Abstract
Rationale
A major challenge in the pharmacological treatment of psychotic disorders is the effective management of the associated cognitive dysfunctions. Novel concepts emphasize a potential benefit of partial agonists acting upon dopamine D2-like receptors in ameliorating these cognitive deficits, and pre-clinical studies suggest that D3-receptor-preferring compounds can exert pro-cognitive effects.
Objective
The objective of the study was to use acute phencyclidine (PCP) treatment to model the cognitive deficits of schizophrenia in mice, and to test the efficacy of the novel, dopamine D3-receptor-preferring drug cariprazine in ameliorating the severity of PCP-triggered cognitive deficits.
Methods
One group of wild-type or D3-receptor knockout mice was acutely treated with either saline or phencyclidine (PCP, 1 mg/kg). A separate group of mice was treated with cariprazine prior to PCP administration. Both groups were then tested in three cognitive tasks: social interaction/recognition and recognition memory, spatial working memory, and attention-set-shifting.
Results
PCP effectively disrupted social recognition and social recognition memory, spatial working memory, and extradimensional attention set-shifting. Cariprazine pretreatment significantly attenuated the emergence of these cognitive deficits in PCP-treated wild-type mice, but not in PCP-treated D3-receptor knockout mice.
Conclusions
In an animal model of PCP-induced cognitive impairment, cariprazine pretreatment significantly diminished PCP-triggered cognitive deficits, and studies on knockout mice show that dopamine D3 receptors contribute to this effect.
Keywords: episodic memory, working memory, attention set-shifting, mouse, phencyclidine, dopamine D3 receptors
INTRODUCTION
Whereas antipsychotic drugs of the first generation are predominantly dopamine D2-like receptor antagonists, the second generation has a broader pharmacological spectrum, ranging from high affinity binding to dopamine D2-like and serotonin (5-HT) 2A receptors to modest D2-like and 5-HT2A receptor affinity and appreciable affinities to many other neurotransmitter receptors (including dopamine D1, 5-HT1, H1 histaminic, M1 muscarinic, and α2-adrenergic receptors; Markowitz et al. 1999). Although both generations of antipsychotic drugs are efficacious in ameliorating positive symptoms of schizophrenia, the second generation is thought to be more effective in the treatment of negative symptoms. However, neither generation effectively improves the cognitive dysfunctions that accompany the disease (Markowitz et al. 1999; Pratt et al. 2008).
At present, all currently prescribed antipsychotic drugs have affinity for dopamine D2-like receptors, and their antagonist action on these receptors is thought to be an essential aspect of their mechanism of action (Mailman and Murthy, 2010). Since recent human genetic and neuroimaging studies pointed to a critical role of dopamine D2 and especially D3 receptors in the control of executive functions that are often disrupted in schizophrenia (Bombin et al. 2008; Lumme et al. 2007), the apparent ineffectiveness of antipsychotic drugs in ameliorating such cognitive deficits led to new investigations on the potential benefit of dopamine-receptor partial agonism. Since the endogenous ligand dopamine has the highest affinity for D3 receptors compared with other dopamine receptors (Sokoloff et al. 1990), it seemed plausible that a partial agonist with high affinity to this receptor subtype may exert stabilizing effects on dopaminergic transmission, either by blocking over-stimulated receptors (via its action as an antagonist) or by stimulating the receptor when the dopaminergic tone is low. Hence, much effort has been made to develop compounds with dopamine D3 receptor-preferring binding characteristics and partial agonist/antagonist effects in functional assays. One such drug candidate, cariprazine, has been shown to exhibit this pharmacological characteristic: it has ~10-fold higher affinity for rat and human D3 receptors compared with D2 receptors, and acts as a partial agonist in functional assays (Kiss et al. 2010). Moreover, positron emission tomography studies showed that cariprazine binds preferentially to dopamine D3/D2 receptors relative to 5-HT1A receptors (Seneca et al. 2011). Additionally, cariprazine did not exert cataleptogenic side effects in rodents (Gyertyán et al. 2011).
Interestingly, in scopolamine-treated rats with impaired learning performance in the water labyrinth test, cariprazine exerted significant cognitive-enhancing effects (Gyertyán et al. 2011). This finding raised the question whether the observed pro-cognitive activity of cariprazine would also improve cognitive deficits characteristic of schizophrenia. The present study examined wild-type C57Bl/6 mice treated with phencyclidine (PCP), a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist that induces a transient state resembling schizophrenia in humans (Javitt and Zukin, 1991). PCP also elicits cognitive deficits that have face validity for cognitive dysfunctions often found in schizophrenic patients (Jentsch and Anzivino, 2004; Marrs et al. 2005; Hashimoto et al. 2005; Egerton et al. 2005; Pratt et al. 2008; Hagiwara et al. 2008; Beraki et al. 2009; Nagai et al. 2009). We show here that PCP effectively disrupts spatial working memory, attention set-shifting, social interaction/recognition, and social recognition memory in mice. This enabled us to examine the effectiveness of cariprazine pretreatment in ameliorating or preventing the effects of PCP on these cognitive functions.
MATERIALS AND METHODS
Animals
Experiments were performed on wild-type C57Bl/6J mice and the 20th generation of congenic C57Bl/6 dopamine D3-receptor knockout mice that were originally generated by Jung et al. (1998) and then back-crossed to wild-type C57Bl/6J mice. The maternal behavior of these congenic D3-receptor knockout mothers does not differ from wild type in terms of litter size, litter survival, and postnatal development of their pups. Moreover, the cognitive phenotypes of the 5th and 10th back-crossed generations of these congenic D3-receptor knockout mice were described previously Glickstein et al., 2002 and Glickstein et al., 2005). Moreover,
Adult (postnatal age P60 to P90) male mice were used in this study. The mice were bred and housed in a temperature-controlled barrier facility (with a 12-hour light/dark cycle (lights on at 6:00 AM)) of the New York State Psychiatric Institute. All experiments involving the animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees at Columbia University and the New York State Psychiatric Institute.
