Abstract
Background:
New-onset diabetes mellitus after a pancreaticoduodenectomy (PD) remains poorly defined. The aim of this study was to define the incidence and predictive factors of immediate post-resection diabetes mellitus (iPRDM).
Methods:
Retrospective review of patients undergoing PD from January 2004 through to July 2010. Immediate post-resection diabetes mellitus was defined as diabetes requiring pharmacological treatment within 30 days post-operatively. Logistic regression was conducted to identify factors predictive of iPRDM.
Results:
Of 778 patients undergoing PD, 214 were excluded owing to pre-operative diabetes (n= 192), declined research authorization (n= 14) or death prior to hospital discharge (n= 8); the remaining 564 patients comprised the study population. iPRDM occurred in 22 patients (4%) who were more likely to be male, have pre-operative glucose intolerance, or an increased creatinine, body mass index (BMI), pre-operative glucose, operative time, tumour size or specimen length compared with patients without iPRDM (P < 0.05). On multivariate analysis, pre-operative impaired glucose intolerance (P < 0.001), pre-operative glucose ≥ 126 (P < 0.001) and specimen length (P= 0.002) were independent predictors of iPRDM. A predictive model using these three factors demonstrated a c-index of 0.842.
Discussion:
New-onset, post-resection diabetes occurs in 4% of patients undergoing PD. Factors predictive of iPRDM include pre-operative glucose intolerance, elevated pre-operative glucose and increased specimen length. These data are important for patient education and predicting outcomes after PD.
Introduction
Post-resection diabetes mellitus (PRDM) is a poorly defined sequela of major pancreatic resection (PR), with a reported incidence ranging from 0% to 50%.1–9 Reasons for this wide range include variable indications for resection, variable follow-up and small sample size. Whereas substantial effort has been expended to define major complications after a pancreaticoduodenectomy (PD) such as pancreatic leak, delayed gastric emptying and post-operative haemorrhage, relatively little has been done to evaluate post-operative diabetes in terms of incidence and predictive factors.
The aetiology of endocrine insufficiency in the post-resection period is likely multifactorial. Contributing factors not only include loss of pancreatic parenchyma and alteration of neurohormonal responses after resection, but also include recurrence or progression of underlying pancreatic disease, progressive gland atrophy and adjuvant treatments such as chemotherapy or radiation. The aim of this study was to evaluate the immediate effects of PD (parenchymal loss and physiologic alterations) on the incidence of post-resection diabetes. This determination is important in that the incidence of PRDM is a consideration in the surgeon's procedure-specific recommendation and the patient's consent to the operation.
Methods
The Mayo Clinic Institutional Review Board approved this study. We performed a retrospective review of all consecutive patients undergoing PD at our institution between January 2004 and July 2010. Data were collected from the medical records with regard to patient demographics, indications for surgery, peri-operative data and outcomes. Patients with a pre-operative diagnosis of diabetes were excluded. Diabetes was defined as elevated serum glucose requiring pharmacological treatment. Patients with a diagnosis of impaired glucose tolerance, impaired fasting glucose or who had elevated fasting blood glucose (FBG) levels but had no formal diabetes evaluation or treatment pre-operatively were included. Patients were followed for 30 days from surgery, recording complications and outcomes including glucose levels and need for pharmacological treatment for elevated glucose. Patients requiring insulin or an oral hypoglycaemic agent within 30 days after operating were considered to have immediate post-resection diabetes mellitus (iPRDM).
Univariate and multivariable associations with iPRDM were evaluated using logistic regression models and summarized with odds ratios (ORs) and 95% confidence intervals (CIs). A multivariable model was developed using a stepwise selection procedure with the P-value as a feature to enter or leave the model set to 0.05. Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC, USA). All tests were two-sided and P-values < 0.05 were considered statistically significant.
Results
Of 778 consecutive patients undergoing PD performed at the Mayo Clinic in Rochester, MN between 1 January 2004 and 31 July 2010, 214 patients were excluded from the analysis because of a pre-operative diagnosis of diabetes (n= 192, 15 diabetes mellitus type 1, 177 diabetes mellitus type 2), having declined Minnesota Research Authorization (n= 14) or peri-operative mortality preventing evaluation of diabetes (n= 8). The remaining 564 patients represent the cohort for this study.
