Abstract
Rationale
The orexin/hypocretin system has recently been implicated in reward-seeking, especially for highly salient food and drug rewards. We reasoned that this system may be strongly engaged during periods of reward restriction, including food restriction.
Objectives
This study examined the involvement of the orexin (Orx) system in responding for sucrose, and in cue-induced reinstatement of extinguished sucrose-seeking, in ad libitum fed vs. food-restricted male subjects.
Methods
Sprague Dawley rats (n=108) were trained to self-administer sucrose, and we determined the effects of pretreatment with the OxR1 receptor antagonist SB 334867 (SB; 10–30 mg/kg) on fixed ratio (FR) or progressive ratio (PR) sucrose self-administration, as well as on cue-induced reinstatement of sucrose-seeking. Finally, expression of the immediate early gene c-fos in Orx neurons was examined after self-administration, late extinction or cue-induced reinstatement of sucrose seeking.
Results
SB decreased lever responding (by about 1/3) and the number of reinforcers earned during FR, and less so during PR, schedules and decreased cue-induced reinstatement to sucrose-seeking to extinction levels, predominately in food-restricted rats. Additionally, Fos expression in Orx neurons in perifornical and dorsomedial hypothalamus was increased during extinction.
Conclusions
These results indicate that signaling at the OxR1 receptor is involved in pronounced sucrose reinforcement, and reinstatement of sucrose-seeking elicited by sucrose-paired cues, in food-restricted subjects. These findings lead us to conclude that conditioned activation of Orx neurons increases motivation for food reward during food restriction.
Keywords: orexin, addiction, obesity, reward-based feeding, palatable food, conditioned stimuli
Introduction
The ability to detect and procure food is an important survival mechanism; however, in the modern environment where food is readily available, this once important survival mechanism may contribute to the obesity epidemic. Animals use cues associated with food to determine where to obtain food, and to identify which foods are safe to eat, most nutritionally valuable and most rewarding. These cues interact with the brain’s reward system to stimulate motivated behavior that in turn may cause excessive food intake and obesity (Castellanos et al. 2009; Nijs et al 2010). Food cues activate specific nuclei within the mesolimbic dopamine and opioid systems, and there is significant overlap of activation within these areas with that produced by drugs of abuse (Avena et al. 2008; Huang et al. 2005; Kelley at al. 2000; Rada et al. 2005; Spangler et al. 2004). Recent findings have implicated lateral orexin (Orx) neurons in preference for cues associated with food or drug reward and in reward-seeking behaviors, including cue-induced reinstatement to extinguished drug seeking (Borgland et al. 2009; Cason et al. 2010; Choi et al. 2010; Smith et al. 2009; Smith and Aston-Jones 2012).
There are two Orx peptides, Orx A and Orx B (also denoted hypocretin 1 and hypocretin 2), and two Orx receptors, termed OxR1 and OxR2 (also denoted HcrtR1 and HcrtR2) (de Lecea et al. 1998; Sakurai et al. 1998). Orexins are synthesized solely in hypothalamic neurons (de Lecea et al. 1998; Sakurai et al. 1998). These neurons send fiber projections throughout the brain (Peyron et al. 1998; Sutcliffe and de Lecea 2002), and Orx receptors are similarly widely distributed although their expression is largely non-overlapping and regionally selective (Kilduff and de Lecea 2001; Lu et al. 2000; Marcus et al. 2001; Trivedi et al. 1998).
Early studies showed that Orx modulated homeostatic behaviors such as arousal and feeding (de Lecea et al. 1998; Sakurai et al. 1998). Central Orx administration stimulated food consumption, and systemic administration of the OxR1 antagonist SB-334867 (SB) reduced feeding (Ishii et al. 2204; Ishii et al. 2005; Rodgers et al. 2000; Sakurai et al. 1998; Thorpe et al. 2005; Yamanaka et al. 1999). Recent data indicate that the orexin system also regulates food reinforced behaviors. Central administration of Orx increased operant responding for sweets, and conversely pharmacological blockade of OxR1s decreased responding for high fat chocolate or sucrose (Borgland et al. 2009; Cason et al. 2010; Choi et al. 2010; Espana et al. 2010). Notably, the effects of Orx signaling on motivated behavior seem to be specific for palatable foods with high reinforcing value (Borgland et al 2009). These studies indicate that the Orx system is involved in conditioned responding for highly rewarding foods. In addition, the Orx system has been implicated in reward-seeking and in approach for environments associated with reward (Borgland et al. 2009; Cason et al. 2010; Smith and Aston-Jones 2012; Harris et al. 2005; Smith et al. 2010). Together, these studies link the Orx system to hedonically motivated eating and stimulus-induced food-seeking.
Previous evidence also indicates that the Orx system may regulate food-seeking behavior associated with food restriction. Food restriction produces changes in hormones that regulate adiposity signals and energy expenditure, and the action of these hormones is mediated at least in part through the Orx system (Lawrence et al. 2003; Lopez et al. 2000). For example, Orx signaling mediates restriction-induced increases in operant responding (Vialou et al. 2011), and Orx is elevated in calorically restricted rats (Pankevich et al. 2010). These studies highlight the potential of Orx signaling to modulate food-seeking behavior in the context of caloric restriction.