Behavioral tests
For each of the behavioral tests described below, a separate group of animals was used, and the experimenters were blind as to the genotypes and treatments of these groups.
Elevated Plus Maze (EPM)
In tests of cognitive functions, it is essential to employ concentrations of drugs that have no effects on emotional behavior and that do not impair locomotor activity. Therefore, we first tested whether cariprazine, administered at a dose range of 0.005 to 0.15 mg/kg, affected the behavior of mice in the EPM, a test of anxiety-related behavior that is also critically dependent upon normal locomotor activity. Animals were exposed to an EPM apparatus designed for mice (Stoelting, Wood Dale, IL; leg height: 45 cm, arm length: 35 cm, lane width: 5 cm, wall height: 15 cm). Testing (under 100 lux lighting) was performed between 1 and 4 PM. Mice were placed in the center of the maze and their time spent in open arms and the number of closed and open arm entries during a 5 min test period was recorded. Measures of the time spent in open arms and the number of open arm entries served as a measure of anxiety-like behavior. The number of closed arm entries served as a measure of locomotor activity.
Social Recognition/Interaction and Social Recognition Memory
This test, originally developed by Engelmann et al. (1995), taxes social recognition/interaction and short-term recognition memory, and was adopted to the mouse as previously described (Mehta and Schmauss, 2011). Briefly, adult mice were singly housed (in standard housing cages (19 × 30 × 15 cm)) 24 h prior to exposure to a non-familiar juvenile mouse. Then, a juvenile mouse was placed into the adult’s housing cage for 5 min and the time the adult interacted with the juvenile (following, sniffing, licking, etc.) was recorded and taken as a measure of social recognition/interaction (T1). At the end of the 5-min exposure, the juvenile was removed. Two hours later, the same juvenile was placed back into the cage housing the adult mouse, and the time of interaction was recorded for another 5-min test period (T2). In mice with intact recognition memory, the time of social interaction during the second exposure should be consistently shorter (i.e., T1–T2 is greater than zero).
Spatial Working Memory (WM)
For these experiments, mice were food restricted such that they gradually (over the period of 5 to 7 days) lost 10% of their free-feeding body weight. Spatial working memory was assessed using a delayed alternation task performed in a T-maze as originally developed for rats by Thomas and Brito (1980). Adjustments were made for the mouse as described in Glickstein et al. (2002). The mouse T-maze was made of 0.6 cm-thick Plexiglas (main alley: 58 cm long and 11 cm wide; side arms: 30 cm long and 11 cm wide, all walls: 18.5 cm high). Briefly, mice were trained for alternate arm entries in the T-maze until they reached 70% correct arm entries (in 10 trials per day) on 2 consecutive days with 5 sec inter-trial delay periods. Then, mice performed the test with 3 longer inter-trial delays (15, 20, and 30 sec, each tested on 2 consecutive days). Only correct arm entries were rewarded with food, and the percentage of correct arm entries in the total number of 10 trials per delay period was taken as a measure of response accuracy.
Attention-Set-Shifting Task (ASST)
Mice were food restricted as described above and tested in the ASST developed for rats by Birrel and Brown (2000) and adjusted for mice as described in Glickstein et al. (2005). Briefly, in the ASST, mice learn to discriminate between 2 stimulus dimensions, odor and texture. The test apparatus, made of Plexiglass (dimensions: 32 × 27 × 15 cm) had a sliding door that separates the holding area from the test area. All mice tested in the ASST proceeded through all 5 consecutive test phases on a single day. Briefly, they first learned to retrieve a food reward deeply buried in unscented terra cotta pots filled with familiar bedding media. Then, they performed the first simple discrimination task (SD) between odor (scented terra cotta pots) or texture (different digging media). Only one stimulus dimension indicated the location of the food pellet, and both dimensions were used in a randomized order. The next test phase was a compound discrimination (CD), in which another stimulus property (a second odor or texture) was introduced that was not a reliable predictor of food reward, i.e., the same positive stimulus (a particular odor or texture) used in the initial SD still guided correct response selection. The next test phase required an intradimensional shift of attention (IDS). In this phase, both relevant and irrelevant stimulus properties were changed, but the relevant stimulus dimension used in the SD and CD (odor or medium texture) remained the same. Then, in the final test phase, the formerly irrelevant stimulus dimension became relevant and thus, required an extradimensional shift of attention (EDS). In all test phases, animals had to reach a criterion of 6 consecutive correct trials, and the number of trials to criterion was referred to as response accuracy.
Drugs
All drugs were administered intraperitoneally. Phencyclidine HCl (PCP) was purchased from Sigma Aldrich (St. Louis, MO) and administered at a dose of 1.0 mg/kg. This dose was selected based on pilot studies on ASST-tested mice showing 0.1 mg/kg PCP was ineffective and 3.0 mg/kg disrupted task performance due to locomotor effects. Cariprazine HCl was synthesized at Gedeon Richter Plc (Budapest, Hungary). Both drugs were dissolved in saline, filter sterilized, and injected at a volume of 0.1ml/10g of body weight.
