Abstract
Multiple genes involved in endocytosis and endosomal protein trafficking in Drosophila have been shown to function as neoplastic tumor suppressor genes (nTSGs), including Endosomal Sorting Complex Required for Transport-II (ESCRT-II) components vacuolar protein sorting 22 (vps22), vps25, and vps36. However, most studies of endocytic nTSGs have been done in mosaic tissues containing both mutant and non-mutant populations of cells, and interactions among mutant and non-mutant cells greatly influence the final phenotype. Thus, the true autonomous phenotype of tissues mutant for endocytic nTSGs remains unclear. Here, we show that tissues predominantly mutant for ESCRT-II components display characteristics of neoplastic transformation and then undergo apoptosis. These neoplastic tissues show upregulation of c-Jun N-terminal Kinase (JNK), Notch, and Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) signaling. Significantly, while inhibition of JNK signaling in mutant tissues partially inhibits proliferation, inhibition of JAK/STAT signaling rescues other aspects of the neoplastic phenotype. This is the first rigorous study of tissues predominantly mutant for endocytic nTSGs and provides clear evidence for cooperation among de-regulated signaling pathways leading to tumorigenesis.
Introduction
Tumor development involves destabilization of the well-controlled processes of cell proliferation, cell polarization, and programmed cell death that are tightly regulated by widely conserved signaling pathways. Therefore, genes that act as regulators of these signaling pathways may behave as nTSGs. In Drosophila, as well as in other organisms, genes that control endocytosis and endosomal protein sorting behave as nTSGs. Such endocytic nTSGs include avalanche (avl) [1], Rab5 [1], vps45 [2], Rabenosyn (Rbsn) [2], tumor suppressor protein 101 (tsg101 aka erupted (ept) or vps23) [3], vps28 [4], vps25 [5]–[7], vps22 (aka larsen (lsn)) [8], vps20 [4], shrub (shrb) [4], vps2 [4], and vps4 [9]. These endocytic nTSGs are involved in endocytosis and endosomal protein sorting of cell signaling receptors and other membrane proteins and inhibit tumor formation by ensuring proper trafficking and collection of cargoes that function in growth control, cell survival, and apical-basal polarity in epithelial tissues.
The ESCRT machinery promotes the maturation of early endosomes into multi-vesicular bodies (MVBs) [10]–[12]. This is a complex process that involves four ESCRT complexes, ESCRT-0, -I, -II and -III. Of interest to this study are the ESCRT-II components vps22, vps25, and vps36. The products of these genes mediate the transfer of cargo from ESCRT-I to ESCRT-III [10]–[12]. Loss-of-function mutations of these genes block this process, which causes abnormal signaling and triggers a complex phenotype composed of autonomous and non-cell autonomous effects [5]–[8].
Previous studies of the mutant phenotypes of ESCRT-II components and other endocytic nTSGs focused on their mosaic phenotype, when mutant clones are surrounded by wild-type cells. Thus, the complex mosaic phenotype of endocytic nTSGs has been well characterized. Epithelial polarity and proliferation control are disrupted in mutant clones [1], [3], [7], [9]. Mutant clones in eye-antennal imaginal discs fail to express the neuronal marker ELAV, indicating that they fail to differentiate [6], [7]. A clear non-cell autonomous effect of mutant clones on proliferation is observed in tissues mosaic for tsg101, vps22, or vps25 [3], [5], [7], [8]. The non-mutant tissues surrounding the mutant clones display increased proliferation [3], [5], [7]. Such tissues form multilayered discs and overgrown adult structures [1], [3], [5]–[7]. vps25 mutant clones also promote non-cell autonomous cell survival through upregulation of the apoptosis inhibitor Diap1 [5], [8], [13].
In mutant clones of endocytic nTSGs, endosomal trafficking is blocked and membrane proteins accumulate in abnormal endosomal compartments [1], [3], [5]–[9], [14]. For example, Notch protein accumulates in abnormally enlarged early endosomes where it undergoes ligand-independent processing and activation [1]–[8]. Active Notch induces non-cell autonomous proliferation in vps22, vps25, and tsg101 mosaic tissues through non-cell autonomous upregulation of JAK/STAT and Yorkie signaling [3], [5], [7], [8], [13].
In mosaic tissues, mutant clones of tsg101 and vps25 are apoptotic [3], [5]–[7]. Apoptosis in these clones is induced by JNK signaling and the canonical apoptotic pathway (Hid/Diap1/Dronc/Ark) [5], [9], [15]. It is commonly believed that JNK signaling and thus apoptosis is induced by cell competition from neighboring non-mutant tissue [6], [16]. Inhibition of apoptosis in vps25 mutant clones unleashes a strong neoplastic phenotype characterized by massive tumorous overgrowth, loss of cell polarity, and invasive properties [5], [6]. Thus, apoptosis serves as a tumor suppressor mechanism. A strong neoplastic phenotype is also observed when the entire tissue is mutant for nTSGs, thus when competitive interactions between mutant and non-mutant tissues are eliminated [8], [17].
From these studies, it is clear that the interactions between the mutant and non-mutant populations of cells greatly influence the final phenotype. However, while the non-cell autonomous mechanisms that cause hyperplastic overgrowth are well characterized, the mechanisms that cause autonomous neoplastic transformation of tissue mutant for endocytic nTSGs are poorly understood. Because endocytic trafficking controls multiple signaling pathways, it is likely that tumors caused by mutations in endocytic nTSGs acquire their neoplastic characteristics through the de-regulation of numerous signaling pathways. In hypomorphic tsg101 and vps25 mutant clones, Yorkie signaling is up-regulated [5], [13], [15]. However, in strong (null) vps25 mosaic discs, Yorkie signaling is only detectable non-cell autonomously in non-mutant neighboring cells [13], suggesting that Yorkie signaling does not significantly contribute to the neoplastic phenotype of these mutant clones.
In endocytic nTSG mutant tissues, the protein levels of the JAK/STAT ligand Unpaired (Upd), the JAK/STAT receptor Domeless (Dome), and the Drosophila STAT, Stat92E, are increased, leading to increased JAK/STAT signaling activity [3], [6], [7], [18]. However, the role of JAK/STAT signaling for the autonomous neoplastic phenotype of nTSG mutant tissue is less clear. Early evidence has indicated that JAK/STAT signaling may be involved in this neoplastic transformation; however, that experiment was done in a heterozygous Stat92E condition throughout the disc that affects both autonomous and non-cell autonomous phenotypes [18]. A rigorous assessment of the neoplastic phenotype in predominantly nTSG mutant tissue in which JAK/STAT signaling is disrupted has not been performed yet.
Here, in order to understand the cause of the neoplastic transformation of these mutant clones, we employed the ey-FLP/cell lethal (cl) system [19] to generate predominantly mutant tissues of the ESCRT-II components vps22, vps25 and vps36. These overgrown, neoplastic tumors display disorganized cellular architecture and disrupted epithelial structures with expanded apical-basal domains. Additionally, these tissues are unable to terminally differentiate and are invasive. Unexpectedly, although competitive cellular interactions have been largely eliminated by the ey-FLP/cl method, these predominantly mutant tissues are also very apoptotic. Within mutant tissues, JNK, Notch, and JAK/STAT signaling are up-regulated. Reducing JNK activity in ESCRT-II mutant tissue partially blocks the overproliferation phenotype and apoptosis but does not otherwise affect neoplastic transformation. In addition, complete loss of JAK/STAT signaling strongly rescues the neoplastic phenotype. Thus, this study supports the idea that de-regulation of signaling pathways, especially JNK and JAK/STAT signaling, in vps22, vps25, and vps36 mutant tissues leads to neoplasia.
Materials and Methods
Drosophila Genetics and Generation of Predominantly Mutant Imaginal Discs
The following mutants and transgenic lines were used: vps225F3-8 [20], vps25N55 [5], vps36Δ69(this study), arkH16 [21], Stat92E397 [22], puc-lacZ [23], Gbe-Su(H)-lacZ [24], E(spl)m8 2.61-lacZ [25], 10X-STAT-GFP [26], UAS-bskDN [27], and ey-Gal4 [28]. vps36Δ69 is a null allele generated by imprecise excision of the P-element transposon inserted in the first exon 29 base pairs upstream of the initiator ATG in the vps36L5212 allele.
