Abstract
Metastasis is a characteristic trait of most tumour types and the cause for the majority of cancer deaths. Many tumour types, including melanoma and breast and prostate cancers, first metastasize via lymphatic vessels to their regional lymph nodes. Although the connection between lymph node metastases and shorter survival times of patients was made decades ago, the active involvement of the lymphatic system in cancer, metastasis has been unravelled only recently, after molecular markers of lymphatic vessels were identified. A growing body of evidence indicates that tumour-induced lymphangiogenesis is a predictive indicator of metastasis to lymph nodes and might also be a target for prevention of metastasis. This article reviews the current understanding of lymphangiogenesis in cancer anti-lymphangiogenic strategies for prevention and therapy of metastatic disease, quantification of lymphangiogenesis for the prognosis and diagnosis of metastasis and in vivo imaging technologies for the assessment of lymphatic vessels, drainage and lymph nodes.
Keywords: lymphangiogenesis, cancer metastasis, cancer metastasis prediction and prevention, imaging of lymphatic vessels
Anatomy and function of the lymphatic system
Tumour-induced lymphangiogenesis promotes metastasis
Tumour-induced lymph node lymphangiogenesis precedes cancer metastasis to lymph nodes
Tumour cell invasion into lymphatic vessels
Peri- and intra-tumoral lymphangiogenesis
Lymphatic vessel markers and lymphangiogenic growth factors
Preventive and therapeutic potential of anti-lymphangiogenic treatment
-
Tumour lymphangiogenesis as a prognostic indicator for lymph node metastasis
- Lymphatic vessel density
- Lymphatic invasion by tumour cells
- Lymphangiogenic growth factors
- Lymph node lymphangiogenesis
Diagnostic value of tumour-associated lymphangiogenesis
Imaging of lymphatic vessels
Imaging lymphatic drainage
Non-invasive imaging of lymph node metastases
Anatomy and function of the lymphatic system
The lymphatic system consists of lymphatic vessels and the lymphoid organs, which include lymph nodes, tonsils, spleen, Peyer's patches and the thymus. This system regulates tissue pressure by draining extravasated fluid back into the blood circulation. Almost all tissues are penetrated by lymphatic capillaries except some avascular tissues such as the epidermis, cartilage, cornea, retina, hair and nails and some vascularized organs including the brain and the retina [1]. Lymphatic capillaries consist of a single layer of overlapping leave-like shaped lymphatic endothelial cells (LECs) and their ends open into the tissue periphery. They are attached to the extracellular matrix by filaments, and it is assumed that through these connections, lymphatic vessels are forced to open when interstitial tissue pressure rises upon fluid extravasation from the blood stream [2]. Interstitial fluid, molecules and cells enter the lymphatic capillaries between discontinuous button like cell junctions [3]. The drained fluid, also known as lymph, is moved through lymphatic capillaries by skeletal muscle action and respiratory movements into larger collecting vessels that are surrounded by a basement membrane and flow-promoting smooth muscle cells. Eventually the lymph is released into the blood stream via the thoracic duct, at the junction of the jugular and subclavian veins. On the way from the peripheral tissues to the blood stream, the lymph fluid passes a cascade of lymph nodes.
In addition to its function in tissue pressure regulation, the lymphatic system is an important component of the immune system. Soluble antigens and antigen-presenting cells are transported via lymphatic vessels into lymphoid organs including the lymph nodes, tonsils, spleen and Peyer's patches, where immune responses are initiated. In addition, specialized lymphatic vessels, the lacteals, are involved in the absorption of dietary fat and fat soluble vitamins [1].
Tumour-induced lymphangiogenesis promotes metastasis
The lymphatic system gained much attention when tumour-associated lymphangiogenesis was correlated with metastasis to draining lymph nodes in several mouse models; in these models, tumours expressed the lymphangiogenic growth factors vascular endothelial growth factor (VEGF)-A, VEGF-C or VEGF-D [4–8]. Since then, VEGF-A, -C and -D have been detected in a range of human tumour types, such as melanoma and breast, cervical, non-small-cell lung, prostate, colorectal and gastric cancers. Furthermore, tumour-associated lymphatic vessel density was correlated with metastasis to the draining lymph nodes, distant metastases and poor prognosis [9–12]. These studies suggested that expression of lymphangiogenic growth factors by tumour cells induces lymphangiogenesis, which promotes metastasis (via lymphatic vessels) to draining lymph nodes and beyond. Furthermore, the initiation of lymphatic metastasis correlated with tumour lymphangiogenesis in a human lung cancer xenograft model in mice (Fig. 1) [13].
Fig. 1.
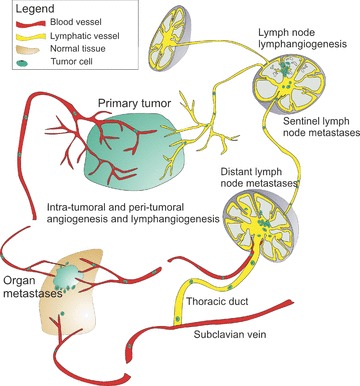
Tumour-induced lymphangiogenesis at the tumour site and in tumour draining lymph nodes promotes metastasis to lymph nodes and beyond.
In addition to increasing the number of lymphatic vessels, tumours also induce lymphatic vessel enlargement, which has been associated with increased passage of tumour cell clusters to sentinel lymph nodes in mouse and human tumours [13–19]. Lymphatic vessel enlargement is at least partly mediated by VEGF-C, produced by tumour cells, which was demonstrated in mouse models of melanoma and lung cancer xenografts [13, 20].
In contrast to angiogenesis, lymphangiogenesis in tumours is likely to be almost exclusively due to the proliferation and sprouting of pre-existing vessels, rather than incorporation of circulating endothelial progenitor cells. In mice that were sub-lethally irradiated and then given grafts of green fluorescent protein (GFP)-expressing bone marrow cells, the GFP-expressing cells did not incorporate into the newly formed lymphatic vessels induced by Lewis lung carcinoma xenotransplants or by VEGF-C delivery [21]. However, studies in inflamed mouse corneas or those exposed to fibroblast growth factor-2 (FGF-2) and human kidney transplants indicate that a minor number of bone-marrow derived cells, proposed to be macrophages, might contribute to the growth of lymphatic vessels [22–24].
