Abstract
A solid state NMR experiment is introduced for probing motions on the millisecond time scale, based on dephasing and refocusing 1H-13C or 1H-15N dipolar couplings. The method is related to the previously described Centerband-Only Detection of Exchange or CODEX experiment. The use of an R-type dipolar recoupling sequence takes advantage of the strong 1H-13C or 1H-15N dipolar coupling, while suppressing the effect of 1H-1H homonuclear coupling. This approach paves the way to detect both the correlation time and reorientational angle of the dynamics in fully protonated samples. The performance of this pulse sequence is demonstrated using imidazole methyl sulfonate.
Keywords: Millisecond Dynamics, Solid state NMR, CODEX, R-CODEX, Reorientation angle, R1871, 1H homonuclear coupling, imidazole
Introduction
Molecular and conformational dynamics on the millisecond scale play important roles in protein function [1–3]. NMR methods provide the opportunity to probe the correlation time and the amplitude of a dynamical process in a site-specific fashion, which has the possibility to provide significant insights into protein function. For example, solution NMR studies, such as CPMG and R1ρ experiments, can provide site-specific correlation times and populations describing the dynamics of proteins in solution [4, 5]. In some cases, the amplitude of the motion can also be obtained quantitatively from for example RDC (residual dipolar coupling) experiments, and insights about the structures of the two sites can be obtained through interpreting chemical shifts [6].
Solid state NMR methods can significantly broaden the range of systems amenable to this kind of analysis, and can be sensitive on a range of timescales. On the scale of milliseconds seconds for example, a family of experiments known as CODEX (Centerband-Only detection of Exchange Experiments) [7–11] exploit anisotropic interactions such as the CSA (chemical shift anisotropy), to encode the orientation of a site and provide the means to detect changes in the orientation, and obtain both the correlation time and reorientational angle. To interpret the data it is necessary to know the principal values and orientations of the CSA, which can be complex for systems like proteins [12, 13]. In addition, most of the CSA tensors are non-uniaxial, which also complicates the analysis.
Therefore, experiments based on heteronuclear dipolar coupling are an attractive alternative. The heteronuclear dipolar coupling is strictly uniaxial, and has a relatively fixed coupling constant with the principal component Dzz along the chemical bond. Schaefer et al. used CODEX with and without 13C-19F dipolar cancellation and demonstrated the presence of a large amplitude motion of a fluorinated aromatic ring [14]. Recently, Dipolar CODEX experiments [15] based on recoupling 13C-15N dipolar couplings using a REDOR (rotational echo double resonance) [16] pulse sequence element, have been reported, and other experiments that utilize 1H-15N dipolar couplings have been implemented with applications on a protein [17]. The 13C-15N Dipolar CODEX requires a long dephasing time due to the weak 13C-15N dipolar interaction, resulting in potential loss of signal. The 1H-15N Dipolar CODEX experiments had potential artifacts from 1H homonuclear coupling and therefore require perdeuteration of the protein.
In this paper, we report a R-CODEX experiment, which recouples the 1H-13C or 1H-15N dipolar couplings via the R187118] recoupling element, a R-type sequence based on symmetry principles [19]. The π flip motion of the imidazolium ring in imidazolium methyl sulfonate was studied with this method to demonstrate properties of the pulse sequence. The results are promising for the detection of protein dynamics in the future. We discuss the advantages of this scheme in terms of experimental results, numerical simulations and theoretical considerations.
Theory
The pulse sequences and experiments shown schematically in Figure 1 are a CODEX like experiment using an R-based recoupling element to develop heteronuclear dipolar coupling; overall the sequence detects reorientation of a 1H-X dipolar bond vector. The advantages of this scheme will be discussed in light of experimental results, numerical simulations and theoretical considerations. Throughout this section, we assume a conformational exchange process for a single 13C-1H pair, that can be described using a two-site jump model, as shown in Scheme 1, and we assume that there is no exchange during the dephasing and refocusing periods (although for numerical simulations the exchange during those periods was included). The normalized populations of the two exchange sites are P1and P2. The exchange rates k1and k−1 satisfy k1 × P1= k−1 × P2.
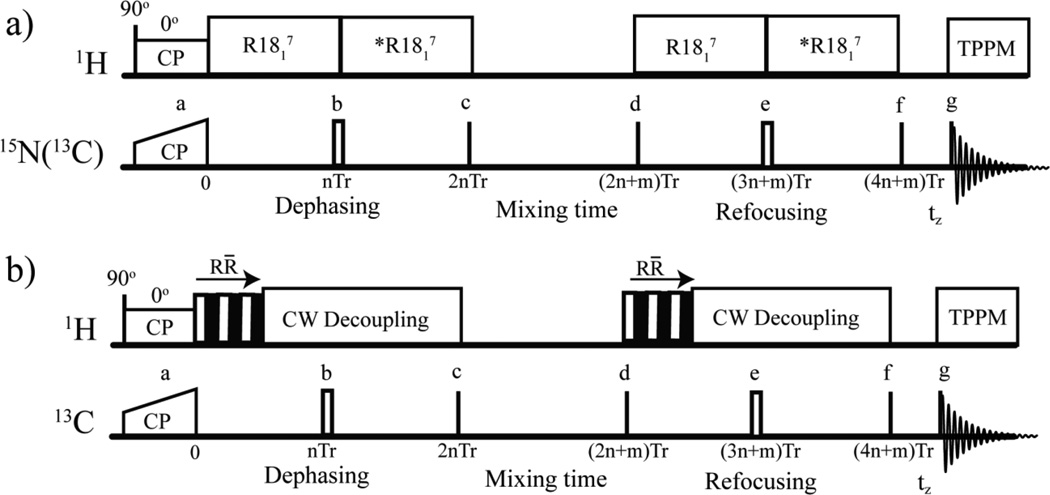
Figure 1.
