Abstract
The purpose of this paper is to determine the active form of matrix metalloproteinases (MMP-2 and MMP-9) in corneal ulcer and ocular surface disorder patients. A total of 35 patients of corneal ulcer, 20 patients of ocular surface disorders and 10 control subjects were included in this study and estimation of active form of MMP-2 and MMP-9 was done by gelatin zymography. Tear samples were collected by capillary tube method. Both pro- and active forms of MMP-9 were detected in 24 out of 35 patients with corneal ulcer and 15 out of 20 patients with ocular surface disorders. None of the patients were showing MMP-2 activity. Neither MMP-2 nor MMP-9 was detected in the control group. Active forms of MMP-9 are present in tears of severe ulcerative and ocular surface disorder patients. Thus, proteinase inhibitors have been recommended for the treatment of corneal ulcer and ocular surface disorders to reduced the progression of stromal ulcer and to minimize corneal scarring.
Keywords: Angiogenesis, Inflammation, Scarring, Wound healing, Re-epithelialization
Introduction
The treatment for severe ocular surface disorders has been a long-standing challenge and has been advocated to a variety of medical techniques and surgical procedures, with only limited success.
The severity of clinical damage in ocular surface disorders is closely related to the amount of scarring and tissue contraction. Matrix metalloproteinases (MMPs) are a family of enzymes that play a vital role in all stages of wound healing response. They degrade extracellular matrix components and also have ability to synthesize collagen and so are important in the remodelling of wounds [1]. Overexpression of MMPs results in excessive extracellular matrix degradation, which leads to tissue destruction and loss of visual function.
The MMP family consist of at least 20 members in humans. They are divided into four classes on the basis of their substrates: collagenases, gelatinases, stromelysins, and membrane-type MMPs [2, 3]. Gelatinases consist of two subtypes (MMP-2 and MMP-9) that degrade type IV collagen, type VII collagen, and laminin. Regulation of MMP expression occurs both at the transcriptional and post-transcriptional levels by control of proenzyme activation and inhibition of the active enzyme by tissue inhibitors of MMPs (TIMPs) [4–7].
In response to injury inflammation, re-epithelialization and angiogenesis occur. There are three main phases: inflammatory, proliferative and remodelling. Granulation tissue formation and matrix deposition are mediated by different cell types (fibroblast, inflammatory and endothelial cells) [8, 9]. MMPs and TIMPs have been implicated in the regulation of angiogenesis [10, 11]. Matrix degradation allows the endothelial cells of the vessel to escape the parent venules and migrate into adjacent tissue towards the angiogenic stimulus, where they form new capillaries [12, 13]. Significant amount of work done by many researchers have demonstrated that there is only a fine line between physiological and pathological conditions which are governed by various mechanism, either directly or indirectly affecting MMP expression.
Material and method
This study was conducted in Department of Ophthalmology in conjunction with the Department of Molecular Biology. Ethics Committee approval has been obtained.
Patients with ocular surface disorders and corneal ulcer were selected on the basis of clinical examination. The patients were then chosen for tear collection. Informed consent was obtained from the volunteers and patients, after the nature and possible consequences of the study explained to them. Tear fluid samples were collected from three groups of subjects. The first group consisted of patients with the diagnosis of corneal ulcer, the second group with ocular surface disorders and the third group consisted of control subjects with no history of eye disorders.
Tear collection
Tear fluid was collected from the inferior tear meniscus, causing the least irritation possible by capillary tube method. The tear samples were then stored at −80 °C until they were used for gelatin zymography.
Gelatin zymography
The technique involves the electrophoresis of secreted protein enzyme through polyacrylamide gels containing enzyme substrates (gelatins). After addition of substrates to the resolving gel mixture (3.85 % dH2O, 20 mg/ml gelatin, 30 % acrylamide, 1.4 M Tris–HCl pH 8.8, 0.5 M Tris–HCl pH 6.8, 10 % SDS, 10 % APS, TEMED), it was poured in between the plates of electrophoresis apparatus. When the resolving gel was set, the stacking gel (6.10 % dH2O, 30 % acrylamide, 0.5 M Tris–HCl, pH 6.8, 10 % SDS, 10 % APS, TEMED) was poured over it. After about 10 min when the stacking gel was set, the comb was removed and gel was assembled onto the electrode section of the gel apparatus.
