Abstract
Objectives
Stressors and emotional distress responses impact chronic fatigue syndrome (CFS) symptoms, including fatigue. Having better stress management skills might mitigate fatigue by decreasing emotional distress. Because CFS patients comprise a heterogeneous population, we hypothesized that the role of stress management skills in decreasing fatigue may be most pronounced in the subgroup manifesting the greatest neuroimmune dysfunction.
Methods
In total, 117 individuals with CFS provided blood and saliva samples, and self-report measures of emotional distress, perceived stress management skills (PSMS), and fatigue. Plasma interleukin-1-beta (IL-1β, IL-2, IL-6, IL-10, and tumor necrosis factor-alpha (TNF-α), and diurnal salivary cortisol were analyzed. We examined relations among PSMS, emotional distress, and fatigue in CFS patients who did and did not evidence neuroimmune abnormalities.
Results
Having greater PSMS related to less fatigue (p = .019) and emotional distress (p < .001), greater diurnal cortisol slope (p = .023) and lower IL-2 levels (p = .043). PSMS and emotional distress related to fatigue levels most strongly in CFS patients in the top tercile of IL-6, and emotional distress mediated the relationship between PSMS and fatigue most strongly in patients with the greatest circulating levels of IL-6 and a greater inflammatory (IL-6):anti-inflammatory (IL-10) cytokine ratio.
Discussion
CFS patients having greater PSMS show less emotional distress and fatigue, and the influence of stress management skills on distress and fatigue appear greatest among patients who have elevated IL-6 levels. These findings support the need for research examining the impact of stress management interventions in subgroups of CFS patients showing neuroimmune dysfunction.
Keywords: Chronic fatigue syndrome, Stress management, Immune dysfunction
1. Introduction
Chronic fatigue syndrome (CFS) is a debilitating disorder characterized by severe, unexplained fatigue that is not alleviated by rest, as well as combinations of other symptoms (e.g., post-exertional malaise) that meet specific diagnostic criteria and that did not predate the onset of fatigue (Fukuda et al., 1994). Prevalence of CFS has been estimated to be from between 0.24% to 0.42% in the United States but up to 0.68% in other regions (Jason et al., 1999; Reyes et al., 2003; Njoku et al., 2007). Disability due to CFS has been estimated to cost $9.1 billion in lost productivity every year in the US (Reynolds et al., 2004). As this syndrome is a public health concern, research has attempted to better understand the physiological mechanisms by which it operates. Much of the ongoing work focuses on putative latent viral infections (Glaser and Kiecolt-Glaser, 1998; Lombardi et al., 2009) and neuroimmune mechanisms (Klimas and Koneru, 2007; Siegel et al., 2006; Tomoda et al., 2005; Torres-Harding et al., 2008; Fletcher et al., 2010) that may underlie the symptom expression and chronicity of this condition. Neuroimmune research has repeatedly implicated dysregulation in the hypothalamic pituitary adrenal (HPA) axis and “overactivation” of some aspects of the immune system as indicated by increased circulating pro-inflammatory cytokines in subgroups of patients diagnosed with CFS (Broderick et al., 2010; Brenu et al., 2011; Heim et al., 2009; Lorusso et al., 2009; Nater et al., 2008; Roberts et al., 2004).
Field work has provided evidence that stressors and emotional distress may exacerbate CFS symptoms (Antoni and Weiss, 2003; Gaab et al., 2005; Nas et al., 2011). Lutgendorf and colleagues (1995) found evidence that emotional distress responses to a major life stressor, Hurricane Andrew, predicted greater likelihood and severity of a subsequent symptom relapse in a cohort of CFS patients residing in South Florida. Fatigue symptoms were the most prevalent among these. Interestingly, emotional distress responses to the storm predicted symptom relapses above and beyond material damage suggesting that individual differences in patients coping responses to this stressor were the key. Having coping resources (optimism and social support) predicted less relapses (Lutgendorf et al., 1995). In a diary study of CFS patients, Faulkner and Smith (2008) found that daily ratings of psychological distress predicted greater physical fatigue during the following week. Together, these findings suggest that emotional distress reactions (to environmental challenges and daily life stress) may trigger physical symptoms in CFS. Conclusions of these studies were that having better stress coping skills may mitigate these emotional distress responses and in turn help to reduce fatigue symptoms.
Having adequate coping skills predicted less illness severity among individuals with CFS (Walker et al., 2009). Conversely, other work found that CFS patients reporting dysfunctional attitudes and automatic thoughts, as well as the use of maladaptive coping strategies, showed greater illness burden (Antoni et al., 1994). While these studies have associated general coping strategies with CFS illness burden, they have not used precise measures of stress-reducing coping strategies nor fatigue severity. In fact, no study to date has examined the influence of a comprehensive set of stress management skills (i.e., including relaxation, tension awareness, cognitive restructuring, coping and interpersonal skills) on fatigue in CFS patients, and no prior work has evaluated whether such an association is actually mediated by reduced emotional distress. Establishing the reliability of these associations could advance our understanding of how stress management skills might mitigate CFS symptomology and justify the use of stress reduction interventions for this population. One focus of the present study is to examine the relationship between perceived stress management skills, emotional distress, and fatigue in a cohort of men and women with CFS.
A second focus of this study is to examine how the association between stress management skills and fatigue operates in the context of neuroimmunologic dysfunction, known to be present in a least some subgroups of patients diagnosed with CFS. Since distress states can disrupt HPA axis functioning (Yehuda et al., 1996; Rosmond et al., 1998) and increase pro-inflammatory processes (Maes et al., 1998; Miller et al., 2002; Steptoe et al., 2001), and since these same neuroimmune processes are believed to contribute to the pathophysiology of CFS in some patients, then stress management processes may have their greatest impact on CFS symptoms in the subgroup of patients who are at the “tipping point” of neuroimmune dysfunction. For CFS patients with no evidence of neuroimmune dysfunction there is no clear rationale for expecting either emotional distress or stress management skills to have an impact on fatigue severity.
Given the heterogeneous nature of populations with CFS in regards to illness onset, disease course, symptom patterns, and immune status, many researchers have attempted to delineate different patient subgroups (Borish et al., 1998; Corradi et al., 2006; Hickie et al., 1995; Porter et al., 2010; Siegel et al., 2006). The notion that CFS patients may be separated into subgroups on the basis of the presence or absence of immunological abnormalities (e.g., low natural killer cell activity, Siegel et al., 2006) is not a new concept. The idea that specific subgroups might be most susceptible to the influence of emotional distress-triggered exacerbation of CFS symptoms such as fatigue is a reasonable and novel sequel to this idea. It then follows that (a) CFS individuals with better stress management skills will reveal less emotional distress and less severe fatigue, and (b) the indirect effect of stress management skills on fatigue by way of emotional distress reduction might be most evident in that subgroup of CFS patients that shows the greatest neuroimmune dysfunction.
Specific abnormalities in HPA axis functioning have been documented in CFS patients in the past few years. CFS patients reveal a flatter cortisol awakening response as well as a flatter diurnal slope of salivary cortisol output compared to healthy individuals (Heim et al., 2009; Jerjes et al., 2005; Nater et al., 2008; Roberts et al., 2004), particularly in patients with early adverse life events (Heim et al., 2009). A qualitative study of CFS patients showed that the more that salivary cortisol diurnal pattern deviated from the normal morning peak and negative slope across the day (as determined by physician classification), the greater was their reported fatigue (Torres-Harding et al., 2008). Together these studies suggest that the severity of the HPA dysregulations—indicated by reduced diurnal variation—a process that may be exacerbated by stress, may relate to greater fatigue in CFS patients.