Tumour-induced lymph node lymphangiogenesis precedes cancer metastasis to lymph nodes
In mouse models of skin carcinogenesis in which VEGF-A or VEGF-C was overexpressed in the skin, lymphangiogenesis was found to occur not only at the tumour site but also in the tumour-draining lymph nodes and to correlate with metastasis to lymph nodes and beyond [7, 8]. Interestingly, the expansion of the lymphatic vasculature in the sentinel lymph node was initiated before cancer cells arrived at these sites, indicating that VEGF-A and VEGF-C from the tumour sites drained to the lymph nodes and induced lymphangiogenesis there. Once metastatic cells reached the lymph nodes, lymphangiogenesis increased (Fig. 1) [7, 8]. Expansion of the lymphatic vasculature in pre-metastatic lymph nodes has been confirmed in mouse models of malignant melanoma, xenographic nasopharyngeal and breast carcinoma [25, 26], indicating that lymphangiogenesis in the pre-metastatic lymph node fosters tumour dissemination by creating a favourable environment for cancer cells. Tumour-induced lymph node lymphangiogenesis also occurs in cancer patients. Prominent hot spots of lymphangiogenesis were detected in metastasized lymph nodes of human melanoma patients and lymphangiogenesis in metastatic auxiliary lymph nodes of breast cancer patients was correlated with metastasis to non-sentinel auxiliary lymph nodes [27, 28].
Tumour cell invasion into lymphatic vessels
Tumour-induced lymphangiogenesis is likely to promote tumour cell dissemination by increasing the number of entry sites into the lymphatic system. Cancer cells seem to exploit a mechanism that immune cells use to gain access to lymphatic vessels: lymphatic entry is induced in activated dendritic cells by interaction of the receptor C-C chemokine receptor type 7 (CCR7) with the chemokine C-C motif chemokine 21 (CCL21), which is constitutively expressed by the lymphatic endothelium and the lymph nodes [29]. CCR7 is as well highly expressed by several breast cancer and malignant melanoma cell lines; metastatic melanoma cell lines that express CCR7 chemotax towards CCL21 in vitro and towards CCL21-secreting LECs in vivo[30, 31]. Importantly, over-expression of CCR7 in murine B16 melanoma cells promoted metastasis to draining lymph nodes [32].
Peri- and intra-tumoral lymphangiogenesis
Lymphangiogenesis occurs at the tumour periphery and in the inner-tumour cell mass. Although it is obvious that peri-tumoral lymphatic vessels are involved in metastasis, it is not clear if, and to what extent, intra-tumoral lymphatic vessels are involved in this process, because they are often collapsed or might be occluded by tumour cells [12]. Ferritin injected into tumours was not efficiently drained into intra-tumoral lymphatic vessels [33] and specific inhibition of intra-tumoral lymphatic vessels did not prevent metastasis to lymph nodes in mouse models of prostate cancer. Also, intra-tumoral lymphatic vessel density did not correlate with lymph node metastasis in oral cavity or laryngeal carcinomas [34, 35]. In contrast, in human malignant melanomas, oropharyngeal carcinomas and head and neck squamous cell carcinomas, intra-tumoral lymphatic vessel density was correlated with lymph node metastasis [27, 35, 36].
Lymphatic vessel markers and lymphangiogenic growth factors
The discovery of novel markers to distinguish blood and lymphatic vessels enabled the investigation of lymphatic vessels and lymphangiogenesis in health and disease. The first markers identified were the homeobox transcription factor Prox1 [37, 38], the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) [39, 40], podoplanin [41] and vascular endothelial growth factor receptor-3 (VEGFR-3) [42]. Prox1, a transcription factor, is activated in a subset of venous endothelial cells during embryogenesis and commits these cells to the lymphatic lineage [37, 43–45]. LYVE-1 is a cell membrane protein of unknown function and is the earliest marker of LECs in development [46, 47] (Fig. 2). Podoplanin, another cell membrane protein, is involved in proper lymphatic vessel development and might have a role in carcinoma formation [48, 49]. VEGFR-3 is a cell membrane receptor for VEGF-C and VEGF-D and is essential for vascular development [42, 50]. These markers are discussed in more detail in a recent publication [51].
Fig. 2.
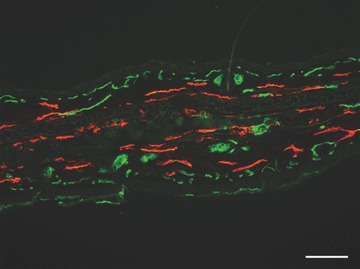
Immunofluorescence staining for markers of lymphatic and blood vessels on a mouse ear section. Lymphatic vessels are stained with an antibody to LYVE-1 (red) and blood vessels are stained with Meca-32 (green). Scale bar = 100 μm
These markers have been used to isolate LECs from human skin. Cultured LECs have been analysed by transcriptional and proteomic profiling to identify additional markers [52–55]. A recent study reported the isolation of mouse LECs from normal skin and from metastatic fibrosarcoma. After in vitro cultivation, transcriptional profiling of these cells revealed markers that were up-regulated on tumour-associated lymphatic vessels, compared to markers of normal vessels such as endothelial-specific adhesion molecule, endoglin and CD200 [55].
A range of soluble factors induce lymphangiogenesis (Table 1). The first lymphangiogenic growth factors identified were VEGF-C and VEGF-D, which bind to VEGFR-3 on LECs and induce receptor phosphorylation and downstream signalling [56, 57]. VEGF-A was shown to induce lymphangiogenesis by binding to VEGFR-2 on LECs [7, 58]. Additional lymphangiogenic growth factors include hepatocyte growth factor, which binds to the c-met receptor [59], angiopoietin-1 (Ang-1) binding to its receptor Tie2 [60, 61], as well as FGF-1 and -2 [62, 63], insulin-like growth factors 1 and 2 (IGF-1 and -2) [64], platelet derived growth factors (PDGF) [65], adrenomedullin (AM) [66], growth hormone (GH) [67] and endothelin-1 (ET-1) [68]. Recently, transforming growth factor-β1 (TGF-β1) has been identified to be a negative regulator of lymphangiogenesis in wound healing [69]. The relative contribution of these different factors towards lymphangiogenesis and their interplay remains to be elucidated.