The present study is the first to investigate the role of the OxR1 in operant responding for sucrose, and in cue-induced reinstatement of extinguished sucrose-seeking, as a function of food restriction. We hypothesized that food restriction would engage the Orx system to increase motivation for food, and therefore that an OxR1 antagonist would decrease food self-administration and reinstatement of food seeking in food-restricted rats.
Methods and Materials
Subjects
Male Sprague Dawley rats (Charles River, Wilmington, MA, USA) were singly housed under a reversed 12h/12h light/dark cycle (lights off 0600 h). Rats were divided into two groups: ad libitum fed and food-restricted. Ad libitum rats had non-restricted access to food and water; food-restricted rats had free access to water and were food-restricted to 85% of the ad libitum rats’ body weight. Food-restricted rats were given their daily food ration at 1500 h. Rats were housed in the animal facility at the Medical University of South Carolina (AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committee and conducted in accordance to the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals.
Experiment 1: Fixed ratio responding for sucrose
Self-administration sessions were conducted in operant conditioning chambers housed in sound attenuating cubicles and controlled via MED-PC IV software (Med-Associates, St Albans, VT, USA). Rats were trained to lever press for sucrose reward (45 mg sucrose pellets, Test Diet, Richmond, IN, USA) on a fixed ratio 1 (FR1) schedule of reinforcement during daily 1 h sessions. Presses on the inactive lever had no programmed consequences. Pellet delivery was simultaneously accompanied by a discrete light + tone cue (78 dB, 2900 Hz; white stimulus light above the active lever) and followed by a 20 s timeout. The red house light (on the wall opposite the levers) was turned off during sucrose reward and time-outs. Rats were given 10 self-administration sessions in which they earned ≥ 10 sucrose pellets. Once stable responding was established, rats were given injections (vehicle or SB) 30 min prior to a self-administration session. Each rat received two injections of SB on different sessions. The doses of SB used in each rat were as follows: 10 and 30 mg/kg, 10 and 20 mg/kg, or 20 and 30 mg/kg. The order of injections was counterbalanced to minimize carryover effects from the previous drug administration such that some rats received the higher dose of SB first while others received the lower dose first. Two or more days of self-administration separated SB injections to allow responding to return to baseline prior to the second drug administration.
Experiment 2: Progressive ratio responding for sucrose
Additional groups of ad libitum fed or food-restricted rats (n = 8/group) were trained to respond for sucrose on an FR1 schedule of reinforcement as described above (Exp. 1). Once stable responding was established, rats were transferred to a progressive ratio (PR) schedule of reinforcement where sucrose reward was dependent upon an increasing number of responses based on the following equation: . This equation produced the following sequence of required lever presses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, etc. (Richardson and Roberts 1996). A single sucrose pellet was delivered each time the required response ratio was achieved. PR test sessions lasted until 1 h elapsed in which the rat did not obtain reward. The last response ratio achieved before 1 h with no reward was considered the breakpoint. Once stable PR responding was established, rats were given injections of vehicle or SB (10 or 30 mg/kg) 30 min prior to PR testing. The order of injections was counterbalanced as described above. Two or more days separated injections to allow responding to return to baseline.
Experiment 3: Cue-induced reinstatement of extinguished sucrose-seeking
Following self-administration sessions, rats from Experiment 1 underwent daily extinction sessions during which sucrose reward and cues were withheld. Presses on either lever had no consequences. These sessions continued until rats met the criteria of two consecutive sessions with < 25 active lever presses. Then, rats were tested for cue-induced reinstatement of sucrose-seeking by delivering the tone + light cues (previously associated with sucrose administration) for active lever presses; no sucrose reward was delivered.
To test the effects of the OxR1 antagonist SB-334867 (SB) on cue-induced reinstatement of sucrose-seeking, rats were given four test sessions in a within-subjects design: two late extinction sessions (i.e., after rats met the extinction criteria) with no cues (vehicle or SB pretreatment) and two cue-induced reinstatement sessions (vehicle or SB pretreatment). The order of sessions was counterbalanced so that the test sessions were presented in different orders to different rats. 30 min prior to test sessions, rats (n=13–15, per group) were pretreated with vehicle or SB (10 or 30 mg/kg).
Experiment 4: Fos expression in orexin neurons following self-administration, extinction or cue-induced reinstatement of extinguished sucrose-seeking
One h following the final self-administration, extinction or cue-induced reinstatement test, rats were anesthetized with an overdose of ketamine/xylazine and perfused transcardially using saline and 4% paraformaldehyde. Brains were collected, post-fixed for 24 hours in 4% paraformaldehyde, transferred to a 20% sucrose solution, and stored at 4 C. Coronal sections (40μm thick) were cut using a cryostat and processed for double label immunohistochemistry (Fos and orexin).
Fos immuohistochemistry
Sections were placed in 0.01M phosphate buffered saline (PBS) solution with 2% sodium azide added and 0.3% hydrogen peroxide for 15 min. After three 5-min washes with PBS, sections were transferred to PBS with 0.3% Triton added (PBST) containing 2% normal donkey serum (NDS) for 2 h. Sections were incubated overnight (16h) in the same solution with the addition of primary antibody (Fos antibody at 1:5000, Santa Cruz Biotechnology, Santa Cruz, CA). Sections were rinsed three times (3x) in PBST and transferred to secondary antibody (biotinylated donkey anti-rabbit, 1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h. Next, sections were rinsed 3x in PBST and transferred to solution containing avidin-biotin complex (1:500, Vector Laboratories, Burlington, CA) for 1.5 h. Sections were rinsed twice with PBST and once with 0.05 M Tris buffer for 5 min. To visualize Fos-related antigen-positive cells, sections were placed in 3′, 3′-diaminobenzidine (DAB) with 0.0002% H2O2 and 0.6% nickel ammonium sulphate in 0.05 M Tris buffer for 4.5 min. The DAB reaction was stopped by immediately transferring sections into Tris buffer. Next, sections were rinsed 3x in PBS for 5 min and transferred to PBS-Az for 45 min.