Several research teams have found that some but not all patients with CFS also evidence an increased pro-inflammatory immune system profile (Broderick et al., 2010; Carlo-Stella et al., 2006; Gaab et al., 2005; Fletcher et al., 2009; Moss et al., 1999). A recent study by Nas and colleagues (2011) found that, compared to control subjects, CFS patients had significantly higher circulating interleukin-6 (IL-6) and IL-2r, which were positively correlated with reported CFS symptoms. Several of the symptoms central to CFS, including fatigue, bear a strong resemblance to “sickness behavior” which can be induced by administration of pro-inflammatory cytokines (Dantzer and Kelley, 2007). However, as noted in a recent review by Bansal and colleagues (2012), there are inconsistencies in research regarding pro-inflammatory cytokines and CFS, as some researchers have noted no differences between CFS patients and healthy controls (Nater et al., 2008; Tomoda et al., 2005). In fact the repeated observation that distinct subgroups of patients diagnosed with CFS do and do not possess marked neuroimmunologic abnormalities has served as the impetus for a reclassification of patients meeting certain immunologic criteria as manifesting “myalgic encephalomyelitis” (Carruthers et al., 2011).
In a separate literature, cancer-related fatigue has been related to alterations in HPA functioning and pro-inflammatory activity (Collado-Hidalgo et al., 2006), and individuals with cancer-related fatigue have demonstrated increased pro-inflammatory immune reactivity in response to stressors (Bower et al., 2007). While differences between cancer-related fatigue and fatigue in CFS have been previously noted (Servaes et al., 2002), there appear to be similar immune and endocrine patterns in these two groups and findings from cancer studies may inform neuroimmune pathways to explore in populations with CFS.
In sum, stress processes well known to affect the HPA axis and cytokine regulation may exacerbate CFS symptoms such as fatigue. Conversely, stress management skills may improve fatigue levels in CFS by mitigating the exacerbating effects of emotional distress on symptoms. The fact that CFS patients may be divisible into subgroups based on the presence of immune abnormalities allows us to test whether the effects of stress management skills on fatigue are especially pronounced in patients who manifest the greatest neuroimmune dysfunction. Demonstrating such an effect would provide evidence that specific subgroups of CFS patients might be particularly likely to benefit from stress management intervention. This would be the first time that any study tested the impact of stress management skills on fatigue in CFS patients who reveal different degrees of neuroimmune dysfunction, perhaps defining a group to target in future intervention work.
In the present study, we aim to examine the relationship between perceived stress management skills, emotional distress, fatigue and immune and neuroendocrine indicators within a population of individuals diagnosed with CFS. First, we hypothesized that greater perceived stress management skills would relate to lower reports of fatigue, and less signs of neuroimmune dysfunction using measures of diurnal cortisol slope and circulating pro-inflammatory cytokines. Second, we hypothesized that the relationship between perceived stress management skills and lower fatigue would be mediated by lower levels of emotional distress. Third, we hypothesized that stress management skills and emotional distress would relate to fatigue most strongly among the subset of patients with evidence of neuroimmune dysfunction.
2. Methods
2.1. Participants
The present study examines baseline data from an ongoing trial testing the effects of a stress management intervention in patients diagnosed with CFS. Participants were primarily recruited via physician referral. Recruitment efforts also included presentations at local CFS conferences, support groups and special events, as well as advertisements on relevant websites. In order to be eligible for the present study, participants were required to have a physician-determined CFS diagnosis according to the Center for Disease Control (CDC) criteria (Fukuda et al., 1994) which was confirmed during a telephone screen interview by a score of 4 or above on a 12-item CDC Symptom Inventory that covers the defining symptoms of CFS. Participants who were not recruited via physician referral had their diagnosis confirmed by a medical doctor from the study after the telephone screen.
Other eligibility requirements included being 21–75 years of age, being fluent in English, and having an active home telephone line. Potential participants were excluded if they met DSM-IV criteria for aschizophrenia, bipolar disorder, or substance abuse. Participants could also be excluded if they were actively suicidal or had a prior psychiatric hospitalization for any thought disorder or affective disorder. These criteria were assessed during the telephone screening interview by a member of the study staff using a brief psychiatric screening adapted from the Structured Clinical Interview for DSM-IV (SCID-I/NP; First et al., 2002). Participants were also excluded if they had been diagnosed with an illness or were receiving medical treatment that would explain chronic fatigue and/or modulate neuroendocrine or immune indicators (e.g., Lyme disease, renal dialysis, cancer, corticosteroids) or if they lived outside of the research area. Finally, to ensure comprehension of study questionnaires, participants were excluded if they demonstrated diminished cognitive capabilities as indicated by having made four or more errors on the Short Portable Mental Status Questionnaire (Pfeiffer, 1975). Of the 161 participants screened for the study, 134 were eligible. Participants were excluded for not having a CFS diagnosis (n = 6), living out of study area (n = 11), being older than 75 years of age (n = 1), not speaking English (n = 1), not having a working phone line (n = 3), and for having an exclusionary medical condition (n = 5). The final enrollment of participants consisted of 117 of the 134 eligible individuals after 10 potential participants indicated that they were no longer interested in participating and seven were lost to follow-up before baseline data was collected.
2.2. Procedures
After enrollment, participants received a home visit by study staff. During this visit, a member of the study team administered a battery of measures regarding psychosocial status and CFS symptoms and provided the participant with eight Salivette® tubes. Participants were instructed to provide saliva samples on two consecutive weekdays within one week of the baseline interview. For each of these days they were requested to take a sample upon awakening, 30 min after awakening, at 4 pm and at 9 pm. Participants were told not to eat or drink before and between the first two samples each day, and to avoid eating a large meal an hour before the afternoon and evening samples. They were also advised to not exercise vigorously on sample collection days and to abstain from alcohol within 12 h prior to sample collection. In order to keep salivary cortisol stable, participants were instructed to refrigerate the Salivette® tubes. Within one week of the initial home visit, participants returned their saliva samples to the study staff and had their blood drawn. Peripheral venous blood samples were taken between 11 am and 3 pm for all participants to minimize extreme diurnal variations and to accommodate patient preferences for scheduling of blood draws. Upon completion of the home visit and blood draw, participants were compensated $50.
2.3. Measures
2.3.1. Perceived stress management skills
Patients with chronic illnesses vary greatly in the degree to which they have accrued stress management techniques through various sources (e.g., books, internet sites, counseling) (Antoni, in press). Perceived stress management skills were measured using the Measure of Current Status (MOCS; Carver, 2006). This measure asks participants to rate their perceived ability to respond to the challenges of daily life on a 5-point response scale with 1 indicating “I cannot do this at all” to 5 indicating “I can do this extremely well.” Items are based on behaviors and personal characteristics that are associated with efficient stress management such as awareness of tension, relaxation skills, cognitive restructuring, coping skills, utilization of social support, and appropriate expression of anger. Items from this measure include “I can clearly express my needs to other people who are important to me” and “I notice right away whenever my body is becoming tense.” In the current study we summed the items on the MOCS to compose an overall stress management skill measure and this score had a high degree of internal consistency (Cronbach’s α = .85). The MOCS has previously been treated as a unifactorial measure in a sample of men with localized prostate cancer (Penedo et al., 2006).