To generate imaginal discs predominantly mutant for vps22, vps25, or vps36, we used the ey-FLP/cl technique [19], [28]. cl indicates an anonymous cell lethal mutation that kills cells when homozygous [19], [28]. The ESCRT-II mutant alleles were crossed to ey-FLP; FRT cl flies. The use of the FRT depended on the location of the ESCRT-II gene in the genome. The complete genotypes are indicated in the legends to the figures.
Immunohistochemistry
Imaginal discs were dissected from third instar larvae and stained using standard protocols. The following antibodies were used: mouse α-Dlg (1∶20), rat α-ELAV (1∶40), mouse α-Mmp1 (catalytic domain; 1∶50), and mouse α-Notchintra (1∶20; DSHB, University of Iowa); mouse α-BrdU (1∶50; Becton Dickinson); rabbit α-cleaved Caspase-3 (Cas-3*; 1∶500; Cell Signaling Technology); mouse α-β-gal (1∶1,000) and rabbit α-pJNK (1∶100; Promega); and rabbit α-aPKC (1∶100; Santa Cruz Biotechnology). AF488-phalloidin and AF546-phalloidin were obtained from Sigma Aldrich. Cy-3 and Cy-5 fluorescently-conjugated secondary antibodies were obtained from Jackson ImmunoResearch. Vectashield with DAPI was obtained from Vector Laboratories. TUNEL kit was obtained from Roche Diagnostics. Images were captured using Olympus Optical FV500 or FV1000 confocal microscopes and processed using Adobe Photoshop CS4.
Results
ESCRT-II Mutant Tissues Show Neoplastic Characteristics
The ey-FLP/cl method generates eye-antennal imaginal discs that are almost entirely composed of mutant tissue in otherwise heterozygous animals (see Methods) [19], [28]. This is accomplished by elimination of the twin-spots after ey-FLP-induced mitotic recombination by a cell lethal (cl) mutation that is present on the homologous chromosome arm. The use of the ey-FLP ensures high FLP activity such that most cells undergo mitotic recombination and only a few heterozygous cells remain. Thus, eye-antennal discs generated by this method are almost entirely mutant for the gene of interest.
We used the ey-FLP/cl system to generate tissues predominantly mutant for ESCRT-II components vps22, vps25, or vps36. These predominantly mutant epithelial tissues have a very striking phenotype: unlike wild-type single-layered eye-antennal imaginal discs, they overgrow into multi-layered, dense “balls” of cells (Figure 1) [7], [8], [17]. These discs also vary considerably in size. Some are about the size of wild-type discs or even slightly smaller while others can be three to five times as large [8], [17]. This was also reported for other endocytic nTSGs [2], [17]. To understand this tumor-like phenotype in more detail, we examined proliferation, cellular architecture, differentiation, and metastatic potential of eye-antennal discs predominantly mutant for vps22, vps25, or vps36.
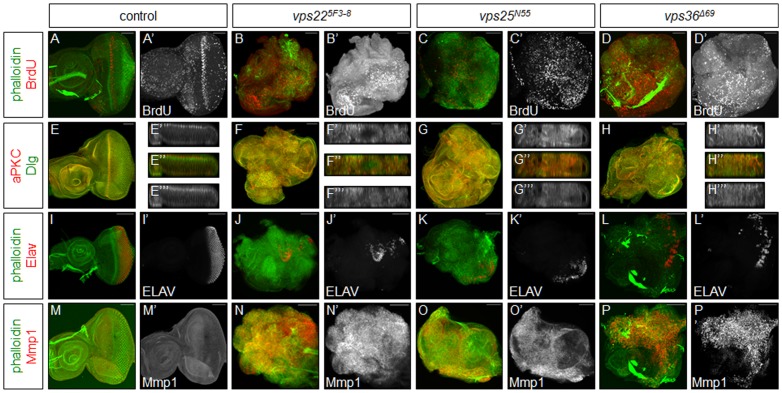
Figure 1. Tissues predominantly mutant for ESCRT-II components vps22, vps25, or vps36 show neoplastic characteristics.
Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A–D) BrdU (red and grayscale) labelings show that proliferation is increased in discs predominantly mutant for vps22 (B,B’), vps25 (C,C’), or vps36 (D,D’), as compared to proliferation in control discs (A,A’). (E–H) aPKC (red and grayscale (E’,F’,G’,H’)) and Dlg (green and grayscale (E’’’,F’’’,G’’’,H’’’)) labelings of discs predominantly mutant for vps22 (F–F’’’), vps25 (G–G’’’), or vps36 (H–H’’’) show that cellular architecture is disrupted, as compared to the architecture of control discs (E–E’’’). (I–L) ELAV (red and grayscale) labelings of discs predominantly mutant for vps22 (J,J’), vps25 (K,K’), or vps36 (L,L’) show that very few cells in the mutant discs differentiate normally, as compared to differentiation in control discs (I,I’). (M–P) Mmp1 (red and grayscale) labelings of discs predominantly mutant for vps22 (N,N’), vps25 (O,O’), or vps36 (P,P’) show that levels of this protein are elevated, as compared to Mmp1 levels in control discs (M,M’). Genotypes: (A) eyFLP;; FRT82B/FRT82B cl. (E,I,M) eyFLP; FRT42D y+/FRT42D cl. (B,F,J,N) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (C,G,K,O) eyFLP; FRT42D vps25N55 y+/FRT42D cl. (D,H,L,P) eyFLP;; vps36Δ69 FRT80B/cl FRT80B.
To assay proliferation in the predominantly mutant tissues, we used Bromodeoxyuridine (BrdU) labeling to mark cells in S-phase. Control discs show the normal BrdU pattern in eye-antennal discs (Figure 1A). Of note is the posterior part of the eye disc in which cells are post-mitotic and differentiate into photoreceptor neurons, cone cells, and other cell types. In discs predominantly mutant for ESCRT-II components, BrdU labeling indicates that proliferation is occurring at elevated levels throughout the entire disc (Figure 1B–D). Post-mitotic areas are not visible or are very small. Thus, proliferation is up-regulated in tissues predominantly mutant for vps22, vps25, or vps36.
To examine cellular architecture of tissues predominantly mutant for ESCRT-II components, we first labeled discs with phalloidin. Phalloidin recognizes cortical actin and thus highlights cellular architecture and organization throughout tissues. Control discs stained with phalloidin show a consistent shape characteristic of Drosophila eye-antennal imaginal discs (Figure 1A,I,M). Discs predominantly mutant for ESCRT-II components trade this characteristic shape for a disorganized, expanded, amorphic structure in which the antennal and eye portions of the disc cannot be distinguished (Figure 1B–D,J–L,N–P). Next, to specifically examine epithelial polarity, we labeled predominantly mutant tissues with antibodies recognizing atypical Protein Kinase C (aPKC) and Discs large (Dlg). In control tissue, aPKC localizes to the polarized apical membrane domain while Dlg is found at the basolateral membrane domain (Figure 1E). In the predominantly mutant tissues, aPKC and Dlg are spread outside of their respective regions of wild-type localization (Figure 1F–H), indicating that apical-basal polarity is disrupted. Together, these data indicate that cellular architecture is disrupted in vps22, vps25, and vps36 mutant tissues, which is consistent with previous reports [7], [8], [17].
It has been shown previously that clones of vps25 mutant cells in mosaics fail to differentiate [6], [7]. Therefore, we were curious to examine the ability of cells to differentiate if almost the entire eye-antennal disc is mutant. Photoreceptor neurons are the first cells that differentiate during eye development. Using ELAV as a neuronal marker, we labeled eye-antennal discs almost entirely mutant for ESCRT-II components to assess differentiation. In the control eye-antennal imaginal disc, cells in the posterior of the disc differentiate into neurons and thus show high expression of ELAV (Figure 1I). In contrast, very few cells in the ESCRT-II predominantly mutant tissues show ELAV expression (Figure 1J–L). The cells that are positive for ELAV are not localized to a specific region of the disc but rather are scattered throughout the tissue. Thus, similar to mutant cells in a mosaic background, cells in predominantly mutant eye-antennal imaginal discs fail to differentiate. The few cells that do differentiate likely correspond to the few heterozygous cells that are present in the disc.