The mean age of this cohort was 64 years, with a mean body mass index (BMI) (kg/m2) of 27, and 55% were male. Comorbidities included hypertension in 243(43%), obesity (BMI ≥30) in 113 (20%), and renal insufficiency (creatinine > 1.2) in 45 patients (9%). A family history of DM was reported in 191 patients (35%). Amongst the 490 patients with available pre-operative glucose levels, pre-operative fasting blood glucose (FBG) values were found to be < 100 mg/dl in 191 patients (39%), between 100–125 mg/dl in 242 (49%), and ≥126 mg/dl in 57 patients (12%). Indications for surgery were malignancy in 421 (75%) and benign in the remaining patients (Table 1). The most common diagnoses included pancreatic adenocarcinoma (n= 241), an intraductal pancreatic mucinous neoplasm (n= 66), ampullary adenocarcinoma (n= 51) and a pancreatic neuroendocrine tumour (n= 36). Only 31 patients (6%) in this series underwent PD for chronic pancreatitis-related symptoms or complications. A pylorus-preserving technique was performed in 455 patients (81%) with the remainder receiving a standard PD. A totally laparoscopic approach for PD was used in 78 patients (14%). The mean operative time and estimated blood loss was 361 min and 646 ml, respectively. A peri-operative blood transfusion was given in 223 patients (40%). On histopathology review, the mean tumour size and specimen length were 3.3 cm and 6.7 cm, respectively.
Table 1.
Indications for operation in patients undergoing a pancreaticoduodenectomy
| Diagnosis | Frequency | Per cent |
|---|---|---|
| Malignant | ||
| Pancreatic ductal adenocarcinoma | 241 | 42.7 |
| Ampullary adenocarcinoma | 51 | 9.04 |
| Duodenal adenocarcinoma | 29 | 5.14 |
| Periampullary adenocarcinoma | 24 | 4.26 |
| Cholangiocarcinoma | 24 | 4.26 |
| Neuroendocrine tumour (malignant) | 23 | 4.08 |
| Other (malignant) | 29 | 5.14 |
| Benign | ||
| Intraductal papillary mucinous neoplasm | 66 | 11.7 |
| Chronic pancreatitis | 31 | 5.5 |
| Neuroendocrine tumour (benign) | 13 | 2.3 |
| Cystadenoma | 9 | 1.59 |
| Other (benign) | 24 | 4.26 |
In the immediate (30 days) post-operative period, 226 patients (40%) had normal FBG levels and required no hyperglycemia treatment. In 316 patients (56%), transiently elevated glucose levels required peri-operative insulin treatment; however, treatment was discontinued during hospitalization because of normalization of FBG levels without treatment. Persistently elevated FBG levels occurred in 22 patients (4%) requiring ongoing treatment with insulin (n= 20) or an oral hypoglycemic agent (n= 2) and were therefore considered to have iPRDM. When evaluating patients with normal pre-operative glucose levels (<100 mg/dl), impaired fasting glucose (100–125 mg/dl) and those with impaired glucose tolerance or FBG ≥126 mg/dl, the incidence of iPRDM increased from 1%, 3% and 19%, respectively (Fig. 1).
Figure 1.
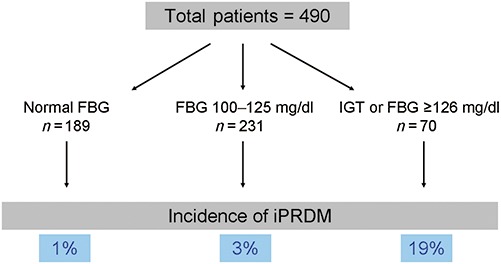
Incidence of immediate post-resection diabetes mellitus after a pancreaticoduodenectomy amongst 490 patients with available pre-operative glucose levels. Overall incidence is 4%. Incidence is low if FBG is normal and increases with abnormal FBG or diagnosis of IGT. FBG, fasting blood glucose; IGT, impaired glucose tolerance
Univariate analysis was performed to identify factors associated with iPRDM (Table 2). Factors with a significant association with iPRDM included male gender (OR 3.88, P= 0.015), pre-operative impaired glucose tolerance (OR 26.11, P < 0.001), pre-operative glucose ≥ 126 mg/dl (OR 20.11, P < 0.001), BMI (5 unit increase, OR 2.0, P < 0.001), pre-operative creatinine (1 unit increase, OR 3.83, P= 0.019), operative time (100 min. increase, OR 1.80, P= 0.013), tumour size (1 cm increase OR, 1.16, P= 0.019) and specimen length (1 cm increase OR, 1.4, P= 0.005). A family history of DM, age, hypertension, indication for surgery, malignancy, laparoscopic approach and pylorus-preserving technique all showed no significant associations with PRDM.
