Abstract
Objectives:
Sarcopenia may negatively affect short-term outcomes after liver resection. The present study aimed to explore whether total functional liver volume (TFLV) is related to sarcopenia in patients undergoing partial liver resection.
Methods:
Analysis of total liver volume and tumour volume and measurements of muscle surface were performed in patients undergoing liver resection using OsiriX® and preoperative computed tomography. The ratio of TFLV to bodyweight was calculated as: [TFLV (ml)/bodyweight (g)]*100%. The L3 muscle index (cm2/m2) was then calculated by normalizing muscle areas (at the third lumbar vertebral level) for height.
Results:
Of 40 patients, 27 (67.5%) were classified as sarcopenic. There was a significant correlation between the L3 skeletal muscle index and TFLV (r= 0.64, P < 0.001). Median TFLV was significantly lower in the sarcopenia group than in the non-sarcopenia group [1396 ml (range: 1129–2625 ml) and 1840 ml (range: 867–2404 ml), respectively; P < 0.05]. Median TFLV : bodyweight ratio was significantly lower in the sarcopenia group than in the non-sarcopenia group [2.0% (range: 1.4–2.5%) and 2.3% (range: 1.5–2.5%), respectively; P < 0.05].
Conclusions:
Sarcopenic patients had a disproportionally small preoperative TFLV compared with non-sarcopenic patients undergoing liver resection. The preoperative hepatic physiologic reserve may therefore be smaller in sarcopenic patients.
Introduction
Over recent decades, liver resection has become increasingly safe as a result of improvements in surgical techniques and perioperative care. At present, mortality rates are reported to be well below 5%.1,2 However, postoperative liver failure remains the most important cause of lethal outcome after liver surgery. Patients with a small postoperative remnant liver volume (RLV) are at higher risk for developing post-resection liver failure. The critical minimum RLV has been estimated to be approximately 25% after resection in normally functioning livers.3 It is still unclear why some patients with smaller hepatic remnants do not develop liver failure or postoperative complications, whereas some with greater residual volumes do. In addition, postoperative morbidity remains a concern in these patients. Therefore, it is of major importance to preoperatively identify patients who are at increased risk for postoperative liver failure and related morbidity. Unfortunately, at present there is no adequate method for assessing this risk in patients undergoing liver resection.
Information on body composition and the metabolic status of patients undergoing liver resection may help to identify patients at increased risk. Patients undergoing liver surgery often suffer from weight loss or even cachexia as a result of malignancies or as a consequence of chemotherapy toxicity. Central to the progress of cancer-related weight loss is the development of sarcopenia. Sarcopenia, defined as the depletion of muscle mass, is proven to affect short-term outcome negatively after liver resection4 and has also been associated with mortality in patients with cirrhosis scheduled for liver transplantation.5 A recent study by van Vledder et al.6 showed that sarcopenia was a strong indicator for a worse prognostic outcome in terms of both disease-free and overall survival after liver resection. It is currently unknown whether sarcopenia is associated with a decrease in total hepatic functional mass. The present study hypothesized that sarcopenic patients may have impaired outcome after liver surgery because their preoperative total functional liver volume (TFLV) is disproportionally small compared with that in non-sarcopenic patients. The present study was intended to explore whether TFLV is related to the level of sarcopenia in patients undergoing partial liver resection.
Materials and methods
Ethics approval
The study was approved by the medical ethics committee of Maastricht University Medical Centre and conducted according to the revised version of the Declaration of Helsinki (October 2008, Seoul). All patients had given written informed consent to the inclusion of their data in a prior study. For the current purpose, no new approval was needed as this prior consent covered the use of these data in related studies conducted within 5 years.
Patients
Patients with primary or secondary liver tumours (mostly colorectal cancer liver metastases) in otherwise normal livers planned for liver resection at Maastricht University Medical Centre between January 2008 and June 2009 were eligible for inclusion in this study. All patients underwent contrast-enhanced computed tomography (CT) within routine preoperative assessment at either the present hospital or at one of the surrounding university-affiliated district general teaching hospitals. Patients were admitted to the hospital 1 day preoperatively and were submitted to routine blood tests.
Surgical procedure
Liver resection was performed as detailed elsewhere.7 In each patient, laparotomy was performed by bilateral subcostal incision, after which intraoperative ultrasound was used to assess the liver, which was then mobilized appropriately prior to hepatic parenchymal transection.