2.3.2. Emotional distress
In order to assess the degree of participants’ emotional distress across the preceding week, the Perceived Stress Scale (PSS; Cohen et al., 1983), the Center for Epidemiologic Studies Depression Scale (CES-D: Radloff, 1977), and the Profile of Mood States (POMS) depression–dejection and anxiety–tension subscales (McNair et al., 1971) were utilized, based on the notion that feelings of perceived stress, depression and anxiety contribute to one’s total experience of emotional distress. The PSS measures participants’ perceptions of life stress by asking them to rate items such as “How often have you been upset because something happened unexpectedly?” on a scale from 0, indicating “never,” to 4, indicating “very often.” The CES-D measures depressive symptomatology by asking participants to rate items such as “I thought my life had been a failure” on a scale from 1, indicating “rarely or none of the time, <1 day” to 4, indicating “most or all of the time, 5–7 days.” The POMS measures various mood states and participants are asked to rate the degree to which they have felt in the past week on a 5-point scale with 0 indicating “not at all” and 4 indicating “extremely”. Sample items from the depression–dejection subscale include “unhappy” and “lonely” and sample items from the tension-anxiety subscale include “on edge” and “nervous.” Collectively, a sum of the scores from the PSS, CES-D and POMS measures demonstrated excellent internal consistency (Cronbach’s α = .89). To create an emotional distress composite score that tapped perceived stress, depressed mood and anxiety, we summed the z-scores of the PSS, CES-D, POMS depression-dejection and POMS tension-anxiety.
2.3.3. Fatigue
We assessed the degree of participants’ fatigue severity across the week prior to data collection with the Fatigue Symptom Inventory severity scale (FSI; Hann et al., 1998) and the POMS fatigue-inertia subscale (McNair et al., 1971). The severity scale of the FSI consists of four items on an 11-point scale where 0 indicates “not at all fatigued” and 10 indicates “as fatigued as I could be”. Sample items on this scale include “rate your level of fatigue on average in the last week”. Similar to the POMS subscales used in the emotional distress composite score, the fatigue-inertia sub-scale is on a 5-point scale with 0 indicating “not at all” and 4 indicating “extremely”. Sample items for the fatigue-inertia subscale include “worn out” and “fatigued.” A fatigue composite score was created by adding the z-scores of the severity subscale of the FSI with the POMS fatigue-inertia subscale (α = .75).
2.3.4. Salivary cortisol
Saliva samples were retrieved from participants by study staff and frozen at −20 °C until assayed in batches. On the day of the assay, saliva samples were thawed, vortexed and centrifuged at 1500 rpm for 15 min prior to being assayed using the Salimetrics high sensitivity ELISA kit (State College, PA). Values from each time point were averaged between the two days of collection and the mean values were used to compute the slope of the regression line fitted through the cortisol data time points as described by Sephton and colleagues (2000).
2.3.5. Pro-inflammatory cytokines
Blood was centrifuged and plasma stored at −80 °C within 4 h of collection until assays were done in batches. All samples were assayed in duplicate. Circulating pro-inflammatory cytokines,IL-1β, IL-2, IL-6 and TNF-α and the an anti-inflammatory cytokine, IL-10, were measured from blood plasma as previously described (Fletcher, et al., 2009) using the Q-Plex™ Human Cytokine –Screen, an ELISA-based test produced by Quansys Biosciences (Logan, Utah). The system uses distinct capture antibodies in a 96-well plate in a defined array. Images were taken of the plate using the Quansys Imager, driven by an 8.4 megapixel Canon 20D digital SLR camera. Once imaged, the plates were analyzed using Quansys Software. In order to assure compatibility with measurements of cytokines in previously published studies in the field (Chiswick et al., 2012; Trune et al., 2011;Wong et al., 2008), the antigen standard concentrations used by Quansys were referenced to “gold standard” for each cytokine represented on the multiplex plate. In this case cytokine standards from R&D Systems, the largest provider of standard ELISA kits, was used. A series dilution was performed on the R&D standard and the Quansys standard supplied in the kit and run side by side on the same plate with all the same conditions and reagents. By this process, the custom multiplex plates used for our study were, in every case, superimposable and ensured that a sample calculated with the reference (R&D) curve gave the same concentration with the Quansys curve.
2.4. Statistical analyses
All data were analyzed using SPSS version 18.0 for Windows. Initially bivariate Pearson correlations and t-tests were performed to examine the relationships between all of the study variables. Undetectable levels of cytokines were substituted for the lowest detectable limit value and analyses were run with and without the undetectable cases to ensure that this data substitution did not affect the pattern of results. We tested our study hypotheses that greater perceived stress management skills (PSMS) were associated with lower fatigue levels via reduced emotional distress (as a mediator). This analysis was conducted using linear regression and an SPSS macro developed by Preacher and Hayes (2008) that allows for the estimation of the indirect effect using both the Sobel test, and bootstrapping to obtain confidence intervals. Next, we examined the moderating role of neuroimmune dysfunction indicators in the relationships between emotional distress and fatigue, and PSMS and fatigue using regression, and then examined a synthesized model in which neuroimmune dysfunction indicators could act to moderate the PSMS-distress–fatigue mediation model tested above. For any pro-inflammatory cytokines shown to moderate the PSMS-fatigue association, we also ran a parallel test using the ratio of the pro-inflammatory cytokine to the anti-inflammatory cytokine, IL-10. We hypothesized that the association between PSMS and fatigue via emotional distress would be most pronounced in cases that presented with the greatest neuroimmune dysfunction. To test this conditional indirect effect, another SPSS macro developed by Preacher and colleagues (2007) was utilized. This macro provides a method for probing the significance of the indirect effect (PSMS to fatigue via emotional distress) at different levels of each neuroimmune indicator showing an omnibus interaction effect and allows for bootstrapping. In order to minimize Type I error, subgroup analyses based on terciles were restricted to the neuroimmune indicators which demonstrated an omnibus interaction effect. We also used a Bonferroni-adjusted significance level (p < .01) based on the number of omnibus interaction tests conducted.
3. Results
3.1. Preliminary analyses
Demographic characteristics of the sample of 117 participants are provided in Table 1. Means and standard deviations of the psychosocial and cortisol variables are provided in Table 2. t-Tests were run to compare males and females on the predictor and outcome variables and no significant differences were found. However, based on the literature indicating the importance of controlling for age and gender, both variables were used as covariates in all analyses (O’Connor et al., 2009). Before examining associations between PSMS and pro-inflammatory cytokines in the CFS sample we compared mean levels of cytokines between the CFS sample and an age (M = 49 years) and gender-equivalent (28% men, 72% women) healthy control comparison group (see Table 3). We found that the CFS patients revealed significantly greater levels of IL-1β (p < .03) and IL-6 (p < .05). Although TNF-α levels were also greater in CFS than controls, the difference was not statistically sig-nificant. There were no other differences in cytokines between CFS patients and healthy controls.
Table 1.
Demographic characteristics of the study sample.
| M | SD | |
|---|---|---|
|
| ||
| Age | 50.71 | 11.49 |
| N | % | |
| Gender | ||
| Female | 97 | 83 |
| Male | 20 | 17 |
| Ethnic identification | ||
| Caucasian (non-Hispanic) | 92 | 79.5 |
| African American | 3 | 2.6 |
| Hispanic | 18 | 15.4 |
| Asian/Asian American | 1 | 0.9 |
| Biracial | 2 | 1.7 |
| Relationship status | ||
| Single | 26 | 22.2 |
| Married or equivalent relationship | 50 | 42.7 |
| Separated | 2 | 1.7 |
| Divorced | 21 | 17.9 |
| Widowed | 6 | 5.1 |
| In a monogamous relationship | 11 | 9.4 |
| Other | 1 | 0.9 |
| Employment status | ||
| Full-time | 20 | 17.1 |
| Part-time | 11 | 9.4 |
| Student | 2 | 1.7 |
| On disability | 47 | 40.2 |
| Retired | 12 | 10.3 |
| Unemployed | 16 | 13.7 |
| Volunteer worker | 1 | 0.9 |
| Other | 8 | 6.8 |
| Education level | ||
| <8th Grade | 1 | 0.9 |
| High school or GED | 13 | 11.1 |
| Trade school | 1 | 0.9 |
| Some college | 37 | 31.6 |
| College graduate | 40 | 34.2 |
| Graduate degree | 25 | 21.4 |
Table 2.