Loss of epithelial integrity and apical-basal polarity, increased proliferation, and loss of differentiation are hallmarks of neoplastic transformation. It has also been demonstrated that vps25 mutant cells have invasive behavior [6]. Matrix metalloprotease 1 (Mmp1) remodels the extracellular matrix and is known to be elevated in and required for metastasis of Drosophila tumors [29]. Therefore, to correlate the metastatic potential of the predominantly mutant vps22, vps25, and vps36 discs with Mmp1 expression, we labeled these discs with an antibody recognizing Mmp1. In control eye-antennal imaginal discs, Mmp1 is present at very low levels (Figure 1M). In contrast, in the predominantly mutant discs, Mmp1 is present at high levels throughout the discs (Figure 1N–P). Taken together, these data demonstrate that ESCRT-II components vps22, vps25, and vps36 are strong nTSGs and that eye-antennal imaginal discs predominantly mutant for these genes display neoplastic characteristics.
Notch, JAK/STAT, and JNK Signaling are Up-regulated in ESCRT-II Mutant Tissues
Due to the endosomal sorting defect in ESCRT-II mutant tissues, multiple signaling pathways are de-regulated [4]–[8], [14], [15]. In discs mosaic for ESCRT-II mutants, it is well understood how de-regulation of signaling contributes to the non-cell autonomous proliferation and survival phenotypes (see Introduction). However, these studies in mosaic tissues fail to answer two important questions: (1) What signaling pathways are de-regulated in predominantly mutant tissues completely independent from interactions with non-mutant populations of cells? (2) Does this autonomous de-regulation of signaling contribute to the autonomous neoplastic phenotype? To answer the first question, we examined levels of Notch, JAK/STAT, and JNK signaling in discs predominantly mutant for ESCRT-II components.
Multiple studies have shown that Notch signaling is up-regulated in tissues mosaic for ESCRT components [3]–[5], [7], [8], [14]. Thus, we were interested to examine levels of the Notch signaling pathway in tissues predominantly mutant for ESCRT-II components. To assess Notch signaling, we used two Notch reporters, the Gbe-Su(H)-lacZ reporter [24] and the E(spl)m8 2.61-lacZ reporter [25]. In control discs, Notch signaling is high in a very stereotypical pattern in the posterior of the eye disc and in the antennal disc (Figure 2A,B). Use of the Gbe-Su(H)-lacZ reporter in vps25 mutant discs showed that Notch signaling is very high throughout the entire disc (Figure 2D). We used the E(spl)-lacZ reporter to examine Notch activity in vps22 and vps36 mutant tissues and found that Notch signaling is indeed very high but only in about half of each mutant disc (Figure 2C,E). To further examine Notch signaling within mutant discs, we assayed levels of the Notch protein using an antibody that recognizes the intracellular portion of the receptor. We found that protein levels are indeed very high throughout mutant discs (Figure S1B-D), supporting the results found with the Gbe-Su(H)-lacZ reporter. From these data, we clearly see that Notch signaling is up-regulated in tissues predominantly mutant for ESCRT-II components.
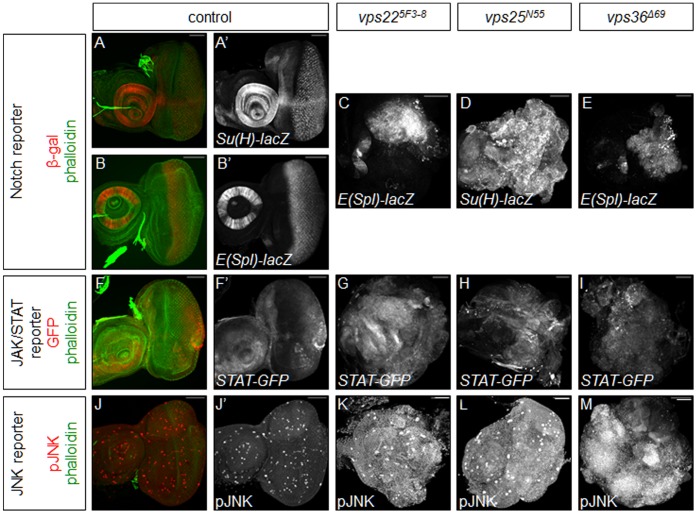
Figure 2. Notch, JAK/STAT, and JNK signaling are upregulated in vps22, vps25, and vps36 mutant tissues.
Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) is used to mark the overall shape of the tissue. Gbe-Su(H)-lacZ, and E(spl)m8 2.61-lacZ are detected by β-gal labeling (red or grayscale). Scale bars represent 50 µm. (A,E) Imaginal discs predominantly mutant for vps25 induce high levels of Gbe-Su(H)-lacZ (E), as compared to control discs (A,A’). (B,C,E) Imaginal discs predominantly mutant for vps22 (C) or vps36 (E) induce high levels of E(spl)m8 2.61-lacZ, as compared to control discs (B,B’). (F–I) Imaginal discs predominantly mutant for vps22 (G), vps25 (H), or vps36 (I) induce high levels of 10X-STAT-GFP, as compared to control discs (F,F’). (J–M) Imaginal discs predominantly mutant for vps22 (K), vps25 (L), or vps36 (M) show high levels of phosphorylated JNK protein, as compared to control discs (J,J’). Genotypes: (A) eyFLP; FRT42D y+/FRT42D cl; Gbe-Su(H)-lacZ/+. (B) eyFLP; E(Spl)m8 2.61-lacZ/+; FRT82B/FRT82B cl. (C) eyFLP; E(spl)m8 2.61-lacZ/+; FRT82B vps225F3-8/FRT82B cl. (D) eyFLP; FRT42D vps25N55 y+/FRT42D cl; Gbe-Su(H)-lacZ/+. (E) eyFLP; E(spl)m8 2.61-lacZ/+; vps36Δ69 FRT80B/cl FRT80B. (F) eyFLP; FRT42D y+/FRT42D cl; 10X-STAT-GFP/+. (G) eyFLP; 10X-STAT-GFP/+; FRT82B vps225F3-8/FRT82B cl. (H) eyFLP; FRT42D vps25N55 y+/FRT42D cl; 10X-STAT-GFP/+. (I) eyFLP; 10X-STAT-GFP/+; vps36Δ69 FRT80B/cl FRT80B. (J) eyFLP; FRT42D y+/FRT42D cl. (K) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (L) eyFLP; FRT42D vps25N55 y+/FRT42D cl. (M) eyFLP;; vps36Δ69 FRT80B/cl FRT80B.
In genetic mosaics, increased JAK/STAT signaling has been observed in tsg101 and vps25 mutant clones, and Notch-induced upregulation of the JAK/STAT ligand Upd has been shown to contribute to the non-cell autonomous increase of proliferation in neighboring non-mutant cells [3], [5], [7], [8], [18]. Thus, we were interested to see if JAK/STAT signaling is affected autonomously in predominantly ESCRT-II mutant tissues. To assess levels of JAK/STAT signaling, we used the well-characterized 10X-STAT-GFP reporter [26]. In control discs, JAK/STAT signaling is only active in the posterior portion of the eye disc and in the antennal disc (Figure 2F). In contrast, JAK/STAT signaling is clearly very elevated throughout ESCRT-II mutant discs (Figure 2G–I).
One additional pathway that is autonomously induced in mutant clones of endocytic nTSG mosaics is JNK signaling [5], [9], [15], [16], [30]. It is assumed that JNK signaling is induced by cell competition between mutant (loser) and non-mutant (winner) cells in the mosaics. In discs predominantly mutant for ESCRT-II genes, the competitive interaction between mutant and non-mutant tissue is removed because most of the non-mutant tissue is eliminated and only mutant tissue remains. We were therefore surprised to see strong labeling with the pJNK antibody, which detects phosphorylated and thus activated JNK, in discs predominantly mutant for ESCRT-II components compared to controls (Figure 2J–M). We also observed a strong induction of puc-lacZ, a JNK-reporter transgene, in discs predominantly mutant for vps25 (Figure S2B). Therefore, JNK activity is induced in ESCRT-II mutant discs independently of cell competition. Taken together, these data show that the Notch, JAK/STAT, and JNK signaling pathways are up-regulated in predominantly ESCRT-II mutant tissues and support a possible role for these conserved signaling pathways in the neoplastic phenotype observed in these tissues.