Image muscle area analysis using OsiriX®
All patients underwent contrast-enhanced CT as part of routine preoperative assessment. Image analysis was conducted using four-phase CT scans provided on CD-ROM. The muscle and liver areas were outlined using the open-source software OsiriX® Version 3.3 (32-bit; http://www.osirix-viewer.com). A 2.8-GHz Intel Core 2 Duo 24″ iMac (Apple, Inc., Cupertino, CA, USA) was used. Muscle areas were measured on transverse slices of the CT scans in a semi-automated fashion by two investigators (SAWGD and TML). The ‘Grow Region (2D/3D Segmentation)’ tool in the ‘Region of Interest’ (RoI) dropdown menu enabled the automatic outlining of skeletal muscles. This modality of OsiriX® is based on differences in Hounsfield units (HU) among muscle, bony structures and body fat. The threshold range for muscle was set between −30 HU and 110 HU. If necessary, the automatically generated outlines were hand-adjusted with the ‘Closed Polygon Selection’ and ‘Repulsor’ tools to optimize the RoI (Fig. 1).
Figure 1.
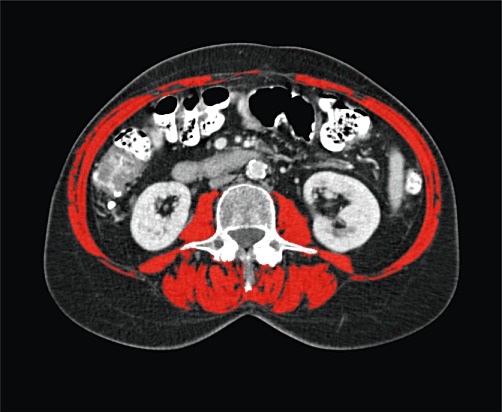
Computed tomography image at the third lumbar vertebral level. The following skeletal muscles are outlined in red: rectus abdominis; oblique and lateral abdominal muscles; paraspinal muscles, and psoas. This female sarcopenic patient had an L3 muscle index of 34.3 cm2/m2
Volumetric liver analysis using OsiriX®
Volumetric analysis using OsiriX® was performed by two investigators (SAWGD and TML). Details on how volumetry was performed with OsiriX® have been described previously.8 The total liver volume (TLV) and the tumour volume (TV) were manually outlined on the preoperative CT scan. The TFLV was calculated using the following formula: TLV − TV = TFLV.
Calculations
Data are expressed as median and range. Skeletal muscle content was assessed by measuring the cross-sectional areas of the muscles at the level of the lumbar 3 (L3) vertebral landmark. Measurements were performed on the first image on which both vertebral spinae were clearly visible and on the following image in the cranial direction. Area measurements were obtained for the following muscles: psoas; paraspinal muscles; transversus abdominis; external and internal oblique abdominis, and rectus abdominis. The mean of the skeletal muscle area was used for calculation. The measured skeletal muscle areas were then normalized for height as is conventional for other body composition measures to derive the L3 skeletal muscle index (L3mi) expressed in cm2/m2. The ratio of TFLV : bodyweight was calculated as: [TFLV (ml)/bodyweight (g)]*100%.
Total body fat-free mass was estimated by the method proposed by Prado et al.9 using the following formula: total body fat-free mass (kg) = 0·30*(skeletal muscle surface area at L3 in cm2) + 6.06. Prado et al.9 constructed this formula with regression equations relating muscle surface area in the lumbar region with total body fat-free mass. Body surface area (BSA) was calculated using the Mosteller formula: BSA (m2) =[(height, cm*weight, kg/3600)0.5].