Statistical Analysis
The effects of cariprazine on EPM performance was assessed using one-way ANOVA followed post hoc by Tukey-Kramer multiple comparisons tests. For a comparison between treatment groups, results obtained with the social recognition/interaction and social recognition memory test were also compared by ANOVA and post hoc by Tukey-Kramer multiple comparisons tests. To evaluate the performance of saline-treated wild-type and D3-receptor knockout mice in the WM test and the ASST, we first conducted a repeated measures ANOVA followed post hoc by Tukey-Kramer multiple comparisons tests. This within-group analysis enabled us to determine differences in performance between the different test phases in the ASST or different delay periods in the WM test. In addition, for each genotype, a one-way ANOVA was used for comparison between treatment groups. All statistical analyses were carried out using Graph Pad InStat Version 3.0 (GraphPad Software, San Diego, CA).
RESULTS
The Effect of Cariprazine on the Behavior in the Elevated Plus Maze (EPM)
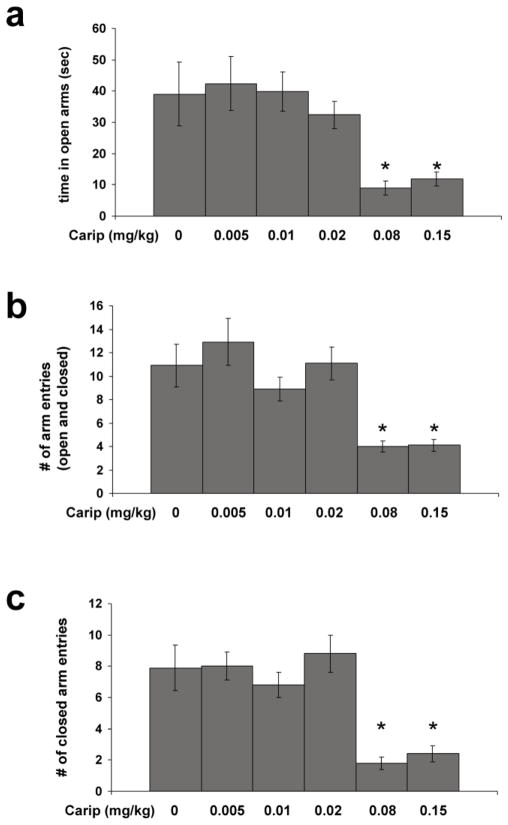
We examined the effects of 5 doses of cariprazine (ranging from 0.005 to 0.15 mg/kg) on EPM behavior of wild-type mice. As shown in Fig. 1a, whereas lower doses of cariprazine (0.005 to 0.02 mg/kg) did not alter the time spent in open arms, the two higher doses (0.08 and 0.15 mg/kg) led to a significant decline of this measure (ANOVA, (F(5,52)=4.20; p=0.0032)). Moreover, the two higher doses of cariprazine also led to a significant decrease in the total number of arm entries (F(5,52)=7.21; p=0.0001)) (Fig. 1b) but, as shown in Fig. 1c, this decrease in the total number of arm entries is largely accounted for by a significant decrease in the number of closed arm entries (F(5,52)=11.75; p=0.0001)). Thus, the two highest doses of cariprazine (0.08 and 0.15 mg/kg) had significant effects on locomotor activity, but doses ranging from 0.005 to 0.02 mg/kg did not affect anxiety-like behavior or locomotor activity in the EPM test. Hence, in the following studies cariprazine was administered at doses ranging from 0.005 to 0.02 mg/kg.
Fig 1. The effect of cariprazine on the behavior of mice exposure to the EPM.
Data are mean (± SEM) of determinations made from 8 to 9 wild-type mice per treatment group. Saline and cariprazine were administered intraperitoneally 1 h prior to EPM testing. a, Time spent in open arms during a 5-min EPM exposure. Significant differences between treatment groups revealed by one-way ANOVA (F(5,52)=4.20; p=0.0032) were resolved post hoc by Tukey-Kramer multiple comparisons tests. *p<0.05 compared with saline and 0.005, 0.01, and 0.02 mg/kg of cariprazine treatment. b, Total number of arm entries. ANOVA revealed significant differences between treatment groups (F(5,52)=7.21; p=0.0001) that were resolved post hoc using Tukey-Kramer multiple comparisons tests. *p<0.001 compared with 0.05 mg/kg of cariprazine treatment, p<0.01 compared with 0.02 mg/kg of carprazine treatment, and p<0.05 compared with saline treatment. c, Number of closed arm entries. Significant differences revealed by ANOVA, (F(5,52)=11.75; p=0.0001) were resolved post hoc by Tukey-Kramer multiple comparisons tests. *p<0.001 compared with saline and 0.005 and 0.02 mg/kg of carprazine treatment, and p<0.01 compared with 0.01 mg/kg of cariprazine treatment. Carip: cariprazine.