Orexin immunohistochemistry
Following Fos immunohistochemistry, the same sections were processed for orexin A immunoreactivity. Sections were transferred to PBST containing 2% NDS and orexin primary antibody (orexin A antibody, 1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) and incubated overnight (16 h). Sections were rinsed 3x in PBST then transferred to a secondary antibody solution (biotinylated donkey anti-goat, 1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2h. Sections were rinsed 3x in PBST and transferred to a solution containing avidin-biotin complex (1:500) for 1.5 h. Sections were rinsed twice with PBST and once with 0.05 M Tris buffer for 5 min. To visualize orexin-positive cells, sections were placed in DAB with 0.0002% H2O2 in 0.05 M Tris buffer. The DAB reaction was stopped after 2.25 min by immediate transfer into Tris buffer. The sections were rinsed in PBS and mounted on gelatin-coated slides, dehydrated and coverslipped.
Fos-positive and orexin-positive cells were quantified using Openlab image processing software (Improvision) on a Macintosh computer connected to a microscope and digital camera. Neurons throughout the orexin cell field were photographed and images saved to the computer. The number of Fos-positive neurons, orexin-positive neurons, and neurons double-labeled for Fos and orexin were quantified using a point-counter tool. Two or three sections per rat were counted; consecutive sections were not analyzed to prevent double counting of cells. Neurons in the left and right hemispheres were counted and averaged across sections for each rat so that each rat produced one mean value. Comparisons between groups were done by analyzing differences between the mean values for rats in each group.
Drugs
SB 334867 (SB) [1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride; purchased from Tocris, Ellisville, MO, USA, or generously donated by Eli Lilly, Indianapolis, IN, USA, or by the National Institute of Drug Abuse, Research Triangle Park, NC, USA] was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-b-cyclodextrin (Sigma) in sterile water; SB was given in a volume of 4 ml/kg (i.p.) 30 min prior to self-administration or cue-induced reinstatement sessions.
Data Analysis
Mixed-model, factorial and one-way ANOVAs were utilized for analyses, with test day as a repeated measure when appropriate. Post hoc analyses were computed with the Tukey-Kramer test. All data are presented as mean ± SEM.
Results
Experiment 1: Fixed ratio responding
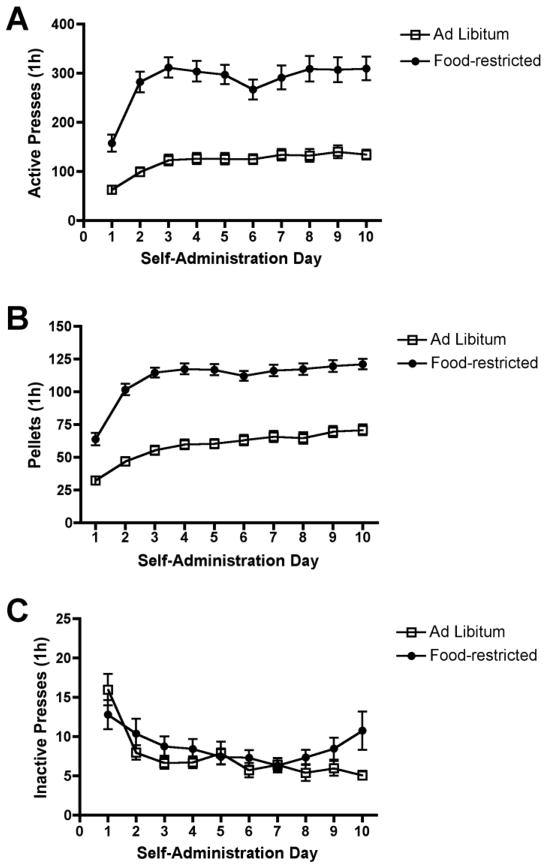
The mean number of lever presses and sucrose pellets during self-administration are shown in Figure 1. As expected, food-restricted rats performed significantly more active lever presses and earned more sucrose reinforcements than ad libitum fed rats. Repeated measures ANOVA revealed a significant group (food-restricted v. ad libitum) x test day (1–10) interaction for active presses [F(9,810) = 3.64, p < 0.001] and number of pellets earned [F(9,810) = 5.04, p < 0.001]. In contrast, there was no significant group x test day interaction or significant main effect of group or test day for inactive lever presses.
Figure 1.
Operant responding for sucrose in food-restricted and ad libitum-fed rats. Food-restricted rats (n = 40) exhibited more active presses (p < 0.001) and obtained more sucrose pellets (p < 0.001) than ad libitum rats (n = 52) during FR1 self-administration. There was no difference in the number of inactive presses between groups.