Table 1.
Lymphangiogenic growth factors
| Growth factor | Receptors on lymphatic vessels | References |
|---|---|---|
| VEGF-A | VEGFR-2 | [58] |
| VEGF-C | VEGFR-2, VEGFR-3 | [56] |
| VEGF-D | VEGFR-2, VEGFR-3 | [57] |
| Hepatocyte growth factor | c-met | [59] |
| Ang-1 | Tie-2 | [60, 61] |
| FGF | FGFR-3 and possibly others | [63] |
| PDGF-BB | PDGFR-α and -β | [65] |
| GH | Growth hormone receptor Calcitonin receptor-like receptor | [67] |
| AM | (calcrl) associated with receptor activity-modifying protein 2 | [148] |
| ET-1 | Endothelin B receptor | [68] |
Lymphangiogenic growth factors are secreted not only by tumour cells but also by tumour-associated stromal cells, in particular tumour-associated macrophages, which has been reported for example in mice bearing human gastric carcinoma xenotransplants [70] and in human melanoma [27]. In human cervical cancer, VEGF-C and VEGF-D expression by macrophages correlated with lymphatic vessel density and frequency of lymph node metastasis [71]. Immunization with the model antigen keyhole limpet haemocyanin in complete Freund's adjuvant was observed to induce B cells to produce VEGF-A [72] whereas dendritic cells, macrophages and neutrophils express VEGF-C in mice with airway inflammation [73].
Preventive and therapeutic potential of anti-lymphangiogenic treatment
There have been several important studies performed to determine whether anti-lymphangiogenic agents can prevent metastasis to lymph nodes. Similar to angiogenesis, lymphangiogenesis does not occur in adults, except in the endometrium during pregnancy and in pathological settings such as chronic inflammation, tissue repair, and tumour growth [1, 74, 75]. Therefore, anti-lymphangiogenic strategies are expected not to interfere with normal lymphatic vessel function. Several studies in experimental cancer models showed that inhibition of lymphangiogenesis reduced the incidence of metastasis to lymph nodes; in most of these studies, the VEGF-C/VEGF-D/VEGFR-3 axis was inhibited before the onset of metastasis. Tumour lymphangiogenesis and lymph node metastasis were reduced by VEGFR-3 blocking antibodies in a mouse model of breast carcinoma and an orthotopic model of gastric cancer [76, 77], by a VEGF-D neutralizing antibody in a tumour model of EBNA 293 (cells expressed VEGF-D) [6], by a soluble VEGFR-3 fusion protein (a ‘VEGF-C/D trap’) in models of melanoma, gastric, prostate, breast and lung cancer [78–80], and by VEGF-C small interfering (si)RNA in a breast cancer model and in gastric cancer xenografts [81, 82]. Recently, an anti-neuropilin-2 antibody was shown to inhibit tumour-induced lymphangiogenesis in experimental mammary and brain tumour models and to delay metastasis to sentinel lymph nodes [83]. Neuropilin-2 is a cell-surface transmembrane protein expressed on tumour-associated but not normal lymphatic vessels [83]. It functions as a co-receptor for VEGF-C and VEGF-A [84] and is not required for developmental lymphangiogenesis [85].
Inhibition of the VEGF-C/-D/VEGFR-3 pathway in adult mice did not affect normal lymphatic vessels [86] and was not reported to induce oedema formation, so this appears to be a relatively safe therapeutic strategy. However, although targeting the VEGF-C/VEGF-D/VEGFR-3 axis efficiently blocks lymphangiogenesis and reduces metastasis to the lymph nodes, it does not completely inhibit formation of metastases in lymph nodes. Furthermore, long-term expression of VEGFR-3-IgG inhibited macrometastasis but not micrometastasis [13]. This indicates that complete blockage of lymphatic metastasis could require targeting of additional lymphangiogenic pathways and of the mechanism of lymphatic vessel invasion by tumour cells. Recently etodolac, an inhibitor of cyclooxygenase-2, inhibited lymphangiogenesis and lymph node metastasis in a mouse model of gastric cancer. Etodolac decreased VEGF-C and – D levels in tumour-associated macrophages, suggesting that reducing macrophage-mediated tumour lymphangiogenesis might help prevent lymph node metastasis [70].
In models of inflammation and wound repair, several anti-lymphangiogenic strategies have been successful – these strategies might also be efficient in inhibiting tumour-induced lymphangiogenesis. A small molecule antagonist of the integrin subunit α5 inhibited inflammation-induced lymphangiogenesis in the mouse cornea [87], antibodies to integrin subunits α1 or α2 blocked VEGF-induced lymphangiogenesis in mouse skin [88], and a specific antagonist of the endothelin receptor type B decreased lymphangiogenesis in a matrigel plug assay in mice [68]. Moreover, siRNA knockdown of endothelial-specific molecule-1 blocked in vivo lymphangiogenesis in matrigel assays [54] and a recent study indicated that transforming growth factor (TGF)-β1 inhibits lymphangiogenesis during wound repair [69]. Additional targets might be revealed by characterizing proteins that transcriptional and proteomic profiling studies showed were expressed on activated LEC [52–55].
The ability of anti-lymphangiogenic agents to prevent the early stages of metastasis has been established in animal models. However, the effect of anti-lymphangiogenic therapies on established metastases is less clear. Blockade of lymphangiogenesis by adenoviral delivery of VEGFR-3-IgG after the tumour cells had already spread out did not suppress lymph node metastasis in lung cancer xenografts in mice [13]. The preventative and interventional effects of a blocking antibody against VEGFR-3 were recently investigated in a mouse xenograft model of melanoma. Although lymph node metastasis was almost completely blocked when the blocking antibody was administered at the time of tumour cell injection, the lymph node tumour burden was 47% lower in mice that were given antibody after the tumour cells had already started to spread [76]. It remains to be determined whether anti-lymphangiogenic treatment can prevent metastasis from lymph nodes to distant organs.