Table 2.
Univariate analysis of potential risk factors for immediate post-resection diabetes mellitus (iPRDM) in patients undergoing a pancreaticoduodenectomy
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Year of operation (1-year increase) | 0.79 (0.62–1.00) | 0.046 |
| Age at operation (10-year increase) | 1.27 (0.88–1.83) | 0.21 |
| Male | 3.88 (1.30–11.62) | 0.015 |
| Pre-operative FBG (20-unit increase) | 1.91 (1.47–2.47) | <0.001 |
| Preoperative FBG | ||
| <100 mg/dl | 1.0 (reference) | |
| 100–125 mg/dl | 3.65 (0.78–17.10) | 0.1 |
| ≥126 mg/dl | 20.11 (4.26–94.87) | <0.001 |
| Impaired glucose tolerance | 26.11 (9.59–71.15) | <0.001 |
| Family history of DM | 0.87 (0.33–2.32) | 0.78 |
| Hypertension | 1.61 (0.68–3.79) | 0.28 |
| BMI (5-unit increase) | 2.00 (1.34–2.99) | <0.001 |
| BMI ≥30 | 2.38 (0.97–5.82) | 0.06 |
| Pre-operative creatinine (1-unit increase) | 3.83 (1.25–11.76) | 0.019 |
| Pre-operative creatinine > 1.2 | 2.67 (0.86–8.32) | 0.09 |
| Laparoscopic approach | 0.61 (0.14–2.68) | 0.52 |
| Pylorus preserving | 0.81 (0.29–2.24) | 0.68 |
| Operative time (100-minute increase) | 1.80 (1.13–2.85) | 0.013 |
| Estimated blood loss (500-cc increase) | 1.21 (0.95–1.54) | 0.12 |
| Transfusion | 1.29 (0.55–3.03) | 0.56 |
| Malignant disease | 0.72 (0.29–1.80) | 0.48 |
| Tumour size (1-cm increase) | 1.16 (1.03–1.32) | 0.019 |
| Specimen length (1-cm increase) | 1.40 (1.11–1.78) | 0.005 |
FBG, fasting blood glucose; DM, diabetes mellitus; BMI, body mass index (kg/m2).
Upon multivariable analysis, only preoperative impaired glucose tolerance (OR 15.5, P < 0.001), pre-operative glucose ≥ 126 (OR 6.43, P < 0.001) and specimen length (OR 1.53, P= 0.002) were independent predictors of iPRDM (Table 3). A predictive model using these three factors demonstrated a c-index of 0.842.
Table 3.
Results of multivariable analysis of factors predictive of immediate post-resection diabetes mellitus (iPRDM) after a pancreaticoduodenectomy
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Impaired glucose tolerance | 15.50 (5.04–47.66) | <0.001 |
| Pre-operative FBG | ||
| ≤125 mg/dl | 1.0 (reference) | |
| ≥126 mg/dl | 6.43 (2.19–18.85) | <0.001 |
| Specimen length (1-cm increase) | 1.53 (1.17–2.01) | 0.002 |
FBG, fasting blood glucose.
Discussion
When counselling patients for a PR, the question is often raised about the risk of developing diabetes post-operatively. The reported incidence of post-resection diabetes mellitus after PD varies in the literature from 0% to 50% and comes from series with various patient populations, small sample size and variable follow-up.1–9 The answer is even more complex acknowledging that the resection itself represents only a portion of the risk, as underlying disease progression, gland atrophy, weight loss or gain, diet and adjuvant chemotherapy or radiation may also contribute to the development of PRDM.
We report a large, single-institutional series of patients undergoing PD where the overall incidence of iPRDM was 4%. This is at the lower end of that reported in most previous studies and is likely accounted for by several factors including a low incidence of patients with chronic pancreatitis, larger sample size and the short-term (30 day) follow-up design of this study. We intentionally limited the evaluation to the first 30 days post-operatively to avoid confounding factors exclusive of resection. This provided the opportunity to attempt to define the independent effect of the loss of pancreatic parenchyma and physiological changes that occur as a result of the pancreatic resection.