The R-CODEX pulse sequences used in these studies to detect conformational exchange rates (a) and reorientation angles (b). 90° pulses are denoted using black lines, and 180° pulses are represented by hollow rectangles. The 180° pulse in the middle of dephasing and refocusing periods refocuses the dephasing due to the isotropic chemical shift. CP: cross polarization. Tr: Rotor period. *R1871: R1817 with 180° phase shift to prevent averaging of the recoupled dipolar coupling by 180° pulse. The mixing time should be an integral number of rotor periods [7]. The dephasing and refocusing periods should be even multiples of the rotor periods to reduce net evolution of other anisotropic interactions. In (b), the mixing time was fixed while the number of R̄R blocks (denoted by one hollow (R) and one solid (R̄) square) of the R1871 pulse was increased systematically. All phases represented by a-g are given in the supplemental materials.
A Simple Description of R-CODEX
During the pulse sequence shown in Figure 1 (a), after cross polarization, the carbon magnetization (Sx) accumulates phase φA under the action of the recoupled single quantum 1H-13C dipolar coupling (I+Sz+I−Sz) during the dephasing time.
| (1) |
A subsequent 90° pulse flips the in-phase magnetization (Sx) to generate the stored longitudinal magnetization. Remaining anti-phase magnetization will disappear because of the choice of phase-cycle. During the mixing time, the exchange process redistributes the magnetization between the two exchange sites. Subsequently a 90° pulse flips the magnetization back to the transverse plane, and the magnetization evolves during the refocusing period under the same recoupling condition as was used for the dephasing period previously, which yields again an accumulated phase φB. The final amplitude of the signal after a z-filter is proportional to
| (2) |
where kex is the sum of the forward and reverse rate constants k1 and k−1, and the angle brackets < > represent a powder average over all molecular (“crystallite”) orientations. The accumulated phases öAand öB will be different if the 1H-13C bond reorients during the mixing time. Due to this effect, the final total (powder averaged) signal will exhibit in a single exponential decay as a function of mixing time tm. The exchange rate kex can be obtained from fitting the decay curve. Moreover, when the dephasing (refocusing) time is long enough, the equation (2) can be approximately simplified as (see details in the supplemental materials)
| (3) |
The population ratios can be obtained by simply comparing the intensities for the short mixing time ((P1+P2)2=1) and the long mixing time (P12+P22).
The pulse sequence designed to extract the reorientation angle (Figure 1 (b)) is in principle similar to the pulse sequences to extract the NHi-NHi+1 projection angle [20]. The length of the heteronuclear dipolar recoupling time increases gradually to form a dephasing curve, which in this case records the information about the projection angle between two exchanging orientations for the 1H-13C (1H-15N) pairs. A constant dephasing (and refocusing) time is used to minimize the effect of T2. The mixing time is long enough as compared with the correlation time of dynamics to make the conformers fully exchanged. Meanwhile, reference experiments with a very short mixing time are carried out as described by Schmidt-Rohr et al. [7]. The normalized intensity is given by
| (4) |
Comparison of the dephasing curve with numerical simulations will yield the reorientation angle. Measurement of the intensity changes at short dephasing times is crucial for determining the reorientational angle, and so the increment time step used during the dipolar recoupling period should be short, due to the strong dipolar coupling of 1H-13C. The R̄R sub-cycle in R1871 allows for taking data points at one ninth of a rotor period [18], making the reorientation angle detection particularly successful with this sequence.
The choice of the coherence used for dynamics detection
In the R-CODEX experiments we stored only the in-phase magnetization, rather than anti-phase part [15] or a combination of both [7–9]. The main concern is that if the 1H spin lattice relaxation time is short, the anti-phase component CzHz or NzHz will lose significant magnetization during mixing time. Moreover, the proton spin lattice relaxation process will modulate some protons’ spin states. This flip of the spin state will change the sign or direction of the dipolar coupling, and spurious decays for the anti-phase magnetization will be exhibited even if the 1H-13C or 1H-15N bond does not reorient. The in-phase component Cz or Nz does not have this problem. It usually develops as the cosine function of the phases as shown in equation 1, which only depends on the absolute value of the dipolar coupling. Furthermore, the in-phase, anti-phase or a combination of them will have similar results when the dephasing time is long enough compared to the inverse of the recoupled anisotropic interaction (as shown in the supplemental materials). In addition, the anti-phase magnetization developed under the recoupled Hamiltonian I+Sz+I−Sz by R1871 is a mixture of zero and double quantum coherence, which is difficult to store efficiently. Therefore, only the in-phase component was kept in these experiments.
Experimental
15N (98%) isotopically enriched imidazole (Cambridge Isotope Laboratories Inc.) was mixed with equimolar methyl sulfonate acid (diluted to 15%, to minimize the release of heat) and crystallized at room temperature. It was crushed into powder form and center-packed in the 4 mm rotor.