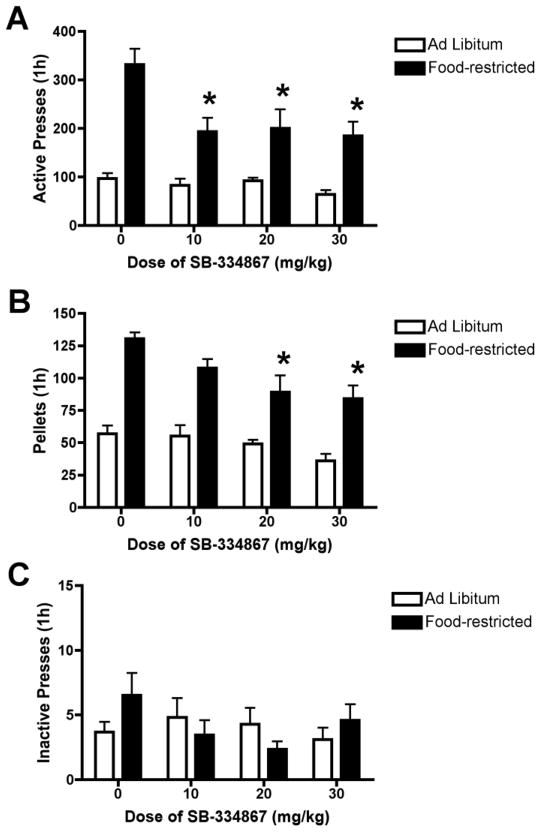
Figure 2 shows the effects of pretreatment with SB on established sucrose self-administration. For active presses, a 2-way ANOVA revealed a significant group (food-restricted v. ad libitum) x dose interaction [F(3,85) = 2.84, p = 0.01]. Post hoc analyses showed that food-restricted animals had more active presses than ad libitum fed rats (p < 0.05), and that pretreatment with SB (10–30 mg/kg) decreased active pressing compared to vehicle pretreatment (p < 0.05), but only in food-restricted rats (Fig 2a). All doses of SB produced approximately a one-third decrease in active presses in deprived animals. There was not a significant group x dose interaction for the number of sucrose pellets earned, but there was a significant main effect of group [F(1,100) = 98.73, p < 0.001] and a significant main effect of dose [F(3,100) = 8.74, p < 0.001]. Therefore, we used separate one-way ANOVAs to evaluate dose effects within food-restricted and ad libitum rats. Pretreatment with 20 or 30 mg/kg SB significantly decreased the number of sucrose reinforcers obtained during self-administration compared to vehicle pretreatment in food-restricted rats [F(3,53) = 7.66, p < 0.01 and p < 0.001, respectively], whereas there was no significant effect of pretreatment with SB in ad libitum rats [F(3,47) = 2.24, p > 0.05; (Fig 2b)]. Responding on the inactive lever was minimal regardless of group, and there was not a significant group x dose interaction or a significant main effect of group or dose for inactive presses (Fig 2c).
Figure 2.
Attenuation of fixed ratio responding for sucrose in food restricted rats by the OxR1 antagonist SB-334867 (SB). Food-restricted (n = 11–13, per dose) and ad libitum (n = 9–11, per dose) rats were pretreated with SB or vehicle 30 min prior to the self-administration session. * p < 0.05 versus vehicle injection.
Experiment 2: Progressive ratio responding for sucrose
The effects of pretreatment with vehicle or SB (10 or 30 mg/kg) on breakpoints for sucrose during PR testing are shown in Figure 3. A 2-way ANOVA for group (ad libitum or restricted) X dose (0,10,30) did not show a significant main effect of group or significant interaction. There was a significant main effect of dose [F(2,42) = 4.24, p < 0.05] on PR breakpoint such that rats pretreated with SB 30 mg/kg (ad libitum and restricted combined) showed decreased breakpoints compared to vehicle pretreatment (p < 0.05). Rats pretreated with SB 10 mg/kg also showed a trend for reduced breakpoints compared to vehicle but the effect did not reach statistical significance (p = 0.08).
Figure 3.
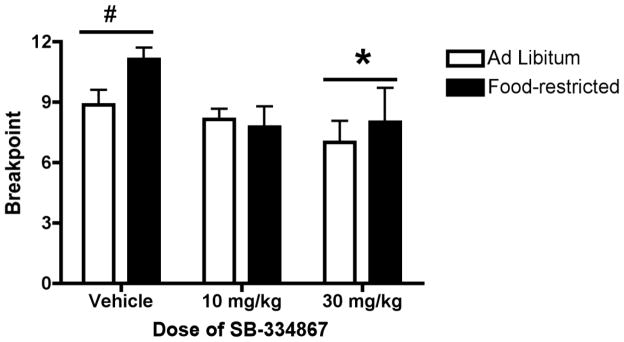
Attenuation of progressive ratio responding for sucrose by the OxR1 antagonist SB-334867 (SB). Rats were pretreated with SB or vehicle 30 min prior to the progressive ratio self-administration session. * p < 0.05 30 mg/kg SB (ad libitum and restricted combined) versus vehicle.