Tear samples were then mixed with an equal volume of 2× non-reducing sample buffer (for 8 ml, 2.8 ml dH2O, 1 ml 0.5 M Tris–HCl, pH 6.8, 0.8 ml glycerol, 3.2 ml 10 % w/v SDS and 0.2 ml 0.2 % bromophenol blue) and 15–20 μl was loaded per well. Gel was electrophoresed at 90 V at 40 °C in 1× running buffer (125 mM Tris–HCl, pH 8.3, 123 M glycerine, 0.5 % SDS) until the bromophenol marker dye reached the bottom of gel. After electrophoresis, SDS was removed from the gel by washing 3 × 10 min in 2.5 % Triton X-100 solution. This allows the MMPs to renature and digest the surrounding substrates when incubated overnight at 37 °C in zymogram incubation buffer (50 mM Tris–HCl, pH 7.6, 10 mM CaCl2·2H2O, 5.0 mM NaCl, 0.05 % Brij 35).
The background stains blue with Coomassie stain as the gel contains gelatin. Where the gelatin degraded, white bands appear indicating the presence of gelatinases. Gelatinase-A (MMP-2) is at about 62 kDa while the gelatinase-B (MMP-9) runs at about 82 kDa.
Results
Both pro- and active forms of MMP-9 were detected in 24 out of 35 (68.5 %) patients of corneal ulcer (Table 1, Fig. 1).
Table 1.
Results of gelatin zymography in corneal ulcer, ocular surface disorders and control subjects
| Aetiology | No. of patients | MMP-9 activity | MMP-2 activity | ||
|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | ||
| Corneal ulcer | 35 | 24 | 68.5 | 0 | 0 |
| Ocular surface disorder | 20 | 15 | 75 | 0 | 0 |
| Control | 10 | 0 | 0 | 0 | 0 |
Fig. 1.
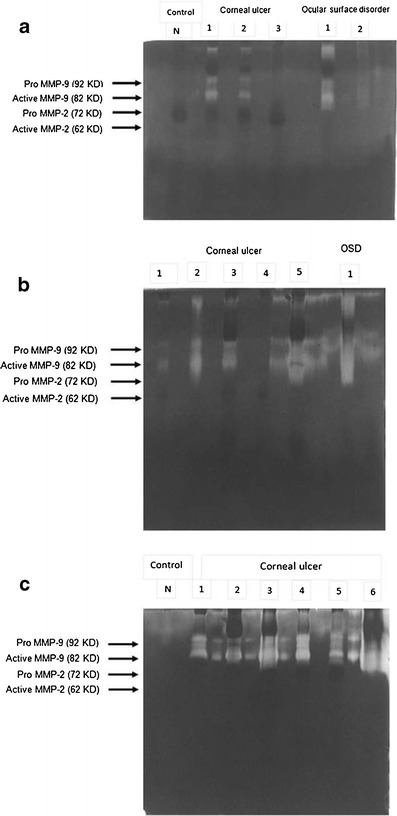
Results of gelatin zymography: a two out of three corneal ulcer patients and one out of two ocular surface disorder patients showing MMP-9 activity (both pro- and active forms). b Four out of five cases of corneal ulcer and one patient of ocular surface disorder showing MMP-9 activity. c All six patients showing MMP-9 activity. We did not find MMP-2 activity in any of our cases. No MMP activity detected in any of the control subjects
Between different aetiologic groups of corneal ulcer, 18 out of 25 (72 %) patients with fungal corneal ulcer, 1 out of 3 (33.3 %) patients with viral keratitis, and 5 out of 7 (71.4 %) patients with bacterial corneal ulcer showed MMP-9 activity (Table 2,Fig 1).
Table 2.
Results of gelatin zymography in corneal ulcer patients with different aetiologies
| Aetiology | No. of patients | Number of positive cases | |||
|---|---|---|---|---|---|
| MMP-9 activity | MMP-2 activity | ||||
| No. of patients | % | No. of patients | % | ||
| Fungal corneal ulcer | 25 | 18 | 72 | 0 | 0 |
| Viral keratitis | 3 | 1 | 33.3 | 0 | 0 |
| Bacterial corneal ulcer | 7 | 5 | 71.4 | 0 | 0 |
| Total | 35 | 24 | 68.5 | 0 | 0 |
| Control | 10 | 0 | 0 | 0 | 0 |
In ocular surface disorders group, MMP-9 activity (both pro and active form) was detected in 15 out of 20 (75 %) patients in gelatin zymography (Table 1, Fig. 1). Between different aetiologies, 3 out of 4 (75 %) patients with Steven Johnson syndrome, 3 out of 3 (100 %) patients with lime burn, 2 out of 2 (100 %) with chemical injury, and 7 out of 11 (63.6 %) patients with dry eye showed MMP-9 activity (Table 3, Fig 1).