All experiments were performed on a Chemagnetics Infinity 300 MHz double resonance instrument, using an APEX double resonance MAS probe in 1H/13C or 1H/15N configuration with a 4 mm rotor. The sample temperature was maintained at 20° C (±0.1 °C). For the 13C R-CODEX experiment, the *R1871 element (180° phase shift relative to R1817) in Figure 1(a) was replaced with 100 kHz CW decoupling because one rotor period was sufficient to fully dephase the 13C magnetization. In the angle detection experiment (Fig 1b), the total time of dephasing (refocusing) element was 2 rotor periods. The CW decoupling time was decreased incrementally with increasing the number of R1871 blocks. The magic angle sample frequency was 11 kHz (±3 Hz). The 1H field strength for the R1871 element was 99 kHz, and the 13C field strength for all the 90° and 180° pulses was 100 kHz. The initial 13C signal was enhanced using adiabatic passage through the Hartmann-Hahn condition [21]. The constant 1H RF field during cross polarization had an amplitude of 52 kHz. The 13C tangential RF field followed equation in the supplemental materials [22], where ωHH was 41 kHz and the tangential parameters Δ and β were 12 kHz and 7 kHz, and the contact time was 3 ms. 60 kHz TPPM 1H decoupling was applied during acquisition.
For the 15N R-CODEX experiment, the magic angle sample frequency was 8.889 kHz, and the dephasing and refocusing time was 4 rotor periods (450 µs). The 1H field strength for the R1871 element was 80 kHz, and the 15N field strength for all 90° and 180° pulses was 40 kHz. The initial 15N signal was enhanced using a flat Hartmann-Hahn cross polarization element, with a 1H RF field had amplitude of 40 kHz and a 15N RF field amplitude of 32 kHz. The contact time was 3 ms. 60 kHz TPPM 1H decoupling was applied during acquisition.
The 13C chemical shift was referenced to the 13C adamantane’s methylene peak at 40.26 ppm [23]. The 15N chemical shift was calibrated indirectly.
All simulations were performed using SPINEVOLUTION 3.3.4 [24].
Results
Correlation times and population ratios of the two exchange sites
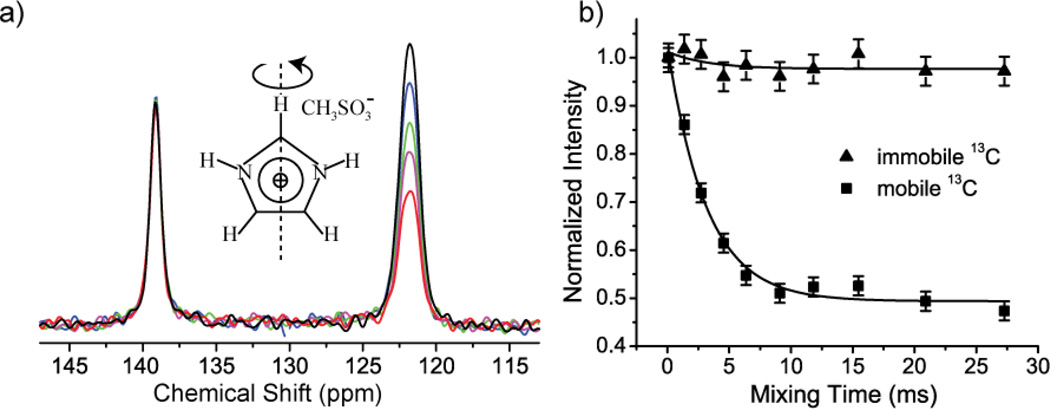
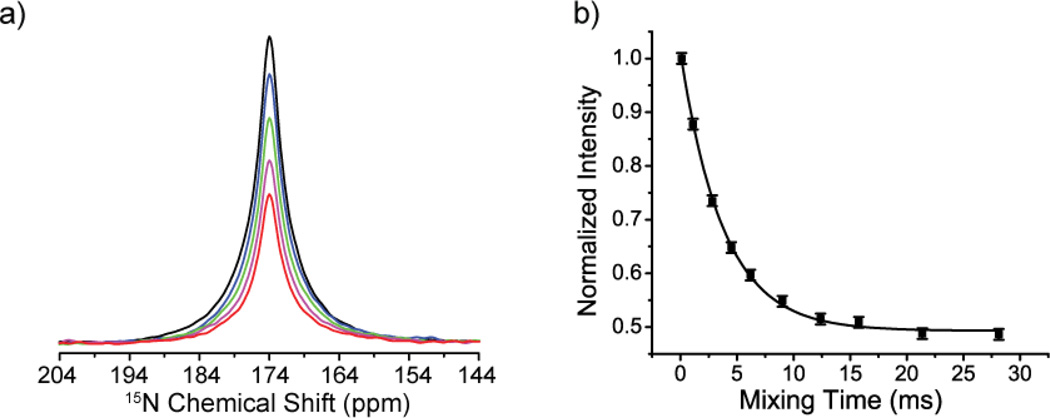
The 180° flip of the imidazolium ring [25, 26] was previously well characterized by 13C and 15N R- CODEX experiments at 20 °C. Spectra from experiments with different mixing times are shown in Figure 2 (a) and Figure 3 (a)). The peak intensities for carbon and nitrogen lines generally decay markedly with increasing mixing time as expected (peak near 122 ppm in Figure 2 (a) and the peak in Figure 3(a)). The peak intensity of the immobile carbon in the rotational axis has only a small amplitude decay with increasing mixing time (peak near 139 ppm in Figure 2 (a)), which can be attributed to the contribution of the motion of the dipolar vector connecting the immobile carbon with remote protons (i.e. the imidic 1H directly bonded to 15N). As shown in Figure 2 (b) and Figure 3 (b), the signal decay was fit well to a single exponential function, and the extracted exchange rates are comparable to previous results from line-shape analysis [25]. Analogous results from the 13C and 15N R-CODEX experiments indicate that the 13C-1H and 15N-1H motions are concerted (or at least have the same time constant) as expected. Moreover, the plateau values in Figure 2 (b) and Figure 3 (b) are 0.5, consistent with the two site jump model proposed with equal populations (Equation 2). We did not need to correct for the T1 of 13C and 15N apparently because they are longer than 5 seconds, i.e. very long as compared with millisecond ring reorientation. The peak intensities of both carbon and nitrogen with the shortest mixing time are about 30% and 20% relative to cross polarization spectrum. This signal loss results from the intrinsic 50% loss of CODEX type experiments [7] and relaxation during the dephasing and refocusing periods.
