Abstract
While early 1990s reports showed the phosphorylation pattern of fetal tau protein to be similar to that of tau in paired helical filaments (PHF) in Alzheimer’s disease (AD), neither the molecular mechanisms of the transient developmental hyperphosphorylation of tau nor reactivation of the fetal plasticity due to re-expression of fetal protein kinases in the aging and AD human brain have been sufficiently investigated. Here, we summarize the current knowledge on fetal tau, adding new data on the specific patterns of tau protein and mRNA expression in the developing human brain as well as on change in tau phosphorylation in the perforant pathway after entorhinal cortex lesion in mice. As fetal tau isoform does not form PHF even in a highly phosphorylated state, understanding its expression and post-translational modifications represents an important avenue for future research towards the development of AD treatment and prevention.
Keywords: Ageing, Brain development, Paired helical filaments-PHF, Mild cognitive impairment, Tau potein kinases, Deafferentation, Entorhinal cortex lesion
1. Introduction
The unique expression pattern of tau protein isoforms in the human central nervous system is considered to be directly linked to the vulnerability of humans to neurodegenerative disorders with tauopathy, especially Alzheimer’s disease (AD) and frontotemporal dementia (Janke et al., 1999). AD is characterized by presence of plaques composed of amyloid beta (Aß) peptide in the brain and presence of various morphological forms of neurofibrillary degeneration in neuronal perikarya (neurofibrillary tangles), dendrites (ribbons of neuritic plaques), axons (neuropil threads and beaded-like axonal accumulations) and oligodendrocytes (coils), all made of highly phosphorylated protein tau. In the fetal human brain, only the shortest tau isoform is expressed, which has three microtubule (MT)-binding domains (R3 tau), 352 amino acids (aa), and a molecular weight of 45 kDa, and is referred to as fetal tau (Andreadis et al., 1992). Adult tau isoforms have four MT-binding domains (R4 tau) and are about 40-fold more efficient at promoting MT assembly (Lindwall and Cole, 1984). The absence of the second MT-binding domain and high level of phosphorylation during fetal development account for enhanced cytoskeletal plasticity required for growing immature neurons (Bramblett et al., 1993; Brion et al., 1993; Goedert et al., 1993). The fact that there is no evidence for MT disarrangement or formation of pathological paired helical filaments (PHF) in the fetal brain, unlike in AD, opens new perspectives on the potential use of fetal tau to prevent neurodegeneration. The role of tau in AD has been extensively studied and reviewed, but there has been little focus on fetal tau as a possible model for understanding AD pathogenesis or regarding its potential therapeutic relevance for treatment and prevention of neurodegeneration in general.
2. Structure
The tau phosphoprotein is a highly extended random coil (“Gaussian”) polymer with a minimal content of ordered secondary structures (Schweers et al., 1994). In PHF, small segments of tau adopt a β-conformation in the second and third MT-binding domains, and interact with other tau molecules, while the remainder of the protein retains largely unfolded structure and gives rise to the fuzzy coat of the filaments (Mandelkow et al., 2007). The human tau protein is encoded by the MAPT gene that comprises 16 exons (14 coding), located on chromosome 17q21 (RefSeq ID: NM_001123066.3, Fig. 1). The alternative splicing of exons 2, 3, and 11 of tau mRNA creates nine protein isoforms (UniProt ID: P10636, Fig. 1), which differ in the number of MT-binding domains (R3–R4) of 31–32 aa in the carboxy-terminal half (coded by exons 10–13), and in the number of amino-terminal inserts (N0–N2) of 29 or 59 aa (coded by exons 2 and 3). This results in a total number of aa ranging from 316 to 776 depending on the isoform. The adult human brain expresses all isoforms with R4 to R3 tau ratio equal to 1 (Goedert et al., 1989; Goedert and Jakes, 1990).
