Abstract
Background and Objective
Subcutaneously injected rapid-acting insulin analogs do not replicate physiologic insulin action due to delays in their onset and peak action resulting in post-prandial glucose excursions. The InsuPatch (IP) is a novel insulin infusion site warming device developed to accelerate insulin action by increasing blood flow to the area of insulin absorption. 13 adolescents with type 1 diabetes (mean age 14±4 years) were enrolled in this study to investigate the effect of the IP on the pharmacodynamics and pharmacokinetics of a 0.2u/kg bolus dose of aspart insulin using the euglycemic clamp technique.
Research Design and Methods
Each subject underwent two euglycemic clamp procedures on separate occasions: one with and one without IP activation in random order.
Results
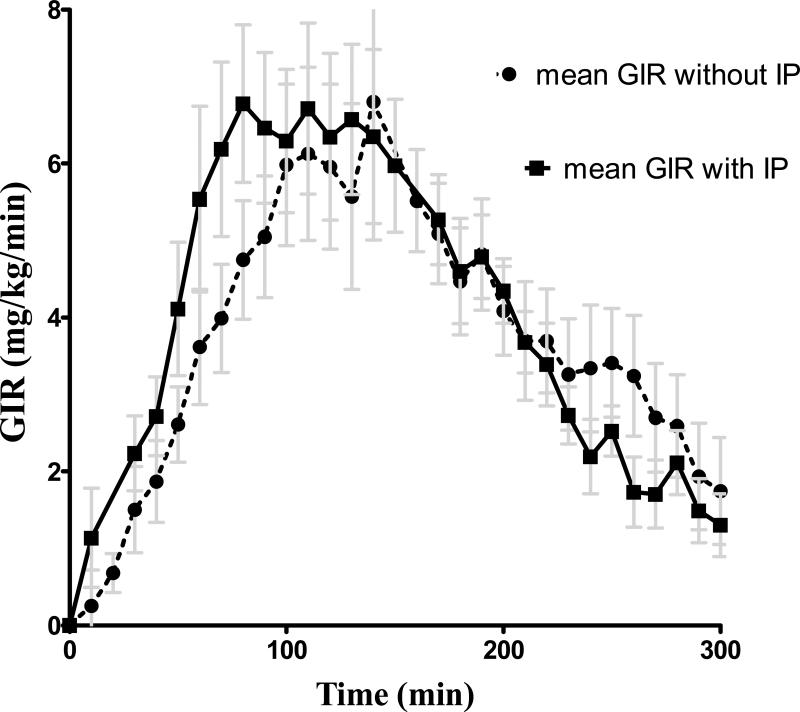
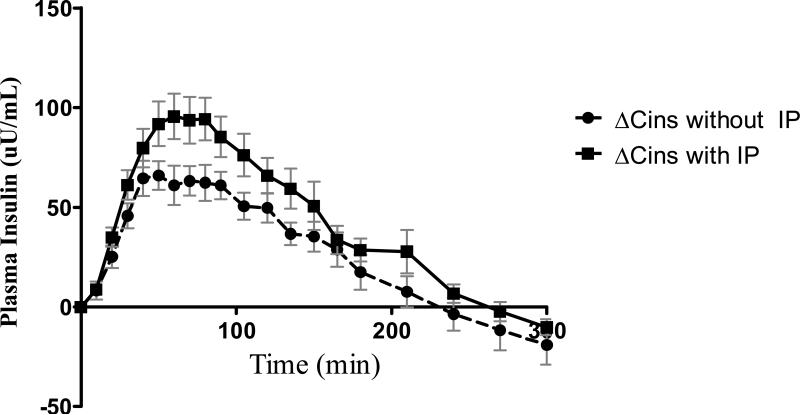
When the insulin bolus was given with IP activation as compared to without IP activation, time to reach maximum insulin action (TGIRmax) and to reach 50% maximum action (T 50%GIRmax) were 35 and 18 minutes earlier (125±8 min vs. 90±6min, p=0.002 and 58±5min. vs. 40±3min, p=0.01, respectively), and the AUC GIR 0-90min, reflecting early glucodynamic action, was significantly greater (p=0.001). IP activation also accelerated the rise in plasma insulin levels after the bolus (p=0.03) and resulted in a higher peak (p=0.04) and greater overall increase (p=0.02) in plasma insulin levels.
Conclusions
Our results demonstrate that insulin infusion site warming with IP activation accelerates the time action profile of aspart insulin which may be of benefit to current open-loop and future closed-loop insulin delivery in patients with type 1 diabetes.