The Effect of Cariprazine on Social Recognition/Interaction and Social Recognition Memory in PCP-treated Mice
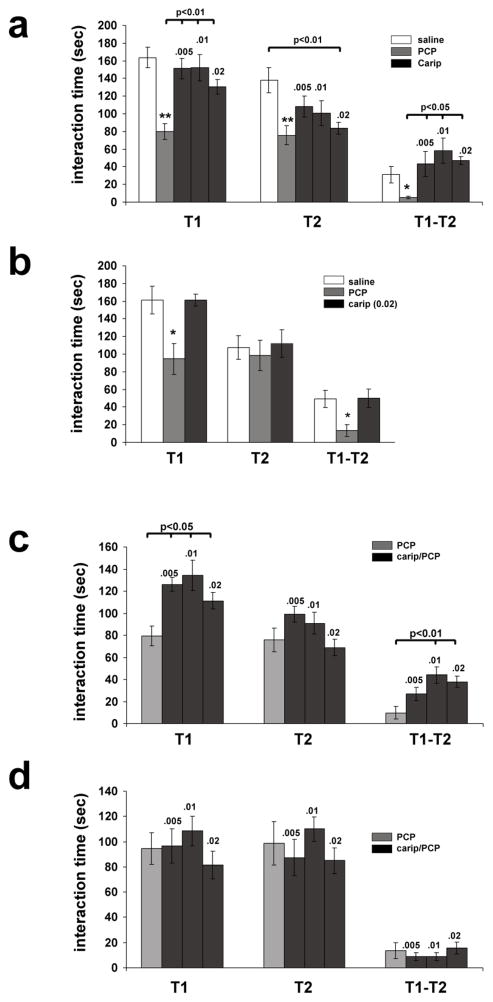
We first examined the effect of PCP (1 mg/kg) and cariprazine (0.005, 0.01, and 0.02 mg/kg) on the social recognition/interaction and social recognition memory of adult wild-type mice exposed to a conspecific juvenile. Either drug was administered 1 h prior to testing for social interaction. For the social interaction time (referred to as T1), ANOVA revealed significant differences (F(4, 35)=10.912; p=0.0001) between the treatment groups. PCP-treated animals exhibited significantly reduced T1 interaction time compared with saline- and all cariprazine-treated animals (Fig. 2a). In contrast, T1 interaction times of cariprazine-treated animals did not significantly differ from saline-treated controls (Fig. 2a).
Fig 2. The effect of cariprazine on PCP-induced impairment of social interaction (T1) and social recognition memory (T2; T1-T2) in wild-type and D3-receptor knockout mice.
PCP (1 mg/kg) was administered 30 min after cariprazine (0.005 to 0.02 mg/kg) pre-treatment. Carip=cariprazine.
a, Comparison of the effects of PCP (1 mg/kg) and cariprazine alone in wild-type mice. Data are mean (± SEM) of results obtained from 7 animals per treatment group. The doses of cariprazine (.005 to .02 mg/kg) are indicated on top of the bars. Significant differences revealed by ANOVA were resolved post hoc using Tukey-Kramer multiple comparisons tests as indicated. *p<0.05 and **p<0.001 compared with saline- and cariprazine-treated mice. b, Comparison of the effects of PCP (1 mg/kg) and cariprazine alone in D3-receptor knockout mice. Similar to wild-type mice, PCP significantly impaired social interaction and social recognition memory in D3-receptor knockout mice, and cariprazine (0.02 mg/kg) had no effect on either measure. Data are mean (± SEM) measures from 7 animals per treatment group and significant differences revealed by ANOVA were resolved post hoc using Tukey-Kramer multiple comparisons tests as indicated. *p<0.05 compared with saline- and cariprazine-treated mice. c, The effect of cariprazine pre-treatment on PCP-induced impairment of social recognition and social recognition memory in wild-type mice. Data are mean (± SEM) of results obtained from 7 animals per treatment group and were compared by ANOVA followed by post hoc Tukey-Kramer multiple comparisons as indicated. The doses of cariprazine (.005 to .02 mg/kg) are indicated on top of the bars. At all doses tested, cariprazine significantly improved social recognition measures (T1) of PCP-treated mice, and 0.01 and 0.02 mg/kg of cariprazine also significantly improved social recognition memory (T1-T2) in PCP-treated mice. d, The effect of cariprazine pretreatment on PCP-induced impairment of social recognition and social recognition memory in D3-receptor knockout mice. Data are mean (± SEM) of measures obtained from 6 animals per treatment group. All doses of cariprazine (indicated on top of the bars) were ineffective in reversing the PCP-induced impairment of social recognition and social recognition memory.
PCP also significantly affected social recognition memory as assessed by the difference between T1 interaction times and the interaction times measured after re-exposure to the same juvenile 2 h later (T2). As shown in Fig. 2a, T2 measures of saline- and cariprazine-treated wild-type mice were substantially shorter than their corresponding T1 measures, but T2 interaction times of PCP-treated mice were very similar to their corresponding T1 measures. As a result, the differences between the T1 and T2 measures (T1-T2) between the treatment groups were significant (F(4, 35)=4.023; p=0.0099). For PCP-treated mice, T1-T2 values were significantly lower compared with saline- and all cariprazine-treated mice. Moreover, T1-T2 values of mice treated with 0.005 to 0.02 mg/kg of cariprazine were significantly higher compared with PCP-treated mice. They were also higher compared to saline-treated controls, but this difference did not reach significance (Fig. 2a).
Similar results were obtained for D3-receptor knockout mice. PCP-treatment significantly reduced T1 interaction times and the T1 measures of cariprazine-treated mice (0.02 mg/kg) did not differ from saline-treated controls (F(2,21)=5.56; p=0.013)) (Fig. 2b). Moreover, in D3-receptor knockout mice, T2 measures of saline- and cariprazine-treated mice differed significantly from PCP-treated mice (F(2,21)=5.33; p=0.014)) with PCP-treated mice exhibiting significantly reduced T1-T2 values compared with saline- and cariprazine-treated mice (Fig. 2b). Moreover, cariprazine (0.02 mg/kg) had no effect on recognition memory in D3-receptor knockout mice when compared with saline-treated controls (Fig. 2b).
In summary, in both genotypes, PCP impaired both social interaction and social recognition memory indicating that expression of D3 receptors is not required for the effects of PCP on social interaction and social memory.