Tissues Predominantly Mutant for ESCRT-II Components are Apoptotic
JNK signaling in nTSG mutant clones in mosaic discs triggers apoptosis [5], [16], [30], [31]. Thus, although competitive interactions are largely abolished in predominantly ESCRT-II mutant discs, which are often overgrown, we examined these discs for apoptosis. We assayed cell death by cleaved Caspase-3 (Cas-3*) and TUNEL labeling in predominantly mutant discs. In control discs, a few Cas-3* positive cells are scattered throughout the tissue, but most cells are not apoptotic (Figure 3A). However, surprisingly, discs predominantly mutant for ESCRT-II genes show high levels of Cas-3* throughout (Figure 3B–D). Similar results were obtained with TUNEL labeling (Figure 3E–H), which detects DNA fragmentation, a hallmark of apoptosis [32], indicating that apoptosis is indeed occurring. Taken together, although competitive interactions between mutant and non-mutant cells are eliminated in discs predominantly mutant for ESCRT-II components, they display high levels of apoptosis.
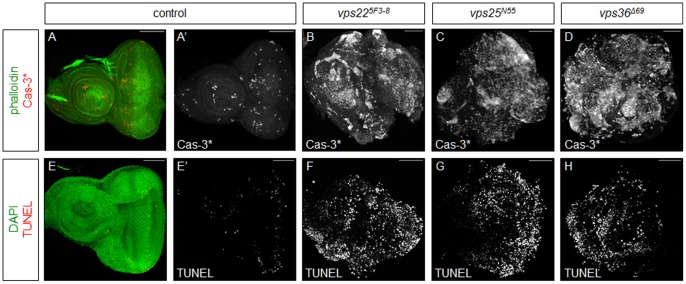
Figure 3. Imaginal discs predominantly mutant for vps22, vps25, or vps36 are apoptotic.
Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) or DAPI (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A–D) Cleaved Caspase-3 (Cas-3*; red and grayscale) labelings show that apoptosis is increased in discs predominantly mutant for vps22 (B), vps25 (C), or vps36 (D), as compared to apoptosis in control discs (A,A’). (E–H) TUNEL (red and grayscale) labelings show that apoptosis is increased in discs predominantly mutant for vps22 (F), vps25 (G), or vps36 (H), as compared to apoptosis in control discs (E,E’). Genotypes: (A) eyFLP; FRT42D y+/FRT42D cl. (B,F) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (C,G) eyFLP; FRT42D vps25N55/FRT42D cl. (D,H) eyFLP;; vps36Δ69 FRT80B/cl FRT80B. (E) eyFLP;; FRT82B/FRT82B cl.
So far, we have analyzed the phenotypes of eye-antennal imaginal discs of ESCRT-II mutants of third instar larvae. We also observed that animals with eye-antennal imaginal discs predominantly mutant for ESCRT-II components die as pharate pupae. Based on our data from imaginal discs, we hypothesized that the apoptosis of the discs may contribute to the death of the pharate pupae. Dissection and examination of the pharate pupae demonstrated that they lack head structures (Figure 4A,B). Thus, it is likely that the apoptosis of the mutant tissues is leading to the death of the animal.
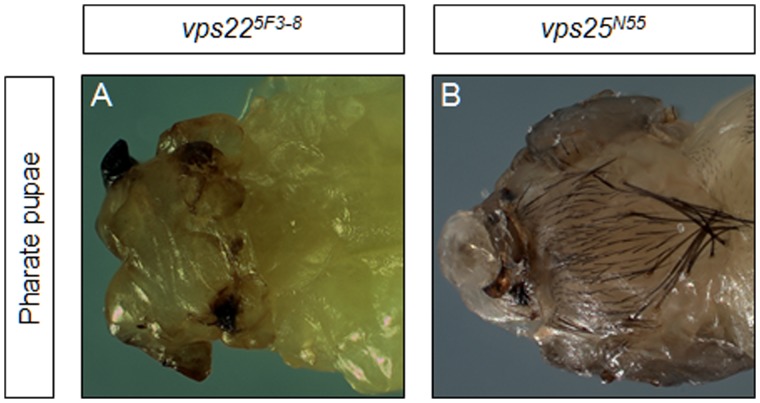
Figure 4. Animals with imaginal discs predominantly mutant for ESCRT-II components die as headless pharate pupae.
Animals with predominantly mutant tissues are generated with the eyFLP-cl system. (A,B) Animals with eye-antennal imaginal discs predominantly mutant for vps22 (A) or vps25 (B) die as pharate pupae that lack heads. Genotypes: (A) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (B) eyFLP; FRT42D vps25N55 y+/FRT42D cl.
Inhibition of JNK Affects the Neoplastic Transformation of ESCRT-II Mutant Tissues
We were curious to examine the role of apoptosis and JNK signaling in these discs. JNK is particularly interesting in this respect because under certain conditions it not only induces apoptosis, but also non-cell autonomous proliferation [33]–[35]. Therefore, we blocked apoptosis and JNK signaling in these mutant tissues and examined the contribution of these pathways to the neoplastic phenotype of imaginal discs predominantly mutant for ESCRT-II components.
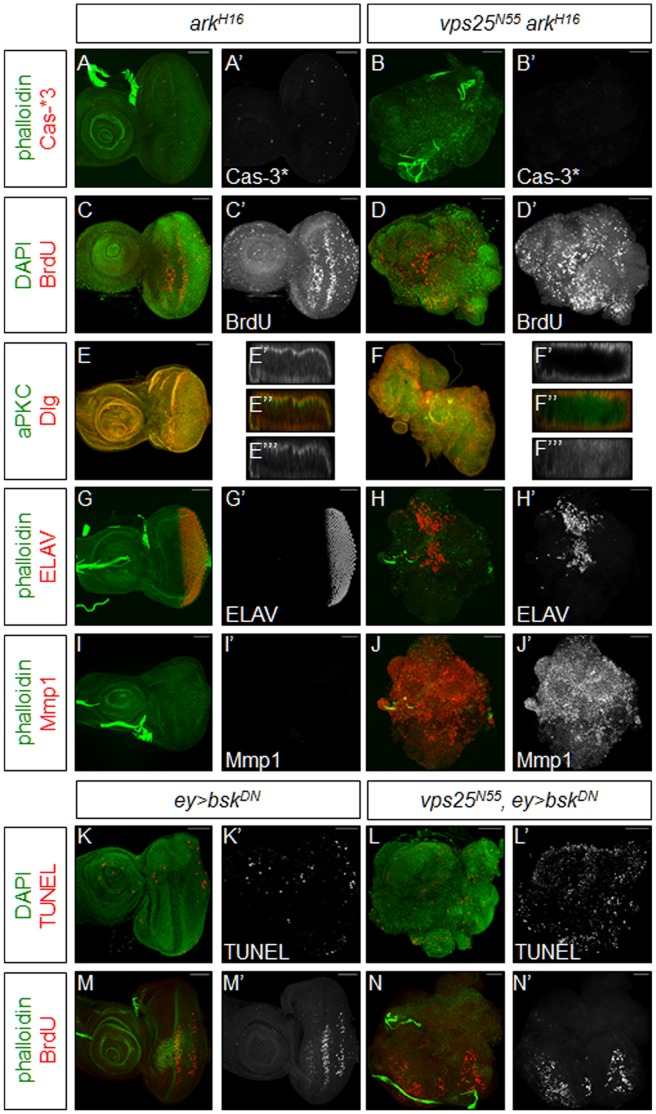
We first blocked apoptosis in mutant discs by generating discs that are predominantly double mutant for vps25 and ark, the Apaf-1 related killer in flies that is an essential component of the cell death pathway [21], [36]–[40]. In vps25 ark double mutant discs, cell death is completely inhibited, as shown by Cas-3* labeling (Figure 5B). In these double mutant discs, the neoplastic phenotype is even more severe. In some animals, the two eye-antennal imaginal discs fuse together into one large epithelial mass (Figure 5F); in a few instances, the two brain lobes and two discs fuse together into a large mass. These tissue fusions were not observed in vps25 single mutant discs and may indicate even more invasive behavior of apoptosis-inhibited vps25 mutant tissue. High levels of proliferation, as indicated by BrdU incorporation, are consistent throughout the entire predominantly mutant tissues (Figure 5D). Cellular architecture is completely disrupted, as shown by the drastic spreading of aPKC and Dlg localization (Figure 5F). A few cells differentiate normally and thus are positive for ELAV, but most cells fail to differentiate (Figure 5H). Finally, there are high levels of Mmp1 throughout the mutant tissue, indicating that the tissue has the potential to be invasive (Figure 5J). Importantly, eye-antennal imaginal discs predominantly mutant for ark alone do not show any neoplastic characteristics (Figure 5C,E,G,I). Therefore, it is clear that cell death is not required for neoplastic transformation in tissues predominantly mutant for ESCRT-II components. In contrast, since the phenotype of vps25 ark double mutant discs is more severe than that of vps25 single mutant discs (Figure 1), apoptosis in these mutant discs serves as a tumor suppressor mechanism to eliminate the cancerous tissue.