Table 3.
Results of gelatin zymography in ocular surface disorders with different aetiologies
| Aetiology | No. of patients | Number of positive cases | |||
|---|---|---|---|---|---|
| MMP-9 activity | MMP-2 activity | ||||
| No. of patients | % | No. of patients | % | ||
| Steven Johnson Syndrome | 4 | 3 | 75 | 0 | 0 |
| Lime burn | 3 | 3 | 100 | 0 | 0 |
| Chemical injury | 2 | 2 | 100 | 0 | 0 |
| Dry eye | 11 | 7 | 63.6 | 0 | 0 |
| Total | 20 | 15 | 75 | 0 | 0 |
| Control | 10 | 0 | 0 | 0 | 0 |
Neither MMP-2 nor MMP-9 activity were detected in the control group. None of the patients showed any MMP-2 activity in gelatin zymography.
On follow-up of four patients with corneal ulcer and two patients with ocular surface disorders, MMP-9 activity decreases with treatment. It indicates that with wound healing, MMPs activity decreases (Table 4, Fig. 2a, b).
Table 4.
MMP activity of four corneal ulcer patients and two ocular surface disorder patients during follow-up (after initiation of treatment)
| Diagnosis | |||||||
|---|---|---|---|---|---|---|---|
| Sample no | First visit | First follow up | Second follow up | ||||
| MMP-2 activity | MMP-9 activity | MMP-2 activity | MMP-9 activity | MMP-2 activity | MMP-9 activity | ||
| Corneal ulcer | 1 | − | ++ | − | + | − | − |
| 2 | − | ++ | − | + | − | − | |
| 3 | − | + | − | − | − | − | |
| 4 | − | + | − | − | − | − | |
| Ocular surface disorders | 1 | − | +++ | − | ++ | − | + |
| 2 | − | +++ | − | ++ | − | + | |
Fig. 2.
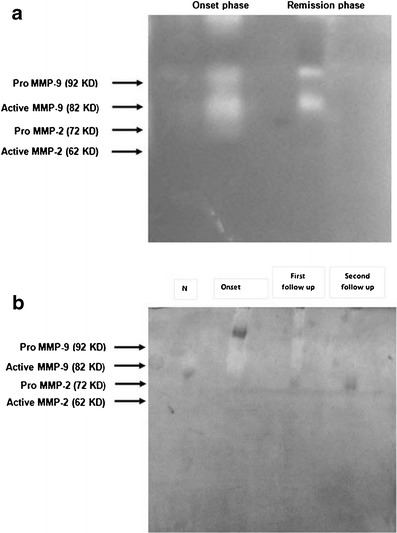
Results of gelatin zymography after initiation of treatment: a in one corneal ulcer patient. b In one ocular surface disorder patient. Results showing decrease in MMP-9 activity as the wound healing proceeded
Discussion
In the present study, detection of active form of MMPs is done with the help of gelatin zymography. MMPs play a vital role in all stages of the corneal wound healing process. They degrade the extracellular matrix and also have ability to synthesize collagen and extracellular matrix, and are therefore play an important role in wound healing. The response to injury includes inflammation, re-epithelialization, angiogenesis, granulation tissue formation and matrix deposition which are mediated by different cell types (fibroblast and inflammatory cells) [8, 9].
In our study, MMP-9 was observed in tear fluid of patients with various corneal ulcer and ocular surface disorders with the help of gelatin zymography. Because the active forms of MMPs are water-soluble enzymes, it is difficult to detect them by immunohistochemical or molecular biological method. Therefore, gelatin zymography (to see the presence of pro- and active forms of MMPs) was performed to evaluate the MMPs activity in the tear fluid. A particular advantage of gelatin zymography compared to immunohistochemistry is that both the latent and active form which can be distinguished on the basis of molecular weight can be detected. For molecular weight markers, we run normal gel whose 92 and 82 kDa corresponded with the white bands of pro and active form of MMP-9.