Figure 2.
(a) 13C-1H R-CODEX spectra with different mixing times (black: 0.09 ms, blue: 1.36 ms, green: 2.73 ms, magenta: 4.55 ms, red: 27.27 ms). The peak near 139 ppm is the apical carbon in the imidazolium ring. The peak near 122 ppm is due to the two basal carbons. The two basal carbons’ peaks are merged and unresolved. (b) Peak intensity vs. mixing time for apical and basal carbons. The black lines are single exponential fits with an exchange rate kex of 0.32+/−0.03 kHz.
Figure 3.
(a) 15N-1H R-CODEX spectra with different mixing times (black: 0.09 ms, blue: 1.12 ms, green: 2.81 ms, magenta: 6.19 ms, red: 28.12 ms). The two 15N peaks are merged and unresolved. (b) Peak intensities (black squares) are plotted vs. mixing time. The black line is a single exponential fit with exchange rate kex of 0.27+/−0.02 kHz.
The Geometry of the Motion
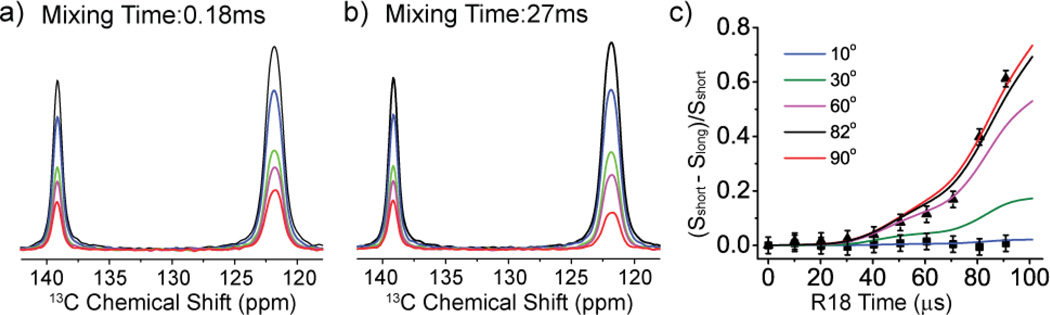
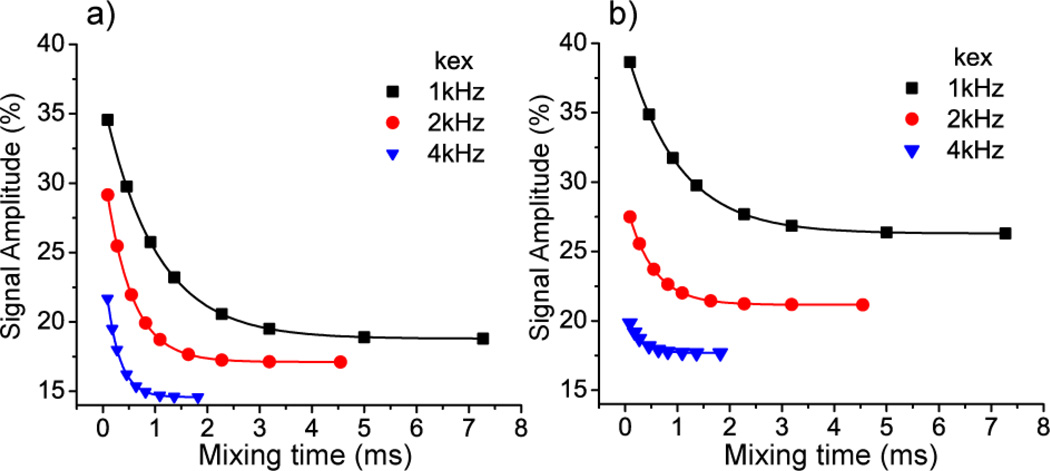
The reorientation angle of the basal 1H-13C bond was characterized as shown in Figure 4. The intensities of both mobile and immobile carbons decay, but the intensities of mobile carbon decay much more in the long mixing time as compared with that in the short mixing time. The build-up curve of the normalized intensities (Sshort – Slong)/Sshort are fit using the corresponding simulations to obtain the reorientational angle as 82° with a 95% confidence interval [70°, 90°] (The RMSD plot is in the supplemental materials). This agrees well with the reorientation angle of 81° determined from the neutron diffraction [27] (Cambridge Structural Database Entry: IMZMAL11). The relatively wide confidence interval partly results from limited S/N (the measurements were performed using natural abundance 13C materials). Also, the build-up curve depends on a sine function of reorientation angle [7], and thus is relatively insensitive at large angles as shown in Figure 4c. Meanwhile, the increment unit of the R1871 pulse in the dephasing and refocusing times is an incomplete cycle, which also will introduce small errors [28]. Use of an accurate dipolar coupling is important for determining the angle accurately in these simulations. An internally consistent method to extract the dipolar coupling constant for these simulations is to detect the 13C-1H dipolar coupling constant on a similar static molecule using a 2D DIPSHIFT [29] experiment, in which the heteronuclear dipolar coupling is reintroduced during the first dimension using the same sequence element [18], R1871. In the current simulations, the dipolar coupling was assumed to be 23.3±0.5 kHz, the value extracted from the 2D experiment on the imidazolium ring of histidine- hydrochloride (described further in the supplemental materials). This dipolar coupling constant (corresponding to 1.09±0.01 Å) is the same as the previous R1871 experiment [18], but is 0.02 Å longer than the neutron diffraction result [30] due to the vibrational averaging effects [31, 32].