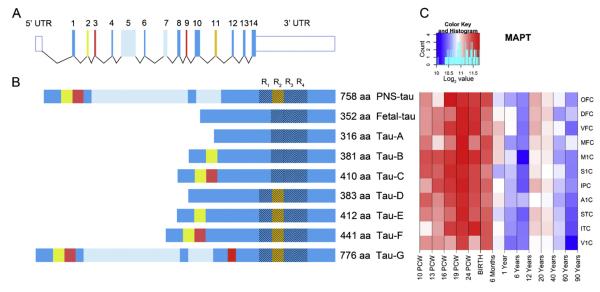
Fig. 1.
Structure of the microtubule associated protein tau
(A) The schematic representation of the longest transcript of the MAPT gene. The MAPT mRNA is composed of 14 exons and two UTRs (16 in total) which are alternatively spliced producing 9 different isoforms. MAPT gene encodes for 4 MT-binding domains (R1–R4). The R2 MT-domain is encoded by exon 11 which is alternatively spliced between different protein isoforms and is missing in fetal tau.
(B) 9 different tau protein isoforms. Fetal-tau is the only isoform expressed in fetal brain tissue.
(C) MAPT gene is expressed in high levels in the all areas of cerebral cortex from the earliest period during entire life (see color code and heat map). The peak expression of fetal tau is during mid-fetal period, with slight decline in expression until birth and decrease after 6th postnatal month. Data for the construction of MAPT mRNA and protein isoforms were obtained from RefSeq ID: NM 001123066.3 and UniProt ID: P10636, while expression data for MAPT transcripts were obtained from the database published in Kang et al. 2011
Abbreviations: OFC – orbital prefrontal cortex, DFC – dorsolateral prefrontal cortex, VFC – ventrolateral prefrontal cortex, MFC – medial prefrontal cortex, M1C – primary motor cortex, S1C – primary somatosensory cortex, IPC – posterior inferior parietal cortex, A1C – primary auditory cortex, STC – superior temporal cortex, ITC – inferior temporal cortex, V1C – primary visual cortex, PCW-postconception weeks.
3. Expression, activation, and turnover
The MAPT transcript is highly expressed during the entire lifespan in all areas and regions of the cerebral cortex (Fig. 1, Kang et al., 2011). The most prominent expression is observed during fetal development, when only fetal tau (N0R3) is expressed. The peak of expression is in the midgestation period and for the frontal region lasts until birth (Fig. 1C). After the sixth postnatal month, 2-fold decrease in the expression levels of the MAPT transcript can be observed. Although there are no significant differences in MAPT transcript expression levels among areas and regions in the human cerebral cortex, it should be noted that the peak expression is prolonged in the frontal brain region (Fig. 1C). The rate of tau protein synthesis is differentially regulated during development. Namely, tau synthesis during neurite development is local in the distal part of the axon. For example, in the mouse cerebellum, after axonal growth ends on the 20th postnatal day, only a limited number of new tau molecules are synthesized (Vilá-Ortiz et al., 2001). The synthesis of tau is not affected in a straightforward manner by MT polymerization or depolymerization. Both the tau mRNA and the tau protein are highly stable molecules and their stability in vivo may predispose tau to accumulate, as it in fact happens in AD (Grundke-Iqbal et al., 1986).