Keywords: insulin action, pharmacokinetic, glycemic control, pediatric diabetes, closed-loop
Introduction
While the time-action profiles of subcutaneous injections of rapid-acting insulin analogs are accelerated in comparison to that of regular insulin1, delays in the absorption and action of these analogs remain important obstacles in attempts to replicate physiologic plasma insulin levels in patients with type 1 diabetes (T1D). In children, adolescents and adults with T1D, we and others have shown that the peak action of 0.2 to 0.3 unit/kg bolus doses of aspart and lispro insulin is not observed until 90-130 minutes after a subcutaneous injection 2,3, and the duration of action often extends beyond 5 hours. Thus, use of current rapid-acting insulin analogs during open-loop therapy in patients with T1D does not fully overcome the tendency of hyperglycemic excursions immediately after a meal and the risk for late post-prandial hypoglycemia.
Problems inherent in the use of the subcutaneous route of insulin administration in T1D are exaggerated further in reactive, closed-loop systems (a.k.a. artificial pancreas system) comprised of external insulin pumps and external glucose sensors 3,4. In these systems, meal-stimulated insulin administration does not even begin until sensor glucose levels start to rise 15-20 minutes after the start of the meal, and it is extended over several hours after the meal to match the physiologic delays in carbohydrate digestion and absorption. While some of the problems in using the subcutaneous route of insulin administration in open and closed-loop insulin delivery can be overcome by pre-meal priming boluses of insulin 3, consistent compliance with early bolus administration has been difficult to achieve in clinical practice.
Recent advances in closed-loop insulin delivery using external pumps and sensors have rekindled interest in the development of new insulin preparations with accelerated rates of insulin absorption and action 2,3,5,6. An alternate approach was suggested a number of years ago by Koivisto and colleagues. These investigators used exposure to a sauna to demonstrate that the rate of insulin absorption could be increased by increasing blood flow to the skin via increasing ambient temperature7. Similar results were obtained in other studies by local application of heat around the site of a bolus injection 8,9. These observations served as the basis of the development of a novel insulin pump infusion set, the InsuPatch (InsuLine Medical Ltd. , Petach-Tikvah, Israel), in which battery-powered warming coils are incorporated into the adhesive layer of the infusion set. When activated around the time of a bolus injection, the device applies controlled heat around the insulin infusion site that, in turn, increases blood flow into the area 10.
The objective of this randomized, crossover study was to investigate the impact of the InsuPatch (IP) on the pharmacodynamics and pharmacokinetics of aspart insulin in youth with T1D by using the euglycemic clamp technique. We hypothesized that warming the insulin infusion site with the IP will increase the rate of absorption and accelerate the action of this rapid-acting insulin analog.
Methods
Subjects
Subjects with T1D who attended the Yale Children's Diabetes Clinic were recruited for this study. Eligibility criteria included a clinical diagnosis of T1D for ≥1 year, age between 12 to 18 years, continuous subcutaneous insulin infusion (CSII) therapy for at least 3 months, HbA1c <10.0% , BMI <95% for age and sex, and the ability to comprehend written and spoken English. Subjects were excluded for any other medical disease aside from T1D or treated hypothyroidism; use of medications that might affect glycemic control; pregnancy or breast-feeding; not consistently using barrier methods or abstinence as contraception; or any other condition that in the judgment of the investigators would interfere with the subject's or parent's ability to provide informed consent or the investigator's ability to perform the study. The Yale University Human Investigation Committee approved the study.
At the initial enrollment visit, the risks and benefits of the study were explained; informed consent was obtained from the parents or subjects who were 18 years of age, and informed assent from the subjects <18 years of age; history and physical examinations were performed and HbA1c was measured.
InsuPatch
The warming element is incorporated into the adhesive layer of the insulin infusion set and connected by wires to batteries that are carried in a modified insulin pump carrying case. The warming element contains a temperature sensor and controller unit that maintains the skin warming at a fixed temperature. In these experiments, the skin warming temperature was set at 38.5 °C.
Procedures
Each subject underwent a glucose clamp study on two separate occasions separated by ≤ 8 weeks; one with the IP activated and the other without IP activation in random order. On each occasion, subjects were admitted on the afternoon prior to the euglycemic clamp procedure. A new insulin infusion set was inserted into the subcutaneous tissue of the anterior abdominal wall and an i.v. catheter was placed for overnight blood sampling. Blood glucose levels were measured hourly during the night and the basal insulin infusion was adjusted as needed to achieve glucose levels between 80-120 mg/dl at the start of the euglycemic clamp procedure the next morning.