Figure 4.
13C-1H R-CODEX spectra with different R1871 recoupling times during the dephasing (and refocusing) period. (black: 0 µs, blue: 20.2 µs, green: 40.4 µs, magenta: 60.6 µs, red: 80.8 µs). The mixing times are 0.18 ms in (a) and 27 ms in (b). In (c), the normalized intensities change as the R1871 recoupling times are simultaneously increased (i.e. both the dephasing and refocusing time). Squares are experimental data for the immobile apical 13C while triangles are experimental data for the basal mobile 13C. The single lines are simulations of 13C-1H bond exchanging with different reorientation angles, which vary from 10° to 90°. The exchange rate in the simulation is 0.3 kHz.
Discussion
Dipolar recoupling via an R-type pulse sequence element
In order to detect the change of the dipolar orientation during the mixing time, the dipolar coupling, which is averaged to zero by magic angle spinning, should be reintroduced. The use of a strong 13C-1H or 15N-1H dipolar coupling and a sequence with a moderately good scaling factor is advantageous because the dephasing and refocusing times can be very short, thus reducing the loss of signal and increasing the maximum detection range of the dynamic rate. Compared with other heteronuclear dipolar recoupling schemes (like T-MREV [33]), the R1871 (and the similar R1852) element can be applied with use of a wide range of magic angle sample spinning frequencies (~10 kHz-20 kHz) while efficiently suppressing the 1H homonuclear coupling [34]. This aspect lends it to practical use in protein studies. Furthermore, the R1871 can be used to better digitize the dephasing curve of the strong 1H-13C or 1H-15N dipolar coupling, because more data points can be collected during a rotor period. These features can result in more accurate determination of the reorientational angle. In addition, the R1871 pulse sequence recouples the single quantum heteronuclear dipolar coupling (I+Sz+I−Sz), which has the property of dipolar truncation [35, 36]. Hence the pulse sequence can specifically detect the motion of the directly connected 1H-15N (1H-13C) bond with little interference of the motion of the remote 1H. These features will be discussed more in the following sections.
Effective range of correlation times that can be detected
CODEX based experiments are very efficient for detecting slow motions. However, spin lattice relaxation and the magnetization exchange due to dipolar spin diffusion complicate the detection of very slow motions. It is generally impossible to distinguish magnetization transfer due to spin diffusion from that due to chemical exchange based only on a single NMR experiment. As a result, the motional rate should ideally be much faster than the spin diffusion rate. The correlation time of 13C spin diffusion can be as short as 100 ms in sparsely labeled proteins [11, 37], while in natural abundance small molecules it can be approximately 1 s [38]. However, fast magic angle spinning (up to 60 kHz) can be used to quench the 13C spin diffusion even in a fully isotopically enriched protein [39, 40], and a variety of R-type pulses (Table 15 and 18 in Ref [28]) can be utilized during the dephasing and refocusing periods even during high frequency magic angle spinning. 15N labeling schemes can also be used to achieve minimal spin diffusion. The dipolar coupling between the closest amides 15N is only 55 Hz (e.g. for α-helix), and for this case negligible spin diffusion is expected during time intervals up to 500 ms at the moderate magic angle spinning frequencies around 10 kHz [11].
The upper limit for the detectable correlation time depends on the effect of the dynamics during the dephasing and refocusing parts. In this paper, we have assumed that there were no reorientation events occurring during the dephasing and refocusing periods. This is of course correct only when the dephasing and refocusing periods are much shorter than the correlation time of dynamics. During the dephasing and refocusing periods, the dynamics will interfere with the evolution of the dipolar couplings. This will have two practical consequences: The first is that the reorientation angle detected by R-CODEX experiment becomes artefactually smaller due to this averaging. The second is that the amplitude of the signal becomes smaller [41, 42] as shown in Figure 5, although the time constant for the motion is still faithfully represented in this experiment. Therefore, stronger anisotropic interactions and high scaling factors for the recoupling are preferred for detecting faster dynamics, because shorter dephasing and refocusing times can be used, and therefore fewer effects are observed from dynamics during the dephasing and refocusing.
Figure 5.
Intensity loss during R-CODEX experiments due to exchange during dephasing is illustrated with simulations that assume different dipolar couplings and motional rate constants. In (a) simulations assumed a two-site jump of a single 13C-1H dipolar pair (with a coupling of 23.3 kHz), a reorientation angle of 120° and equal site populations. The solid lines are the single exponential fits to the discrete simulation results; rate constants so derived were perfectly consistent with the exchange rates assumed in the simulations. In (b) the parameters were the same as those used in (a) except that the dipolar coupling was reduced to 4.6 kHz. Note that the amplitude of magnetization is reduced, particularly for the faster motions, but the simulated decay constants are again perfectly consistent with the exchange rates assumed in the simulations.