Sarcopenia
The definition of sarcopenia was based on a method validated in patients with cancer.9 In this method, cut-off values associated with mortality are determined by optimal stratification. The L3mi values associated with mortality determined in this way were 55.4 cm2/m2 for men and 38.9 cm2/m2 for women. Patients with an L3mi lower than these values were considered sarcopenic.9
Statistics
Data are presented as median (range). Correlations were calculated using Pearson's test. A P-value of <0.05 was considered to indicate statistical significance. The resulting regression line was described as a linear equation, and the correlation coefficient (r) was calculated. The Mann–Whitney U-test was applied to compare continuous data between groups. Statistical analysis was performed using Prism 5.0 for Windows (GraphPad Software, Inc., San Diego, CA, USA) and spss Version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patients
A total of 40 patients were evaluated in the present study. Their median age was 62 years (range: 41–80 years) and median body mass index (BMI) was 25.2 kg/m2. Thirty-seven patients underwent liver resection because of colorectal liver metastases, two patients because of hepatocellular carcinoma and one patient because of a carcinoid metastasis. Patients were not infectious and did not have liver dysfunction. The median L3mi was 49.5 cm2/m2 (range: 35.5–70.8 cm2/m2) in men and 38.4 cm2/m2 (range: 29.4–61.0 cm2/m2) in women. Based on the predefined criteria, sarcopenia was identified in 27 patients (67.5%), including 17 of 24 (70.8 %) men and 10 of 16 (62.5%) women (Table 1).
Table 1.
Patient characteristics at the time of muscularity assessment (n= 40 patients)
| Variables | Values |
|---|---|
| Age, years, median (range) | 62 (41–80) |
| Sex, male, n (%) | 24 (60.0) |
| Weight, kg, median (range) | 76 (49–127) |
| Height, cm, median (range) | 172 (155–195) |
| BMI, kg/m2, median (range) | 25 (19.3–42.9) |
| Fat-free body mass, kg, median (range) | 48 (31–66) |
| BSA, m2, median (range) | 1.9 (1.47–2.46) |
| Preoperative laboratory tests, median (range) | |
| Bilirubin, µmol/l (normal < 20) | 11.8 (6.9–9.5) |
| ALT, U/l (normal < 50) | 26 (7–67) |
| AST, U/l (normal < 38) | 21 (7–52) |
| LDH, U/l (normal 120–250) | 367 (263–595) |
| Ggt, U/l (normal < 55) | 45 (13–258) |
| CRP (normal < 10) | 3 (1–88) |
| Creatinine, µmol/l (normal 60–115) | 87 (46–287) |
| Preoperative muscle status, cm2/m2, median (range) | |
| L3 muscle index in all patients | 46.4 (29.4–70.8) |
| L3 muscle index in men | 49.5 (35.5–70.8) |
| L3 muscle index in women | 38.4 (29.4–61.0) |
| Sarcopenic patients, n (%) | 27 (62.5) |
BMI, body mass index; BSA, body surface area; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; Ggt, γ-glutamyl transferase; CRP, C-reactive protein.
Features associated with sarcopenia
Total liver volume, TFLV and fat-free body mass were significantly lower in the sarcopenia group than in the non-sarcopenia group (P < 0.05). The ratio of TFLV : bodyweight was also significantly lower in the sarcopenia group than in the non-sarcopenia group [2.0% (range: 1.4–2.5%) and 2.3% (range: 1.5–2.5%), respectively; P < 0.05] (Fig. 2). Age, sex, weight and height did not significantly differ between the sarcopenia and non-sarcopenia groups. The association between sarcopenia and BMI did not attain statistical significance (P= 0.06) (Table 2). There was no significant correlation between age and L3mi (r= 0.006, P= 0.97).
Figure 2.
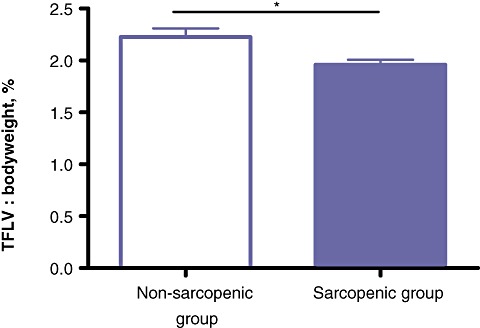
The calculated total functional liver volume (TFLV) : bodyweight ratio. *P < 0.05
Table 2.