Tumour lymphangiogenesis as a prognostic indicator for lymph node metastasis
Malignant tumours, including melanoma and breast and prostate cancers, often metastasize first via lymphatic vessels to their regional lymph nodes [89]. The status of regional lymph nodes is an important parameter to determine the stage of disease progression in different tumour types, including breast cancer and melanoma. Lymph node status is used to predict patient survival times and determine their clinical management strategy [10, 90]. To determine the node status, regional lymph nodes (or only sentinel lymph nodes in the case of breast cancer and melanoma patients) are removed and sections are analysed to detect metastases. The procedure of lymph node dissection and examination is elaborate and is associated with significant morbidity and costs [91–93]. In addition, tumour-draining lymph nodes can be missed, resulting in false prognoses. Several non-invasive methods are currently available to discern normal lymph nodes from those with metastases; however, they are not sensitive enough to detect micrometastases [94]. In addition, there is no solid method for predicting disease progression in patients with small tumours without lymph node involvement. Therefore, more reliable markers that can predict lymph node metastasis in early and advanced cancers are needed for therapeutic decision-making [10]. Several novel lymphangiogenesis parameters might be more sensitive prognostic indicators of metastasis to lymph nodes and disease progression than current methods. These include lymphatic vessel density at tumour sites and in tumour draining lymph nodes, expression of lymphangiogenic growth factors, invasion of cancer cells into lymphatic vessels and altered lymph flow and volume in tumour draining lymph nodes.
Lymphatic vessel density
An increase in tumour-associated lymphatic vessel density correlates with lymph node metastasis and unfavourable prognosis in a number of human cancer types, including melanoma and breast, gastric, colorectal, bladder, cervical, ovarian, head and neck, and non-small cell lung cancers [9, 10, 12]. In particular, in studies of human cutaneous melanoma, lymphangiogenesis was the most sensitive prognostic indicator for lymph node metastasis – detection of lymphangiogenesis was more sensitive prognostic factor than measurement of tumour thickness [15, 27]. The density of intra- and peri-tumoral lymphatic vessels and the lymphatic vessel area correlated with shortened disease-free and overall survival times [15, 27]. Thus, quantification of tumour-associated lymphatic vessel density is a promising tool for predicting metastasis of various cancers. However, this method could vary in reproducibility; it relies on counting lymphatic vessels in ‘hot spot’ regions of lymphangiogenesis in tissue sections, but finding the relevant hot spots depends on the training and experience of the investigator [10]. Recently, guidelines for standardized assessment of lymphangiogenesis in human beings have been recommended [10]. By applying these guidelines, intra- and inter-observer variability might decrease and thus quantification of lymphatic vessel density might become a reliable prognostic for metastasis. However, the most sensitive and reliable method for quantifying tumour lymphangiogenesis remains to be determined.
Lymphatic invasion by tumour cells
Studies in human breast cancer revealed that tumour cell invasion into lymphatic vessels predicted lymph node involvement and was a prognostic factor for overall and disease-free survival [95, 96]. In accordance with these findings, lymphatic vessel invasion was found to be a prognostic indicator of lymph node metastasis in human melanoma [97] and of adverse outcome in several studies of gastric cancer [98–101] and node-negative carcinomas of bladder and oesophagus [102, 103]. These findings indicate that quantification of tumour cell invasion of lymphatic vessels might be used in prognosis in cancer patients with uninvolved lymph nodes.
Lymphangiogenic growth factors
Several factors are being investigated as prognostic indicators of metastasis. The most studied factor is VEGF-C. In a range of human tumours, including melanoma, breast, gastric, colorectal, cervical, endometrial, ovarian and non-small cell lung cancer, it was shown that expression of VEGF-C by tumour or tumour-associated cells correlated with lymph node metastasis and poor prognosis [9, 11, 12]. Recently, a meta-study analysed a large number of published human cancer studies that found VEGF-C expression in tumours and lymph node metastases. In 56 of these 73 studies (77%), VEGF-C expression correlated with lymph node metastasis [12]. Interestingly, VEGF-C levels change with tumour progression; this was shown in human melanomas in which mRNA levels of VEGF-C were higher during the vertical growth phase (advanced melanoma) compared to the horizontal growth phase. The greatest levels of VEGF-C expression were found in nodal metastases, followed by regional dermal metastases and then primary tumours [104].
Fewer studies investigated tumour VEGF-A and VEGF-D expression and lymph node metastasis [12]. VEGF-D expression was correlated with lymph node metastases in 14 of 32 studies (44%) and VEGF-A was in 6 of 16 studies (38%) [12]. Further elucidation of lymphangiogenic growth factors might reveal additional suitable prognostic factors.
Several methods were applied to quantify lymphangiogenic growth factor expression in cancer patients, including detection of protein levels in serum or plasma, and mRNA or protein levels in tumour biopsies or tumour tissue sections [12].
Quantification of circulating lymphangiogenic growth factor levels in human blood would be the preferred biomarker for prognosis, because sample collection is minimally invasive and levels can be measured repeatedly. However, the validity of the method has not been established. Circulating levels of VEGF-C correlated with lymph node metastasis and differed between healthy persons and patients with oesophageal squamous cell carcinoma, malignant melanoma, cervical carcinoma, non-small cell lung carcinoma or colorectal cancer [105–109]. However, this difference was not confirmed in studies assessing circulating VEGF-C or VEGF-D levels patients with ovarian carcinoma, breast carcinoma, cervix adenocarcinoma, colorectal cancer or head and neck carcinoma [105, 110–113]. A potential reason for this discrepancy could be the fact that serum levels of lymphangiogenic growth factors vary among patients. For instance, high levels of serum VEGF-C in patients with non-metastatic cancers are in the range of those of patients with metastatic tumours [107]. Therefore, it is a challenge to interpret the levels of circulating VEGF-C for prognostic purposes without knowing each patient's pre-disease level. This issue probably also affects measurement of other lymphangiogenic factors. Furthermore, lymphangiogenic growth factors such as VEGF-C are produced during other conditions, such as inflammation [1], which could affect the reliability of this prognostic method.
Lymphangiogenic growth factors have been quantified in tumour biopsy samples using RT-PCR and ELISA analyses. Increased expression of VEGF-C and VEGF-D mRNA and protein in tumour biopsies has been associated with lymph node metastasis [114–117]. However, to perform these analyses, biopsies need to be carefully collected, because intra-tumoral VEGF-C and VEGF-D levels were found to be lower than peri-tumoral levels [118–122]; biopsies derived from the tumour centre therefore might not represent VEGF-C or VEGF-D levels at the hot spots of lymphangiogenic activity.