4. Biological functions
Under normal circumstances, the main biological function of tau is to assemble and stabilize MT, primarily in axons, and to regulate neuritic growth and shortening dynamics (Weingarten et al., 1975). This function is mediated by the R2 and R3 MT-binding domains. The absence of R2, N1 and N2 in fetal tau causes weakening of its binding ability for tubulin, and makes it least effective in promoting MT assembly and stabilization. The biological activity of tau is controlled mostly by phosphorylation and, to a lesser degree, by glycosylation (Lindwall and Cole, 1984; Buée et al., 2000). The longest tau isoform (tau 40), contains 45 presumed serine (Ser) phosphorylation sites, 35 presumed threonine (Thr) phosphorylation sites, and 5 presumed tyrosine (Tyr) phosphorylation sites (Takashima, 2011). The functional impact of phosphorylation state of fetal tau depends also on the specific phosphorylation sites (Liu et al., 2007). Studies employing phosphorylation site-specific anti-tau antibodies have confirmed that PHF-tau contains at least 30 phosphorylated Ser and Thr residues (Kopke et al., 1993). Although only a single isoform of tau is expressed in the fetal human brain, two species can be distinguished due to the differential extent of their phosphorylation, where the heavier species is being recognized by all PHF specific antibodies in a phosphorylation-dependent manner (Brion et al., 1993). Phosphorylation near the Ser202 distinguishes fetal tau from normal adult tau and corresponds to one of the abnormally phosphorylated sites during the early stages of AD (Goedert al., 1993). In the human fetal brain, the spatial distribution of fetal tau was evaluated over several developmental stages (Fig. 2A–E, Šimić et al., 2009), using the AT8 antibody, which recognizes phosphorylation of multiple sites around Ser202 (Ser199, Ser202, and Thr205) (Goedert et al., 1995). The spatiotemporal distribution of AT8 immunoreactivity in the human fetal brain suggests that hyperphosphorylation of fetal tau occurs in the distal portion of growing axons (Fig. 2B–E, Šimić et al., 2009). After the majority of axonal terminals reach their synaptic targets, hyperphosphorylation of fetal tau is downregulated, resulting in absence of AT8 immunoreactivity along axonal tracts after 35 weeks of gestation. The abundance of fetal tau (short and highly phosphorylated) concurrently with intensive axonal growth/retraction (in processes of pathways, areas and synaptic targets selection), supports the idea that fetal tau satisfies requirements for flexible/plastic MT system. Unlike conspicuous continuous AT8 immunoreactivity along axons of the human fetal brain, AT8 immunoreactivity in aged and AD brain is more discrete/punctuate (with “dotted” or “beaded-like” hyperphosphorylated tau deposits) (Šimić et al., 2009). It seems that the extent of tau phosphorylation decreases with advancing age due to activation of phosphatases. The phosphorylation sites in fetal tau could be more rapidly dephosphorylated by phosphatase 1, phosphatase 2A, and calcineurin than the same sites in pathological aggregations of hyperphosphorylated tau in the form of PHF (Yamamoto et al., 1995).
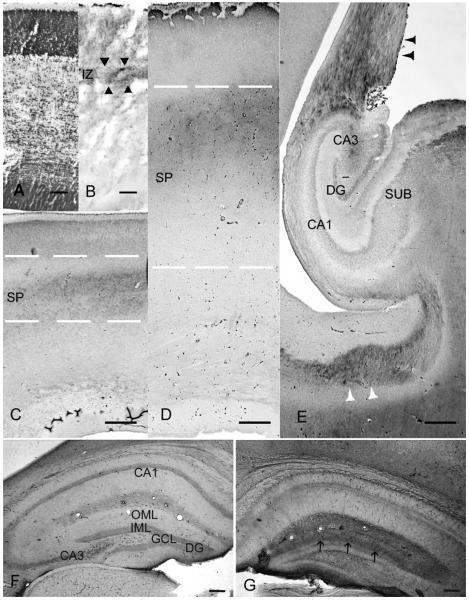
Fig. 2.
Function of fetal tau in assembling and stabilization of microtubules in growing cortical afferents. (A–E) (Šimić et al., 2009), and tau in entorhinal fibers in the normal (F) and deafferented (G) mouse dentate gyrus (DG) 7 days after entorhinal cortex lesion (ECL), revealed by AT8 immunoreactivity (AT8-ir) (previously shown in rat, Mudher et al., 2001).
(A) Coronal section of the fetal brain stained with Nissl showing cytoarchitectonic of the dorsolateral telencephalic wall at the 10th week of gestation (wg).
(B) AT8-ir fetal tau in growing monoaminergic and cholinergic fiber systems (arrowheads) in the superficial intermediate zone (IZ) at 10th wg.