Features associated with sarcopenia in 40 patients
| Variables | No sarcopenia (n= 13) | Sarcopenia (n= 27) | P-value |
|---|---|---|---|
| Age, years, median (range) | 60 (43–80) | 64 (41–79) | 0.735 |
| Sex, male, n (%) | 7 | 17 | 0.733 |
| Weight, kg, median (range) | 85 (49–127) | 74 (54–106) | 0.363 |
| Height, m, median (range) | 1.7 (1.6–1.9) | 1.7 (1.6–2.0) | 0.108 |
| Body mass index, kg/m2, median (range) | 29 (20–43) | 25 (19–31) | 0.06 |
| Fat-free body mass, kg, median (range) | 57 (36–67) | 48 (31–60) | <0.05 |
| Body surface area, m2, median (range) | 2.0 (1.5–2.5) | 1.9 (1.5–2.3) | 0.709 |
| L3 muscle index, cm2/m2, median (range) | 59 (40–71) | 42 (29–53) | <0.005 |
| Total liver volume, ml, median (range) | 1841 (919–2417) | 1402 (1138–2630) | <0.05 |
| Tumour volume, ml, median (range) | 13 (0–112) | 8 (1–37) | <0.05 |
| Total functional liver volume, ml, median (range) | 1840 (867–2404) | 1396 (1129–2625) | <0.05 |
Relationship between body composition and TFLV
The average time required to measure the L3 muscle area was 4 min per measurement per patient. There was a significant correlation between skeletal L3mi and TFLV (r= 0.64, P < 0.001). There were also significant correlations between TFLV and fat-free body mass (r= 0.74, P < 0.001) and BSA (r= 0.78, P < 0.001) (Fig. 3a–c).
Figure 3.
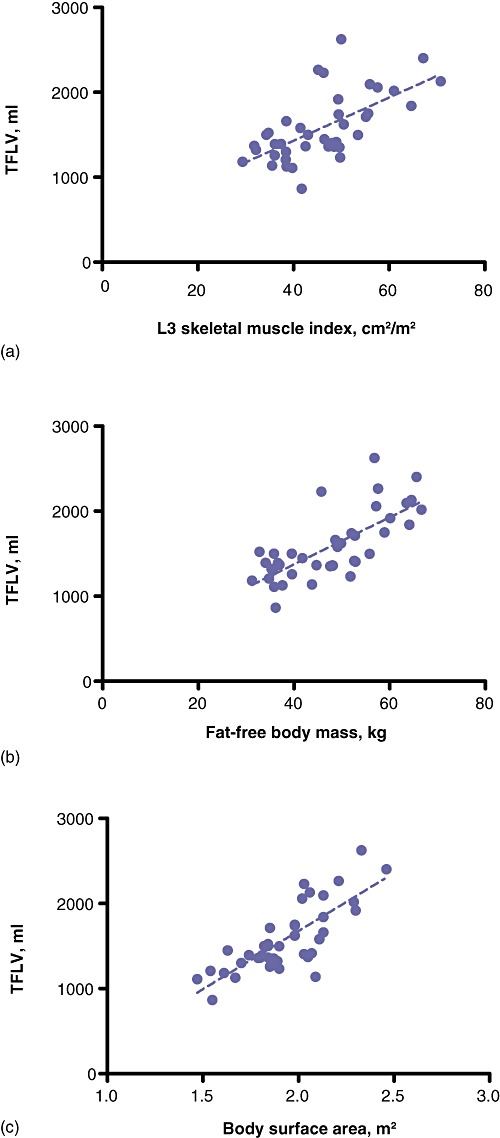
Correlations between total functional liver volume (TFLV) in 40 patients and (a) L3 muscle index (r= 0.64, P < 0.0001), (b) fat-free body mass (r= 0.74, P < 0.0001), and (c) body surface area (r= 0.78, P < 0.0001)
Discussion
The aim of the present study was to investigate whether TFLV was influenced by the presence or absence of sarcopenia in patients undergoing liver surgery. There was a significant correlation between the L3mi and preoperative TFLV. The relationship between body composition and TFLV was confirmed by significant correlations between preoperative TFLV and both fat-free body mass and BSA. Sarcopenic patients had a significantly lower preoperative TFLV compared with non-sarcopenic patients. Because a relationship between bodyweight and TLV is known to exist, the TFLV : bodyweight ratio was subsequently calculated.10 The TFLV : bodyweight ratio was significantly lower in the sarcopenia group than in the non-sarcopenia group, providing evidence that liver weight is reduced to a disproportionally greater extent in sarcopenic patients.