Descriptive statistics for the psychosocial and cortisol variables.
| M | SD | Range | |
|---|---|---|---|
| FSI Intensity | 26.74 | 5.3 | 14–38 |
| POMS fatigue–inertia | 26.67 | 5.01 | 14–35 |
| Fatigue composite | 0.01 | 1.79 | −4.48–3.66 |
| PSS | 31.2 | 9.12 | 10–56 |
| CES-D | 25.37 | 12.85 | 2–57 |
| POMS depression–dejection | 33.82 | 14.2 | 15–70 |
| POMS anxiety–tension | 18.28 | 7.88 | 4–38 |
| Emotional distress composite | 0.12 | 3.58 | −6.13–8.61 |
| perceived stress management skills | 42.09 | 8.83 | 21–63 |
| Diurnal cortisol slope | −0.12 | 0.15 | −1.19–0.37 |
| Awakening cortisol | 0.43 | 0.57 | 0.023–4.760 |
| Awakening + 30 min cortisol | 0.51 | 0.48 | 0.021–4.065 |
| 4 pm Cortisol | 0.21 | 0.37 | 0.004–2.414 |
| 9 pm Cortisol | 0.14 | 0.21 | 0.007–1.380 |
Abbreviations: FSI (Fatigue Symptom Inventory), POMS (Profile of Mood States), PSS (Perceived Stress Scale), CES-D (Center for Epidemiologic Survey-Depression).
Table 3.
Cytokine levels in CFS participants and age- and gender-equivalent healthy controls.
| CFS (N = 117)
|
Controls (N = 95)
|
|||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| IL-1β | 38.8 (70.2) | 4.30–316.85 | 20.9 (38.5) | 0.0–203.7 |
| IL-2 | 7.4 (8.6) | 0.00–37.65 | 7.00 (15.6) | 0.5–52.7 |
| IL-6 | 11.9 (19.4) | 0.80–94.08 | 7.2 (12.3) | 0.0–8.90 |
| IL-10 | 10.9 (12.8) | 2.72–73.77 | 10.2 (14.8) | 7.10–93.0 |
| TNF-α | 25.8 (46.2) | 0.00–215.31 | 18.2 (39.1) | 0.0–40.3 |
Control values were obtained from a mixed gender (28% men, 72% women) and middle-aged (M = 49.0 yrs) sample of healthy controls.
3.2. Associations among study variables
Fatigue, emotional distress and PSMS all significantly correlated with each other (p’s < .01). Controlling for age and gender, there was a significant association between PSMS and diurnal cortisol slope (r =−.23, p = .023), and PSMS and IL-2 (r =−.19, p = .043). PSMS scores were not related to IL-1β, IL-6, IL-10, TNF-α levels or the IL-6/IL-10 ratio, all p’s > .10. There were no direct associations between fatigue and diurnal cortisol slope or cytokines, nor were there direct associations between emotional distress and diurnal cortisol slope or cytokines, all p’s > .10.
Since cytokines were measured at a single point in time whereas cortisol was sampled across the di-urnal period we wondered whether a single point-in-time cortisol measurement might map better onto cytokine values. Assuming that elevated evening cortisol levels may be the best single-point-in-time indicator of abnormal HPA regulation we examined whether greater evening (9 pm) cortisol levels may relate to greater levels of circulating pro-inflammatory cytokines. We found significant associations between greater TNF-α and greater evening cortisol (β = .20, p = .048) and a marginally significant association between greater IL-6 and greater evening cortisol (β = .18, p = .084).
3.3. Perceived stress management skills and fatigue mediation model analyses
Results of a linear regression provided support for the hypothesized mediation model, such that greater PSMS was related to less emotional distress (β = −.18, p < .001), and lower levels of reported fatigue (β = −.46, p = .019). Fig. 1 presents the result of the mediation analyses showing that emotional distress mediated the relationship between PSMS and fatigue. The standardized regression coefficient between PSMS and fatigue significantly decreased (β = .12, p = .98) when controlling for levels of emotional distress. Using Preacher and Hayes (2008) bootstrapping methodology based on 5000 bootstrap resamples, the 95% confidence interval for emotional distress as a mediator was −0.1365 to −0.0361. Because this interval did not contain zero, it provided further support for the mediation model.
Fig. 1.
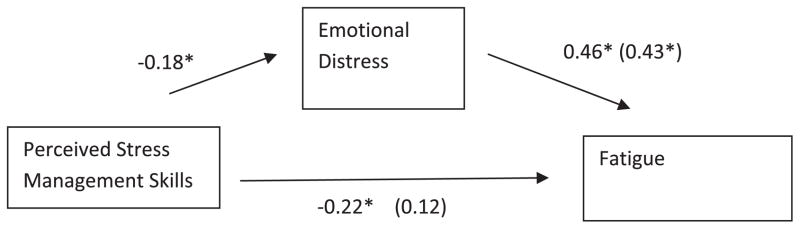
Regression coefficients for the relationship between perceived stress management skills and fatigue as mediated by emotional distress. The values outside of parentheses are the simple regression coefficients and the values in parentheses are the regression coefficients when for the full mediation model *p < .05.
3.4. Perceived stress management skills mediation model in subgroups of CFS patients
Given that stress (and perceived stress management skills) effects on fatigue symptoms may be more apparent in CFS patients with greater background levels of neuroimmune dysfunction (Borish et al., 1998; Corradi et al., 2006; Porter et al., 2010; Siegel et al., 2006), we hypothesized that the effects of PSMS on fatigue might be greater among patients with greater levels of circulating pro-inflammatory cytokines and lower diurnal cortisol slope. We first examined each arm of the mediation model for moderated effects. IL-6 was seen to moderate the relationship between PSMS and emotional distress (p = .02) such that greater PSMS was significantly associated with less emotional distress (β = −.77, p < .01) only in the subgroup of patients in the top tercile of IL-6 (high IL-6 subgroup) and not among those patients in the lower two terciles of IL-6. The relationship between emotional distress and fatigue was moderated by IL-6 level (p = .04) such that greater emotional distress related to greater fatigue in the high IL-6 subgroup (β = .65, p < .01). Finally the PSMS and fatigue association was moderated by IL-6 (p < .01) such that greater PSMS related to less fatigue (β = −.60, p < .01), only in the high IL-6 subgroup.
As a last step in this series of analyses, we tested the overall model for moderated mediation using the methods outlined in Preacher et al. (2007). These results are presented in Table 4. Controlling for age and gender, the omnibus interaction between PSMS and IL-6 was significant (p < .01), indicating that the indirect effect of PSMS on fatigue through emotional distress is moderated by levels of IL-6. Given the positive sign of the interaction, it can be interpreted that the indirect effect is larger for participants with higher circulating levels of IL-6. Table 4 also presents conditional indirect effects at each tercile of the moderator, IL-6. It can be seen that at bottom and middle tercile of IL-6, there is not an indirect effect between PSMS and fatigue via emotional distress, but in the top tercile of IL-6, the indirect effect is significant. Using bootstrapping based on 5000 bootstrapped re-samples, the 95% bias corrected and accelerated confidence interval for the conditional indirect effect in the top tercile was −0.1987 to −0.0007. Because this interval did not contain zero, the significant nature of the moderated mediation model was further supported.