The effect of vicinal protons
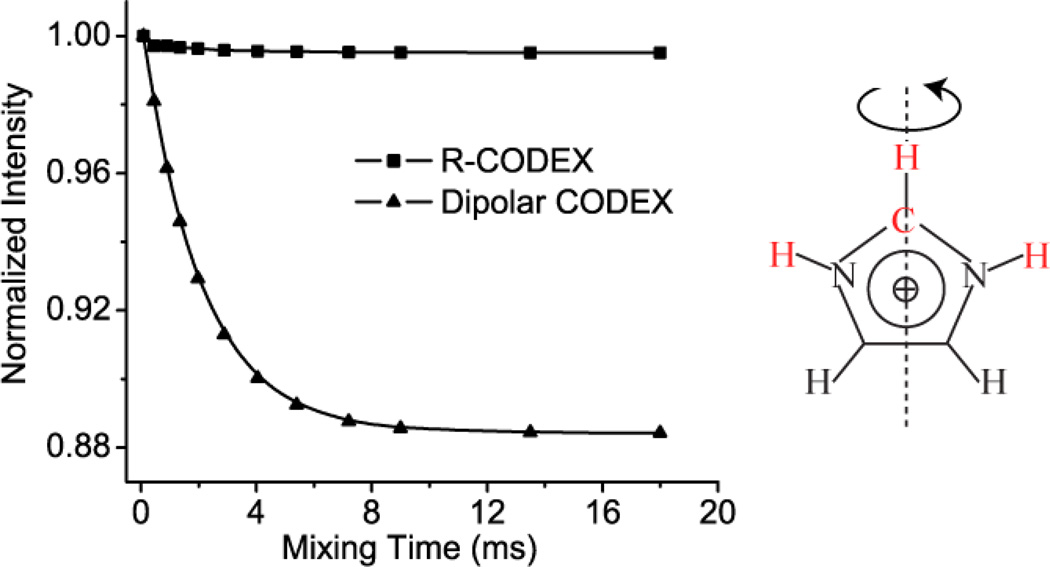
In most protein samples, the 1H-15N (or 1H-13C) bond of interest is located in a dense 1H network. The R1871 pulse sequence element used in the R-CODEX experiment, which can effectively suppress the 1H homonuclear dipolar couplings during the dephasing and recoupling time, can retain larger amplitude of signal (as shown in the simulations in the supplemental materials). Moreover, R-CODEX experiments minimize the effects from the motions of additional nearby 1Hs. The single quantum dipolar spin operators generated in this R-CODEX experiment corresponding to various neighboring spin pairs (H+CzSITE1+H−CzSITE1 compared to H+CzSITE2+H−CzSITE2) do not commute, so the weak dipolar interaction between a 13C and a remote 1H is truncated by the directly bonded 1H-13C coupling. Therefore, in a R-CODEX the artefactual decay due to the motion of the remote 1H has a very small amplitude (as shown in the Figure 6).
Figure 6.
The effect of motion of a remote 1H on 13C-1H R CODEX and Dipolar CODEX (which uses a REDOR element to recouple the 1H-13C dipolar interaction). The simulation system contains the apical carbon, the 1H bonded to it and the imidic 1H bonded to 15N (depicted as red in the right figure). We assume the imidazolium ring has a 180° flip with kex of 0.5 kHz. The left figure shows the remote 1Hs (imidic 1H bonded to 15N) have a substantial effect for dipolar CODEX and a modest one for R-CODEX.
In summary, in this study we used an R element to dephase and rephase 13C or 15N magnetization via the H-X heteronuclear dipolar coupling, in the context of a CODEX like pulse sequence for characterization of slow dynamics. This scheme has a number of useful features. The use of a strong 13C-1H or 15N-1H dipolar coupling and a sequence with a moderately good scaling factor is advantageous because the dephasing and refocusing times can be very short, thus reducing the loss of signal and increasing the maximum detection range of the dynamic rate (elaborated further in the supplemental material). Compared with other heteronuclear dipolar recoupling schemes (like T-MREV [33]), the R1871 (and the similar R1852) can be applied with use of a wide range of magic angle sample spinning frequencies (~10 kHz-20 kHz) while efficiently suppressing the 1H homonuclear coupling [34]. This aspect lends it to practical use in protein studies. Furthermore, the R1871 can be used to better digitize the dephasing curve of the strong 1H-13C or 1H-15N dipolar coupling, because more data points can be collected during a rotor period. These features can result in more accurate determination of the reorientational angle. In addition, the R1871 pulse sequence recouples the single quantum heteronuclear dipolar coupling (I+Sz+I−Sz), which has the property of dipolar truncation [35, 36]. Hence the pulse sequence can specifically detect the motion of the directly connected 1H-15N (1H-13C) bond with little interference of the motion of the remote 1H.
Conclusions
We have developed and demonstrated a new pulse sequence, R-CODEX, for characterization of slow molecular conformational dynamics. As for other dipolar based CODEX type sequences, the uniaxial dipolar coupling tensor and relatively fixed dipolar coupling constant provide a convenient probe for the reorientation angle analysis. The use of a strong heteronuclear coupling (e.g. 1H-13C or 1H-15N) is advantageous, because less signal loss occurs during the sequence, making faster motions amenable to study and making angle determination potentially more accurate. We demonstrate that with this sequence quantitative measurement can be carried out with fully protonated material. All of these properties make it a convenient method to detect slow dynamics in proteins. A model motional system, the π flipping of imidazolium ring was characterized in imidazolium methyl sulfonate. The motional rate constants and reorientational angle were consistent with previous results and models [25, 26].
Supplementary Material
Highlights.
An approach to detect millisecond-second dynamics
Both correlation time and reorientation angle can be detected
The effect of vicinal protons is suppressed in the dense 1H environment.
Acknowledgement
We thank Professor Kristin Kumashiro, Dr. Benjamin Wylie and Dr. Segolene Laage for critical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wenbo Li, Columbia University, Department of Chemistry, 3000 Broadway, New York, NY, 10027, USA, wl2215@columbia.edu.