All subjects fasted overnight and were kept fasting until the end of the 5-hour clamp study. A second i.v. catheter was placed on the contralateral arm for infusion of exogenous glucose on the morning of the clamp study, and subjects were randomized to receive 0.2unit/kg body weight bolus of aspart insulin with or without the IP activation. Subjects who were studied with IP activation during the first clamp were given the same dose of aspart insulin without IP activation during the second euglycemic clamp and vice versa. During IP activation studies, IP was activated to warm the insulin infusion site for 15 minutes prior to the bolus and was kept on for 60-90 minutes after administration of the bolus. In all studies, the basal infusion of insulin via the insulin pump was suspended after the bolus was given.
A variable rate of 20% dextrose was infused and adjusted every five minutes based on bedside measurements of plasma glucose to maintain levels between 90-100 mg/dl during the study for a period of five hours 11,12. Blood was collected at 10-minute intervals to measure insulin plasma levels for the first 90 minutes, at 15-minute intervals for the next 90 minutes, and then every 30 minutes for the last 120 minutes.
Biochemical methods
HbA1c was measured by the DCA Vantage Analyzer (Siemens Medical Equipment, Malvern, PA, USA), plasma glucose by the YSI Glucose Analyzer (Yellow Springs Instrument Life Sciences Incorporated, Yellow Springs, Ohio, USA), and plasma insulin by the Mercodia iso-insulin ELISA test, with a reported cross reactivity of 80% with insulin aspart (MercodiaAB, Uppsala, Sweden).
Statistical Analyses
The exogenous glucose infusion rate (GIR) was analyzed every 10 minutes and adjusted for changes in the glucose space, as previously described 11. The pharmacodynamic parameters that were calculated for each clamp study included the maximum glucose infusion rate (GIRmax), time to maximum glucose infusion rate (TGIRmax) and time to half maximum glucose infusion rate (T50%GIRmax). In addition to total AUCGIR 0-300 min, partial AUCGIR values from 0-90 min (AUCGIR 0-90min) were also analyzed to determine early glucodynamic action.
The mean baseline plasma insulin concentrations were similar between the two studies (62±10 uU/mL vs. 71±18 uU/mL with and without IP respectively, p=0.4), but the within subject absolute difference between the two studies averaged 27uU/mL. To adjust for these within subject differences in baseline insulin levels, the increments in plasma insulin levels from baseline were used for analysis. Mean increment (ΔCins), maximum increment (ΔCins max), total area under curve for ΔCins (AUC ΔCins 0-300min) and the AUC ΔCins0-90min (an index of early insulin exposure) were calculated. Comparisons of pharmacokinetic parameters between IP and no IP studies are based on data from 12 of the 13 subjects, since there were assay problems in insulin samples from one of the clamp studies in one of the subjects.
Statistical comparisons were performed using GraphPad Prism version 5.0 (GraphPad Software Inc. La Jolla, CA). Paired t-tests were used where applicable to compare plasma glucose and glucose clamp data, which are reported as mean ± SEM.
Results
This study included 4 girls and 9 boys with type 1 diabetes, totally 13 subjects aged 14±4 years, Tanner stage II-V and with a mean HbA1c of 7.3±0.2%. Mean plasma glucose concentrations did not differ between the two studies during the 5-hour clamps (96±1mg/dL with IP and 98±1mg/dL without IP, p=0.2).
As illustrated in Figure 1 and the Table, IP activation shifted the time action curve of aspart insulin to the left, with an earlier T GIRmax (p=0.002) and T 50%GIRmax (p=0.01). While the total AUCGIR 0-300min and GIRmax did not differ significantly between the two studies, the AUCGIR0-90min was much greater with IP activation (p=0.001).
Figure 1.
Aspart insulin time action profiles with (squares and solid line) and without (circles and dashed line) the InsuPatch activation. Data are shown as mean ± SEM.
Table.
The pharmacodynamic and pharmacokinetic parameters (mean±SEM) with and without the InsuPatch activation after the same bolus dose of aspart insulin investigated by euglycemic clamp study.