Table 4.
Multiple regression analysis for the conditional indirect effect of perceived stress management skills on fatigue through emotional distress as a function of circulating level of IL-6..
| B | SE | t | p | |
|---|---|---|---|---|
| Mediator variable model | ||||
| Constant | −5.589 | 4.932 | −1.133 | 0.260 |
| PSMS | 0.045 | 0.096 | 0.466 | 0.642 |
| IL-6 | 13.058 | 4.812 | 2.714 | 0.008 |
| IL-6 × PSMS | −0.280 | 0.108 | −2.581 | 0.011 |
| Age | 0.001 | 0.028 | 0.034 | 0.973 |
| Gender | 1.486 | 0.824 | 1.805 | 0.074 |
| Dependent variable model | ||||
| Constant | −5.399 | 2.422 | −2.230 | 0.028 |
| PSMS | 0.136 | 0.047 | 2.892 | 0.005 |
| Emotional distress | 0.201 | 0.049 | 4.124 | 0.000 |
| IL-6 | 7.573 | 2.433 | 3.113 | 0.002 |
| IL-6 × PSMS | −.176 | 0.055 | −3.228 | 0.002 |
| Age | −0.030 | 0.014 | −2.200 | 0.030 |
| Gender | 0.608 | 0.408 | 1.489 | 0.140 |
| Conditional indirect effects of IL-6 at each tercile | ||||
| IL-6 group | Indirect effect | SE | Z | p |
|
| ||||
| First tercile | −.0194 | 0.019 | −1.01 | 0.40 |
| Second tercile | −0.006 | 0.022 | −.784 | 0.43 |
| Third tercile | −0.0827 | 0.049 | −2.15 | 0.03 |
The indirect effect of PSMS on fatigue through emotional distress was also moderated by the ratio of IL-6 to IL-10 (p = .01), following a similar pattern to that observed for IL-6 values alone. Given the positive sign of the interaction, it can be interpreted that the indirect effect is larger for participants with higher ratio of IL-6 to IL-10. Bootstrapping analyses revealed that the 95% bias corrected and accelerated confidence interval for the conditional indirect effect was −0.2688 to −0.0199. Because this interval did not contain zero, the significant nature of the moderated mediation model was further supported. We found no evidence that levels of other neuroimmune indicators moderated the PSMS-fatigue model.
3.5. Sickness behavior model
Results of a series of linear regressions did not provide support for a sickness behavior mediation model in which emotional distress has an indirect effect on fatigue via levels of pro-inflammatory cytokines. None of the cytokines demonstrated a significant direct relationship with fatigue (all p’s > .05). Therefore tests of mediation could not be conducted.
4. Discussion
4.1. Stress management skills and fatigue
The present study examined whether having greater perceived stress management skills (PSMS) related to less fatigue through their influence on emotional distress levels within a sample of individuals diagnosed with CFS. We also tested whether this association varied among subgroups of patients with and without the presence of neuroimmune dysfunction. The results of this study indicate that greater PSMS relate to lower levels of fatigue which may be mediated by lower levels of emotional distress. Previous research has revealed a link between emotional distress levels and subsequent CFS symptom exacerbation, including increased fatigue (Faulkner and Smith, 2008; Lutgendorf et al., 1995). The impact that heightened stress has on health outcomes may be mitigated by coping mechanisms and other stress management skills that an individual possesses. In a study of cancer patients, Faul and colleagues (2010) found that greater perceived stress management skills (relaxation, coping skills, cognitive restructuring skills) were associated with less distress and higher quality of life ratings. Similar findings have emerged with breast cancer (Antoni et al., 2006) and prostate cancer (Penedo et al., 2004, 2006) patients. The present work extends this line by indicating that stress management skills such as relaxation, ability to cognitively reappraise stressful situations, and having good interpersonal skills, may be able to modify the influence of emotional distress on fatigue symptoms in CFS. Having these skills also appears to relate to some of the neuroimmune parameters that may maintain or exacerbate the course of CFS. This work provides justification for the use of stress management interventions in this population. These relationships were maintained irrespective of patient age or gender thus providing support for the potential generalizability of stress management interventions in CFS.
4.2. Stress management skills and neuroimmune dysfunction
We found that greater PSMS related to lower IL-2 levels and a greater cortisol slope. Given past research findings on high IL-2 levels in CFS patients (Patarca et al., 1994) and that higher levels of IL-2 could potentially indicate greater inflammatory activity (Smith, 1988) the participants with greater PSMS may have had an immune profile reflecting less underlying dysfunction. However, since we did not find consistent associations between PSMS and other pro-inflammatory cytokines that are more commonly used to reflect immune dysfunction, this isolated finding should be interpreted with caution. Given the association between greater PSMS and greater negative diurnal cortisol slope it is plausible that patients with greater PSMS may have evinced better hypothalamic pituitary adrenal (HPA) regulation (Sephton et al., 2000; Stone et al., 2001). Dysfunction of the HPA axis has been hypothesized to be associated with symptoms of CFS, including fatigue, and dysregulated cortisol production has been noted repeatedly in comparisons of CFS patients with healthy controls as observed by hypocortisolism, decreased awakening response, and a flatter diurnal cortisol slope (Addington, 2000; Demitrack and Crofford, 1998; Heim et al., 2009; Nater et al., 2008; Roberts et al., 2004). It is noteworthy that we did not find that degree of diurnal cortisol slope was associated with fatigue, though there have similarly been inconsistent findings regarding cortisol levels and fatigue within populations with CFS (Torres-Harding et al. 2008;Gaab et al., 2004; Rubin et al., 2005; ter ter Wolbeek et al., 2007). It is possible that HPA axis dysfunction is more relevant in the development of CFS than it is in the maintenance of CFS symptoms (Heim et al., 1999). It is possible that there is an aspect of endocrine-immune system modulation dependent on stress management skills that was not revealed in the present analyses. For instance it is plausible that persons with better stress management skills have less anxiety and negative mood, which in turn relates to lower evening cortisol levels and better HPA control over pro-inflammatory cytokine production. It is also possible that these relationships are bi-directional with mood or HPA functioning secondary to an inflammatory profile accompanying fatigue fluctuations. Prospective, time-lagged designs may help to elucidate the temporality of these processes.