Ann McDermott, Columbia University, Department of Chemistry, MC 3113, 3000 Broadway, New York, NY, 10027, USA, Tel: + 1 212 854 8393, Fax: + 1 212 932 1289, aem5@columbia.edu.
References
- 1.Vale RD, Milligan RA. The Way Things Move: Looking Under the Hood of Molecular Motor Proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 2.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 3.Benkovic SJ, Hammes-Schiffer S. A Perspective on Enzyme Catalysis. Science. 2003;301:1196–1202. doi: 10.1126/science.1085515. [DOI] [PubMed] [Google Scholar]
- 4.Palmer AG, Massi F. Characterization of the Dynamics of Biomacromolecules Using Rotating-Frame Spin Relaxation NMR Spectroscopy. Chemical Reviews. 2006;106:1700–1719. doi: 10.1021/cr0404287. [DOI] [PubMed] [Google Scholar]
- 5.Palmer AG. NMR Characterization of the Dynamics of Biomacromolecules. Chemical Reviews. 2004;104:3623–3640. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]
- 6.Salmon L, Bouvignies G, Markwick P, Lakomek N, Showalter S, Li DW, Walter K, Griesinger C, Bruschweiler R, Blackledge M. Protein Conformational Flexibility from Structure-Free Analysis of NMR Dipolar Couplings: Quantitative and Absolute Determination of Backbone Motion in Ubiquitin. Angew. Chem.-Int. Edit. 2009;48:4154–4157. doi: 10.1002/anie.200900476. [DOI] [PubMed] [Google Scholar]
- 7.DeAzevedo ER, Hu WG, Bonagamba TJ, Schmidt-Rohr K. Principles of centerband-only detection of exchange in solid-state nuclear magnetic resonance, and extension to four-time centerband-only detection of exchange. Journal of Chemical Physics. 2000;112:8988–9001. [Google Scholar]
- 8.Reichert D, Pascui O, Bonagamba TJ, Belton P, Schmidt A, deAzevedo ER. CONTRA: Improving the performance of dynamic investigations in natural abundance organic solids by mirror-symmetric constant-time CODEX. Journal of Magnetic Resonance. 2008;191:141–147. doi: 10.1016/j.jmr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Reichert D, Pascui O, Bonagamba TJ, DeAzevedo ER, Schmidt A. Scaling-down the CSA recoupling in S-CODEX 1D-MAS exchange experiments. Chemical Physics Letters. 2003;380:583–588. [Google Scholar]
- 10.Kennedy SB, DeAzevedo ER, Petka WA, Russell TP, Tirrell DA, Hong M. Dynamic structure of a protein hydrogel: A solid-state NMR study. Macromolecules. 2001;34:8675–8685. [Google Scholar]
- 11.Deazevedo ER, Kennedy SB, Hong M. Determination of slow motions in extensively isotopically labeled proteins by magic-angle-spinning 13C -detected 15N exchange NMR. Chemical Physics Letters. 2000;321:43–48. [Google Scholar]
- 12.Wylie BJ, Franks WT, Rienstra CM. Determinations of 15N Chemical Shift Anisotropy Magnitudes in a Uniformly 15N,13C-Labeled Microcrystalline Protein by Three-Dimensional Magic-Angle Spinning Nuclear Magnetic Resonance Spectroscopy. The Journal of Physical Chemistry B. 2006;110:10926–10936. doi: 10.1021/jp060507h. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Tasayco ML, Polenova T. Dynamics of Reassembled Thioredoxin Studied by Magic Angle Spinning NMR: Snapshots from Different Time Scales. Journal of the American Chemical Society. 2009;131:13690–13702. doi: 10.1021/ja9037802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weldeghiorghis TK, Stueber D, Schaefer J. Slow motion in [ring-fluoro]polycarbonate by CODEX. Journal of Polymer Science, Part B: Polymer Physics. 2008;46:1062–1066. [Google Scholar]
- 15.Li W, McDermott AE. Characterization of slow conformational dynamics in solids: Dipolar CODEX. Journal of Biomolecular NMR. 2009;45:227–232. doi: 10.1007/s10858-009-9353-8. [DOI] [PubMed] [Google Scholar]
- 16.Gullion T, Schaefer J. Rotational-echo double-resonance NMR. Journal of Magnetic Resonance. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Krushelnitsky A, DeAzevedo E, Linser R, Reif B, Saalwächter K, Reichert D. Direct observation of millisecond to second motions in proteins by dipolar CODEX NMR spectroscopy. Journal of the American Chemical Society. 2009;131:12097–12099. doi: 10.1021/ja9038888. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Edén M, Levitt MH. Recoupling of heteronuclear dipolar interactions in solid-state NMR using symmetry-based pulse sequences. Chemical Physics Letters. 2001;342:353–361. [Google Scholar]
- 19.Brinkmann A, Levitt MH. Symmetry principles in the nuclear magnetic resonance of spinning solids: Heteronuclear recoupling by generalized Hartmann-Hahn sequences. Journal of Chemical Physics. 2001;115:357–384. [Google Scholar]
- 20.Reif B, Hohwy M, Jaroniec CP, Rienstra CM, Griffin RG. NH-NH Vector Correlation in Peptides by Solid-State NMR. Journal of Magnetic Resonance. 2000;145:132–141. doi: 10.1006/jmre.2000.2067. [DOI] [PubMed] [Google Scholar]
- 21.Hediger S, Meier BH, Ernst RR. Adiabatic passage Hartmann-Hahn cross polarization in NMR under magic angle sample spinning. Chemical Physics Letters. 1995;240:449–456. [Google Scholar]