Lymphangiogenic growth factors can also be detected on tissue sections. This method has the advantage of preserving the histological environment and allows discrimination between intra- and peri-tumoral structures and hot spots of lymphangiogenesis, as well as cell-type specific expression of lymphangiogenic growth factors [12].
Lymph node lymphangiogenesis
In mouse models of skin carcinogenesis, malignant melanoma, breast and nasopharyngeal carcinoma, the lymphatic vasculature of the lymph nodes expanded prior to tumour metastasis [7, 8, 25, 26]. Once cancer cells arrived in the lymph node, lymphangiogenesis was increased [7, 8]. Hot spots of lymphangiogenesis have been found in metastases-bearing lymph nodes in human melanoma patients [27] and lymphangiogenesis in sentinel lymph nodes of breast cancer patients was associated with metastases to non-sentinel lymph nodes [28]. These studies supported the concept that lymph node lymphangiogenesis occurs in cancer patients.
Interestingly, pre-metastatic lymphatic vessel expansion in tumour-draining lymph nodes was associated with increased lymph flow to the lymph nodes in a mouse model of metastatic melanoma [26]. Lymph flow imaging was performed in vivo using near infrared quantum dots or Cy5.5 labelled magnetic nanoparticles. An increased amount and rate of lymph flow through draining lymph nodes in the same mouse model was also shown by magnetic resonance imaging (MRI) after injection of a gadolinium-based contrast agent [123]. These experiments suggest that detecting increased lymph flow to sentinel lymph nodes, or increased lymph node volumes might predict metastasis to lymph nodes. Further studies on this subject are needed to confirm this hypothesis.
Diagnostic value of tumour-associated lymphangiogenesis
Although quantification of tumour-induced lymphangiogenesis is a valuable method for predicting lymph node metastasis, it is not clear whether detection of lymphangiogenesis might be used in the early detection of metastases in lymph nodes or elsewhere. Because lymphangiogenesis has been found in lymph nodes of several experimental cancer models, as well as in patients, it might be prognostic factor for metastasis, especially because it has been shown to precede cancer metastasis in mice [7, 8, 25, 26]. If pre-metastatic lymphangiogenesis is as well a feature in human cancer patients, it remains to be determined whether pre-metastatic and metastatic lymph node lymphangiogenesis can be used to develop reliable diagnostics. It may be a challenge, however, to distinguish between lymph node lymphangiogenesis evoked by cancer metastasis or by inflammation [124].
Imaging of lymphatic vessels
To analyse lymphatic vessels and their function in patients, to monitor anti-lymphangiogenic treatment, and to use lymphatic vessel density as a prognostic or diagnostic indicator, methods are needed to image lymphatic vessel structure and their function in vivo. Agents to image lymphatic vessels have been administered intradermally, subcutaneously, intravenously or directly into lymphatic vessels. Dyes such as Evans Blue or colloidal carbon particles and fluorescent dyes such as fluorescein were injected intradermally or subcutaneously into the interstitial tissue of mice or human beings, to obtain indirect micro-lymphangiographies of superficial lymphatic vessels [58, 125, 126]. For example, micro-lymphangiography with fluorescin isothiocyanate (FITC)-dextran allowed non-invasive estimation of the width of lymph channels in human patients suffering from oedema [126]. Interstitial administration of the contrast agent also enabled lymphangiography of larger and deep lymphatic vessels by MRI in mice and human beings [94, 127, 128] and, less successfully, by X-ray scans [94].
Contrast agents can be delivered intravenously, via hyperpermeable blood vessels, through interstitial tissue, into the lymphatic vessels [94]. Particles up to several nanometres in size can extravasate from blood vessels and pool into lymphatic vessels through gap junctions between the endothelial cells. Particles up to 100 nm in diameter extravasate from the blood into the interstitial space, where they are phagocytosed by macrophages and are further transported to the lymph nodes. Particles larger than 100 nm typically remain trapped in the interstitial tissue [94].
Imaging lymphatic drainage
The draining capacity of lymphatic vessels has been assessed by tracking injected radioactive colloids and fluorescent dyes. Lymphoscintigraphy is used to diagnose lymphedema, a condition characterized by non-functional lymphatic vessels. Typically, 99m-technetium (99mTc)-labelled sulphur colloid or 99mTc-human serum albumin is administered, either intradermally or subcutaneously [129]. A scintigraphic camera that detects γ emission is used to visualize the radiotracer's flow pattern. More recently, indocyan green (IC-green), an albumin-binding near-infrared dye was investigated for assessing lymphatic function and lymphedema. Using IC-green, pulsative lymph flow has been shown in pigs [130] and subcutaneous IC-green injection revealed dilated lymphatic channels and lymph flow obstruction in human patients suffering from secondary lymphedema [131]. Furthermore, lymph flow has been quantified in breast cancer patients using IC-green [132].
To identify tumour-draining lymph nodes in human cancer patients, Tc-99m-labelled sulphur colloid, albumin nanocolloid, antimony colloid or other soluble non-colloids are injected intradermally or subcutaneously around the tumour and their drainage to lymph nodes is detected by a γ scintigraphic camera [94]. A range of further radiographic contrast agents and dyes have been tested in experiments with mice and in clinical trials to track sentinel lymph nodes of tumours [133–139]. Optical methods to follow drainage patterns are increasingly considered, because of the possibility to track differently labelled dyes simultaneously. However, these methods are currently limited in imaging of nodes in deeper tissues, and in the case of quantum dots, bear toxicity issues. In mice, near-infrared dye-labelled IgGs [138], dendrimers [137], magnetic nanoparticles [26], indocyan green and different quantum dots [26, 134–137, 139] were found in lymph nodes following injection into the interstitial tissue. Lymph flow to lymph nodes could also be tracked and quantified by MRI using a gadolinium-based contrast agent [123, 140].