4.3. Distress sensitive subgroups
Because prior work has identified immune-based subgroups of CFS patients (Siegel et al., 2006) we examined whether indicators of neuroimmune dysfunction could act as moderators of the PSMS-fatigue mediation model established in our initial observations. Specifically we hypothesized that the association between PSMS and fatigue via emotional distress would be most pronounced in cases that presented with the greatest neuroimmune dysfunction as indicated by elevated pro-inflammatory cytokine levels and reduced diurnal cortisol slope. Patients’ level of IL-6, as well as the ratio of IL-6:IL-10were found to moderate the effects of PSMS on fatigue such that our model was only significant in the subgroup of patients in the top tercile of IL-6 and IL-6:IL-10 ratio but not in those in the lower terciles (corresponding to normal IL-6 levels). There has been a well-documented elevation of IL-6, in some samples of CFS patients compared to healthy controls (Broderick et al., 2010; Carlo-Stella et al., 2006; Gaab et al., 2005). Given the consistently observed relationship between IL-6 and health status indicators in the literature (Collado-Hidalgo et al., 2006; Dantzer and Kelley, 2007; Davis et al., 2008; Lutgendorf et al., 1999; Thomas et al., 2011; Vollmet-Conna et al., 2004), it appears that IL-6 may be a plausible cytokine “biomarker” for designating a neuroimmune dysfunction subgroup of CFS patients who are especially sensitive to emotional distress responses as they relate to symptom exacerbation. Specifically, we found that the PSMS-Emotional Distress-Fatigue mediation model was increasingly significant in patients with IL-6 levels >11.9 pg/ml, which is clearly elevated relative to our sample of healthy controls (mean = 7.2 pg/ml). Cytokine assays were run in duplicate to ensure reliability within the present study. This is the first study to demonstrate the utility of creating subgroups of CFS patients defined byIL-6 levels alone and in combination with IL-10 levels, and may pave the way for more effort in the subgrouping line of work.
It should be kept in mind that there was no support that other neuroimmune indicators that we measured functioned in such a moderating role. It is plausible that patients with elevated IL-6 levels differ in some systematic way from CFS patients with other neuroimmune abnormalities, and that the IL-6-by-PSMS interaction effect on fatigue noted here may illuminate future pathways to pursue. It may be that these patients have co-morbid medical conditions, chronic stressors, or share demographic similarities. However, these findings demand replication before these avenues are pursued. It would have been interesting to examine the moderated mediation model in a sample of healthy controls but we did not have psychosocial information for the control sample. Finally, it should be kept in mind that we did not exhaust all of the ways in which one might examine immunologic subgroups of CFS patients. Because many different inflammatory cytokines are likely to act in concert, future work in systems biology may help to use cytokine network analyses (e.g., Broderick et al., 2010) to delineate other subgroups of CFS patients.
Results from the present study did not support the hypothesis that the relationship between emotional distress and fatigue is mediated by alterations in pro-inflammatory cytokines. However, we do not believe that the sickness behavior model (Kelley et al., 2003) has been disproven in patients with CFS, and assert that this model demands further examination. It is possible that the lack of findings is in part due to the fact that this study examined subjective emotional distress over the prior week, rather than having examined a more objective measure of stressors.
4.4. Limitations
Limitations of the study include the highly educated nature of the sample, the cross-sectional design, the lack of data for all potential covariates, the assessment of immune parameters at a single time point, and the reliance on self-report measures. While the criteria established by Fukuda and colleagues has been seen as more stringent than the criteria established by Reeves and colleagues (2005) and has been the diagnostic standard for the past two decades, CFS researchers have questioned the specificity of the Fukuda diagnostic system (Jason et al., 2004). An international case definition of myalgic encephalomyelitis (ME) was recently established that involves additional neurological and immunological criteria beyond those established by Fukuda and colleagues (Carruthers et al., 2011). Given the heterogeneous nature of illness in CFS patients, the definition published by Carruthers and colleagues aims to select a more homogenous group that has a common underlying pathophysiology (e.g., neuroimmune dysfunction). The number of participants in the current study who also met the new international case definition in addition to meeting the Fukuda case definition is unknown, but future work should include such criteria.
The patients included in the present study composed a highly educated sample. While this is not atypical of research studies on CFS (Jason et al., 2007; Servaes et al., 2000), it is possible that the findings may not generalize to a less educated sample. Additionally, we did not collect data regarding participants’ prior experiences in psychotherapy so we were unable to include past experience in therapy as a covariate for perceived stress management skills.
The measurement of immune parameters in the current study has a few limitations. While a single time point measurement of cytokines is commonly used for the sake of participant comfort and cost-effectiveness, there is a mixed literature on the presence of diurnal variations in cytokines such as IL-6. Some research has indicated that IL-6 is fairly stable throughout the day (Haack et al., 2002; Bollinger et al., 2010) while others have found that it fluctuates (Alesci et al., 2005; Bonda et al., 2010). Although the blood draw window from 11 am to 3 pm used in the present study was designed to limit potential diurnal variations, it is possible that variations existed within this window. Additionally, while age and gender were controlled for in our analyses, we did not have information regarding women’s menstrual status or body mass index (BMI) and were therefore unable to assess either as potential covariates. High BMI has been repeatedly linked to high levels of circulating pro-inflammatory cytokines, so the results of this study should be interpreted as preliminary findings that demand replication with more extensive controls (Himmerich et al., 2006; Panagiotakos et al., 2005). The lack of information regarding hormonal factors in our female participants may be considered a minor limitation as several studies have not found differences in many pro-inflammatory cytokines based upon menstrual phase, menopausal status, or oral contraceptive use (Al-Harthi et al., 2000; O’Brien et al., 2007; Willis et al., 2003).
Since all symptom and psychological measures were based on self-reports at the same point in time it is unclear whether stress management skills predated fluctuations in distress and fatigue. Because it is possible that fatigue levels could affect both perceptions of stress management skills and emotional distress, future studies should explore the longitudinal effects of changes in stress management skills on changes in emotional distress and fatigue over time. Similarly it would be fruitful to examine the relationship between cytokine levels and changes in PSMS, distress and fatigue in time-lagged designs to clarify the temporality of the relationships reported here.
5. Conclusion
In summary, these findings provide initial support for the notion that CFS patients having more confidence in their stress management skills may show less emotional distress and fatigue and that these associations are most pronounced in a subgroup of CFS patients presenting with indicators of neuroimmune dysfunction. This raises the possibility that teaching stress management skills to individuals with CFS may help them to reduce emotional distress, and reduce fatigue. While cognitive-behavioral therapy (CBT) interventions have long been used in the treatment of CFS and have been seen to reduce fatigue, they have generally been aimed at modifying illness beliefs in order to increase levels of physical activity among participants. However, a recent meta-analysis by Wibourg and colleagues (2010) demonstrated that physical activity does not mediate the effect of cognitive-behavioral therapy (CBT) on reductions in fatigue in CFS patients. Moreover, recent critiques of graded exercise interventions for CFS patients (White et al., 2011) have noted that using overly inclusive diagnostic criteria for CFS studies has created confusion in the literature concerning the efficacy of physical activity-based interventions for symptom management (Lancet, 2011). This lack of physical activity mediation found in Wibourg’s meta-analysis, and the ongoing controversy concerning diagnostic criteria in the CFS literature, together with the results from the present study, suggest that emotional distress reduction, rather than modification of beliefs surrounding physical activity, may mediate the relationship between CBT and fatigue reductions in certain subgroups of CFS patients.
Cognitive-behavioral stress management (CBSM) interventions have been shown to be effective in lowering emotional distress and modulating quality of life and health indicators in a variety of medical populations including individuals with HIV, men with prostate cancer, and women with breast cancer (Antoni and Weiss, 2003; Antoni et al., 2006a,b, 2009; Cruess et al., 2000; McGregor et al., 2004; Penedo et al., 2007). A small pilot study of CBSM for individuals with CFS in a separate sample from the present one, demonstrated CBSM’s ability to decrease symptom severity and perceived stress, in conjunction with improved self-reported quality of life (Lopez et al., 2011). Future research should examine whether such interventions can modulate stress management skills to lower emotional distress and fatigue symptoms in CFS patients, particularly in subgroups of individuals likely to derive the greatest benefits.
Acknowledgments
This work was supported by a Grant from the National Institutes of Health to MHA (1R01 NS055672).