| Pharmacodynamic Parameters | No InsuPatch | With InsuPatch | P |
|---|---|---|---|
| GIRmax (mg/kg/min) | 7.2±0.7 | 8.0±1 | 0.4 |
| T GIRmax (min) | 125±8 | 90±6 | 0.002 |
| T 50%GIRmax (min) | 58±5 | 40±3 | 0.01 |
| AUC GIR 0-300min (mg/kg) | 1141±107 | 1280±118 | 0.1 |
| AUC GIR 0-90min (mg/kg) | 260±30 | 354±40 | 0.001 |
| Pharmacokinetic Parameters* | P | ||
| ΔCins (uU/mL) | 32±3 | 45±7 | 0.08 |
| ΔCmax (uU/mL) | 81±8 | 106±11 | 0.04 |
| AUC ΔCins 0-300 min (uU.min/ml) | 10470±1202 | 13380±1670 | 0.02 |
| AUC ΔCins 0-90 min (uU.min/ml) | 4342±441 | 5846±698 | 0.04 |
All values calculated as increments above baseline (ΔC)
The increments in plasma insulin that were achieved after the 0.2 unit/kg bolus of aspart insulin are shown in Figure 2. As shown in the Table, while differences in mean ΔCins levels between the two studies did not achieve statistical significance (p=0.08), the maximal increment in insulin (ΔCmax) was higher with the IP versus without the IP activation (p=0.04). The time to reach maximum increments in plasma insulin averaged 67±10min without the IP. In contrast, the same increment in plasma insulin level was achieved at 41±4 min with the IP activation (p=0.03). The overall bioavailability of insulin, as indicated by AUC ΔCins 0-300min was 28 % greater with the IP activation versus without IP activation. It is especially noteworthy that skin warming with the IP increased exposure to insulin during the first 90 minutes after the insulin bolus (AUC ΔCins0-90min) by 35% versus without IP (p=0.04). All subjects tolerated the IP well without any site irritation or discomfort.
Figure 2.
The change in plasma insulin concentrations from the baseline (ΔCins) during studies with (squares and solid line) and without (circles and dashed line) the InsuPatch activation, presented as mean ± SEM.
Discussion
These data demonstrate that warming the skin around an insulin infusion site to 38.5°C with the IP accelerates the time action profile of a standard bolus dose of aspart insulin. Notably, the peak action of the bolus was observed 35 minutes sooner with IP activation and the glucodynamic effects of the bolus were increased by nearly 40% during the 90 minutes following the bolus (AUC GIR 0-90 min). On the other hand, the peak action and total glucodynamic effects of the bolus (AUC GIR 0-300min) did not differ significantly between the two studies. The pharmacokinetic analyses demonstrated that skin warming with the IP accelerated the absorption of the bolus and increased the magnitude of the rise in plasma insulin concentrations (ΔCmax). In addition, the increase in AUC ΔCins 0-300 min suggests that use of the IP enhanced the bioavailability of the aspart bolus, as well. This modest discrepancy between the magnitude of the pharmacokinetic and pharmacodynamic effects of the IP may partly relate to the fact that almost all of the subjects in this study were teenagers in puberty. As we have previously demonstrated, the insulin resistance of puberty is likely to have blunted some of the glucodynamic enhancements that would have otherwise resulted from the higher insulin concentrations that were achieved with the IP3.
Our results are in agreement with previous studies that demonstrated faster insulin kinetics and dynamics by increasing ambient temperature and local warming of the skin around an insulin infusion site 7-9. The results also correspond with a recent meal study investigating the effect of the IP on rapid acting insulin action in the adults with T1D 10. Moreover, our results with the IP compared favorably to those of other studies that used alternate technologies aimed at increasing the rate of absorption and action of subcutaneously injected insulin tested only in adults 6,13-15. The acceleration of the insulin action profiles of bolus dose of rapid acting insulins have been reported with intradermal micro-needle insulin delivery 15 and mixing lispro insulin with recombinant human hyaluronidase, which breaks down barriers to fluid dispersion in the subcutaneous space 16. The major advantages of the IP are that it is well tolerated, non-invasive, and doesn't involve injecting pharmacologic agents with potential adverse side effects.
The duration of heating before the bolus injection was based on previous studies demonstrating that it takes up to 10 minutes for increases in blood flow to subcutaneous tissues to reach maximum values after skin surface warming17. We activated the IP 15 minutes prior to the insulin bolus in this study to give us a 5 minute margin of error. As previously shown3 and demonstrated in the control studies without IP activation in this study, peak plasma insulin concentrations are observed up to ~90 minutes after the 0.2 u/kg body weight bolus injection. We chose to keep the warming active for 60-90min to ensure that we maintained increased blood flow during anticipated period of maximum insulin absorption. It remains to be determined whether shorter warming periods are equally effective. The anterior abdominal wall was used for placement of the infusion set in all of these experiments for consistency with our previous studies and to provide better site visibility and temperature control. It will be intriguing to investigate the rate of acceleration of insulin absorption from different sites (e.g., arm versus leg versus abdomen) after warning the skin with the IP in a future research study.