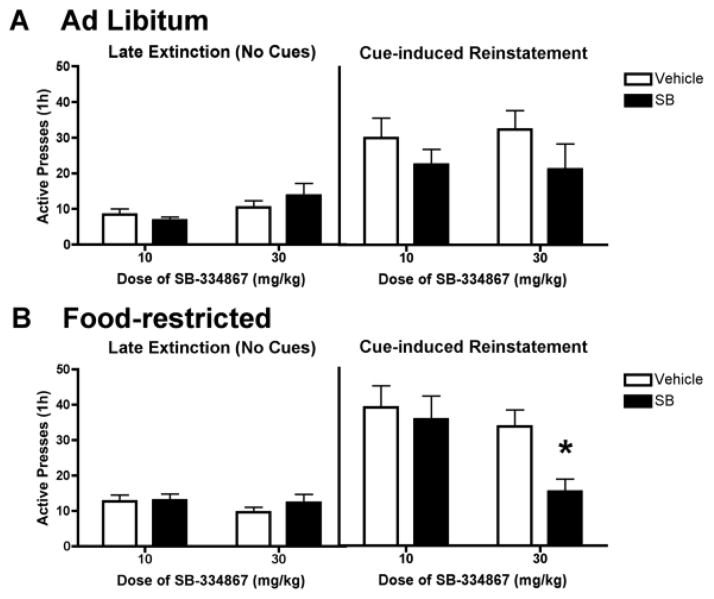
Experiment 3: Cue-induced reinstatement of extinguished sucrose-seeking
Both ad libitum fed and food-restricted rats extinguished within five extinction sessions. Figure 4 shows the mean number of active lever presses during late extinction sessions vs. during cue-induced reinstatement of sucrose-seeking following pretreatment with SB (10 or 30 mg/kg) or vehicle. There was not a significant group (ad libitum vs food-restricted) x treatment (vehicle vs SB) interaction during extinction regardless of SB dose. There was a significant main effect of group on active pressing during extinction when rats were pretreated with SB 10 mg/kg [F(1,52) = 11.52, p < 0.01]. Post hoc analysis showed that food-restricted rats pressed more (although minimally) during extinction than ad libitum fed rats.
Figure 4.
Attenuation of cue-induced reinstatement of sucrose-seeking in food-restricted rats by the OxR1 antagonist SB-334867 (SB). In a within-subject design, rats were pretreated with SB (10 or 30 mg/kg) or vehicle 30 min prior to late extinction sessions (no cues or sucrose) or cue-induced reinstatement (tone + light cues). * indicates p < 0.01 versus vehicle + cue.
During reinstatement, there was not a significant group (ad libitum vs restricted) X treatment (vehicle vs SB) interaction regardless of SB dose. There was a significant main effect of dose on active presses when rats were pre-treated with SB 30 mg/kg [F(1,52) = 7.79, p < 0.01]. Post hoc analysis showed that rats pretreated with SB 30 mg/kg had fewer active presses. Therefore, we compared vehicle with SB 30 mg/kg pretreatment within each group (ad libitum vs. food-restricted) using paired t-tests. These tests revealed that only food-restricted rats pretreated with SB 30 mg/kg had decreased active pressing during reinstatement. There were no significant effects of test session on inactive presses in any group tested.
Experiment 4: Fos expression in hypothalamus following self-administration, extinction or cue-induced reinstatement of extinguished sucrose-seeking
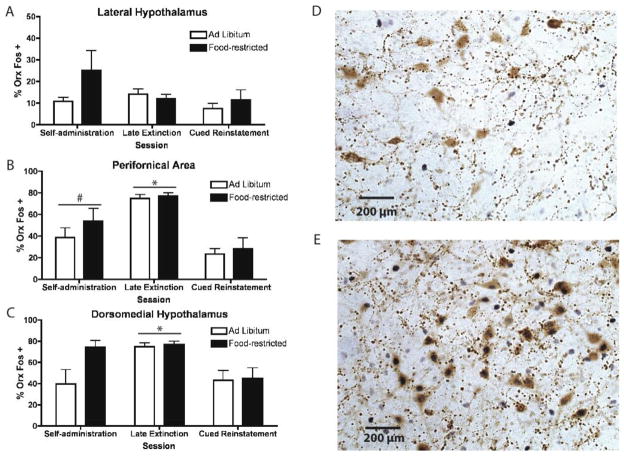
The percentages of orexin neurons that were Fos-positive (% Orx Fos+) in lateral hypothalamus (LH), perifornical area (PFA), and dorsomedial hypothalamus (DMH) following self-administration, late extinction or cue-induced reinstatement to sucrose-seeking are illustrated in Figure 5. The corresponding mean raw numbers of Orx (single and double-labeled) cells are summarized in Table 1.
Figure 5.
Percentages of Orx neurons that were Fos+ (% Orx Fos+) following self-administration, late extinction or cue-induced reinstatement of sucrose-seeking in (A) lateral hypothalamus, LH, (B) perifornical area, PFA or (C) dorsomedial hypothalamus, DMH. * = p < 0.001, late extinction versus self-administration or cue-induced reinstatement; # = p < 0.05, self-administration versus cue-induced reinstatement. Self-administration: n=5 ad libitum, n=5 food-restricted; Extinction: n=7 ad libitum, n=4 food-restricted; Cue-induced Reinstatement: n=10 ad libitum, n=6 food-restricted. Panels D and E are representative images of immunohistochemical double-labeling of orexin expression (brown) and c-Fos (dark purple) in D) LH and in E) PFA and DMH, respectively, during late extinction.
Table 1.