The current literature suggests that patients undergoing PD continue to be at risk for diabetes mellitus in the months and years after the operation, whereas conflicting series show no significant changes over time.7,8 Seiler and colleagues reported a 6.4% incidence of iPRDM after PD at 12 months follow-up.9 In a previous study utilizing oral glucose tolerance testing (OGTT) pre-operatively, 3 months post-operatively, and yearly thereafter, Ishikawa and colleagues demonstrated that patients with a normal preoperative OGTT showed no decline over time, whereas those with abnormal OGTT patterns were likely to demonstrate a decline in glucose tolerance.8 These differences may be attributed to the multiple confounding factors independent of PR alone. Long-term procedure-related factors such as stricturing of the pancreatic anastomosis and delayed effects of altered neurohormonal responses may also play a role. In a randomized, controlled trial comparing pancreaticojejunostomy versus pancreatic duct ligation, a two-fold incidence of PRDM was observed in patients with duct ligation.10
In the present study, the incidence of iPRDM was not different for patients with malignant indications for resection compared with those with benign indications. Resection for pancreatic adenocarcinoma may actually have a beneficial effect on ameliorating diabetes or reducing its likelihood, as a modest causal association has been demonstrated between pancreatic cancer and type II diabetes.11 In patients with new-onset diabetes and pancreatic adenocarcinoma, PR has been shown to ameliorate diabetes in more than 50% of patients, suggesting a diabetogenic influence of cancer.12 However, for patients with a diagnosis of chronic pancreatitis, the incidence of PRDM may reflect the underlying disease progression in addition to the effects of resection.2,4
We identified three factors predictive of iPRDM: impaired glucose tolerance, FBG ≥126 mg/dl and increasing specimen length. In this three-factor model, a favourable predictive ability was observed with a c-index of 0.842. The first two of these factors suggest that impaired glucose control increases the risk for diabetes after PR. This has been demonstrated in several smaller series.7,8 It is plausible that in this ‘pre-diabetic’ state, further loss of insulin, glucagon and the altered neurohormonal response to glucose induced by resection would increase the risk of progressing to diabetes mellitus.13–15 Alternatively, these diagnoses also suggest the possibility that some patients in this and other series my actually have diabetes mellitus but are lacking a formal evaluation and diagnosis. We acknowledge this as a limitation in this study as we were unable to identify clear documentation that these patients had a formal diabetes evaluation; this is in part reflective of our tertiary practice where patients' primary care evaluations may have been done elsewhere.
According to the most recent publication of American Diabetes Association (ADA), a FBG of ≥126 mg/dl is an independent criteria for the diagnosis of DM.16 In the present study, if a patient had a new finding of elevated FBG yet no other evaluation or clinical documentation of DM, we elected to include them in this study. The rationale for this is that the ADA criteria have largely been established in asymptomatic patients without pancreatic disease requiring operation, whereas many of the patients in our cohort had possible confounding factors such as obstructing pancreatic lesions, recent endoscopic procedures, biliary stenting or cholangitis at the time of the FBG measurement. Furthermore, justification of including these patients in this study is that a minority of these patients had persistently elevated FBG post-operatively. It is also noteworthy that this group was less likely to develop iPRDM than those with impaired glucose tolerance (OR 6.4 vs. 48.8). When grouped together, the incidence of iPRDM for a patient with either condition was 19%.
With the possibility of including patients with undiagnosed DM in this study, one would expect the observed incidence of iPRDM of 4% to be an overestimate of the true risk of iPRDM after PD. Perhaps the most important and useful finding in this study is that for patients with a normal pre-operative FBG, the incidence of iPRDM is only 1%.
As of 2010, the ADA has included an HbgA1c of ≥6.5% as an independent criterion for establishing the diagnosis of DM.17 During the time period of this study, HbgA1c was not a standard part of the pre-operative evaluation of patients with elevated FBG and therefore could not be evaluated as a predictor of iPRDM. It has been shown that an elevated HgbA1C is predictive of PRDM with a positive predictive value, negative predictive value, sensitivity and specificity of 82%, 94%, 90% and 88%, respectively.18 Importantly, these authors reported the incidence of PRDM to be very low if the HgbA1c is normal pre-operatively.