In the next experiments, we asked whether cariprazine pretreatment would alter PCP-induced impairments of social recognition and social recognition memory. In these experiments, cariprazine was administered 1 h prior to the T1 exposure, and PCP was administered 30 min later.
As shown in Fig. 2c, 0.005 to 0.02 mg/kg of cariprazine significantly reduced the effects of PCP on social interaction measures obtained from wild-type mice (T1; F(3, 28)=6.63; p=0.002)). Cariprazine pre-treatment also significantly affected PCP effects on T1-T2 values (F(3, 28)=8.297; p=0.0009)) with 0.01 to 0.02 mg/kg of cariprazine (but not 0.005 mg/kg) significantly increasing T1-T2 differences compared with PCP-treated mice (Fig. 2c).
In contrast to the results obtained with PCP-treated wild-type mice, cariprazine had no effect in PCP-treated D3-receptor knockout mice. Neither dose of cariprazine affected the T1 measures of PCP-treated mice (F(3, 24)=1.08; p=0.3812)), and T2 measures (F(3,24)=1.093; p=0.375)) as well as T1-T2 values (F(3, 24)=0.148; p=0.93)) were also unaffected (Fig. 2d) suggesting the effect of cariprazine is mediated by D3 receptors. However, it is also recognized that even studies on knockout mice cannot definitively exclude the possibility that the effect of cariprazine is mediated by another, yet to be identified mechanism that is also impaired in D3-receptor knockout mice.
The Effect of Cariprazine on Spatial Working Memory (WM) in PCP-treated Mice
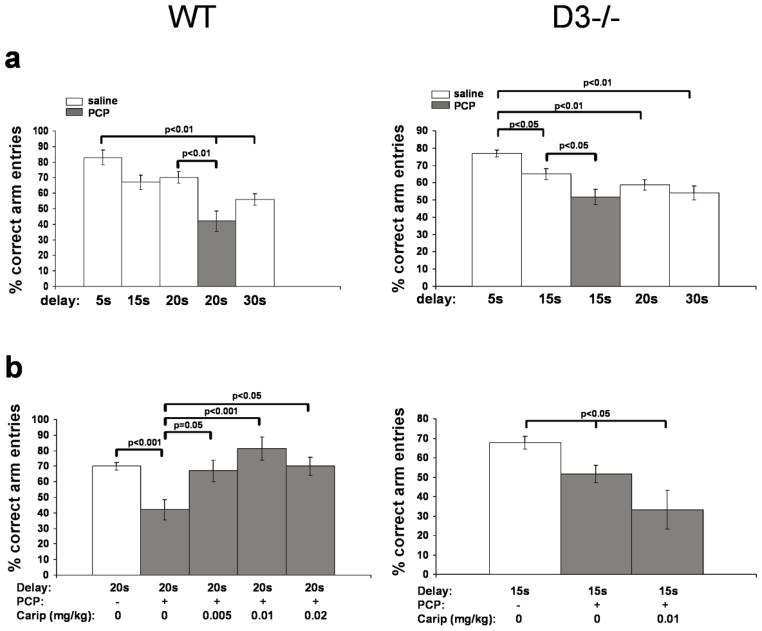
Spatial WM was assessed using a delayed alternation test performed in a T-maze. The results are shown in Figure 3. For saline-treated wild-type mice, repeated measures ANOVA (F(3,39)=4.047, p=0.017) revealed significant differences in test performance at different delay periods, and post hoc Tukey-Kramer multiple comparisons revealed a significant decline of task performance in tests with 30 sec inter-trial delay when compared to 5 sec delay (p<0.05). For saline-treated D3-receptor knockout mice, repeated measures ANOVA (F(3,39)=7.083, p=0.0012) also revealed significant differences in test performance at different delay periods and, compared with the performance at 5 sec delay, post hoc Tukey-Kramer multiple comparisons revealed significantly lower performance already at 15 sec (p<0.05) inter-trial delay, and even more significant impairment at 20 (p<0.01) and 30 sec (p<0.01) delays. These results are consistent with results of our previous study showing that D3-receptor knockout mice exhibited impaired spatial working memory compared with wild-type mice. This deficit, however, is due to the reduced dopamine D1-receptor activity found in D3-receptor knockout mice (Glickstein et al. 2002).
Fig 3. The effect of cariprazine on PCP-induced impairment of spatial working memory.
a, In both wild-type (WT) and D3-receptor knockout mice (D3−/−), PCP significantly impaired the performance in the delayed alternation test at 20 and 15 sec delays, respectively. Data are mean (± SEM;) of results obtained from 9 (WT) or 8 (D3−/−) animals per delay and treatment. Significant differences revealed by one-way ANOVA were resolved post hoc using Tukey-Kramer multiple comparisons tests as indicated. b, The effect of cariprazine on spatial working memory in WT (left) and D3−/− mice (right) tested at 20 and 15 sec delay, respectively. Data are mean (± SEM) of measures obtained from 9 (WT) and 6 (D3−/−) animals per treatment group. Significant differences revealed by ANOVA were resolved post hoc using Tukey-kramer multiple comparisons tests as indicated. Carip= cariprazine.
In view of the results summarized above for drug-naive mice, we tested the effects of PCP in wild-type and D3-receptor knockout mice at 20 and 15 sec delay, respectively. The results are shown in Fig. 3a. One-way ANOVA indicated significant differences for wild-type mice (F(4, 45)=10.93; p=0.0001)), and post hoc Tukey-Kramer multiple comparisons tests revealed that PCP treatment significantly impaired the performance of wild-type mice at 20 sec delay. Similarly, ANOVA indicated significant differences for D3-receptor knockout mice (F(4, 40)=7.09; p=0.0002)), and PCP treatment significantly impaired their performance at 15 sec delay (Fig. 3a).