(C) AT8-ir fetal tau in growing thalamocortical afferent fibers in the deep subplate zone (SP) at 18th wg.
(D) AT8-ir fetal tau in the waiting cortical afferents in the superficial SP at 22th wg.
(E) The fornix (black arrowheads) as well as a subset of commissural fibers (white arrowheads) are heavily labeled by AT8, whereas the hippocampus is unstained at 22th wg.
(F) Coronal sections of the mouse hippocampal formation in the normal circumstances, outer molecular layer (OML) of the non-denervated dentate gyrus (DG) is not labeled by AT8.
(G) AT8-ir of deafferented mouse DG, 7 days after ECL. The denervated OML is heavily labeled by AT8. Note the sharp border (arrows) between the denervated OML and the non-denervated inner molecular layer (IML) of the DG. White dashed lines delineate the superficial (upper) and deep (lower) border of the transient subplate zone (SP). Immunohistochemical staining with monoclonal mouse anti-human paired helical filaments-tau, is done using antibody AT8 (Innogenetics, Belgium, 1:200), method described in Jovanov-Milošević et al., 2010. ECL was performed as described in Deller and Frotscher, 1997. Scale bars: 100 μm in A, B, F and G; 500 μm in C, D, E.
Abbreviations: DG, dentate gyrus; CA1, CA3, hippocampal subfields; SUB, subiculum; GCL, granule cell layer; IML, inner molecular layer; OML, outer molecular layer.
5. Relevance of fetal tau for AD treatment
Based on the discovery that mutations in the APP (amyloid ß precursor protein) gene induce AD in familial early-onset cases of the disease (that comprise about 0.45% of all cases), the dominant view on AD pathogenesis for the last 20 years has been the amyloid hypothesis. This led to a somewhat premature conclusion that Aß is the main trigger of all AD. However, Braak and Braak (1997) have shown that tau pathology in the entorhinal and hippocampal brain regions precedes Aß accumulation (by at least 27 years). Therefore it is not surprising that over 20 recent clinical trials of potential disease-modifying drugs based on manipulation of Aß have failed (Mangialasche et al., 2010). To date, there are also no approved and established pharmacologic treatment options for either primary (frontotemporal dementia, progressive supranuclear palsy, corticobasal degeneration) or secondary tauopathies like AD (Karakaya et al., 2012). Moreover, although a variety of therapeutics targeting tau hyperphosphorylation and aggregation in AD have been recently proposed, examined in pre-clinical models (such as phenylthiazolyl hydrazide, rhodanines, minocycline, N-744, and a variety of anti-tau antibodies) and some even introduced into clinical trials (clinical phase 3: valproate; clinical phase 2: AL-108, AL-208, MTC/LMT, nicotinamide, N-031112/NP12, lithium), none of them succeeded to provide significant therapeutic benefit (Mangialasche et al., 2010; Herrmann et al., 2011). Therefore, in addition to questions pertaining to the timing of therapeutic administration, concerns over the adequacy of currently used preclinical models for AD have been raised (Hass, 2012).