Increasing specimen length was also identified as a predictor of iPRDM in our patient cohort. We did not evaluate remnant pancreatic volume in this study, but it is intuitive that patients with a proportionally large pancreatic head or that required transection into the pancreatic body would be at an increased risk for PRDM.
We identified several other factors associated with iPRDM on univariate analysis such as elevated BMI and creatinine levels that are known risk factors for the development of DM in the general population, yet these failed to demonstrate any independent predictive ability for iPRDM. However, the low incidence of iPRDM in this study limits the ability to perform a more complex statistical evaluation and modelling of predictive factors.
In summary, iPRDM occurs in 4% of all patients undergoing PD. Factors predictive of iPRDM include impaired glucose tolerance, elevated glucose and increasing specimen length. The last two factors are easily determined pre-operatively and represent patients at risk for progression to DM as well as patients with potentially undiagnosed new-onset DM. The incidence of iPRDM in patients with normal pre-operative FBG levels is only 1%; this information is helpful for surgeons and their patients when considering PD as a treatment option for pancreatic disease. Further studies to address the multifactorial aetiology of delayed onset DM after PR are needed.
Conflicts of interest
None declared.
References
- 1.Lemaire E, O'Toole D, Suvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434–438. doi: 10.1046/j.1365-2168.2000.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Riediger H, Adam U, Fischer E, Keck T, Pfefer F, Hopt UT. Long-term outcome after resection for chronic Pancreatitis in 224 Patients. J Gastrointest Surg. 2007;11:949–960. doi: 10.1007/s11605-007-0155-6. et al. [DOI] [PubMed] [Google Scholar]
- 3.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez RE, Fernandez-del Castillo C, Rattner DW, Change Y, Warshaw AL. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300. doi: 10.1097/00000658-200003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone WM, Sarr MD, Nagorney DM, McIlrath DC. Chronic pancreatitis. Results of Whipple's resection and total pancreatectomy. Arch Surg. 1988;123:815–819. doi: 10.1001/archsurg.1988.01400310029004. [DOI] [PubMed] [Google Scholar]
- 6.Sohn T, Campbell K, Pitt H, Sauter PK, Coleman J, Lillemoe KD. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg. 2000;4:355–365. doi: 10.1016/s1091-255x(00)80013-x. et al. [DOI] [PubMed] [Google Scholar]
- 7.Litwin J, Dobrowolski S, Orlowska-Kunikowska E, Sledzinski Z. Changes in glucose metabolism after kausch-whipple pancreatectomy in pancreatic cancer and chronic pancreatitis patients. Pancreas. 2008;36:26–30. doi: 10.1097/mpa.0b013e318137aa61. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa O, Ohigashi H, Eguchi H, Yokoyama S, Yamada T, Takachi K. Long-term follow-up of glucose tolerance function after pancreaticoduodenectomy: comparison between pancreaticogastrostomy and pancreaticojejunostomy. Surgery. 2004;136:617–623. doi: 10.1016/j.surg.2004.01.006. et al. [DOI] [PubMed] [Google Scholar]
- 9.Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection – long term results. Br J Surg. 2005;92:547–556. doi: 10.1002/bjs.4881. et al. [DOI] [PubMed] [Google Scholar]
- 10.Tran K, Van Eijck C, Di Carlo V, Hop WC, Zerbi A, Balzano G. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422–428. doi: 10.1097/00000658-200210000-00004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevelance and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrader H, Menge BA, Breuer TG, Ritter PR, Uhl W, Schmidt WE. Impaired glucose-induced glucagon suppression after partial pancreatectomy. J Clin Endocrinol Metab. 2009;94:2857–2863. doi: 10.1210/jc.2009-0826. et al. [DOI] [PubMed] [Google Scholar]
- 14.Menge BA, Schrader H, Breuer TGK, Dabrowski Y, Uhl W, Schmidt W. Metabolic consequences of a 50% partial pancreatectomy in humans. Diabetologia. 2009;52:306–307. doi: 10.1007/s00125-008-1219-1. et al. [DOI] [PubMed] [Google Scholar]
- 15.Slezac A, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes – 2012 position statement. Diabetes Care. 2012;25(1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;34(1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton L, Jeyarajah DR. HbA1c can be helpful in predicting progression to diabetes after Whipple procedure. HPB. 2007;9:26–28. doi: 10.1080/13651820600917286. [DOI] [PMC free article] [PubMed] [Google Scholar]