Figure 5. Inhibition of apoptosis affects the neoplastic transformation of ESCRT-II mutant tissue.
Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) or DAPI (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A–J) Apoptosis is inhibited by removal of ark. (A,B) Cleaved Caspase-3 (Cas-3*; red and grayscale) labelings show that apoptosis is completely inhibited in discs predominantly mutant for vps25 and ark (B,B’). Cell death is also absent in discs predominantly mutant for ark (A,A’). (C,D) BrdU (red and grayscale) labelings show that proliferation is elevated in tissues predominantly mutant for vps25 and ark (D,D’). Proliferation is not affected in control tissues predominantly mutant for ark (C,C’). (E,F) aPKC (red and grayscale (E’,F’)) and Dlg (green and grayscale (E’’’,F’’’)) labelings of discs predominantly mutant for vps25 and ark show that cellular architecture is disrupted (F–F’’’). Cellular architecture is not disrupted in control discs predominantly mutant for ark (E–E’’’). (G,H) ELAV (red and grayscale) labelings of discs predominantly mutant for vps25 and ark show that very few cells in the double mutant discs differentiate normally (H,H’). Differentiation occurs normally in control discs predominantly mutant for ark (G,G’). (I,J) Mmp1 (red and grayscale) labelings of discs predominantly mutant for vps25 and ark show that levels of this protein are increased (J,J’). Mmp1 levels are not affected in control discs predominantly mutant for ark (I,I’). (K–N) Apoptosis is reduced by inhibition of JNK signaling by expression of the UAS-bskDN transgene using ey-Gal4. Phalloidin (green) or DAPI (green) is used to mark the overall shape of the tissue. (K,L) TUNEL (red and grayscale) labelings show that apoptosis is reduced in discs predominantly mutant for vps25 in which JNK signaling is inhibited (L,L’). Apoptosis is also low in control discs in which JNK signaling is inhibited (K,K’). (M,N) BrdU (red and grayscale) labelings show that proliferation is reduced in tissues predominantly mutant for vps25 in which JNK signaling is inhibited (N,N’). Proliferation is not affected in control discs in which JNK signaling is inhibited (M,M’). Genotypes: (A,C,E,G,I) eyFLP; FRT42D arkH16/FRT42D cl. (B,D,F,H,J) eyFLP; FRT42D vps25N55 arkH16/FRT42D cl. (K,M) eyFLP/UAS-bskDN; FRT42D y+/FRT42D cl; ey-Gal4/+. (L,N) eyFLP/UAS-bskDN; FRT42D vps25N55 y+/FRT42D cl; ey-Gal4/+.
We also examined the role of JNK signaling in apoptosis, proliferation and neoplastic characteristics in discs predominantly mutant for ESCRT-II genes by inhibiting JNK signaling through overexpression of a dominant negative form of the Drosophila JNK homologue basket (bsk), bskDN [27], using ey-Gal4. In control discs, overexpression of bskDN in otherwise wild-type discs has no apparent effect on architecture, polarity, differentiation, and Mmp1 expression (Figure 5K,M, Figure S3A,C,E). However, compared to the apoptosis observed in vps25 mutant discs (Figure 3C,G), TUNEL-positive cell death is strongly suppressed by expression of bskDN in discs predominantly mutant for vps25 (Figure 5L) suggesting that JNK signaling contributes to the apoptotic phenotype of predominantly mutant ESCRT-II eye discs. Intriguingly, the proliferation pattern is also reduced in these discs, as assayed by BrdU labeling (Figure 5N), implying that JNK-induced proliferation at least partially contributes to the strong proliferation phenotype of vps25 mutant discs. Labeling with phalloidin (Figure 5N, Figure S3D,F) and staining with antibodies recognizing aPKC and Dlg (Figure S3B) both indicate that cellular architecture remains disrupted even when JNK signaling is inhibited. Mutant discs have lost their characteristic shape and instead are simply dense “balls” of cells. aPKC and Dlg are both spread outside of their normal domains of localization. Only a few cells in the disc are positive for the differentiation marker ELAV, and they are spread throughout the disc (Figure S3D). Finally, despite a report that JNK can induce Mmp1 expression [35], expression of bskDN in discs predominantly mutant for vps25 does not suppress the elevated levels of Mmp1 expression (Figure S3F), suggesting that other mechanisms can also induce Mmp1. Thus, while inhibition of JNK signaling partially blocks apoptosis and proliferation, is has no effect on the other neoplastic characteristics seen in ESCRT-II mutant cells.
Inhibition of JAK/STAT Signaling Significantly Rescues the Neoplastic Transformation of ESCRT-II Mutant Tissues
Since we saw increased levels of JAK/STAT signaling in ESCRT-II mutant tissues, we investigated the possible autonomous role of JAK/STAT signaling in predominantly mutant tissues. A previous study examined tsg101 mutant discs in a heterozygous Stat92E mutant background and reported a genetic interaction [18], but due to the heterozygous Stat92E condition, a rigorous analysis of the role of JAK/STAT signaling in the neoplastic transformation of nTSG mutant tissue has not been completed. To accomplish this, we completely inhibited JAK/STAT signaling in vps22 mutant tissues using the null allele Stat92E397. We used vps22 in these experiments because vps22 and Stat92E both map to the same chromosome arm (3R), allowing a convenient double mutant analysis. It was recently shown that Stat92E mutant clones are eliminated by cell competition [41]. Interestingly, control discs predominantly mutant for Stat92E in which competitive interactions are eliminated reveal only weak abnormalities (Figure 6A,C,E,G). The proliferation pattern appears slightly abnormal (Figure 6A), and discs of slightly reduced size are generated. Importantly, overall tissue architecture (Figure 6A,E,G), apical-basal polarity (Figure 6C), and differentiation (Figure 6E) are normal in predominantly mutant Stat92E discs. There is also no Mmp1 expression in these discs (Figure 6G).
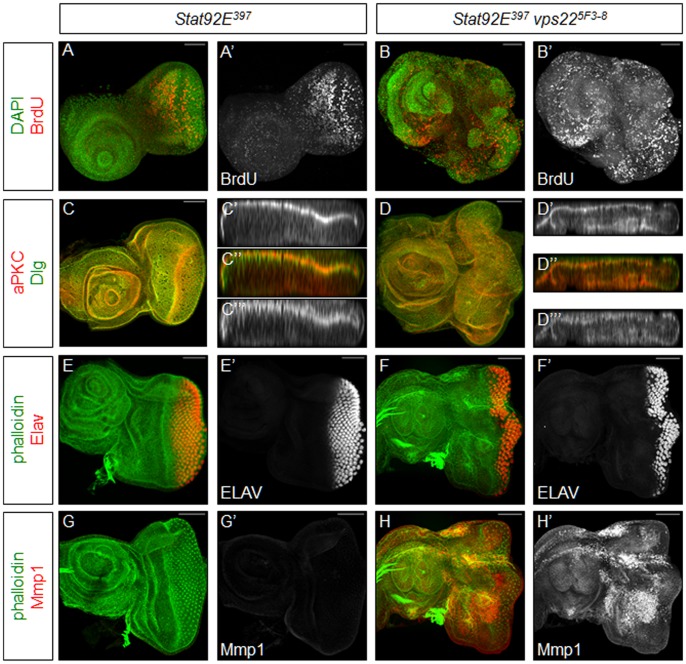
Figure 6. Inhibition of JAK/STAT signaling partially rescues the neoplastic transformation of ESCRT-II mutant tissue.
Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A,B) BrdU (red and grayscale) labelings show that proliferation is elevated in tissues predominantly mutant for vps22 and Stat92E (B,B’) Proliferation is slightly abnormal in control tissues predominantly mutant for Stat92E (A,A’). (C,D) aPKC (red and grayscale (C’,D’)) and Dlg (green and grayscale (C’’’,D’’’)) labelings of discs predominantly mutant for vps22 and Stat92E show that cellular architecture is largely intact (D–D’’’). Cellular architecture is not disrupted in control discs predominantly mutant for Stat92E (C–C’’’). (E,F) ELAV (red and grayscale) labelings of discs predominantly mutant for vps22 and Stat92E show that differentiation is completely rescued (F,F’) from the loss of differentiation seen in ESCRT-II mutant discs. Differentiation occurs normally in control discs predominantly mutant for Stat92E (E,E’). (G,H) Mmp1 (red and grayscale) labelings of discs predominantly mutant for vps22 and Stat92E show that levels of this protein are increased (H,H’). Mmp1 levels are not affected in control discs predominantly mutant for Stat92E (G,G’). Genotypes: (A,C,E,G) eyFLP;; FRT82B Stat92E397/FRT82B cl. (B,D,F,H) eyFLP;; FRT82B vps225F3-8 Stat92E397/FRT82B cl.
However, loss of JAK/STAT signaling in vps22 mutant discs (vps22 Stat92E double mutants) strongly rescues the neoplastic characteristics seen in vps22 single mutant tissues. The disorganization of cellular architecture observed in vps22 mutant discs is significantly rescued by removal of JAK/STAT signaling. Labeling with phalloidin shows that double mutant discs retain their characteristic eye-antennal imaginal disc shape (Figure 6B,F,H). Staining with antibodies recognizing aPKC and Dlg reveals that spreading of these two proteins outside their wild-type domains of localization is minimized with most aPKC localized to the apical membrane domain and most Dlg localized to the basolateral membrane domain (Figure 6D). Thus, removal of JAK/STAT signaling leads to rescue of the disorganization of cellular architecture observed in vps22 mutant tissues.
Loss of JAK/STAT signaling in discs predominantly mutant for vps22 also significantly rescues the failure of differentiation seen in vps22 mutant discs (Figure 6F). Few cells are positive for ELAV in vps22 mutant discs, and cells that are differentiating normally are scattered throughout the tissue (Figure 1J–L). In striking contrast, when JAK/STAT signaling is inhibited, the entire posterior domain of the disc is positive for ELAV (Figure 6F), indicating that many cells are undergoing normal differentiation. This ELAV pattern is hardly distinguishable from the wild-type pattern (Figure 1I), implying that hyperactive JAK/STAT signaling in vps22 mutant cells inhibits differentiation.
Loss of JAK/STAT signaling in vps22 mutant discs, however, has little to no effect on Mmp1 expression. Mmp1 levels remain elevated throughout the tissue (Figure 6H), suggesting that JAK/STAT signaling is not required for Mmp1 expression and for possible metastatic capability. Thus, elevated JAK/STAT signaling in ESCRT-II mutant tissue plays a very important role in the neoplastic transformation, leading to both disorganization of cellular architecture and failure of differentiation.
Discussion
While it is well established how de-regulated signaling pathways in ESCRT-II mutant clones mediate non-cell autonomous interactions with neighboring non-mutant cells to contribute to hyperplastic overgrowth and increased cell survival [1], [3], [5]–[9], [13], it was largely unknown which signaling pathways trigger neoplastic transformation autonomously. To address this question, we generated predominantly mutant eye-antennal imaginal discs in which competitive interactions are eliminated so that we could examine the autonomous results of de-regulated signaling.
Overall, it appears that the same signaling pathways that are induced in mosaic clones are also activated in predominantly mutant tissues. However, two results of this study are noteworthy. First, it is surprising that JNK activity is strongly induced in tissues predominantly mutant for ESCRT-II genes. This is surprising because JNK signaling was believed to be induced by cell competition from neighboring non-mutant cells in mosaic tissues [6], [16], [30]. However, non-mutant tissue is largely eliminated by the ey-FLP/cl method and thus competitive interactions are eliminated. Therefore, it is not known how JNK signaling is induced in these tissues. Nevertheless, JNK signaling is critical for the overgrowth phenotype of predominantly ESCRT-II mutant eye discs as inhibition of this pathway partially blocks cell proliferation. Second, de-regulation of the JAK/STAT signaling pathway is critical for the neoplastic transformation of vps22 mutant discs. Loss of JAK/STAT signaling dramatically normalizes the neoplastic phenotype of vps22 mutant cells. In addition to JNK and JAK/STAT activity, we also found Notch activity increased in discs predominantly mutant for ESCRT-II genes. Therefore, we tested a genetic requirement of Notch signaling for neoplastic transformation of ESCRT-II mutant cells. However, loss of Notch was inconclusive because even the wild-type control discs did not grow when Notch was inhibited (data not shown).
Interestingly, although ESCRT-II mutant tissues undergo neoplastic transformation, they also show high levels of apoptosis. Animals with predominantly mutant eye-antennal imaginal discs die as headless pharate pupae, a phenotype likely caused by the apoptosis of the imaginal discs before the adult stage. Reduction of JNK signaling in vps22, vps25, or vps36 mutant discs leads to lower levels of apoptosis, supporting a role for JNK signaling in the cell death of the predominantly mutant tissues. More excitingly, JNK also controls proliferation in these tissues, as shown by the reduction of proliferation seen when JNK signaling was down-regulated. This observation is consistent with previous findings that JNK can induce non-cell autonomous proliferation [35] and that apoptosis-induced proliferation is mediated by JNK activity [33], [34], [42]. While inhibition of JNK signaling reduces proliferation in predominantly mutant ESCRT-II mutant discs, it does not affect other aspects of the neoplastic phenotype.
The role of JAK/STAT signaling in these mutants is complex. In mutant clones of ESCRT-II mosaic discs, Notch-induced secretion of the JAK/STAT ligand Upd triggers non-cell autonomous proliferation [5], [7]. However, we observed that autonomous de-regulated JAK/STAT signaling in predominately mutant discs is critical for the neoplastic transformation of vps22 mutants. In vps22 Stat92E double mutant discs, organization of cellular architecture is definitively rescued with the layout of the tissue closely resembling that of a wild-type eye-antennal imaginal disc. In addition, apical-basal polarity markers are localized more-or-less correctly in these tissues, indicating that epithelial polarity is more intact. Finally, differentiation in the posterior portion of the eye disc is preserved when JAK/STAT signaling is inhibited. Thus, de-regulation of JAK/STAT signaling in vps22 mutant discs contributes to the cellular disorganization and the lack of differentiation seen in the tissues, which is consistent with a previous study that implicated JAK/STAT signaling in cell cycle control, cell size, and epithelial organization in tsg101 mutant tissues [18].
It was recently shown that cells with strong gain of JAK/STAT activity transform into supercompetitors and eliminate neighboring cells with normal JAK/STAT activity by cell competition [41]. However, in mosaic discs, a supercompetitive behavior of ESCRT-II mutant cells has not been observed. In fact, these mutant cells are eliminated by apoptosis. Only if apoptosis is blocked in these cells, is a strong overgrowth phenotype with neoplastic characteristics observed [5], [6]. Thus, apoptosis can serve as a tumor suppressor mechanism to remove cells with potentially malignant JAK/STAT activity.
How endosomal trafficking specifically regulates JAK/STAT signaling and, thus, how blocking trafficking leads to increases in signaling pathway activity are interesting questions to answer in the future. It is possible that, like endocytic regulation of the Notch receptor, the endosomal pathway tightly regulates Domeless (Dome), the JAK/STAT pathway receptor. It has been shown previously that Dome is trafficked through the endocytic machinery and that this trafficking of Dome can affect the downstream output of the JAK/STAT signaling pathway [43], [44]. It is also possible that Notch-induced Upd secretion causes autocrine JAK/STAT signaling in these mutants. However, technical problems (knocking down Notch function both in wild-type and mutant tissue causes general problems in tissue growth) prevented us from examining this possibility.