In the next series of experiments, animals received cariprazine 1 h prior to testing either at 20 sec (wild-type) or 15 sec (D3-receptor knockout) delay followed by PCP 30 min later. As shown in Fig. 3b, in wild-type mice (F(4, 45)=6.124; p=0.0008)), cariprazine pretreatment blocked the effects of PCP at all doses tested (0.005 to 0.02 mg/kg); the 0.01 mg/kg dose exerted the strongest effect. In contrast, in PCP-treated D3-receptor knockout mice tested at the 15 sec inter-trial delay, 0.01mg/kg of cariprazine had no effect (F(2,18)=4.1238; p=0.04) (Fig. 3b)).
In summary, cariprazine significantly reduced the PCP-induced impairment of spatial working memory in wild type, but not in D3-receptor knockout mice, again suggesting that this effect is mediated by D3 receptors.
The Effect of Cariprazine on the Performance of PCP-treated Mice in the ASST
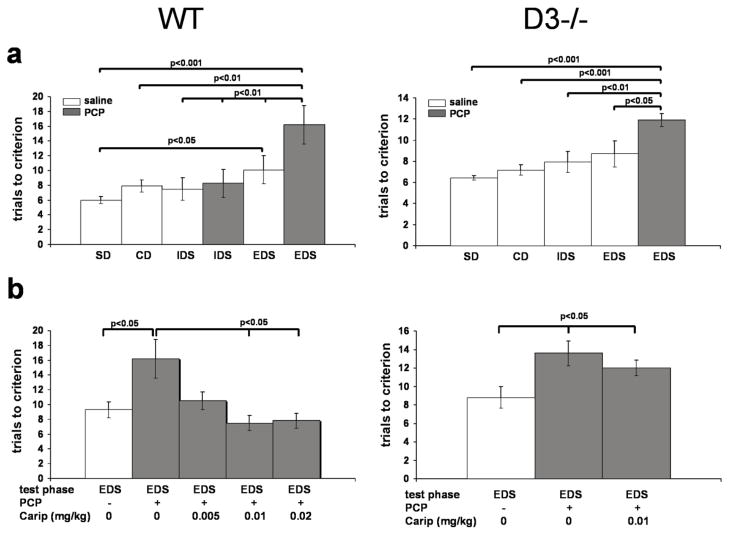
For ASST-tested wild-type and D3-receptor knockout mice treated with saline, repeated measures ANOVA revealed significant differences in the number of trials to criterion between the last 4 test phases (wild-type: F(3,27)=5.09, p=0.01; D3-receptor knockout: F(3,23)=5.65, p=0.006). In wild-type mice, post hoc Tukey-Kramer multiple comparisons revealed a significantly larger number of trials to criterion for the extra-dimensional set-shifting phase (EDS) compared to the simple and compound discriminations phases (SD, CD) and the intradimensional set-shifting phase (IDS) (p<0.05). Although D3-receptor knockout mice outperform their wild-type littermates in the EDS phase (see also Glickstein et al. 2005), the number of trials to criterion is still significantly higher in the EDS phase than in the SD- or CD-phase performances of these mice (p<0.01).
To test the effect of PCP on set-shifting performance, wild-type mice that completed the SD and CD phases of the ASST received PCP 1 h prior to IDS testing, and mice that completed the SD, CD, and IDS phases received PCP 1 h prior to EDS testing. Control animals received saline injections 1 h prior to IDS or EDS testing. As shown in Fig. 4a, there were significant differences between these treatment groups (F(5, 36)=5.03; p=0.0015), and post hoc Tukey-Kramer multiple comparisons tests revealed that PCP treatment effectively impaired the performance in the EDS, but not the IDS phase of the ASST (Fig. 4a). This finding is consistent with a previous report of Egerton et al. (2005) showing the PCP specifically affects EDS performance.
Fig 4. The effect of cariprazine on PCP-induced impairment of performance in the EDS phase of the ASST.
a, In both wild-type (WT) and D3-receptor knockout mice (D3−/−), PCP significantly impaired the performance in the EDS phase of the ASST. Data are mean (± SEM) of measures obtained 6 animals per treatment group and genotype. Significant differences revealed by ANOVA were resolved post hoc using Tukey-Kramer multiple comparisons tests as indicated. b, The effect of cariprazine on EDS performance in PCP-treated WT (left) and D3−/− mice (right). Data are mean (± SEM) of measures obtained from 6 animals per treatment group and genotype. Significant differences between treatment groups were resolved post hoc using Tukey-Kramer multiple comparisons tests as indicated. Carip= cariprazine.
There were also significant differences between EDS-tested D3-receptor knockout mice treated with saline or PCP (F(4, 30)=6.278; p=0.001); post hoc analysis revealed that also PCP-treated D3-receptor knockout mice exhibited significantly impaired performance in the EDS phase (Fig. 4a), albeit to a somewhat lesser extent when compared with the effect of PCP in wild-type mice (Fig. 4a).
In the next series of experiments, animals that completed the SD, CD, and IDS phases of the ASST received cariprazine 1 h prior to EDS testing, followed by PCP 30 min later. As shown in Fig. 4b, in wild-type mice, cariprazine pretreatment significantly reduced the effects of PCP at doses of 0.01 and 0.02 mg/kg; the effect at 0.005 mg/kg did not reach significance (F(4, 30)=5.06; p=0.0038)). In contrast, in PCP-treated D3-receptor knockout mice, 0.01 mg/kg of cariprazine pre-treatment had no effect on the EDS performance (F(2, 18)=3.89; p=0.04)) (Fig. 4b).