Mean +/− SEM numbers of Orx neurons per section following self-administration, extinction or cue-induced reinstatement to extinguished sucrose-seeking.
| Region | Session | Ad Libitum | Food-restricted |
|---|---|---|---|
| LH | Self-administration | 36.03 ± 2.67 | 43.38 ± 7.72 |
| Late extinction | 48.38 ± 2.66 | 37.58 ± 5.00 | |
| Cued reinstatement | 50.79 ± 4.57 | 49.45 ± 2.91 | |
| PFA | Self-administration | 40.62 ± 5.73* | 46.51 ± 8.64* |
| Late extinction | 75.42 ± 4.22 | 78.13 ± 6.80 | |
| Cued reinstatement | 74.82 ± 8.94 | 72.3 ± 3.23 | |
| DMH | Self-administration | 13.57 ± 3.10 | 18.45 ± 6.44 |
| Late extinction | 12.15 ± 2.07 | 17.92 ± 2.68 | |
| Cued reinstatement | 11.20 ± 2.26 | 12.08 ± 2.68 |
p < 0.001, self-administration versus late extinction or cue-induced reinstatement.
% Orx Fos+
For LH, the 2-way ANOVA did not reveal a significant effect of group, session, or significant interaction, on the mean % Orx Fos+ (Fig 5a). In PFA and DMH, 2-way ANOVAs revealed a significant effect of session [F(2,26) = 22.71, p < 0.001; F(2,26) = 10.89, p < 0.001], but did not reveal a significant effect of group or a significant interaction on the mean % Orx Fos + (Fig 5b, c). Post hoc analyses revealed that the % Orx Fos + was significantly higher during late extinction than during self-administration or cue-induced reinstatement in both PFA and DMH. Additionally, the % Orx Fos+ in PFA was higher during self-administration than during reinstatement.
Mean numbers of Orx neurons (Table 1)
Two-way ANOVAs did not reveal significant group x session interactions or significant main effects of group or session on the mean numbers of Orx neurons in LH or DMH. In PFA, there was a significant main effect of session [F(2,26) = 12.65, p < 0.001]. Post hoc analysis showed increased numbers of Orx neurons in PFA during late extinction and cue-induced reinstatement compared to during self-administration.
Correlations
Correlation analyses were used to compare % Orx Fos+ in LH, PFA or DMH with the number of active presses and sucrose pellets obtained during self-administration and with reinstatement scores for each animal following cue-induced reinstatement. None of these analyses revealed significant correlations between the % Orx Fos+ and reinstatement scores following vehicle + cue presentation in any region or group tested.
Discussion
The findings from the present study indicate that signaling at the OxR1 receptor is involved in sucrose seeking and reinforcement, particularly in food-restricted subjects. These findings are consistent with the view that Orx is engaged in the motivation induced by highly salient rewards; here, reward salience is increased by food restriction. Notably, recent findings also show that the Orx system mediates the antidepressant effect of food restriction (Lutter et al. 2008; Scott et al. 2011). This leads us to speculate that elevated conditioned responses of Orx neurons to reward-related stimuli during food restriction, as seen here, may contribute to positive mood and overeating. In the present study, pretreatment with the OxR1 antagonist (SB) significantly attenuated operant responding for sucrose during self-administration and dose dependently decreased the number of sucrose pellets obtained in food-restricted rats; these effects were not significant in ad libitum-fed subjects. Similarly, pretreatment with SB (30 mg/kg) decreased cue-induced reinstatement of extinguished sucrose-seeking in food-restricted rats but not in ad libitum rats. During PR responding, pretreatment with SB (30 mg/kg) attenuated breakpoints independent of food-restriction. There was a large increase in the percentage of Orx neurons that were Fos activated in PFA and DMH during late extinction, indicating that these regions may play an important role in abstinence or extinction processes.
SB effects on responding for sucrose
In our study, SB was only effective at attenuating FR responding for sucrose in food-restricted rats. These findings are consistent with previous results showed that SB did not decrease FR responding for a sucrose solution in ad libitum fed animals (Richards et al., 2008). These results are also consistent with studies demonstrating that SB decreased FR responding for high fat food (Nair et al., 2010), and reduced PR responding for chocolate and sweets (Borgland et al. 2009; Cason et al. 2010) in food-restricted rats, but had no affect on responding for regular chow (Borgland et al. 2009).
Our finding that SB decreased PR breakpoint independent of food-restriction is in contrast to findings by Espana et al (2010) that reported SB decreased PR responding for sucrose in ad libitum fed, but not food-restricted, rats. However, in that study ad libitum rats were initially food-restricted and then re-fed prior to PR testing (Espana et al. 2010). Previous studies show that restriction produces long-lasting changes in the Orx system that persist after re-feeding (Pankevich et al. 2010), and it seems possible that this may contribute to the differences between their results and ours.
A within subject design was used to test the effects of SB on FR responding for sucrose; therefore, there could be carryover effects of giving multiple drug administrations. To minimize potential carryover effects, the doses of SB administered were counterbalanced such that some rats received the lower dose of SB first and a separate group received the higher dose first. Drug administration sessions were separated by at least two sessions of self-administration with no drugs to allow responding on the active lever to return to baseline prior to the second drug administration.
Taken together, our results support previous findings that signaling at the OxR1 is involved in reinforcement of salient food rewards, but its involvement is specific to palatable foods and level of food restriction (however, see Sharf et al., 2010). These findings are consistent also with studies demonstrating that food deprivation increases the palatability of sucrose (Berridge 1991; Rothemund et al 2007) and produces increased activation of LH orexin neurons (Kurose et al., 2002).