It will be important to examine how de-regulated JAK/STAT signaling in ESCRT-II mutants causes neoplastic transformation. JAK/STAT signaling is known to be an oncogenic pathway in Drosophila [45]–[49] and in humans [50] but its downstream targets that promote tumorigenesis are not yet clear. JAK/STAT signaling may be feeding into other pathways that promote tumorigenesis, such as dpp signaling [45], or may be targeting other proteins involved in transformation, such as Cyclin D [46], [51]–[54].
A number of studies have implicated genes that function in endocytosis and endosomal protein sorting as tumor suppressors in human cancers. Most well known is Tsg101, as early studies showed that downregulation of Tsg101 promotes the growth of mouse 3T3 fibroblasts in soft agar [55]. When these cells were injected into nude mice, they formed metastatic tumors [55]. However, later studies have shown conflicting results [56]–[58], and it is still unclear if Tsg101 functions as a tumor suppressor in metazoans. Importantly, a number of studies have shown changes in expression of ESCRT components in human cancer cells, including changes in expression of ESCRT-I components Tsg101 [59]–[62] and Vps37A [63], [64] and ESCRT-III components Chmp1A and CHMP3 [63], [65] . Since the primary proteins that function in endocytosis and endosomal trafficking are conserved from yeast to humans, it is likely that our findings in Drosophila may have important implications for human disease.
Supporting Information
Notch protein levels are upregulated in vps22 , vps25 , and vps36 mutant tissues. Shown are predominantly mutant eye-antennal imaginal discs. Scale bars represent 50 µm. Phalloidin (green) is used to mark the overall shape of the tissue. Notch protein levels (red, grayscale) are shown by staining with an antibody recognizing the intracellular domain of the protein (α-Nintra). Notch protein levels are increased in imaginal discs predominantly mutant for vps22 (B), vps25 (C), or vps36 (D), as compared to Notch protein levels in control discs (A,A’). Genotypes: (A) eyFLP;; FRT82B/FRT82B cl. (B) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (C) eyFLP; FRT42D vps25N55 y+/FRT42D cl. (D) eyFLP;; vps36Δ69 FRT80B/cl FRT80B.
(TIF)
JNK signaling is upregulated in tissues predominantly mutant for vps25. Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) is used to mark the overall shape of the tissue. puc-lacZ is detected by β-gal labeling (red or grayscale). (A-B) Imaginal discs predominantly mutant for vps25 induce high levels of puc-lacZ (B), as compared to puc-lacZ expression in control discs (A,A’). Scale bars represent 50 µm. Genotypes: (A) eyFLP; FRT42D/FRT42D cl; puc-LacZ/+. (B) eyFLP; FRT42D vps25N55 y+/FRT42D cl; puc-LacZ/+.
(TIF)
Inhibition of JNK signaling does not affect the disorganization of cellular architecture, the failure of differentiation, and the invasive potential of ESCRT-II mutant tissue. Shown are predominantly mutant eye-antennal imaginal discs. JNK signaling is inhibited by expression of the UAS-bskDN transgene using ey-Gal4. Phalloidin (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A,B) aPKC (red and grayscale (A’,B’)) and Dlg (green and grayscale (A’’’,B’’’)) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that cellular architecture is disrupted (B-B’’’). Cellular architecture is not disrupted in control discs in which JNK signaling is inhibited (A-A’’’). (C,D) ELAV (red and grayscale) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that very few cells in the mutant discs differentiate normally (D,D’). Differentiation occurs normally in control discs in which JNK signaling is inhibited (C,C’). (E,F) Mmp1 (red and grayscale) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that levels of this protein are increased (F,F’). Mmp1 levels are not affected in control discs in which JNK signaling is inhibited (E,E’). Genotypes: (A,C,E) eyFLP/UAS-bskDN; FRT42D y+/FRT42D cl; ey-Gal4/+. (B,D,F) eyFLP/UAS-bskDN; FRT42D vps25N55 y+/FRT42D cl; ey-Gal4/+.
(TIF)
Acknowledgments
We would like to thank Erika Bach (NYU), Hugo Bellen (Baylor College of Medicine), Sarah Bray (University of Cambridge), Uwe Irion (Max-Planck-Institute Tübingen), the Bloomington Drosophila Stock Center at Indiana University and the Developmental Studies Hybridoma Bank at the University of Iowa for fly stocks and reagents. We are indebted to Georg Halder and Michael Galko in whose labs part of this work was performed at MD Anderson Cancer Center. We acknowledge Clare Richards, Zhihong Chen and Christian Antonio for excellent technical assistance.
Funding Statement
This work was supported by R01 GM068016 from the National Insitutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lu H, Bilder D (2005) Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol 7: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 2. Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, et al. (2008) Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell 19: 4167–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moberg KH, Schelble S, Burdick SK, Hariharan IK (2005) Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell 9: 699–710. [DOI] [PubMed] [Google Scholar]
- 4. Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, et al. (2009) Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci 122: 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, et al. (2006) vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development 133: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, et al. (2005) Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 9: 711–720. [DOI] [PubMed] [Google Scholar]
- 7. Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9: 687–698. [DOI] [PubMed] [Google Scholar]
- 8. Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A (2009) Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS ONE 4: e4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodahl LM, Haglund K, Sem-Jacobsen C, Wendler F, Vincent JP, et al. (2009) Disruption of Vps4 and JNK function in Drosophila causes tumour growth. PLoS One 4: e4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henne WM, Buchkovich NJ, Emr SD (2011) The ESCRT pathway. Dev Cell 21: 77–91. [DOI] [PubMed] [Google Scholar]
- 11.Teis D, Saksena S, Emr SD (2009) SnapShot: the ESCRT machinery. Cell 137: 182–182 e181. [DOI] [PubMed]
- 12. Hurley JH, Emr SD (2006) The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct 35: 277–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graves HK, Woodfield SE, Yang CC, Halder G, Bergmann A (2012) Notch signaling activates Yorkie non-cell autonomously in Drosophila. PLoS One 7: e37615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D (2008) Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol 180: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinson BS, Moberg KH (2011) Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway. Cell Cycle 10: 4110–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T (2009) Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell 16: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menut L, Vaccari T, Dionne H, Hill J, Wu G, et al. (2007) A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics 177: 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert MM, Beam CK, Robinson BS, Moberg KH (2009) Genetic interactions between the Drosophila tumor suppressor gene ept and the stat92E transcription factor. PLoS ONE 4: e7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stowers RS, Schwarz TL (1999) A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irion U, St Johnston D (2007) bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature 445: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srivastava M, Scherr H, Lackey M, Xu D, Chen Z, et al. (2007) ARK, the Apaf-1 related killer in Drosophila, requires diverse domains for its apoptotic activity. Cell Death and Differentiation 14: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silver DL, Montell DJ (2001) Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell 107: 831–841. [DOI] [PubMed] [Google Scholar]
- 23.Ring JM, Martinez Arias A (1993) puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev Suppl: 251–259. [PubMed]
- 24. Furriols M, Bray S (2001) A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol 11: 60–64. [DOI] [PubMed] [Google Scholar]
- 25. Kramatschek B, Campos-Ortega JA (1994) Neuroectodermal transcription of the Drosophila neurogenic genes E(spl) and HLH-m5 is regulated by proneural genes. Development 120: 815–826. [DOI] [PubMed] [Google Scholar]
- 26. Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, et al. (2007) GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns 7: 323–331. [DOI] [PubMed] [Google Scholar]
- 27. Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400: 166–169. [DOI] [PubMed] [Google Scholar]
- 28. Newsome TP, Asling B, Dickson BJ (2000) Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127: 851–860. [DOI] [PubMed] [Google Scholar]
- 29. Beaucher M, Hersperger E, Page-McCaw A, Shearn A (2007) Metastatic ability of Drosophila tumors depends on MMP activity. Dev Biol 303: 625–634. [DOI] [PubMed] [Google Scholar]