The data from the present study provide novel background information that may help to explain the results reported by Peng et al.4 and van Vledder et al.6 Both of these studies showed that sarcopenia has a negative impact on postoperative outcome after liver resection. However, the reason why outcomes are worse in sarcopenic patients compared with non-sarcopenic patients remains unclear. One explanation may be that the metabolic physiologic reserve in sarcopenic patients is decreased. The revelation by CT volumetric analysis of the liver in the present study that preoperative TFLV is smaller in sarcopenic patients than in non-sarcopenic patients supports this hypothesis. It may very well be that patients with sarcopenia have worse outcomes after liver surgery because their preoperative TFLV/hepatocyte mass is disproportionally small compared with that in non-sarcopenic patients. These findings underline the importance of assessing for sarcopenia in addition to liver volumetry in patients scheduled for liver resection.
It has been suggested that in healthy individuals the level of sarcopenia is related to age.11 This was not found to be true in the patients enrolled in the present study because there was no significant difference in age between groups. There was also no significant correlation between age and L3 muscle mass index. This provides circumstantial evidence that the L3 muscle mass index reflects the extent of sarcopenia in patients with liver tumours and is not determined by age per se. Further, there was no significant correlation between TV and level of sarcopenia.
The percentage future RLV is usually expressed as the ratio of RLV : TLV (RLV/TLV*100%). The critical minimum RLV has been estimated to be approximately 25% after resection.3,12–14 Truant et al.10 showed that the ratio of RLV to bodyweight (RLV : BW) was more specific than RLV alone in predicting postoperative course after extended hepatectomy. These authors showed that a cut-off RLV : BW value of 0.5% can be used as a critical point associated with postoperative outcome.10 As the L3 muscle mass index is probably a more robust indicator of physiologic reserves and cachexia than bodyweight, perhaps an RLV : L3mi ratio would be even more accurate in predicting outcome after hepatectomy.
The extent of muscle wastage in sarcopenia is often underestimated in cancer patients because a substantial proportion of patients with sarcopenia have a BMI within the normal range and some sarcopenic patients are even overweight.9 For instance, in the present study none of the sarcopenic patients were underweight (BMI < 18.5 kg/m2) and one sarcopenic patient was overweight. Sarcopenic obese patients will probably be even more vulnerable in major surgery because they are subject to the health risks of both obesity and depleted lean body mass.9 The L3 muscle mass index is a valuable tool for diagnosing frailty in patients with a normal or high BMI. Assessing muscle mass preoperatively in patients undergoing liver surgery, in order to estimate physiologic metabolic reserve, may soon become even more important as there are simultaneous incremental increases in the incidences of obesity and primary and secondary liver tumours in the Western world.
In conclusion, sarcopenic patients had a disproportionally small preoperative TFLV compared with non-sarcopenic patients undergoing liver resection. The preoperative hepatic physiologic reserve may therefore be disproportionally low in sarcopenic patients compared with non-sarcopenic patients. The L3 muscle mass index is easily obtainable and may represent a valuable additional tool for preoperative risk assessment in patients undergoing partial liver resection.
Conflicts of interest
None declared.
References
- 1.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. et al. [DOI] [PubMed] [Google Scholar]
- 2.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. et al. [DOI] [PubMed] [Google Scholar]
- 3.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng Y. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB. 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;10:166–173. doi: 10.1016/j.cgh.2011.08.028. et al. [DOI] [PubMed] [Google Scholar]
- 6.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, IJzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2011;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 7.Dejong CH, Garden OJ. Neoplasms of the liver. In: Majid AA, Kingsnorth A, editors. Greenwich Medical Media; 2003. pp. 146–156. . In: , eds. Advanced Surgical Practice. London: , pp. [Google Scholar]
- 8.van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010;34:2426–2433. doi: 10.1007/s00268-010-0663-5. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. et al. [DOI] [PubMed] [Google Scholar]
- 10.Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O. Remnant liver volume to body weight ratio > or = 0.5%: a new cut-off to estimate postoperative risks after extended resection in non-cirrhotic liver. J Am Coll Surg. 2007;204:22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. et al. [DOI] [PubMed] [Google Scholar]
- 11.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 12.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. et al; discussion 406407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. et al. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero A, Vigano L, Polastri R, Muratore A, Eminefendic H, Regge D. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–1651. doi: 10.1007/s00268-007-9123-2. et al. [DOI] [PubMed] [Google Scholar]