This study shows both the pro- and active forms of MMP-9 were present in gelatin zymography. Width and intensity of MMP-9 activity bands in gelatin zymography increase with severity of diseases. None of the patients with a healthy eye showed any MMP activity in gelatin zymography.
In this study MMP-9 (82 kDa) were present at a higher level in the tear fluid of patients with corneal ulcer and ocular surface disorders versus patients with healthy eyes. In contrast to previous studies [14, 15], we did not find any increase in the level of MMP-2 activity (neither pro- nor active forms) in any of our patients, this may be due to high tear fluid flow in the diseased eye causing a dilution effect.
Follow-up of four patients with corneal ulcer and two patients with ocular surface disorders showed that both the pro- and active forms of MMP-9 decreases as the wound healing proceeded.
Our data support that the use of aggressive therapy rapidly reduces the activity of tear proteases, both in cases of corneal ulcer and ocular surface disorders. Medical and surgical treatments of these patients lead to reduction in tear film proteolytic activity that corresponded with the improvement in the clinical signs of corneal ulceration. Normalizing proteolytic activity in the tear film is an objective sign of effectiveness of treatment of corneal ulcer and ocular surface disorder. Thus, proteinase inhibitors have been recommended for the treatment of corneal ulcer and ocular surface disorders to reduce the progression of stromal ulcer and to minimize corneal scarring.
In conclusion, in spite of various limitations, it could be confirmed that active MMPs are present in tears of severe ulcerative and ocular surface disorders patients. The active form of gelatinases, especially MMP-9, which is a consistent finding in all our cases, may be closely related with these diseases, and use of protease inhibitors can reduce the progression of stromal ulcer and can minimize corneal scarring.
References
- 1.Birkedal-Hansen H. From tadpole collagenase to a family of matrix metalloproteinases. J Oral Pathol. 1988;17:445–451. doi: 10.1111/j.1600-0714.1988.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LL, Dyer R, Hupe DJ. Matrix metalloproteinases. Curr Opin Chem Biol. 1998;2:466–471. doi: 10.1016/S1367-5931(98)80122-1. [DOI] [PubMed] [Google Scholar]
- 3.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structure, evolution and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 4.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinases genes in normal and neoplastic cells. Enzyme Protein. 1996;49:20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 5.Bode W, Fernandez-Catalan C, Grams F, Gomis-Ruth FX, Nagase H, Tschesche H, et al. Insights into MMP–TIMP interaction. Ann NY Acad Sci. 1999;878:73–91. doi: 10.1111/j.1749-6632.1999.tb07675.x. [DOI] [PubMed] [Google Scholar]
- 6.Kleiner DE, Jr, Stetler-Stevenson WG. Structural biochemistry and activation of matrix metalloproteinases. Curr Opin Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-W. [DOI] [PubMed] [Google Scholar]
- 7.Woessner JF., Jr Matrix metalloproteinase inhibition. From the Jurassic to the third millennium. Ann NY Acad Sci. 1999;878:388–403. doi: 10.1111/j.1749-6632.1999.tb07697.x. [DOI] [PubMed] [Google Scholar]
- 8.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblast during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 9.Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 10.Langer R, Conn H, Vacanti J, Haudenschild C, Folkman J. Control of tumor growth in animals by infusion of an angiogenesis inhibitors. Proc Natl Acad Sci U S A. 1980;77:4331–4335. doi: 10.1073/pnas.77.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 12.Das A, McLamore A, Song W, McGuire PG. Retinal neovascularisation is suppressed with matrix metalloproteinase inhibitor. Arch Ophthalmol. 1999;117:498–503. doi: 10.1001/archopht.117.4.498. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/S0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 14.Sakimoto T, Sholi J, Sawa M. Active form of gelatinases in tear fluid in patients with corneal ulcer or ocular burn. Jpn J Ophthalmol. 2003;47:423–426. doi: 10.1016/S0021-5155(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Pan Q, Xue Q, Cui J, Qi C. Evaluation of matrix metalloproteinase concentration in precorneal tear film from dogs with pseudomonas aeruginosa associated keratitis. AmJ Vet Res. 2008;69:1341–1345. doi: 10.2460/ajvr.69.10.1341. [DOI] [PubMed] [Google Scholar]