- 30. Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, et al. (2011) Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev Cell 20: 315–328. [DOI] [PubMed] [Google Scholar]
- 31. Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G (2012) Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci U S A 109: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7: 491–501. [DOI] [PubMed] [Google Scholar]
- 34. Shlevkov E, Morata G (2012) A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ 19: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uhlirova M, Bohmann D (2006) JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. Embo J 25: 5294–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanuka H, Sawamoto K, Inohara N, Matsuno K, Okano H, et al. (1999) Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4-related caspase activator. Mol Cell 4: 757–769. [DOI] [PubMed] [Google Scholar]
- 37. Mills K, Daish T, Harvey KF, Pfleger CM, Hariharan IK, et al. (2006) The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. J Cell Biol 172: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez A, Chen P, Oliver H, Abrams JM (2002) Unrestrained caspase-dependent cell death caused by loss of Diap1 function requires the Drosophila Apaf-1 homolog, Dark. EMBO J 21: 2189–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez A, Oliver H, Zou H, Chen P, Wang X, et al. (1999) Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat Cell Biol 1: 272–279. [DOI] [PubMed] [Google Scholar]
- 40. Zhou L, Song Z, Tittel J, Steller H (1999) HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol Cell 4: 745–755. [DOI] [PubMed] [Google Scholar]
- 41. Rodrigues AB, Zoranovic T, Ayala-Camargo A, Grewal S, Reyes-Robles T, et al. (2012) Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development 139: 4051–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suissa Y, Ziv O, Dinur T, Arama E, Gerlitz O (2011) The NAB-Brk signal bifurcates at JNK to independently induce apoptosis and compensatory proliferation. J Biol Chem 286: 15556–15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Devergne O, Ghiglione C, Noselli S (2007) The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci 120: 3457–3464. [DOI] [PubMed] [Google Scholar]
- 44. Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, et al. (2002) The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 129: 5437–5447. [DOI] [PubMed] [Google Scholar]
- 45. Bach EA, Vincent S, Zeidler MP, Perrimon N (2003) A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics 165: 1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai YC, Sun YH (2004) Long-range effect of upd, a ligand for Jak/STAT pathway, on cell cycle in Drosophila eye development. Genesis 39: 141–153. [DOI] [PubMed] [Google Scholar]
- 47. Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N (1995) Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. Embo J 14: 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hanratty WP, Dearolf CR (1993) The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet 238: 33–37. [DOI] [PubMed] [Google Scholar]
- 49. Luo H, Rose PE, Roberts TM, Dearolf CR (2002) The Hopscotch Jak kinase requires the Raf pathway to promote blood cell activation and differentiation in Drosophila. Mol Genet Genomics 267: 57–63. [DOI] [PubMed] [Google Scholar]
- 50. Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, et al. (2003) STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 197: 157–168. [DOI] [PubMed] [Google Scholar]
- 51. Meyer CA, Jacobs HW, Datar SA, Du W, Edgar BA, et al. (2000) Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. Embo J 19: 4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Datar SA, Jacobs HW, de la Cruz AF, Lehner CF, Edgar BA (2000) The Drosophila cyclin D-Cdk4 complex promotes cellular growth. Embo J 19: 4543–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malumbres M, Barbacid M (2007) Cell cycle kinases in cancer. Curr Opin Genet Dev 17: 60–65. [DOI] [PubMed] [Google Scholar]
- 54. Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, et al. (1999) Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. Embo J 18: 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li L, Cohen SN (1996) Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 85: 319–329. [DOI] [PubMed] [Google Scholar]
- 56. Bache KG, Slagsvold T, Cabezas A, Rosendal KR, Raiborg C, et al. (2004) The Growth-regulatory protein HCRP1/h Vps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation. Molecular Biology of the Cell 15: 4337–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krempler A, Henry MD, Triplett AA, Wagner KU (2002) Targeted deletion of the Tsg101 gene results in cell cycle arrest at G1/S and p53-independent cell death. J Biol Chem 277: 43216–43223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner KU, Krempler A, Qi Y, Park K, Henry MD, et al. (2003) Tsg101 is essential for cell growth, proliferation, and cell survival of embryonic and adult tissues. Mol Cell Biol 23: 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, et al. (2004) Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut 53: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu RT, Huang CC, You HL, Chou FF, Hu CC, et al. (2002) Overexpression of tumor susceptibility gene TSG101 in human papillary thyroid carcinomas. Oncogene 21: 4830–4837. [DOI] [PubMed] [Google Scholar]
- 61. Oh KB, Stanton MJ, West WW, Todd GL, Wagner KU (2007) Tsg101 is upregulated in a subset of invasive human breast cancers and its targeted overexpression in transgenic mice reveals weak oncogenic properties for mammary cancer initiation. Oncogene 26: 5950–5959. [DOI] [PubMed] [Google Scholar]
- 62. Young TW, Mei FC, Rosen DG, Yang G, Li N, et al. (2007) Up-regulation of tumor susceptibility gene 101 protein in ovarian carcinomas revealed by proteomics analyses. Mol Cell Proteomics 6: 294–304. [DOI] [PubMed] [Google Scholar]
- 63. Li J, Belogortseva N, Porter D, Park M (2008) Chmp1A functions as a novel tumor suppressor gene in human embryonic kidney and ductal pancreatic tumor cells. Cell Cycle 7: 2886–2893. [DOI] [PubMed] [Google Scholar]
- 64. Xu Z, Liang L, Wang H, Li T, Zhao M (2003) HCRP1, a novel gene that is downregulated in hepatocellular carcinoma, encodes a growth-inhibitory protein. Biochem Biophys Res Commun 311: 1057–1066. [DOI] [PubMed] [Google Scholar]
- 65. Walker GE, Antoniono RJ, Ross HJ, Paisley TE, Oh Y (2006) Neuroendocrine-like differentiation of non-small cell lung carcinoma cells: regulation by cAMP and the interaction of mac25/IGFBP-rP1 and 25.1. Oncogene 25: 1943–1954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Notch protein levels are upregulated in vps22 , vps25 , and vps36 mutant tissues. Shown are predominantly mutant eye-antennal imaginal discs. Scale bars represent 50 µm. Phalloidin (green) is used to mark the overall shape of the tissue. Notch protein levels (red, grayscale) are shown by staining with an antibody recognizing the intracellular domain of the protein (α-Nintra). Notch protein levels are increased in imaginal discs predominantly mutant for vps22 (B), vps25 (C), or vps36 (D), as compared to Notch protein levels in control discs (A,A’). Genotypes: (A) eyFLP;; FRT82B/FRT82B cl. (B) eyFLP;; FRT82B vps225F3-8/FRT82B cl. (C) eyFLP; FRT42D vps25N55 y+/FRT42D cl. (D) eyFLP;; vps36Δ69 FRT80B/cl FRT80B.
(TIF)
JNK signaling is upregulated in tissues predominantly mutant for vps25. Shown are predominantly mutant eye-antennal imaginal discs. Phalloidin (green) is used to mark the overall shape of the tissue. puc-lacZ is detected by β-gal labeling (red or grayscale). (A-B) Imaginal discs predominantly mutant for vps25 induce high levels of puc-lacZ (B), as compared to puc-lacZ expression in control discs (A,A’). Scale bars represent 50 µm. Genotypes: (A) eyFLP; FRT42D/FRT42D cl; puc-LacZ/+. (B) eyFLP; FRT42D vps25N55 y+/FRT42D cl; puc-LacZ/+.
(TIF)
Inhibition of JNK signaling does not affect the disorganization of cellular architecture, the failure of differentiation, and the invasive potential of ESCRT-II mutant tissue. Shown are predominantly mutant eye-antennal imaginal discs. JNK signaling is inhibited by expression of the UAS-bskDN transgene using ey-Gal4. Phalloidin (green) is used to mark the overall shape of the tissue. Scale bars represent 50 µm. (A,B) aPKC (red and grayscale (A’,B’)) and Dlg (green and grayscale (A’’’,B’’’)) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that cellular architecture is disrupted (B-B’’’). Cellular architecture is not disrupted in control discs in which JNK signaling is inhibited (A-A’’’). (C,D) ELAV (red and grayscale) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that very few cells in the mutant discs differentiate normally (D,D’). Differentiation occurs normally in control discs in which JNK signaling is inhibited (C,C’). (E,F) Mmp1 (red and grayscale) labelings of discs predominantly mutant for vps25 in which JNK signaling is inhibited show that levels of this protein are increased (F,F’). Mmp1 levels are not affected in control discs in which JNK signaling is inhibited (E,E’). Genotypes: (A,C,E) eyFLP/UAS-bskDN; FRT42D y+/FRT42D cl; ey-Gal4/+. (B,D,F) eyFLP/UAS-bskDN; FRT42D vps25N55 y+/FRT42D cl; ey-Gal4/+.
(TIF)