In summary, cariprazine pretreatment blocked PCP-induced impairment of extradimensional attention set-shifting in wild-type, but not in D3-receptor knockout mice. Also these results support the conclusion that the effect of cariprazine is mediated by D3 receptors.
DISCUSSION
Deficits in cognitive functions are prominent endophenotypes of manifest schizophrenia and also found in children and adolescents at risk for developing schizophrenia (Park and Holzman, 1992; Zihl et al. 1998; Erlenmeyer-Kimling et al. 2000; Gottesman and Erlenmeyer-Kimling, 2001; Nuechterlein et al. 2004; Carriòn et al. 2011). Although first and second generation antipsychotic drugs can effectively ameliorate the positive symptoms, the need for improved treatment of cognitive deficits is clearly recognized (Green, 1996; Pratt et al. 2008).
One animal model successfully used to reproduce cognitive abnormalities found in schizophrenia is based on acute or subacute treatment with the NMDA receptor antagonist PCP, a treatment that induces a transient state resembling schizophrenia in humans (Javitt and Zukin, 1991). We show here that a single, low and non-toxic dose of PCP (1 mg/kg) is capable of impairing social interaction and social recognition memory, working memory, and extradimensional attention set-shifting in mice. These PCP-induced impairments were detected in both wild-type and dopamine D3-receptor knockout mice, indicating that D3 receptors are not critical mediators of the effects of PCP on these cognitive functions. However, cariprazine, a dopamine D3-receptor preferring drug with partial agonist pharmacology, effectively blocked the deleterious effect of PCP on these functions in wild-type, but not in D3-receptor knockout mice. This finding suggests that, despite the complex mechanisms by which PCP elicits cognitive deficits, D3-receptor preferring partial agonists can effectively ameliorate their severity.
One reason for the effectiveness of cariprazine in ameliorating PCP-induced cognitive deficit could reside in its ability to exert pro-cognitive effects. Such cognitive enhancing effects of cariprazine have previously been demonstrated in rats with scopolamine-induced learning impairments (Gyertyán et al. 2011). However, we show here that cariprazine alone only marginally increased social recognition memory in wild-type mice. Rather, its pro-cognitive effect is only apparent in PCP-treated animals with disrupted social recognition memory. Nevertheless, the lack of a corresponding effect in PCP-treated D3-receptor knockout mice suggests that expression of D3 receptors is necessary for cariprazine to exert its effect in PCP-treated animals.
The effects of cariprazine are not delimited to social recognition memory (a hippocampal/perirhinal function) in PCP-treated animals. Cariprazine also mitigated the effects of PCP on two other cognitive domains, spatial working memory and attention set-shifting, i.e., executive functions governed by the prefrontal cortex (PFC) that are dependent upon normal dopaminergic function. Acute administration of PCP, however, elicits a robust increase in cortical dopamine output (Jentsch et al. 2008). Moreover, activation of the mesolimbic dopaminergic pathway is critically dependent upon the effect of PCP acting in the prefrontal cortex (Jentsch et al. 1998) and thought to be associated with the working memory deficits triggered by PCP and other congeners (Verma and Mogadam, 1996). Hence, our finding that cariprazine pretreatment was also capable of attenuating the PCP-induced impairments of cognitive functions governed by the PFC in wild-type mice (but not in D3-receptor knockout mice) suggests yet another mechanism by which cariprazine could protect cognitive functions in PCP-treated mice whose cognitive dysfunctions are due to increased dopaminergic output: The high affinity of cariprazine for dopamine D3 receptors along with its partial agonist activity at this receptor should enable cariprazine to normalize signaling through dopamine D3 receptors during periods of imbalanced (increased) dopaminergic tone, especially since D3 receptors have highest affinity for dopamine (Sokoloff et al. 1990). Hence, as a partial dopamine D3-receptor agonist, cariprazine could be in a strong position to stabilize prefrontal cortical circuitries in the face of stimuli that can perturb them.
Our study on D3-receptor knockout mice points to an essential role of dopamine D3 receptors in mediating cariprazine’s effects on preserving cognitive functioning in PCP-treated mice. This does, however, not exclude the possibility that other targets of cariprazine contribute to this effect. Cariprazine also has appreciable affinity to dopamine D2 receptors, high affinity to 5-HT2B receptors, and low to moderate affinity to 5-HT1A, 5-HT2C, and histamine H1 receptors (Kiss et al. 2010). Clearly, more studies are needed to further elucidate the role of these receptors in modulating the severity of PCP-triggered cognitive dysfunctions in animal models, and ultimately in patients with prodromal symptoms or manifest psychosis.
Of particular interest will be to discern whether effects of D3-receptor preferring partial agonists on cognitive function differ from those exerted by D2-receptor preferring partial agonists. Previous studies on knockout mice lacking dopamine D2 and D3 receptors uncovered distinct roles played by D2 and D3 receptors in cognitive function governed by the medial prefrontal and orbital frontal cortex, namely working memory, attention set-shifting, and reversal learning (Glickstein et al. 2002; Glickstein et al. 2005; DeSteno and Schmauss, 2009). Moreover, studies on rats found pro-cognitive effects of D3, but not D2, receptor blockade in tests of social recognition memory (a hippocampal/perirhinal function; Loiseau and Millan, 2009), and in a rat model of impaired visual recognition memory (post-weaning isolation rearing; Watson et al. 2011). Altogether, these studies support the idea that targeting dopamine D3-receptors is a promising new avenue for the treatment of cognitive deficits that accompany psychotic disorders.