Non-invasive imaging of lymph node metastases
The removal of tumour-draining lymph nodes, in particular the complete dissection of auxiliary lymph node that is performed in breast cancer patients, can trigger serious adverse reactions, including lymphedema. Questions have been raised about the benefits of routinely performing auxiliary lymph node dissection [94]. Thus, non-invasive methods were developed to detect metastasis-containing lymph nodes and avoid the unnecessary dissection of metastases-free lymph nodes. Ultrasound (US) can detect changes in shape, size and echogenicity induced by metastases. Because ultrasound does not employ a contrast agent, it might be used to detect metastases in lymph nodes that obstruct the drainage of radiotracer to sentinel lymph nodes [94]. However, ultrasound cannot image deep-lying lymph nodes [94]. Conflicting results have been published regarding the sensitivity and specificity of the combination of ultrasound and fine-needle aspiration biopsy in detection of tumour cells [141, 142]. One study reported that lymph nodes could be visualized by ultrasound upon interstitial injection of microbubbles [143]. Metastasis-induced enlargement of lymph nodes can also be imaged by computed tomography (CT) or MRI [94, 129]. In addition, positron emission tomography (PET) can be used after intravenous injection of 18F-fluorodeoxyglucose for direct detection of metastases in lymph nodes [94, 129]. Often PET imaging is combined with CT or X-ray analyses to combine PET data with structural information [144].
CT, MRI and US are routinely used in clinical settings to evaluate lymph nodes. However, none of these methods is sensitive enough to detect micrometastases. Micrometastases, particularly those from breast tumours, are often found in normal-sized and normal-shaped lymph nodes [145, 146]. Furthermore, lymph node enlargement is not necessarily a result of malignancy but can also be caused by hyperplasia or infections [129]. Several attempts have been made to increase the sensitivity of metastasis detection. Whereas conventional MRI is able to detect nodal metastasis as small as 8–10 mm, MRI, after injection of lymphotropic monocrystalline iron oxide nanoparticles, detects nodal metastases as small as 2 mm [146, 147]. Further improvements in sensitivity might be achieved by the combination of different imaging modalities [129, 146]. Non-invasive imaging of lymph node lymphangiogenesis in tumour-draining lymph nodes could be more sensitive than current methods of detecting metastases.
Acknowledgments
Research from the authors’ laboratory is supported by National Institutes of Health grant CA69184, Swiss National Fund grant 3100A0–108207, Austrian Science Foundation grant S9408-B11, Cancer League Zurich, Oncosuisse and Commission of the European Communities grant LSHC-CT-2005–518178 (M.D.).
References
- 1.Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–22. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerli R, Solito R, Weber E, et al. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–57. [PubMed] [Google Scholar]
- 3.Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–82. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 6.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 7.Hirakawa S, Kodama S, Kunstfeld R, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirakawa S, Brown LF, Kodama S, et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–7. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–54. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Auwera I, Cao Y, Tille JC, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006;95:1611–25. doi: 10.1038/sj.bjc.6603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper MS, Tille JC, Nisato R, et al. Lymphangiogenesis and tumor metastasis. Cell Tissue Res. 2003;314:167–77. doi: 10.1007/s00441-003-0748-7. [DOI] [PubMed] [Google Scholar]
- 12.Rinderknecht M, Detmar M. Lymphangiogenesis in cancer metastasis. In: Stacker SA, Achen MG, editors. Cancer metastasis – biology and treatment. Berlin: Springer: Science+Business Media B.V; 2009. in press. [Google Scholar]
- 13.He Y, Rajantie I, Pajusola K, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 14.Nathanson SD, Anaya P, Avery M, et al. Sentinel lymph node metastasis in experimental melanoma: relationships among primary tumor size, lymphatic vessel diameter and 99mTc-labeled human serum albumin clearance. Ann Surg Oncol. 1997;4:161–8. doi: 10.1007/BF02303800. [DOI] [PubMed] [Google Scholar]
- 15.Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi A, Gallo O, Massi D, et al. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer. 2004;101:973–8. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Auwera I, Van Den Eynden GG, Colpaert CG, et al. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res. 2005;11:7637–42. doi: 10.1158/1078-0432.CCR-05-1142. [DOI] [PubMed] [Google Scholar]
- 18.Liang P, Hong JW, Ubukata H, et al. Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch. 2006;448:570–5. doi: 10.1007/s00428-006-0166-9. [DOI] [PubMed] [Google Scholar]
- 19.Massi D, Puig S, Franchi A, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Pathol. 2006;59:166–73. doi: 10.1136/jcp.2005.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Rajantie I, Ilmonen M, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–40. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 22.Religa P, Cao R, Bjorndahl M, et al. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–90. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–72. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–4. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 25.Qian CN, Berghuis B, Tsarfaty G, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–76. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 26.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–86. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–42. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 28.Van Den Eynden GG, Vandenberghe MK, van Dam PJ, et al. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13:5391–7. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- 29.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 30.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 31.Shields JD, Emmett MS, Dunn DB, et al. Chemokine-mediated migration of melanoma cells towards lymphatics – a mechanism contributing to metastasis. Oncogene. 2006;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 32.Wiley HE, Gonzalez EB, Maki W, et al. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–43. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 33.Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–6. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 34.Wong SY, Haack H, Crowley D, et al. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–98. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 35.Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–20. [PubMed] [Google Scholar]
- 36.Maula SM, Luukkaa M, Grenman R, et al. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–6. [PubMed] [Google Scholar]
- 37.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 38.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–83. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 39.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson DG, Prevo R, Clasper S, et al. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–21. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 41.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong YK, Foreman K, Shin JW, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–5. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan RS, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gale NW, Prevo R, Espinosa J, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nightingale TD, Frayne ME, Clasper S, et al. A mechanism of sialylation functionally silences the hyaluronan receptor LYVE-1 in lymphatic endothelium. J Biol Chem. 2009;284:3935–45. doi: 10.1074/jbc.M805105200. [DOI] [PubMed] [Google Scholar]