Cue-induced reinstatement of extinguished food-seeking
The present study is the first to investigate a role of Orx in cue-induced reinstatement of extinguished food-seeking. The findings demonstrate that signaling at the Ox1R is necessary for cued reinstatement of sucrose-seeking in food-restricted rats. Recent studies showed that SB decreases reinstatement of extinguished cocaine-seeking induced by cues or context (Mahler et al., 2012; Smith et al. 2009; Smith et al. 2010; Smith and Aston-Jones 2012) and reinstatement of extinguished sucrose- or alcohol-seeking induced by yohimbine (Richards et al. 2008). In contrast, SB did not affect yohimbine-induced or pellet primed reinstatement of high-fat food-seeking (Nair et al. 2008), indicating that orexin’s influence on reinstatement may be sensitive to the specific reward and reinstatement modality.
Our results for reinstatement of food seeking are particularly interesting given that other studies link cue-induced feeding with obesity in humans (Castellanos et al. 2009; Rothemund et al. 2007; Stice et al. 2008). Imaging studies show activation in caudate nucleus, gustatory cortex, somatosensory regions and dorsal striatum in obese individuals compared to lean controls in anticipation of food consumption (Stice et al. 2008) or in response to high-calorie food images (Rothemund et al. 2007). Similarly, obese individuals show increased visual attention to food-related images when fasted or sated whereas non-obese individuals only show a similar increase when fasted (Castellanos et al. 2009; Nijs et al. 2010).
Fos induction in orexin neurons
There was a large increase in the percentage of orexin neurons that were Fos+ in PFA and DMH during late extinction, indicating that these regions may play a role in extinction processes. To our knowledge, neither PFA nor DMH has been previously implicated in extinction processes. Our laboratory proposed a dichotomy of Orx function, such that Orx in LH is associated with rewarding processes and Orx in PFA and DMH is involved in arousal and responses to stress (Aston-Jones et al. 2010; Harris and Aston-Jones 2006). In this view, the increase in Fos expression in PFA and DMH during extinction may reflect increased arousal or stress associated with the absence of sucrose during extinction sessions. In agreement, Choi et al (2010) reported an increase in Fos expression in PFA, but not LH, Orx neurons of rats when a meal was expected but not delivered in a context previously associated with meal delivery (Choi et al. 2010). Additionally, recent studies have identified hypothalamic neurons, including Orx neurons, that serve as central glucose sensors to promote adaptive behaviors such as increased wakefulness, locomotor activity and foraging that in turn contribute to feeding behavior (Burdakov et al., 2005; Gonzalez et al., 2008). Therefore, it seems plausible that manipulations to the normal feeding regimen (i.e., the availability of sucrose during self-administration and absence of sucrose during extinction) may alter the responsiveness of Orx neurons and contribute to their increased activity during extinction as seen here.
The manner in which Orx neurons may be involved in extinction is unclear, but it is notable that Orx neurons are strongly connected with brain regions previously associated with extinction processes, including the nucleus accumbens shell and infralimbic cortex (Milan et al. 2011; Moore et al. 1993; Peters et al., 2009). Interestingly, our results showed that there were more Orx neurons in PFA following extinction and cue-induced reinstatement than following self-administration. The mechanism causing these differences is unknown; however, it seems plausible that the higher numbers of orexin neurons during extinction and reinstatement might be associated with increased arousal when a reward is expected but not obtained and is consistent with the studies described above.
Orexin and reward centers
As described above, Orx neurons send projections throughout the CNS. It is unknown where Orx acts within the CNS to influence sucrose-motivated behavior, but the midbrain dopaminergic or basal forebrain cholinergic system seems plausible. Previous studies have shown that presentation of food or associated stimuli elicits cortical acetylcholine release (Fadel et al. 1996; Frederick-Duus et al. 2001; Inglis et al. 2004) that requires signaling via the OxR1 (Frederick-Duus et al. 2007). Other studies have investigated neural sites involved in cue- and context-induced reinstatement of food-seeking but have not examined the role of Orx inputs to these areas (Petrovich et al. 2002; McLaughlin and Floresko 2007; Floresko et al. 2008; Marchant et al. 2009; Stratford and Kelley 1997).
Importantly, connections between nucleus accumbens and hypothalamus activate Orx neurons that, in turn, increase ingestion of palatable food (Stratford et al. 1997; Stratford and Kelley 1999; Zhang and Kelley 2000; Zheng et al. 2007). Zheng et al (2007) demonstrated that intra-accumbens DAMGO induced high-fat diet appetite through activation of hypothalamic Orx neurons and OxR1 signaling in VTA. Similarly, OxR1 signaling in the paraventricular nucleus of the thalamus (PVT) increases reward-driven feeding and dopamine levels in nucleus accumbens through direct connections between PVT and nucleus accumbens (Choi et al., 2012). Finally, our laboratory has demonstrated that Orx projections to VTA mediate Orx-A effects on drug reward including morphine conditioned place preference, and that cue-induced reinstatement of cocaine-seeking is dependent upon OxR1-AMPA receptor interactions in VTA (Aston-Jones et al. 2009; Harris et al. 2005; Mahler et al. 2012). Taken together, these findings indicate that Orx targets in the midbrain dopaminergic or basal forebrain cholinergic system are potential sites of action where Orx may act to influence sucrose-motivated behavior reported in this manuscript. Future studies examining the effects of SB on Fos expression in Orx targets following cue-induced reinstatement of extinguished sucrose-seeking will further elucidate Orx’s specific sites of action. Our preliminary findings (not shown here) indicate that prefrontal cortex and amygdala might be important sites where SB modulates Fos expression in Orx targets.