- 22.Detken A, Hardy EH, Ernst M, Kainosho M, Kawakami T, Aimoto S, Meier BH. Methods for sequential resonance assignment in solid, uniformly 13C, 15N labelled peptides: Quantification and application to antamanide. Journal of Biomolecular NMR. 2001;20:203–221. doi: 10.1023/a:1011212100630. [DOI] [PubMed] [Google Scholar]
- 23.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. Journal of Biomolecular NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 24.Veshtort M, Griffin RG. SPINEVOLUTION: A powerful tool for the simulation of solid and liquid state NMR experiments. Journal of Magnetic Resonance. 2006;178:248–282. doi: 10.1016/j.jmr.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Goward GR, Saalwächter K, Fischbach I, Spiess HW. Reorientation phenomena in imidazolium methyl sulfonate as probed by advanced solid-state NMR. Solid State Nuclear Magnetic Resonance. 2003;24:150–162. doi: 10.1016/S0926-2040(03)00043-2. [DOI] [PubMed] [Google Scholar]
- 26.Fischbach I, Spiess HW, Saalwächter K, Goward GR. Solid state NMR spectroscopic investigations of model compounds for imidazole-based proton conductors. Journal of Physical Chemistry B. 2004;108:18500–18508. [Google Scholar]
- 27.Hsu B, Schlemper EO. X-N deformation density studies of the hydrogen maleate ion and the imidazolium ion. Acta Crystallographica Section B. 1980;36:3017–3023. [Google Scholar]
- 28.Levitt MH. Encyclopedia of Magnetic Resonance. John Wiley & Sons, Ltd; 2007. Symmetry-Based Pulse Sequences in Magic-Angle Spinning Solid-State NMR. [Google Scholar]
- 29.Munowitz MG, Griffin RG, Bodenhausen G, Huang TH. Two-dimensional rotational spin-echo nuclear magnetic resonance in solids: correlation of chemical shift and dipolar interactions. Journal of the American Chemical Society. 1981;103:2529–2533. [Google Scholar]
- 30.Fuess H, Hohlwein D, Mason SA. NEUTRON-DIFFRACTION STUDY OF L-HISTIDINE HYDROCHLORIDE MONOHYDRATE. Acta Crystallographica Section B-Structural Science. 1977;33:654–659. [Google Scholar]
- Ying J, Bax A. NMR Determination of Amide N-H Equilibrium Bond Length from Concerted Dipolar Coupling Measurements. Journal of the American Chemical Society. 2008;130:16518–16520. doi: 10.1021/ja805654f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Case DA. Calculations of NMR dipolar coupling strengths in model peptides. Journal of Biomolecular NMR. 1999;15:95–102. doi: 10.1023/a:1008349812613. [DOI] [PubMed] [Google Scholar]
- 33.Hohwy M, Jaroniec CP, Reif B, Rienstra CM, Griffin RG. Local structure and relaxation in solid-state NMR: Accurate measurement of amide N-H bond lengths and H-N-H bond angles. Journal of the American Chemical Society. 2000;122:3218–3219. [Google Scholar]
- 34.Schanda P, Meier BH, Ernst M. Accurate measurement of one-bond H-X heteronuclear dipolar couplings in MAS solid-state NMR. Journal of Magnetic Resonance. 2011;210:246–259. doi: 10.1016/j.jmr.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann A, Kentgens AP. Sensitivity enhancement and heteronuclear distance measurements in biological 17O. J Phys Chem B. 2006;110:16089–16101. doi: 10.1021/jp062809p. [DOI] [PubMed] [Google Scholar]
- 36.Hohwy M. Band-selective homonuclear dipolar recoupling in rotating solids. J. Chem. Phys. 2002;117:4973. [Google Scholar]
- 37.Luo W, Hong M. Determination of the oligomeric number and intermolecular distances of membrane protein assemblies by anisotropic 1H-driven spin diffusion NMR spectroscopy. Journal of the American Chemical Society. 2006;128:7242–7251. doi: 10.1021/ja0603406. [DOI] [PubMed] [Google Scholar]
- 38.Pascui O, Beiner M, Reichert D. Identification of slow dynamic processes in poly(n-hexyl methacrylate) by solid-state 1D-MAS exchange NMR. Macromolecules. 2003;36:3992–4003. [Google Scholar]
- 39.Lewandowski JR, Sein J, Sass HJ, Grzesiek S, Blackledge M, Emsley L. Measurement of site-specific 13C spin-lattice relaxation in a crystalline protein. Journal of the American Chemical Society. 2010;132:8252–8254. doi: 10.1021/ja102744b. [DOI] [PubMed] [Google Scholar]
- 40.Reichert D, Bonagamba TJ, Schmidt-Rohr K. Slow-down of 13C spin diffusion in organic solids by fast MAS: A CODEX NMR study. Journal of Magnetic Resonance. 2001;151:129–135. doi: 10.1006/jmre.2001.2337. [DOI] [PubMed] [Google Scholar]
- 41.Hackel C, Franz C, Achilles A, Saalwächter K, Reichert D. Signal loss in 1D magic-angle spinning exchange NMR (CODEX): Radio-frequency limitations and intermediate motions. Physical Chemistry Chemical Physics. 2009;11:7022–7030. doi: 10.1039/b906527j. [DOI] [PubMed] [Google Scholar]
- 42.Saalwächter K, Fischbach I. The application of MAS recoupling methods in the intermediate motional regime. Journal of Magnetic Resonance. 2002;157:17–30. doi: 10.1006/jmre.2002.2552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.