One limitation of this study is that the full potential of the IP may even be greater than what we observed by warming the skin only to 38.5°C. Other devices that warm the skin to 40-42°C are well tolerated, approved by the FDA and, as shown in Doppler-flow ultrasound studies, cause a greater increase in local blood flow than skin warming to 38.5°C. The optimal timing of IP activation (e.g., manually 15 minutes before versus automatically with the bolus) and the duration of IP activation also remain to be determined. Once these parameters of use are clarified, we look forward to future studies where this novel insulin infusion set is incorporated into external close-loop systems to improve system performance in managing meal-stimulated glucose excursions.
Acknowledgements
This work was made possible by grants from the National Institutes of Health (Clinical and Translational Science Award 5 KL2 RR024138 and R01 DK085618), Juvenile Diabetes Research Foundation (JDRF 17-2010-426), and the assistance from the staff of the Yale-New Haven Hospital Research Unit who are supported by NIH CTSA grant RR 023423. The abstract was published in Diabetes, 916-P, A-250, American Diabetes Association 71st Scientific Meeting.
Dr. Eda Cengiz, Dr. Jennifer Sherr, Eileen Tichy, Melody Martin, Amy Steffen and Lori Carria have no disclosures. Dr. William Tamborlane is a consultant for Medtronic, Novo Nordisk, Eli Lilly, Bayer, Unomedical, Insuline and a speaker for Novo Nordisk and Eli Lilly. Dr. Stuart Weinzimer is a consultant for Medtronic, Animas, BD Medical, Insuline and a speaker for Eli Lilly.
We thank Yale Pediatric Diabetes Clinic & Research Center Team members, our patients and their families.
Abbreviations
- CSII
Continuous subcutaneous insulin infusion
- FDA
Federal Drug Administration
- GIR
Glucose infusion rate
- IP
InsuPatch
- T1D
Type 1 diabetes
- YSI
Yellow Springs Instrument
References
- 1.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396–402. doi: 10.2337/diab.43.3.396. [DOI] [PubMed] [Google Scholar]
- 2.Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32:240–4. doi: 10.2337/dc08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care. 2008;31:44–6. doi: 10.2337/dc07-0737. [DOI] [PubMed] [Google Scholar]
- 4.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–50. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 5.Morrow L, Muchmore DB, Ludington EA, Vaughn DE, Hompesch M. Reduction in intrasubject variability in the pharmacokinetic response to insulin after subcutaneous co-administration with recombinant human hyaluronidase in healthy volunteers. Diabetes Technol Ther. 13:1039–45. doi: 10.1089/dia.2011.0115. [DOI] [PubMed] [Google Scholar]
- 6.Steiner S, Hompesch M, Pohl R, et al. A novel insulin formulation with a more rapid onset of action. Diabetologia. 2008;51:1602–6. doi: 10.1007/s00125-008-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koivisto VA. Sauna-induced acceleration in insulin absorption from subcutaneous injection site. Br Med J. 1980;280:1411–3. doi: 10.1136/bmj.280.6229.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger M, Cuppers HJ, Hegner H, Jorgens V, Berchtold P. Absorption kinetics and biologic effects of subcutaneously injected insulin preparations. Diabetes Care. 1982;5:77–91. doi: 10.2337/diacare.5.2.77. [DOI] [PubMed] [Google Scholar]
- 9.Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E. Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia. 1994;37:377–80. doi: 10.1007/BF00408474. [DOI] [PubMed] [Google Scholar]
- 10.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31:980–7. doi: 10.1016/j.clinthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 12.Cengiz E, Swan KL, Tamborlane WV, Steil GM, Steffen AT, Weinzimer SA. Is an automatic pump suspension feature safe for children with type 1 diabetes? An exploratory analysis with a closed-loop system. Diabetes Technol Ther. 2009;11:207–10. doi: 10.1089/dia.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow L, Muchmore DB, Ludington EA, Vaughn DE, Hompesch M. Reduction in intrasubject variability in the pharmacokinetic response to insulin after subcutaneous co-administration with recombinant human hyaluronidase in healthy volunteers. Diabetes Technol Ther. 2011;13:1039–45. doi: 10.1089/dia.2011.0115. [DOI] [PubMed] [Google Scholar]
- 14.Pettis RJ, Ginsberg B, Hirsch L, et al. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13:435–42. doi: 10.1089/dia.2010.0184. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann L, Nosek L, Flacke F, et al. U-100, pH-Neutral formulation of VIAject((R)) : faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- 16.Hompesch M, Muchmore DB, Morrow L, Vaughn DE. Accelerated insulin pharmacokinetics and improved postprandial glycemic control in patients with type 1 diabetes after coadministration of prandial insulins with hyaluronidase. Diabetes Care. 2011;34:666–8. doi: 10.2337/dc10-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic proceedings Mayo Clinic. 2003;78:603–12. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]