- 48.Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–21. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Villar E, Scholl FG, Gamallo C, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 50.Partanen TA, Arola J, Saaristo A, et al. VEGF-C and VEGF-D expression in neuroen-docrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–96. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 51.Jurisic G, Detmar M. Lymphatic endothelium in health and disease. Cell Tissue Res. 2009;335:97–108. doi: 10.1007/s00441-008-0644-2. [DOI] [PubMed] [Google Scholar]
- 52.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roesli C, Mumprecht V, Neri D, Detmar M. Identification of the surface-accessible, lineage-specific vascular proteome by two-dimensional peptide mapping. FASEB J. 2008;22:1933–44. doi: 10.1096/fj.07-100529. [DOI] [PubMed] [Google Scholar]
- 54.Shin JW, Huggenberger R, Detmar M. Transcriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesis. Blood. 2008;112:2318–26. doi: 10.1182/blood-2008-05-156331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clasper S, Royston D, Baban D, et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- 56.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95:548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kajiya K, Hirakawa S, Ma B, et al. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–95. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–56. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 61.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 62.Kubo H, Cao R, Brakenhielm E, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibrob-last growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–73. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin JW, Min M, Larrieu-Lahargue F, et al. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphan-giogenesis. Mol Biol Cell. 2006;17:576–84. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bjorndahl M, Cao R, Nissen LJ, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–8. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–45. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 66.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banziger-Tobler NE, Halin C, Kajiya K, Detmar M. Growth hormone promotes lymphangiogenesis. Am J Pathol. 2008;173:586–97. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spinella F, Garrafa E, Di Castro V, et al. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–76. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- 69.Clavin NW, Avraham T, Fernandez J, et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–27. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 70.Iwata C, Kano MR, Komuro A, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181–9. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- 71.Schoppmann SF, Birner P, Stockl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angeli V, Ginhoux F, Llodra J, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–57. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Red-Horse K, Rivera J, Schanz A, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–52. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Proulx ST, Kwok E, You Z, et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum. 2007;56:4024–37. doi: 10.1002/art.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts N, Kloos B, Cassella M, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–7. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu K, Kubo H, Yamaguchi K, et al. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328–33. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin J, Lalani AS, Harding TC, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 79.He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 80.Krishnan J, Kirkin V, Steffen A, et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–22. [PubMed] [Google Scholar]
- 81.He XW, Liu T, Chen YX, et al. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008;15:193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- 82.Chen Z, Varney ML, Backora MW, et al. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–11. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 83.Caunt M, Mak J, Liang WC, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–42. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 84.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–50. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 85.Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 86.Karpanen T, Wirzenius M, Makinen T, et al. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169:708–18. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–72. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–3. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 89.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413–23. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 90.Dadras SS, Detmar M. Angiogenesis and lymphangiogenesis of skin cancers. Hematol Oncol Clin North Am. 2004;18:1059–70. doi: 10.1016/j.hoc.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Blanchard DK, Donohue JH, Reynolds C, Grant CS. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–7. doi: 10.1001/archsurg.138.5.482. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 92.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 93.Campbell SC, Klein EA, Levin HS, Piedmonte MR. Open pelvic lymph node dissection for prostate cancer: a reassessment. Urology. 1995;46:352–5. doi: 10.1016/S0090-4295(99)80219-2. [DOI] [PubMed] [Google Scholar]
- 94.Sharma R, Wendt JA, Rasmussen JC, et al. New horizons for imaging lymphatic function. Ann N Y Acad Sci. 2008;1131:13–36. doi: 10.1196/annals.1413.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schoppmann SF, Bayer G, Aumayr K, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–12. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee AH, Pinder SE, Macmillan RD, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42:357–62. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 97.Doeden K, Ma Z, Narasimhan B, et al. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J Cutan Pathol. 2009;36:772–80. doi: 10.1111/j.1600-0560.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 98.Hyung WJ, Lee JH, Choi SH, et al. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–7. doi: 10.1007/BF02573892. [DOI] [PubMed] [Google Scholar]
- 99.Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–35. doi: 10.1097/01.SLA.0000072260.77776.39. discussion 35–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dicken BJ, Graham K, Hamilton SM, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64–73. doi: 10.1097/01.sla.0000194087.96582.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arigami T, Natsugoe S, Uenosono Y, et al. Lymphatic invasion using D2–40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer. 2005;93:688–93. doi: 10.1038/sj.bjc.6602739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vazquez-Sequeiros E, Wang L, Burgart L, et al. Occult lymph node metastases as a predictor of tumor relapse in patients with node-negative esophageal carcinoma. Gastroenterology. 2002;122:1815–21. doi: 10.1053/gast.2002.33665. [DOI] [PubMed] [Google Scholar]
- 103.Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533–9. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 104.Goydos JS, Gorski DH. Vascular endothelial growth factor C mRNA expression correlates with stage of progression in patients with melanoma. Clin Cancer Res. 2003;9:5962–7. [PubMed] [Google Scholar]