References
- Addington JW. Chronic fatigue syndrome: a dysfunction of the hypothalamic–pituitary–adrenal axis. J Chron Fatigue Synd. 2000;7 (2):63–73. [Google Scholar]
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Al-Harthi L, Wright DJ, Anderson D, Cohen M, Matity, Ahu D, Cohn J, Cu-Unvin S, Burns D, Reichelderfer P, Lewis S, Beckner S, Kovacs A, Landay A. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res. 2000;20:719–724. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- Antoni MH. Stress management, psychoneuroimmunology and disease. In: Segerstrom S, editor. Psychoneuroimmunology. Oxford; NY: pp. 1121–1228. in press. [Google Scholar]
- Antoni MH, Weiss D. Stress, immunity, and chronic fatigue syndrome: a conceptual model to guide the development of treatment and research. In: Jason LA, Fennell PA, Taylor RR, editors. Handbook of Chronic Fatigue Syndrome and Fatiguing Illnesses. Wiley; New York: 2003. [Google Scholar]
- Antoni MH, Brickman A, Lutgendorf S, Klimas N, Imia-Fins A, Ironson G, Quillan R, Miguez MJ, van Riel F, Morgan R, Patarca R, Fletcher MA. Psychosocial correlates of illness burden in Chronic Fatigue Syndrome. Clin Infect Dis. 1994;18 (Suppl 1):S73–S78. doi: 10.1093/clinids/18.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Carrico AW, Duran RE, Spitzer S, Penedo F, Ironson G, Fletcher MA, Klimas N, Schneiderman N. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in. Psychosom Med. 2006a;68:143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, Phillips K, Gluck S, Carver CS. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006b;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor BA, Carver CS, Blomberg B. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23:580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26:24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Bollinger T, Bollinger A, Naujoks J, Lange T, Solbach W. The influence of regulatory T cells and diurnal hormone rhythms on T helper cell activity. Immunology. 2010;131:488–500. doi: 10.1111/j.1365-2567.2010.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda T, Kaminski KA, Kozuch M, Kozieraszka A, Wojtkowska I, Dobrzycki S, Kralisz P, Nowak K, Prokopczuk P, Musial WJ. Circadian variations of interleukin 6 in coronary circulations of patients with myocardial infarction. Cytokine. 2010;50:204–209. doi: 10.1016/j.cyto.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Borish L, Schmaling K, DiClementi JD, Streib J, Negri J, Jones JF. Chronic fatigue syndrome: identification of distinct subgroups on the basis of allergy and psychologic variables. J Allergy Clin Immunol. 1998;102 (2):222–230. doi: 10.1016/s0091-6749(98)70090-9. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21 (3):251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Brenu EW, van Driel ML, Staines DR, Ashton KJ, Ramos SB, Keane J, Klimas NG, Marshall-Gradisnik SM. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J Transl Med. 2011;9:81. doi: 10.1186/1479-5876-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in Chronic Fatigue Syndrome. Brain Behav Immun. 2010;24:1209–1217. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo-Stella N, Badulli C, De Silvestri A, Bazzichi L, Martinetti M, Lorusso L, Bombardieri S, Salvaneschi L, Cuccia M. A first study of cytokine genomic polymorphisms in CFS: positive association of TNF-857 and IFNgamma 874 rare alleles. Clin Exp Rheumatol. 2006;24 (2):179–182. [PubMed] [Google Scholar]
- Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles ACP, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisbik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;4:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. Measure of Current Status. 2006 < http://www.psy.miami.edu/faculty/ccarver/sclMOCS.html>.
- Chiswick EL, Duffy E, Japp B, Remick D. Detection and quantification of cytokines and other biomarkers. Methods Mol Biol. 2012;844:15–30. doi: 10.1007/978-1-61779-527-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers of persistent fatigue in breast cancer. Clin Cancer Res. 2006;12 (9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Corradi KM, Jason LA, Torres-Harding SR. Exploratory subgrouping in CFS: infectious, inflammatory, and other. In: Columbus A, editor. Adv Psychol Res. Vol. 41. Nova Science Publishers; Hauppauge, NY: 2006. pp. 115–127. [Google Scholar]
- Cruess DG, Antoni MH, McGregor BA, Boyers A, Kumar M, Kilbourn K. Cognitive-behavioral stress management reduces serum cortisol by enhancing positive contributions among women being treated for early stage breast cancer. Psychosom Med. 2000;62:304–308. doi: 10.1097/00006842-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demitrack M, Crofford L. Evidence for and pathophysiological implications of hypothalamic pituitary adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci. 1998;840:684–697. doi: 10.1111/j.1749-6632.1998.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Faul LA, Jim HS, Williams C, Loftus L, Jacobsen PB. Relationship of stress management skill to psychological distress and quality of life in adults with cancer. Psychooncology. 2010;19:102–109. doi: 10.1002/pon.1547. [DOI] [PubMed] [Google Scholar]
- Faulkner S, Smith A. A longitudinal study of the relationship between psychological distress and recurrence of upper respiratory tract infections in chronic fatigue syndrome. Br J Health Psychol. 2008;13 (1):177–186. doi: 10.1348/135910706X171469. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP): Biometrics. Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. doi: 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher MA, Rosenthal M, Antoni M, Ironson G, Zeng XR, Barnes Z, Harvey JM, Hurwitz B, Levis S, Broderick G, Klimas NG. Plasma neuropeptide Y: a biomarker for symptom severity in chronic fatigue syndrome. Behavioral and Brain Functions. 2010;6:76. doi: 10.1186/1744-9081-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A International Chronic Fatigue Syndrome Study Group. The Chronic Fatigue Syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121 (12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gaab J, Engert V, Heitz V, Schad T, Schürmeyer TH, Ehlert U. Associations between neuroendocrine responses to the Insulin Tolerance Test and patient characteristics in Chronic Fatigue Syndrome. J Psychosom Res. 2004;56 (4):419–424. doi: 10.1016/S0022-3999(03)00625-1. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schürmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation: relevance to viral infections and chronic fatigue syndrome. Am J Med. 1998;105 (3):35S–42S. doi: 10.1016/s0002-9343(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, Greenberg H, Lyman G. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 1999;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. 2009;66:72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- Hickie I, Lloyd A, Hadzi-Pavlovic D, Parker G, Bird K, Wakefield D. Can the chronic fatigue syndrome by defined by distinct clinical features? Psychol Med. 1995;25:925–935. doi: 10.1017/s0033291700037417. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Pollmacher T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17:196–201. [PubMed] [Google Scholar]
- Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, McCready W, Huang CF, Plioplys S. A community-based study of Chronic Fatigue Syndrome. Arch Intern Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- Jason LA, Torres-Harding SR, Jurgens A, Helgerson J. Comparing the Fukuda et al criteria and the Canadian case definition for Chronic Fatigue Syndrome. J Chron Fatigue Synd. 2004;12 (1):37–52. [Google Scholar]
- Jason LA, Torres-Harding SR, Friedberg F, Corradi K, Njoku MG, Donalek J, Reynolds N, Brown M, Weitner BB, Rademaker A. Non-pharmacologic interventions for CFS: a randomized trial. J Clin Psychol Med Settings. 2007;14:275–296. [Google Scholar]
- Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005;87:299–304. doi: 10.1016/j.jad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17:S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Klimas NG, Koneru AO. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr Rheumatol Rep. 2007;9 (6):482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- Lancet. Patients’ power and PACE. The Lancet. 2011;377:1808. doi: 10.1016/S0140-6736(11)60696-X. [DOI] [PubMed] [Google Scholar]