It seems unlikely that the effects of SB in the present studies were due to a generalized locomotor effect. SB had no effect on inactive presses, or on FR or reinstatement responding in ad libitum rats. Similarly, other studies have shown that SB has no effect on responding for certain other rewards (Borgland et al. 2009; Smith et al. 2009). These findings indicate that SB does not substantially impair lever-pressing behavior. Furthermore, greater suppression of locomotor activity is produced by an OxR2 antagonist than the OxR1 antagonist (Smith et al. 2009), consistent with the view that arousal effects of Orx are linked primarily to OxR2s (Akanmu and Honda 2005; Lin et al. 1999; Marcus et al. 2001; Willie et al. 2003).
Our finding that the Orx system is involved in cue-induced reinstatement of sucrose-seeking is particularly interesting given that recent studies have linked visual attention bias for food cues, and increased activation of brain reward centers in anticipation of food reward, to obesity (Castellanos et al. 2009; Nijs et al. 2010; Rothemund et al. 2007; Stice at al. 2008). Additionally, our data support previous findings that show the Orx system drives increased food seeking that occurs during food restriction (Pankevich et al. 2010; Vialou et al. 2011). This is not surprising given that the Orx system influences hormones that regulate adiposity signals and energy expenditure during caloric restriction (Lawrence et al. 2003; Lopez et al. 2000) and that these gut hormones increase the rewarding value of palatable food via Orx dependent mechanisms (Perello et al., 2010). Together, these studies indicate that obesity is associated with increased responses to salient food rewards and that the Orx system may be involved in cue-induced responding for palatable foods under conditions that stimulate feeding. The ineffectiveness of dieting in obese humans may reflect persistent conditioned responses of Orx neurons, as seen here during food restriction. Treatments that decrease such conditioned Orx signaling may provide a novel therapeutic approach to ameliorating obesity.
Acknowledgments
This research was supported by the National institute of Drug Abuse grants F32 DA023354, R01 DA017289 and R37 DA06214.
Footnotes
Dr. Cason has no biomedical financial interests or potential conflict of interests to disclose. Dr. Aston-Jones has no biomedical financial interests or potential conflict of interests to disclose.
References
- Akanmu MA, Honda K. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Res. 2005;1048:138–145. doi: 10.1016/j.brainres.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharm. 2009;56:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156(4):865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16(2):103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/Hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, de Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent hypothalamus: relationship to neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100(5):419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes. 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167(1):11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264(1):R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. The hypocretin-orexin system regulated cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31(2):336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Moore H, Sater M, Bruno JP. Trans-synaptic stimulation of cortical acetylcholine release after partial 192 IgG-saporin-induced loss of cortical cholinergic afferents. J Neurosci. 1996;16(20):6592–6600. doi: 10.1523/JNEUROSCI.16-20-06592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149(3):499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143(1):25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Harris G, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Huang XF, Yu Y, Zavitsanou K, Han M, Storlien L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135(1–2):150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 2004;62(4):1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Differential effects of the selective orexin-1 receptor anagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Satiety enhancement by selective orexin-1 receptor anagonist SB-334867: influence of test context and profile comparison with CCK8-S. Behav Brain Res. 2005;160:11–24. doi: 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Fleming S, Holahan MR. A pharmacological analysis of the substrates underlying conditioned feeding induced by repeated opioid stimulation of the nucleus accumbens. Neuropsychopharm. 2000;23(4):455–467. doi: 10.1016/S0893-133X(00)00117-2. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2003;143(1):155–62. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lopez M, Seoane L, Garcia MC, Lago F, Casanueva FF, Senaris R, et al. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun. 2000;269:41–45. doi: 10.1006/bbrc.2000.2245. [DOI] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29(5):1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking. Neuroscience. 2007;146(4):1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosc. 2011;30(13):4626–35. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Sarter M, Bruno JP. Bidirectional modulation of stimulated cortical acetylcholine release by benzodiazepine receptor ligands. Brain Res. 1993;627(2):67–74. doi: 10.1016/0006-8993(93)90330-p. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154(2):406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–54. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci. 2010;30(48):16399–16407. doi: 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in the prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evan rats. Psychopharm. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, Roberts Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficancy. J Neurosci methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Arch JR, Blundell JE. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96:71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, et al. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, et al. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222(2):288–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35(5):798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, See R, Aston-Jones G. Orexin/hypocretin signaling at the OX1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58(1):179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the brain. Brain Res Mol Brain Res. 2004;124(2):134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2008;31(12):4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17(11):4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharm. 2005;182(1):75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Vialou V, Cui H, Perello M, Mahgoub M, Yu HG, Rush AJ, et al. A role for delta FosB in calorie restriction-induced metablic changes. Biol Psychiatry. 2011;70(2):204–207. doi: 10.1016/j.biopsych.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, et al. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Wiliams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38(5):715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Kunii K, Nambu T, Tsujino N, Sakai S, Matsuzaki I, et al. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000;859:404–409. doi: 10.1016/s0006-8993(00)02043-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–77. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]