Whereas highly phosphorylated fetal tau retains its MT-binding ability, PHF-tau completely loses the ability to bind to MT (Bramblett et al., 1993; Yoshida and Ihara, 1993). Additionally, the high expression level of Ser/Thr protein kinase glycogen synthase kinase-3β (GSK-3β) in developing brains decreases with advancing age (Takahashi et al., 1994). Searching the USA patent and trademark office database (http://patft.uspto.gov), using search terms ‘fetal tau’ and ‘Alzheimer’s disease’ on 3 January 2012, reveals 25 patents from which about a third is related to inhibitors of GSK-3ß. Another yet not well investigated possibility could be to try to promote fetal plasticity mechanisms by inducing the re-expression of, for example, embryonic kinases that phosphorylate tau protein. This potential avenue of research is based on the fact that, in normal circumstances, the tau phosphorylation decreases postnatally (in part also due to phosphatases activation). It seems that during lifetime period, the different multiple factors, such as genetic dysfunction (mutational and genetic susceptibility) as well as environmental impact (e.g., oxidative stress, inflammation, traumatic brain lesion) may lead to common pathogenic vicious circle, which causes the increase of phosphorylation of tau again, with consequent increase in tau polymerization, misfolding and formation of PHF (Iqbal et al., 2009). Indeed, the denervation of dentate gyrus (DG) by entorhinal cortex lesion (ECL) in rat and mouse, as a classical in vivo model of brain trauma, shows de novo AT8 immunoreactivity in entorhinal fibers 7 day after lesion, probably representing an attempt of neuronal circuitry reorganization (Fig. 2F–G). It was suggested that ECL in rodents induces morphological changes similar to those observed in AD patients, as the deterioration of the entorhino-hippocampal projecting neurons in AD causes loss of synapses in the DG similar to ECL (Deller and Frotscher, 1997). In addition, transneuronal changes, alterations in synaptic proteins and an increased synthesis of tau-α-tubulin were described both in Alzheimer’s disease and following ECL (Deller and Frotscher, 1997). The trans-synaptic spread of tau pathology is recently further substantiated in a transgenic mouse model that differentially expresses pathological human tau in the entorhinal cortex (Liu et al., 2012). In humans, after three or more decades of age, many factors that interfere with synaptic plasticity (synaptic long-term potentiation, dendritic remodelling, reactive synaptogenesis) and axoplasmic transport, seem to converge onto a single common pathogenic pathway that causes the phosphorylation of tau to increase again. As we previously reported, in such a situation, hyperphosphorylation of tau protein in AD might result from the inapt reactivation of developmental plasticity mechanisms (Šimić et al., 2009). In other words, to induce higher levels of MT plasticity, tau proteins need to be in a higher phoshorylated state, as we found that the same epitope (Ser199/Ser202/Thr205) revealed by the antibody AT8 is phosphorylated in growing fetal axons (Fig. 2, Šimić et al., 2009), in degenerating axons of AD brains (Šimić et al., 2009) and in entorhinal fibers in plastic response to ECL in small rodents (Fig. 2F–G, Mudher et al., 2001).
6. Conclusions
Under the premise that AT8 immunoreactivity permits the evaluation of neuronal changes well before the actual formation of tangles, neuritic plaques and neuropil threads, we propose that at least in the earliest stages, neurofibrillary degeneration includes pathological activation of fetal plasticity mechanisms. As fetal brain isoform of tau protein, even in a highly phosphorylated state, does not form PHF, understanding its expression and post-translational modifications represents and interesting and potentially important field for future research towards the development of AD treatment and prevention.
Acknowledgements
This work was supported by grant no. 108-1081870-1872 (“Development of cortical connections in man”) and grant no. 108-1081870-1942 (A Phosphorylation of tau proteins in development and Alzheimer’s disease”) from the Croatian Ministry of Science Education and Sports of Republic of Croatia, project no. 09/16 (“Detection and tracking of biological markers for early therapeutic intervention in sporadic Alzheimer’s disease”) from Croatian Science Foundation, and in part by NIH grant AG05138.
