, Zhang J, et al. (2011) Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol 26: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 14. Castera L, Forns X, Alberti A (2008) Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 48: 835–847. [DOI] [PubMed] [Google Scholar]
- 15. Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, et al. (2012) Noninvasive elastography-based assessment of liver fibrosis progressionand prognosis in primary biliary cirrhosis. Hepatology 56(1): 198–208. [DOI] [PubMed] [Google Scholar]
- 16. Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24: 289–293. [DOI] [PubMed] [Google Scholar]
- 17. Cao Q, Mak KM, Lieber CS (2007) Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol 46(1): 124–33. [DOI] [PubMed] [Google Scholar]
- 18. Alberti A, Caporaso N (2011) HBV therapy: guidelines and open issues. Dig Liver Dis 43 Suppl 1S57–63. [DOI] [PubMed] [Google Scholar]
- 19. Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, et al. (2011) Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepato 26 (10): 1536–1543. [DOI] [PubMed] [Google Scholar]
- 20. Tian L, Wang Y, Xu D, Gui J, Jia X, et al. (2011) Serological AFP/golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer 129(8): 1923–1931. [DOI] [PubMed] [Google Scholar]
- 21. Gu Y, Chen W, Zhao Y, Chen L, Peng T (2009) Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Ann Clin Biochem 46: 38–43. [DOI] [PubMed] [Google Scholar]
- 22. Wright LM, Huster D, Lutsenko S, Wrba F, Ferenci P, et al. (2009) Hepatocyte GP73 expression in Wilson disease. J Hepatol 51: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maitra A, Thuluvath PJ (2004) GP73 and liver disease: a (Golgi) complex enigma. Am J Gastroenterol 99(6): 1096–1098. [DOI] [PubMed] [Google Scholar]
- 24. Zoulim F, Perrillo R (2008) Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol 48 Suppl 1S2–19. [DOI] [PubMed] [Google Scholar]
- 25. Sebagh M, Samuel D, Antonini TM, Coilly A, Degli Esposti D, et al. (2012) Twenty-year protocol liver biopsies: Invasive but useful for the management of liver recipients. J Hepatol 56: 840–847. [DOI] [PubMed] [Google Scholar]
- 26. Poynard T, Ngo Y, Perazzo H, Munteanu M, Lebray P, et al. (2011) Prognostic value of liver fibrosis biomarkers: a meta-analysis. Gastroenterol Hepatol (N Y) 7: 445–454. [PMC free article] [PubMed] [Google Scholar]
- 27. Guha IN, Myers RP, Patel K, Talwalkar JA (2011) Biomarkers of liver fibrosis: what lies beneath the receiver operating characteristic curve? Hepatolog 54: 1454–1462. [DOI] [PubMed] [Google Scholar]
- 28. Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, et al. (2011) High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir The 16: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fung J, Lai CL, But D, Wong D, Cheung TK, et al. (2008) Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol 103: 1421–1426. [DOI] [PubMed] [Google Scholar]
- 30. Wong GL, Wong VW, Chim AM, Yiu KK, Chu SH, et al. (2011) Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol 26: 300–305. [DOI] [PubMed] [Google Scholar]
- 31. Wright LM, Yong S, Picken MM, Rockey D, Fimmel CJ (2009) Decreased survival and hepato-renal pathology in mice with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol 2: 34–47. [PMC free article] [PubMed] [Google Scholar]