Acknowledgments
This study was supported by Forest Laboratories, Inc. and Gedeon Richter. Both companies participated in the study design.
References
- Beraki S, Diaz-Heijtz R, Tai F, Ogren SO. Effect of repeated treatment of phencyclidine on cognition and gene expression in C57Bl/6 mice. Int J Neuropsychopharmacology. 2009;12:243–255. doi: 10.1017/S1461145708009152. [DOI] [PubMed] [Google Scholar]
- Birrel JM, Brown VJ. Medial frontal cortex mediates perceptual attention set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombin I, Arango C, Mayoral M, Castro-Fornieles J, Gonzales-Pinto A, Gonzales-Gomez C, Moreno D, Parellada M, Beaza I, Graell M, Ptero S, Saiz PA, Patiño-Garcia A. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:873–879. doi: 10.1002/ajmg.b.30710. [DOI] [PubMed] [Google Scholar]
- Carrión R, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiat. 2011;168:806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSteno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual atttentional set-shifting following PCP administration: a rodent model of set-shifting deficits. Psychopharmacology. 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Beh. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Jamal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychosis: The New York High Risk Project. Am J Psychiat. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein SB, DeSteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during task requiring attention. Cer Cor. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Erlenmeyer-Kimling L. Family and twin strategies as a head start in defining prodromes and endophenotypes for hypothetical early interventions in schizophrenia. Schizophrenia Res. 2001;51:93–102. doi: 10.1016/s0920-9964(01)00245-6. [DOI] [PubMed] [Google Scholar]
- Green ME. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiat. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Kiss B, Sághy K, Laszy J, Szabó G, Szabados T, Gémesi LI, Pásztor G, Zájer-Balázs M, Kapás A, Csongor EA, Domány G, Tihanyi, Szombathelyi Z. Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int. 2011;59:925–935. doi: 10.1016/j.neuint.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Fujita Y, Ishima T, Kunitachi S, Shirayama Y, Iyo A, Hashimoto K. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antipsychotic drug perospirone: Role of serotonin 5-HT1A receptors. Eur Neuropsychopharmacol. 2008;18:448–454. doi: 10.1016/j.euroneuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur J Pharmacol. 2005;519:114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiat. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: behavioral and neurochemical evidence. Psychopharmacology (1998) 1998;138:89–95. doi: 10.1007/s002130050649. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Anzivino LA. A low dose of the alpha2 agonist clonidine ameliorates the visual attention and spatial working memory deficits produced by phencyclidine administration to rats. Psychopharmacology. 2004;175:76–83. doi: 10.1007/s00213-004-1772-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Sanchez D, Elsworth JD, Roth RH. Clonidine and guanfacine attenuate phencyclidine-induced dopamine overflow in rat prefrontal cortex: Mediating influence of the alpha-2A adrenoreceptor subtype. Brain Res. 2008;1246 :41–46. doi: 10.1016/j.brainres.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M-Y, Skryabin BV, Arai M, Abbondanzo S, Fu D, Robakis NK, Brosius J, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2-mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- Kiss B, Horváth A, Némethy Z, Schmidt E, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyán I, Agai-Csongor E, Domány G, Tihanyi K, Adham N, Szombathelyi Z. Cariprazine (RGH-188), a dopamine D3 receptor-preferring, D3/D2 dopamine receptor antagonist–partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333:328–40. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyán I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl) 2005;179:567–575. doi: 10.1007/s00213-004-2096-z. [DOI] [PubMed] [Google Scholar]
- Loiseau F, Milan MJ. Blockade of dopamine D3 receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: Similar actions of D1 receptor agonists, but not D2-receptor antagonists. Neuropsychopharmacology. 2009;19:23–33. doi: 10.1016/j.euroneuro.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Lumme V, Aalto S, Ilonen T, Någren K, Hietala J. Dopamine D2/D3 receptor binding in the anterior cingulate cortex and executive functioning. Psychiatry Res. 2007;156:69–74. doi: 10.1016/j.pscychresns.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JS, Brown CS, Moore TR. Atypical antipsychotics. Part I: Pharmacology, Pharmacokinetics, and Efficacy. Annals Pharmacother. 1999;33:73–85. doi: 10.1345/aph.17215. [DOI] [PubMed] [Google Scholar]
- Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoreceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- Mehta M, Schmauss C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Beh Neurosci. 2011;125:29–36. doi: 10.1037/a0021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology. 2009;202:315–328. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg JM, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Res. 2004;15:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiat. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modeling prefrontal cortex deficits in schiziphrenia: implications for treatment. Br J Pharmacol. 2008;153:5465–5470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca N, Finnema SJ, Laszlovszky I, Kiss B, Horváth A, Pásztor G, Kapás M, Gyertyán, Farkas S, Innis RB, Halldin C, Gulyás B. Occupancy of dopamine D2 and D3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology (Berl) 2011;218:579–587. doi: 10.1007/s00213-011-2343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Brito GN. Recovery of delayed alternation in rats after lesions in medial frontal cortex and septum. J Comp Physiol Psychol. 1980;94:808–818. doi: 10.1037/h0077834. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Marsden CA, Millan MJ, Fone KC. Blockade of dopamine D3 but not D2 receptor reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacology. 2012;37:770–786. doi: 10.1017/S1461145711000435. [DOI] [PubMed] [Google Scholar]
- Zihl J, Gron G, Brunnauer A. Cognitive deficits in schizophrenia and affective disorders: evidence for a final common pathway disorder. Acta Psychiat Scan. 1998;97:351–357. doi: 10.1111/j.1600-0447.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]