A major hindrance in the paediatric HIV field is the limited evidence-base to guide practice. Large randomised paediatric studies are difficult to do, because of the complex medical and social issues and limited global expertise. The randomised Children with HIV Early Antiretroviral Therapy Study showed benefit with the early treatment of children under 1 year of age,1 but no randomised study results are available for children older than this.2 The Thai and Cambodian guidelines follow those of WHO, which are based on data from non-randomised large cohorts, and expert advice.3
The Comprehensive International Program of Research on AIDS (CIPRA) supports research in resource-limited countries to prevent and treat HIV/AIDS. Such support provides a unique opportunity for countries to develop their research infrastructure and expertise, while undertaking studies that are relevant to the country and the region. Our group, the HIV Netherlands Australia Thailand Research Collaboration, in partnership with the National Center for HIV/AIDS Dermatology and STDs in Cambodia, was awarded the CIPRA grant by the US National Institute of Allergy and Infectious Diseases (NIAID) to study when to start antiretroviral therapy in children aged between 1 year and 12 years. Under current Thai guidelines, antiretrovirals are started when CD4 is below 20% in children ages 1 year to 3 years and below 15% in older children. We hypothesise that antiretrovirals could be deferred until CD4+ cell-count falls below 15% in both asymptomatic and mildly symptomatic children with moderate immune suppression (CD4+ 15–24%), without affecting their prognosis. 300 children have been randomised to immediate antiretroviral therapy in the control group (CD4+ 15–24%) versus deferred therapy (CD4+ <15%). PREDICT (Paediatric Randomised to Early versus Deferred Antiretroviral Initiation in Cambodia and Thailand) will complete enrolment in July of this year, and results are expected in 2011.
Here we share our experience in doing a large randomised paediatric study. With the support of NIAID, we built a network of eight Thai and two Cambodian sites to achieve international research standards. Although our site had experience in HIV clinical trials, we encountered a difficult learning curve; and coordinating the PREDICT study taught important lessons.
There were concerns among local community groups on the randomised nature of the trial, the differences between country guidelines and the trial’s protocol about when to start antiretrovirals, and on the enrolment of orphans. Thai law allows orphans to participate in clinical trials as long as they have a primary caregiver to give consent on their behalf. Cambodian law does not address this issue, and therefore we chose not to enroll Cambodian orphans. Community involvement throughout the process, from the early steps of protocol and preparation of the consent form to recruitment and trial conduct, helped to resolve these issues. We also greatly underestimated the time it would take to start the study. We allowed for 6 months of preparation in the grant proposal. In reality, from the award’s announcement, it took 15 months for protocol development and ethics committee approval, 22 months for first enrolment, and 30 months for NIAID approval of all sites (figure). By the time we received NIAID authorisation to begin enrolment, 6550 Thai and 648 Cambodian children had received antiretroviral therapy under the national programmes, which greatly affected the number of participants eligible for inclusion in the study. Furthermore, the preparation process was expensive.
Figure.
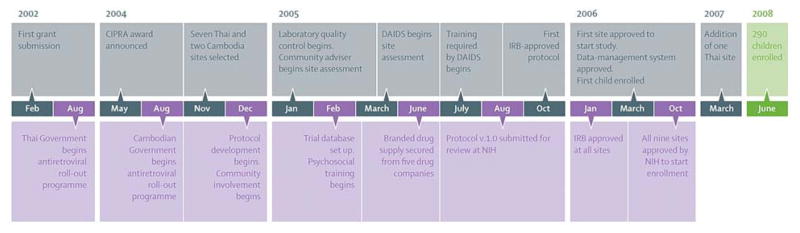
Time line for the PREDICT study
DAIDS=US NIH Division of AIDS. IRB=institutional review board. NIH=US National Institutes of Health.
Since PREDICT started, treatment guidelines continue to change. Regular discussions of the trial’s relevance and need for modifications, among investigators, the community, funding agencies, and the data-safety and monitoring board, are important. We feel that there should be a forum for researchers and funding agencies to set goals and practices, for best use resources and time, to move forward the paediatric HIV research agenda in developing countries. Furthermore, we suggest grant agencies should provide new grantees with written information on procedures, timelines for protocol and site approval, and a separate budget for the preparation stage to allow research sites in resource-limited settings to build their capacity to do studies at international standards. Mentorship from grant agencies to new grantees will also prove invaluable to advance paediatric HIV research.
Acknowledgments
We thank the US National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health and the National Institute of Child Health and Human Development for funding the PREDICT study. We also thank GlaxoSmithKline, Boehringer Ingelheim, Abbott, Merck, and Roche for donating antiretroviral drugs.
Footnotes
We declare that we have no conflict of interest.
References
- 1.Violari A, Cotton M, Gibb D, et al. on behalf of the CHER Study Team. Antiretroviral therapy initiated before 12 weeks of age reduces early mortality in young HIV-infected infants: evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study. 4th IAS Conference on HIV Pathogenesis and Treatment; Sydney, Australia. July 22–25, 2007; [accessed July 9, 2008]. http://www.ias2007.org/pag/Abstracts.aspx?AID=5557. [Google Scholar]
- 2.UNAIDS. [accessed Dec 12, 2007];2006 report on the global AIDS epidemic. 2006 May 30; http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
- 3.WHO. [accessed July 9, 2008];Antiretroviral therapy of HIV infection in infants and children in resource-limited settings, towards universal access: recommendations for a public health approach. 2006 http://www.who.int/hiv/mediacentre/fs_2006guidelines_paediatric/en/index.html.