- Lombardi VC, Ruscetti FW, Gupta JD, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petro-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA. Detection of an infectious retrovirus, XMRV, in the blood cells of patients with Chronic Fatigue Syndrome. Science. 2009;326 (5952):585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- Lopez C, Antoni M, Penedo F, Weiss D, Cruess S, Segotas MC, Helder L, Siegel S, Klimas N, Fletcher MA. A pilot study of cognitive behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. J Psychosom Res. 2011;70 (4):328–334. doi: 10.1016/j.jpsychores.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G. Immunological aspects of chronic fatigue syndrome. Autoimmun Rev. 2009;8 (4):287–291. doi: 10.1016/j.autrev.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Antoni MH, Fletcher MA, Penedo F, Baum A, Schneiderman N, Klimas N. Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew. Psychosom Med. 1995;57 (4):310–323. doi: 10.1097/00006842-199507000-00002. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol. 1999;54:M434–439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Song C, Lin C, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10 (4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppelman LF. Manual: Profile of Mood States. Educational and Industry Testing Service (EDITS); San Diego, CA: 1971. [Google Scholar]
- Moss RB, Merchandetti A, Vojdani A. TNF-a and chronic fatigue syndrome. J Clin Immunol. 1999;19:314–316. doi: 10.1023/a:1020595709352. [DOI] [PubMed] [Google Scholar]
- Nas K, Cevik R, Batum S, Sarac AJ, Acar S, Kalkanli S. Immunologic and psychosocial status in Chronic Fatigue Syndrome. Bratislava Med J. 2011;112 (4):208–212. [PubMed] [Google Scholar]
- Nater UM, Youngblood LS, Jones JF, Unger ER, Miller AH, Reeves WC, Heim C. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with Chronic Fatigue Syndrome. Psychosom Med. 2008;70 (3):298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- Njoku MG, Jason LA, Torres-Harding The prevalence of chronic fatigue syndrome in Nigeria. J Health Psychol. 2007;12 (3):461–474. doi: 10.1177/1359105307076233. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuro Immuno Modulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Yannakoulia M, Crysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Patarca R, Klimas NG, Lutgendorf S, Antoni M, Fletcher MA. Dysregulated expression of tumor necrosis factor in Chronic Fatigue Syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin Infect Dis. 1994;18:S147–S153. doi: 10.1093/clinids/18.supplement_1.s147. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR, Molton I, Gonzalez JS, Roos BA, Carver CS, Schneiderman N, Antoni M. Cognitive-behavioral stress management improves stress-management skills and quality of life in men recovering from treatment of prostate carcinoma. Cancer. 2004;100 (1):192–200. doi: 10.1002/cncr.11894. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Molton I, Dahn JR, Shen BJ, Kinsinger D, Traeger L, Siegel S, Schneiderman N, Antoni M. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31 (3):261–270. doi: 10.1207/s15324796abm3103_8. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Traeger L, Dahn JR, Molton I, Gonzalez JS, Schneiderman N, Antoni M. Cognitive behavioral stress management intervention improves quality of life in Spanish monolingual Hispanic men treated for localized prostate cancer: results of a randomized control trial. Int J Behav Med. 2007;14 (3):164–172. doi: 10.1007/BF03000188. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23 (10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Porter N, Lerch A, Jason LA, Sorenson M, Fletcher MA, Herrington J. A comparison of immune functionality in viral versus non-viral CFS subtypes. J Behav Neurosci Res. 2010;8 (2):1–8. [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40 (3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivar Behav Res. 2007;42 (1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1 (3):385–401. [Google Scholar]
- Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, Papanicolaou DA, Unger ER, Vernon SD, Heim C. Chronic fatigue syndrome – a clinically empirical approach to its definition and study. BMC Med. 2005;3(19) doi: 10.1186/1741-7015-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Nisenbaum R, Hoaglin DC, Unger ER, Emmons C, Randall B, Stewart JA, Abbey S, Jones JF, Gantz N, Minden S, Reeves WC. Prevalence and incidence of Chronic Fatigue Syndrome in Wichita, Kansas. Arch Intern Med. 2003;163:1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- Roberts ADL, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. 2004;184:135–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic, and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;86 (6):1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Rubin GJ, Hotopf M, Papadopoulos A, Cleare A. Salivary cortisol as a predictor of postoperative fatigue. Psychosom Med. 2005;67 (3):441–447. doi: 10.1097/01.psy.0000161207.73744.4e. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Servaes P, van der Werf S, Prins J, Verhagen S, Bleijenberg G. Fatigue in disease-free cancer patients compared with fatigue in patients with Chronic Fatigue Syndrome. Support Care Cancer. 2000;9:11–17. doi: 10.1007/s005200000165. [DOI] [PubMed] [Google Scholar]
- Servaes P, Prins J, Verhagen S, Bleigenberg G. Fatigue after breast cancer and in chronic fatigue syndrome: similarities and differences. J Psychosom Res. 2002;52 (6):453–459. doi: 10.1016/s0022-3999(02)00300-8. [DOI] [PubMed] [Google Scholar]
- Siegel S, Antoni MH, Fletcher MA, Maher K, Segota MC, Klimas N. Impaired natural Immunity, cognitive dysfunction, and physical symptoms in patients with Chronic Fatigue Syndrome: preliminary evidence for a subgroup? J Psychosom Res. 2006;60:559–566. doi: 10.1016/j.jpsychores.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240 (4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci. 2001;101:185–192. [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirshbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- ter Wolbeek M, van Doornen LJ, Coffeng LE, Kavelaars A, Heijnen CJ. Cortisol and severe fatigue: a longitudinal study in adolescent girls. Psychoneuroendocrinology. 2007;32 (2):171–182. doi: 10.1016/j.psyneuen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun. 2011;25:53–58. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A, Joudoi T, Rabab EM, Matsumoto T, Park TH, Miike T. Cytokine production and modulation: comparison of patients with chronic fatigue syndrome and normal controls. Psychiatry Res. 2005;134 (1):101–104. doi: 10.1016/j.psychres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Torres-Harding S, Sorenson M, Jason L, Reynolds N, Brown M, Maher K, Fletcher MA. The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res. 2008;13 (3):157–180. doi: 10.1111/j.1751-9861.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Larrain BE, Hausman FA, Kempton JB, MacArthur CJ. Simultaneous measurement of multiple ear proteins with multiplex ELISA assays. Hear Res. 2011;275:1–7. doi: 10.1016/j.heares.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, Davenport T, Wakefield D, Hickie J, Lloyd A. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med. 2004;34:1289–1297. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- Walker K, Lindner H, Noonan M. The role of coping in the relationship between depression and illness severity in Chronic Fatigue Syndrome. J Allied Health. 2009;38 (2):91–99. [PubMed] [Google Scholar]
- White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, Baber HL, Burgess M, Clark LV, Cox DL, Bavinton J, Angus BJ, Murphy G, Murphy M, O’Dowd H, Wilks D, McCrone P, Chalder T, Sharpe M. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. The Lancet. 2011;377:823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibourg JF, Knoop H, Stulemeijer M, Prins JB, Bleijenberg G. How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychol Med. 2010;40 (8):1281–1287. doi: 10.1017/S0033291709992212. [DOI] [PubMed] [Google Scholar]
- Willis C, Morris JM, Danis V, Gallery ED. Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod. 2003;18:1173–1178. doi: 10.1093/humrep/deg231. [DOI] [PubMed] [Google Scholar]
- Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–3456. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40 (2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]