References
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–33. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer related lesions in different age categories. Neurobiology of Aging. 1997;18:351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–99. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Brion JP, Smith C, Couck AM, Gallo JM, Anderton BH. Developmental changes in tau phosphorylation: fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer’s disease. Journal of Neurochemistry. 1993;61:2071–80. doi: 10.1111/j.1471-4159.1993.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Research Reviews. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Deller T, Frotscher M. Lesion-induced plasticity of central neurons: sprouting of single fibers in the rat hippocampus after unilateral entorhinal cortex lesion. Progress in Neurobiology. 1997;53:687–727. doi: 10.1016/s0301-0082(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, et al. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5066–70. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–26. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO Journal. 1990;9:4225–30. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognizes tau protein phosphorylated at both serine 202 and threonine 205. Neuroscience Letters. 1995;189:167–9. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. Journal of Biological Chemistry. 1986;261:6084–9. [PubMed] [Google Scholar]
- Hass C. Strategies, development, and pitfalls of therapeutic options for Alzheimer’s disease. Journal of Alzheimer’s Disease. 2012;28:241–81. doi: 10.3233/JAD-2011-110986. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Chau SA, Kircanski I, Lanctôt KL. Current and emerging drug treatment options for Alzheimer’s disease: a systematic review. Drugs. 2011;71:2031–65. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathologica. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Beck M, Stahl T, Holzer M, Brauer K, Bigl V, et al. Phylogenetic diversity of the expression of the microtubule-associated protein tau: implications for neurodegenerative disorders. Molecular Brain Research. 1999;68:119–28. doi: 10.1016/s0169-328x(99)00079-0. [DOI] [PubMed] [Google Scholar]
- Jovanov-Milošević N, Petanjek Z, Petrović D, Judaš M, Kostović I. Morphology, molecular phenotypes and distribution of neurons in developing human corpus callosum. European Journal of Neuroscience. 2010;32:1423–32. doi: 10.1111/j.1460-9568.2010.07400.x. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–9. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaya T, Fußer F, Prvulovic D, Hampel H. Treatment options for tauopathies. Current Treatment Options in Neurology. 2012;14:126–36. doi: 10.1007/s11940-012-0168-7. [DOI] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. Journal of Biological Chemistry. 1993;268:24374–84. [PubMed] [Google Scholar]
- Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. Journal of Biological Chemistry. 1984;259:5301–5. [PubMed] [Google Scholar]
- Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. European Journal of Neuroscience. 2007;26:3429–36. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Duff K. Trans-synaptic spread of tau pathology in vivo. PloS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathology. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurology. 2010;9:702–16. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Mudher AK, Yee B, Smith AD, Perry VH. Deafferntation of the hippocampus results in the induction of AT8 positive ‘granules’ in the rat. Neuroscience Letters. 2001;301:5–8. doi: 10.1016/s0304-3940(01)01593-2. [DOI] [PubMed] [Google Scholar]
- Schweers O, Schönbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. Journal of Biological Chemistry. 1994;269:24290–7. [PubMed] [Google Scholar]
- Šimić G, Stanić G, Mladinov M, Jovanov-Milošević N, Kostović I, Hof PR. Does Alzheimer’s disease begin in the brainstem. Neuropathology and Applied Neurobiology. 2009;35:532–54. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Tomizawa K, Kato R, Sato K, Uchida T, Fujita SC, et al. Localization and developmental changes of tau protein kinase I/glycogen synthase kinase-3 beta in rat brain. Journal of Neurochemistry. 1994;63:245–55. doi: 10.1046/j.1471-4159.1994.63010245.x. [DOI] [PubMed] [Google Scholar]
- Takashima A. Tauopathy and brain aging. In: Nixon RA, Yuan A, editors. Cytoskeleton of the nervous system. Springer; New York, Dordrecht, Heidelberg, London: 2011. pp. 133–50. [Google Scholar]
- Vilá-Ortiz GJ, Santa-Coloma TA, Carminatti H, Radrizzani M. The rate of tau synthesis is differentially regulated during postnatal development in mouse cerebellum. Cellular and Molecular Neurobiology. 2001;21:535–43. doi: 10.1023/A:1013823524302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Hasegawa M, Ono T, Tashima K, Ihara Y, Miyamoto E. Dephosphorylation of fetaltau and paired helical filaments-tau by protein phosphatases 1 and 2A and calcineurin. Journal of Biochemistry. 1995;118:1224–31. doi: 10.1093/oxfordjournals.jbchem.a125011. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Ihara Y. Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. Journal of Neurochemistry. 1993;61:1183–6. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]