- 105.Mathur SP, Mathur RS, Gray EA, et al. Serum vascular endothelial growth factor C (VEGF-C) as a specific biomarker for advanced cervical cancer: Relationship to insulin-like growth factor II (IGF-II), IGF binding protein 3 (IGF-BP3) and VEGF-A [corrected] Gynecol Oncol. 2005;98:467–83. doi: 10.1016/j.ygyno.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Krzystek-Korpacka M, Matusiewicz M, Diakowska D, et al. Up-regulation of VEGF-C secreted by cancer cells and not VEGF-A correlates with clinical evaluation of lymph node metastasis in esophageal squamous cell carcinoma (ESCC) Cancer Lett. 2007;249:171–7. doi: 10.1016/j.canlet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Tamura M, Ohta Y. Serum vascular endothelial growth factor-C level in patients with primary nonsmall cell lung carcinoma: a possible diagnostic tool for lymph node metastasis. Cancer. 2003;98:1217–22. doi: 10.1002/cncr.11529. [DOI] [PubMed] [Google Scholar]
- 108.Vihinen PP, Hilli J, Vuoristo MS, et al. Serum VEGF-C is associated with metastatic site in patients with malignant melanoma. Acta Oncol. 2007;46:678–84. doi: 10.1080/02841860600965020. [DOI] [PubMed] [Google Scholar]
- 109.Duff SE, Li C, Renehan A, et al. Immunodetection and molecular forms of plasma vascular endothelial growth factor-C. Int J Oncol. 2003;22:339–43. doi: 10.3892/ijo.22.2.339. [DOI] [PubMed] [Google Scholar]
- 110.Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogen-esis and immune tolerance. Med Sci Monit. 2005;11:BR280–92. [PubMed] [Google Scholar]
- 111.Duff SE, Saunders M, McCredie V, et al. Pre-operative plasma levels of vascular endothelial growth factor A, C and D in patients with colorectal cancer. Clin Oncol (R Coll Radiol) 2005;17:367–71. doi: 10.1016/j.clon.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Hoar FJ, Lip GY, Belgore F, et al. Circulating levels of VEGF-A, VEGF-D and soluble VEGF-A receptor (sFIt-1) in human breast cancer. Int J Biol Markers. 2004;19:229–35. doi: 10.1177/172460080401900308. [DOI] [PubMed] [Google Scholar]
- 113.Mitsuhashi A, Suzuka K, Yamazawa K, et al. Serum vascular endothelial growth factor (VEGF) and VEGF-C levels as tumor markers in patients with cervical carcinoma. Cancer. 2005;103:724–30. doi: 10.1002/cncr.20819. [DOI] [PubMed] [Google Scholar]
- 114.Nakamura Y, Yasuoka H, Tsujimoto M, et al. Flt-4-positive vessel density correlates with vascular endothelial growth factor-d expression, nodal status, and prognosis in breast cancer. Clin Cancer Res. 2003;9:5313–7. [PubMed] [Google Scholar]
- 115.Choi WW, Lewis MM, Lawson D, et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol. 2005;18:143–52. doi: 10.1038/modpathol.3800253. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura Y, Yasuoka H, Tsujimoto M, et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125–32. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 117.Li YS, Kaneko M, Amatya VJ, et al. Expression of vascular endothelial growth factor-C and its receptor in invasive micropapillary carcinoma of the breast. Pathol Int. 2006;56:256–61. doi: 10.1111/j.1440-1827.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- 118.Gombos Z, Xu X, Chu CS, et al. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res. 2005;11:8364–71. doi: 10.1158/1078-0432.CCR-05-1238. [DOI] [PubMed] [Google Scholar]
- 119.Kurahara H, Takao S, Maemura K, et al. Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res. 2004;10:8413–20. doi: 10.1158/1078-0432.CCR-04-0379. [DOI] [PubMed] [Google Scholar]
- 120.Onogawa S, Kitadai Y, Tanaka S, et al. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32–9. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shintani S, Li C, Ishikawa T, et al. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 2004;40:13–20. doi: 10.1016/s1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 122.Zhang B, Zhao WH, Zhou WY, et al. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev. 2007;31:436–42. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 123.Ruddell A, Harrell MI, Minoshima S, et al. Dynamic contrast-enhanced magnetic resonance imaging of tumor-induced lymph flow. Neoplasia. 2008;10:706–13. doi: 10.1593/neo.08342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji RC. Lymph node lymphangiogenesis: a new concept for modulating tumor metastasis and inflammatory process. Histol Histopathol. 2009;24:377–84. doi: 10.14670/HH-24.377. [DOI] [PubMed] [Google Scholar]
- 125.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-a mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mellor RH, Stanton AW, Azarbod P, et al. Enhanced cutaneous lymphatic network in the forearms of women with postmastec-tomy oedema. J Vasc Res. 2000;37:501–12. doi: 10.1159/000054083. [DOI] [PubMed] [Google Scholar]
- 127.Kobayashi H, Kawamoto S, Brechbiel MW, et al. Detection of lymph node involvement in hematologic malignancies using micromagnetic resonance lymphan-giography with a gadolinum-labeled dendrimer nanoparticle. Neoplasia. 2005;7:984–91. doi: 10.1593/neo.05454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi H, Kawamoto S, Sakai Y, et al. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Natl Cancer Inst. 2004;96:703–8. doi: 10.1093/jnci/djh124. [DOI] [PubMed] [Google Scholar]
- 129.Barrett T, Choyke PL, Kobayashi H. Imaging of the lymphatic system: new horizons. Contrast Media Mol Imaging. 2006;1:230–45. doi: 10.1002/cmmi.116. [DOI] [PubMed] [Google Scholar]
- 130.Sharma R, Wang W, Rasmussen JC, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292:H3109–18. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 131.Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg. 2007;45:1016–21. doi: 10.1016/j.jvs.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 132.Sevick-Muraca EM, Sharma R, Rasmussen JC, et al. Imaging of lymph flow in breast cancer patients after micro-dose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246:734–41. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer – a multi-center validation study. N Engl J Med. 1998;339:941–6. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 134.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ballou B, Ernst LA, Andreko S, et al. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug Chem. 2007;18:389–96. doi: 10.1021/bc060261j. [DOI] [PubMed] [Google Scholar]
- 136.Hama Y, Koyama Y, Bernardo M, et al. Spectral near-infrared fluorescence imaging of curved surfaces using projection reconstruction algorithms. Contrast Media Mol Imaging. 2007;2:82–7. doi: 10.1002/cmmi.129. [DOI] [PubMed] [Google Scholar]
- 137.Kobayashi H, Hama Y, Koyama Y, et al. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007;7:1711–6. doi: 10.1021/nl0707003. [DOI] [PubMed] [Google Scholar]
- 138.Hama Y, Koyama Y, Urano Y, et al. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Cancer Res Treat. 2007;103:23–8. doi: 10.1007/s10549-006-9347-0. [DOI] [PubMed] [Google Scholar]
- 139.Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–5. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 140.Proulx ST, Kwok E, You Z, et al. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci. 2007;1117:106–23. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 141.Motomura K, Inaji H, Komoike Y, et al. Gamma probe and ultrasonographically-guided fine-needle aspiration biopsy of sentinel lymph nodes in breast cancer patients. Eur J Surg Oncol. 2001;27:141–5. doi: 10.1053/ejso.2000.1059. [DOI] [PubMed] [Google Scholar]
- 142.Bedrosian I, Bedi D, Kuerer HM, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol. 2003;10:1025–30. doi: 10.1245/aso.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 143.Choi SH, Kono Y, Corbeil J, et al. Model to quantify lymph node enhancement on indirect sonographic lymphography. AJR Am J Roentgenol. 2004;183:513–7. doi: 10.2214/ajr.183.2.1830513. [DOI] [PubMed] [Google Scholar]
- 144.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244:39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 145.Cserni G. Metastases in axillary sentinel lymph nodes in breast cancer as detected by intensive histopathological work up. J Clin Pathol. 1999;52:922–4. doi: 10.1136/jcp.52.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. Jama. 2005;293:855–62. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 147.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 148.Zudaire E, Martinez A, Cuttitta F. Adrenomedullin and cancer. Regul Pept. 2003;112:175–83. doi: 10.1016/s0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]


